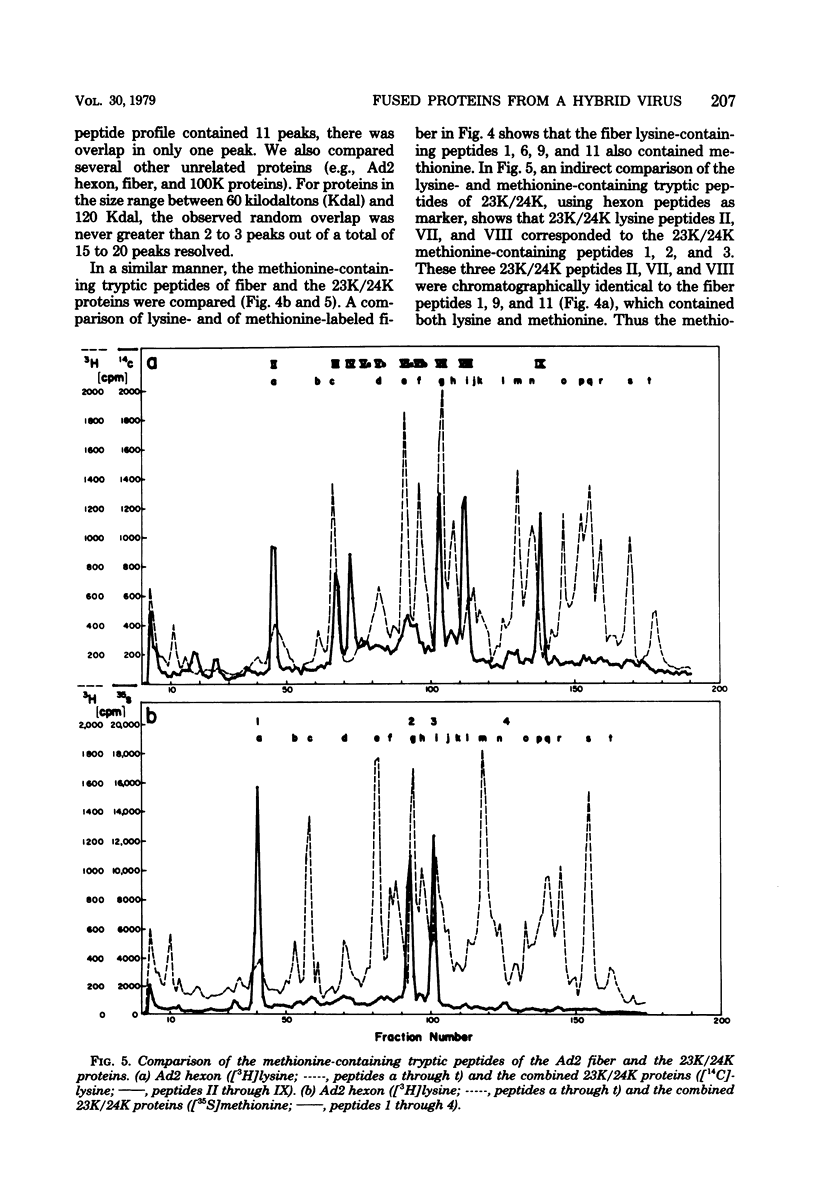
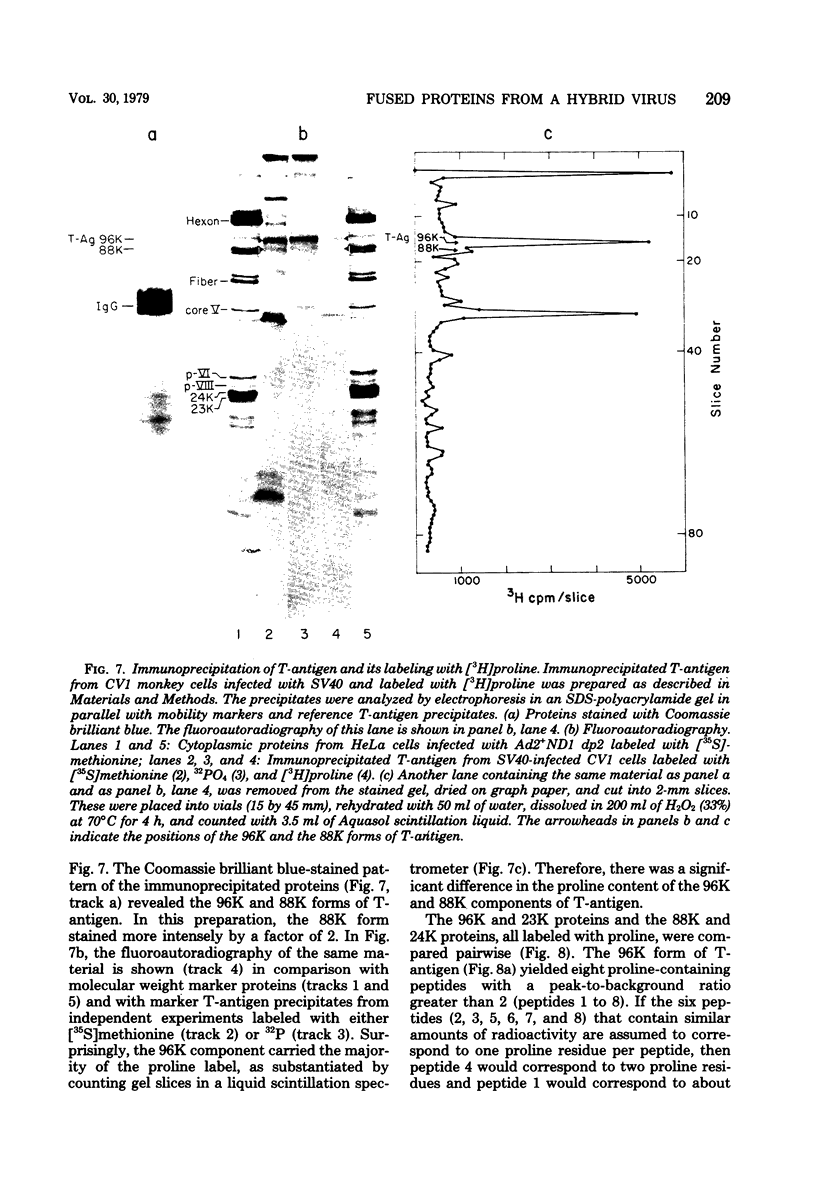
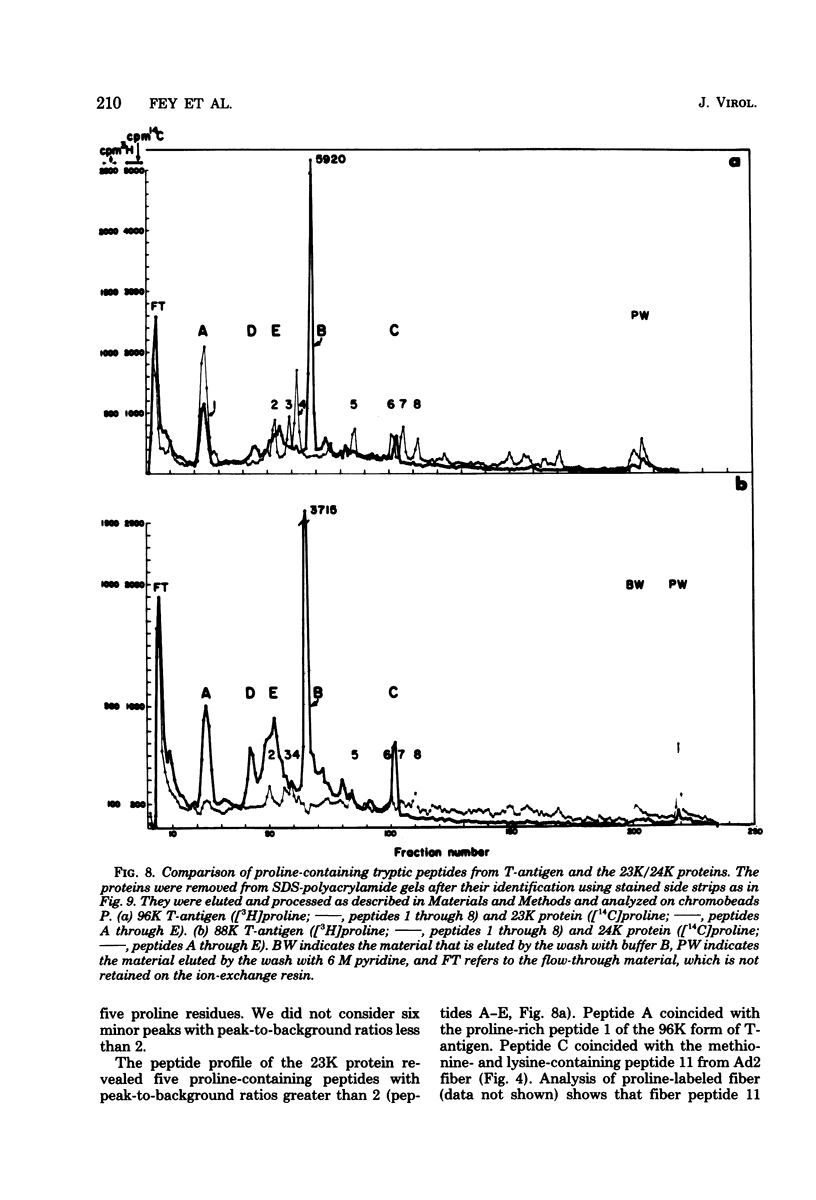
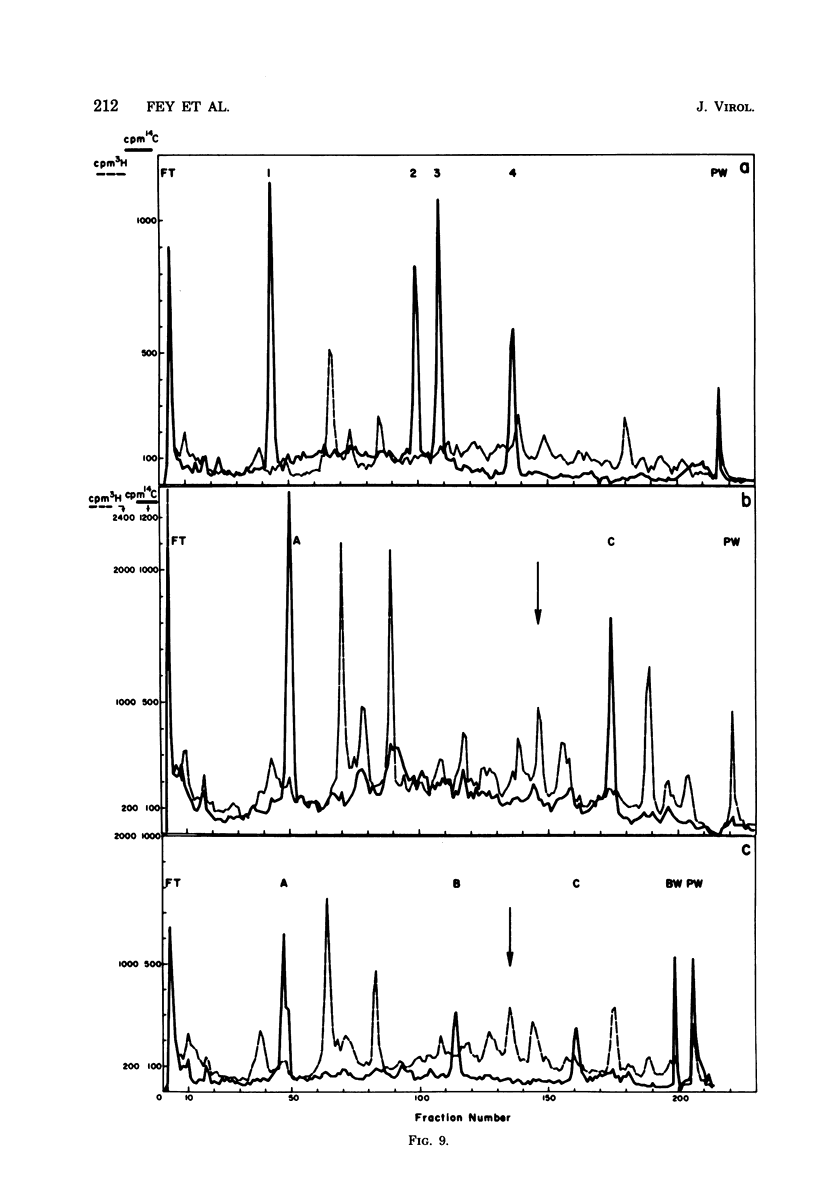
Abstract
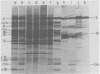
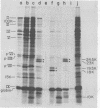
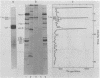
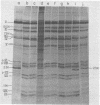
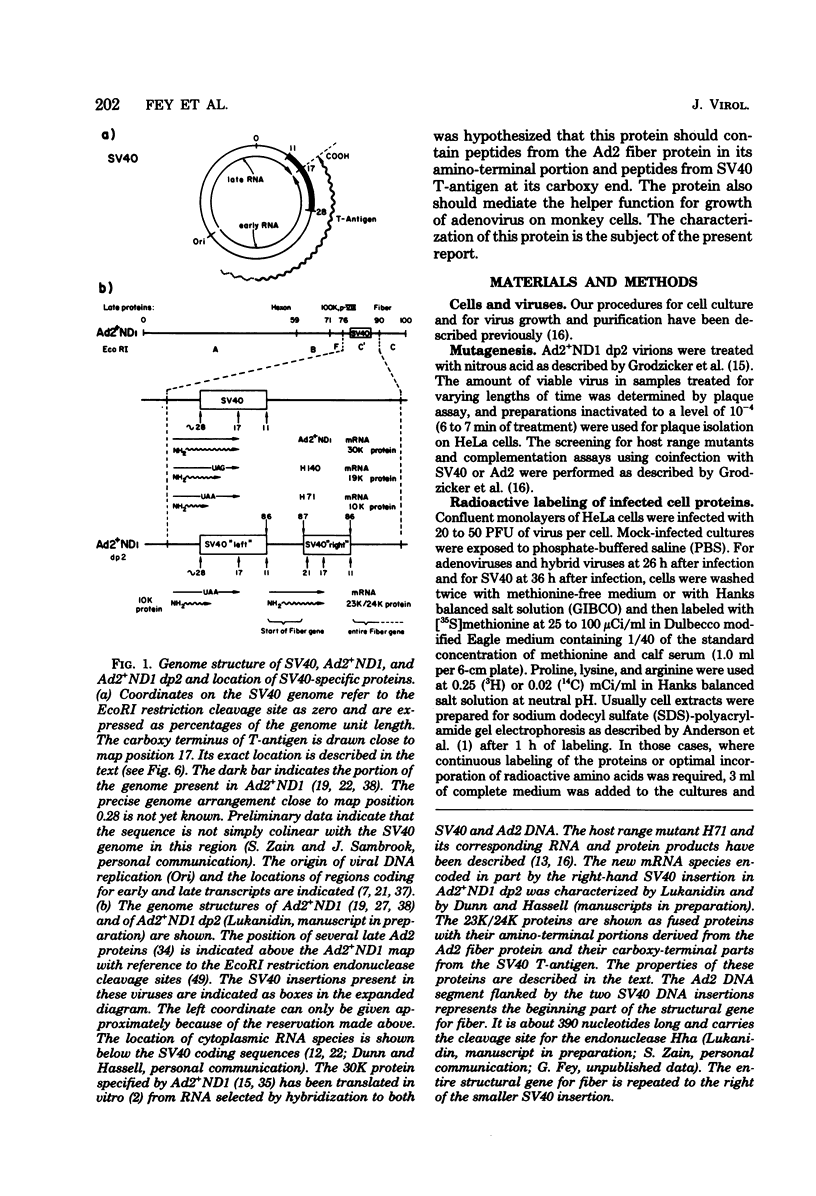
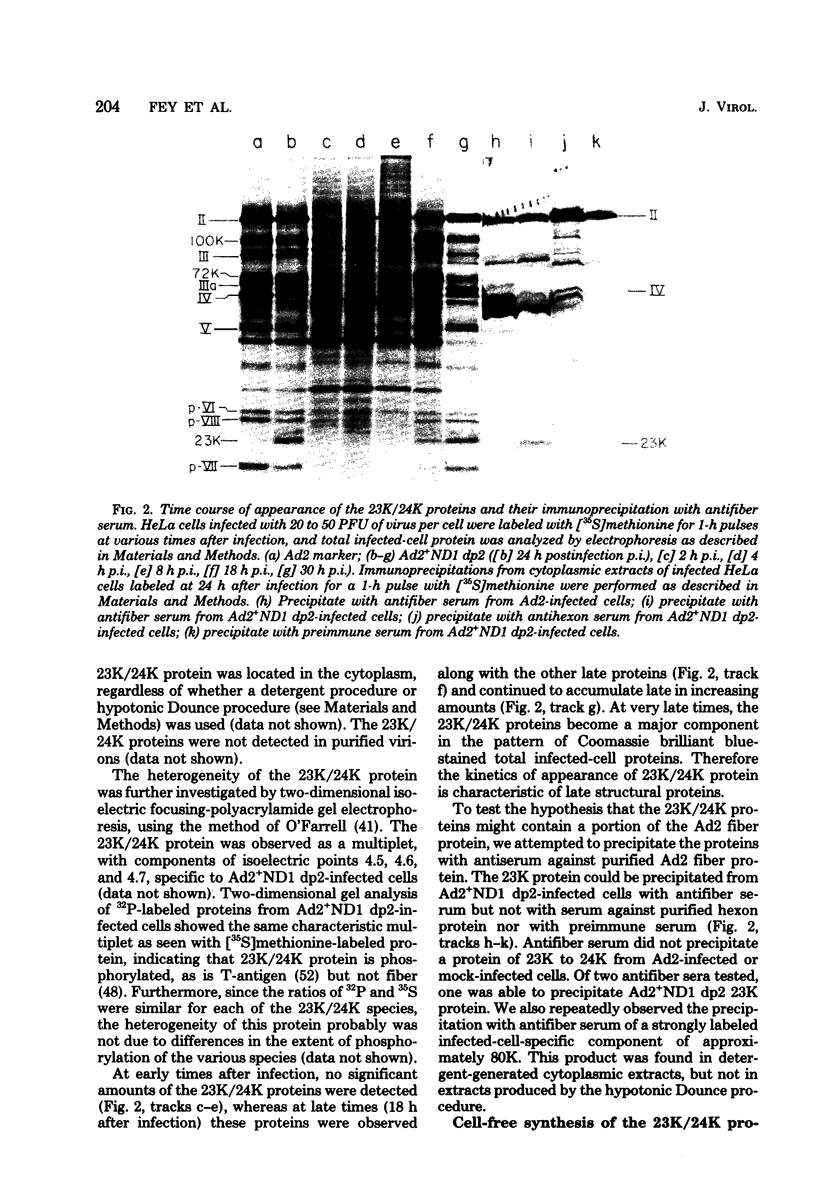
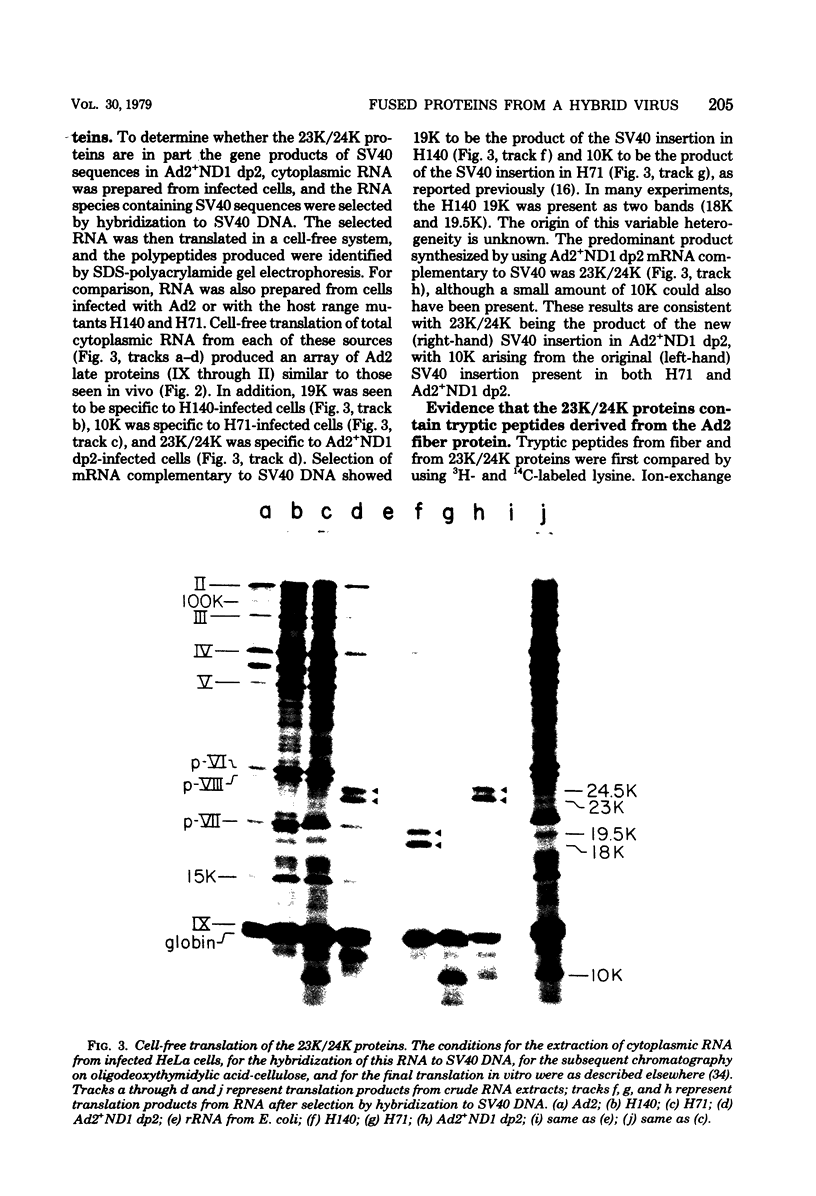
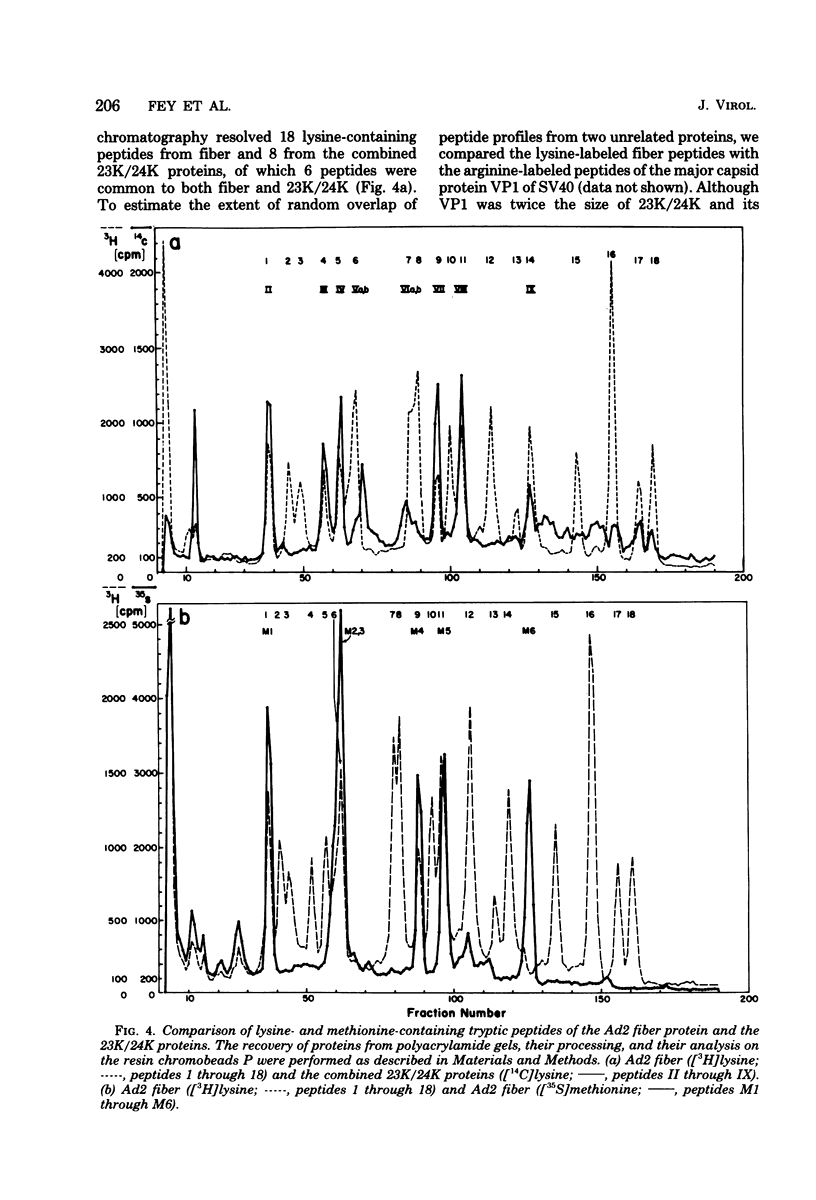
The adenovirus type 2-simian virus 40 (SV40) hybrid virus Ad2+ND1 dp2 (E. Lukanidin, manuscript in preparation) specified two proteins (molecular weights, 24,000 and 23,000) that are, in part, products of an insertion of SV40 early DNA sequences. This was demonstrated by translation in vitro from viral mRNA that had been selected by hybridization to SV40 DNA. These two phosphorylated, nonvirion proteins were produced late in infection in amounts similar to adenovirus 2 structural proteins and were closely related to each other in tryptic peptide composition. The portion of SV40 DNA (map units 0.17 to 0.22 on the SV40 genome) coding for these proteins was joined to sequences coding for the amino-terminal part of the adenovirus type 2 structural protein IV (fiber). The Ad2+ND1 dp2 23,000- and 24,000-molecular-weight proteins were hybrid polypeptides, with about two-thirds of their tryptic peptides contributed by the fiber protein and the remainder contributed by SV40 T-antigen. They shared with T-antigen (molecular weight, 96,000) a carboxy-terminal proline-rich tryptic peptide. Together, the tryptic peptide composition of these proteins and the known SV40 DNA sequences suggested the reading frame for the translation of T-antigen. The carboxy terminus for T-anigen would then be located on the SV40 genome map next to the TAA terminator triplet at position 0.175, 910 bases away from the cleavage site of the restriction endonuclease EcoRI. Seven host range mutants from Ad2+ND1 dp2 were isolated that had lost the capacity to propagate on monkey cells. They did not induce detectable levels of the hybrid proteins. Three of these mutants had lost the SV40 DNA insertion that codes in part for these proteins. Thus, in analogy to the Ad2+ND1 30,000-molecular-weight protein, the presence of these proteins correlates with the presence of the helper function for adenovirus replication on monkey cells.
Full text
PDF




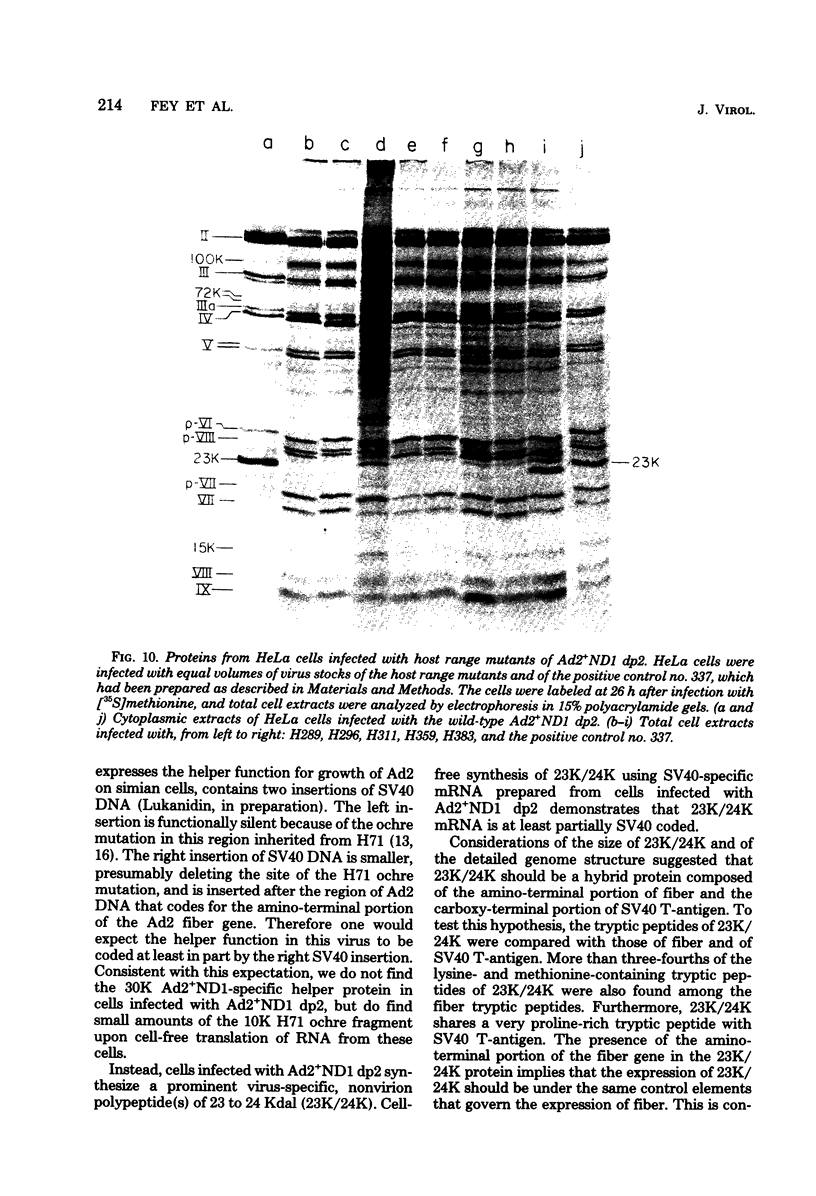



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Lewis J. B., Baum P. R., Gesteland R. F. Simian virus 40-specific polypeptides in AD2+ ND1- and Ad2+ ND4-infected cells. J Virol. 1976 May;18(2):685–692. doi: 10.1128/jvi.18.2.685-692.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum S. G., Horwitz M. S., Maizel J. V., Jr Studies of the mechanism of enhancement of human adenovirus infection in monkey cells by simian virus 40. J Virol. 1972 Aug;10(2):211–219. doi: 10.1128/jvi.10.2.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Hager L., Dulbecco R. Simian virus 40 T antigen binds to DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3754–3757. doi: 10.1073/pnas.71.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. B., Smith A. E. Monomer molecular weight of T antigen from simian virus 40-infected and transformed cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2254–2258. doi: 10.1073/pnas.73.7.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Weissman S. M., Zain B. S., Pan J., Lewis A. M., Jr The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 2. The sequence of the early strand transcript. Nucleic Acids Res. 1974 Apr;1(4):595–611. doi: 10.1093/nar/1.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A. R., Hassell J. A. A novel method to map transcripts: evidence for homology between an adenovirus mRNA and discrete multiple regions of the viral genome. Cell. 1977 Sep;12(1):23–36. doi: 10.1016/0092-8674(77)90182-9. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Gall W. E. The antibody problem. Annu Rev Biochem. 1969;38:415–466. doi: 10.1146/annurev.bi.38.070169.002215. [DOI] [PubMed] [Google Scholar]
- Fey G., Hirt B. Fingerprints of polyoma virus proteins and mouse histones. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):235–241. doi: 10.1101/sqb.1974.039.01.030. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Wewerka-Lutz Y., Levine A. S., Sambrook J., Sharp P. A. Adenovirus transcription. II. RNA sequences complementary to simian virus 40 and adenovirus 2DNA in AD2+ND1- and AD2+ND3-infected cells. J Virol. 1975 Sep;16(3):662–673. doi: 10.1128/jvi.16.3.662-673.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesteland R. F., Wills N., Lewis J. B., Grodzicker T. Identification of amber and ochre mutants of the human virus Ad2+ND1. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4567–4571. doi: 10.1073/pnas.74.10.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicker T., Anderson C., Sharp P. A., Sambrook J. Conditional lethal mutants of adenovirus 2-simian virus 40 hybrids. I. Host range mutants of Ad2+ND1. J Virol. 1974 Jun;13(6):1237–1244. doi: 10.1128/jvi.13.6.1237-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicker T., Lewis J. B., Anderson C. W. Conditional lethal mutants of adenovirus type 2-simian virus 40 hybrids. II. Ad2+ND1 host-range mutants that synthesize fragments of the Ad2+ND1 30K protein. J Virol. 1976 Aug;19(2):559–571. doi: 10.1128/jvi.19.2.559-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Nakajima K., Oda K., Shimojo H. Complementation of translational defect for growth of human adenovirus type 2 in Simian cells by a Simian virus 40-induced factor. J Mol Biol. 1973 Dec 5;81(2):207–223. doi: 10.1016/0022-2836(73)90190-3. [DOI] [PubMed] [Google Scholar]
- Hassell J. A., Lukanidin E., Fey G., Sambrook J. The structure and expression of two defective adenovirus 2/simian virus 40 hybrids. J Mol Biol. 1978 Apr 5;120(2):209–247. doi: 10.1016/0022-2836(78)90065-7. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr, Levine A. S., Siegel S. Structure of two adenovirus-simian virus 40 hybrids which contain the entire SV40 genome. J Mol Biol. 1974 Oct 15;89(1):113–126. doi: 10.1016/0022-2836(74)90165-x. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lewis A. M., Jr Use of nondefective adenovirus-simian virus 40 hybrids for mapping the simian virus 40 genome. J Virol. 1973 Sep;12(3):643–652. doi: 10.1128/jvi.12.3.643-652.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Howley P., Nathans D., Martin M. Posttranscriptional selection of simian virus 40-specific RNA. J Virol. 1975 Feb;15(2):433–437. doi: 10.1128/jvi.15.2.433-437.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Lewis A. M., Jr, Oxman M. N., Levine A. S. Strand orientation of SV40 transcription in cells infected by non-defective adenovirus 2-SV40 hybrid viruses. Nat New Biol. 1973 Dec 19;246(155):202–205. doi: 10.1038/newbio246202a0. [DOI] [PubMed] [Google Scholar]
- Kirschner K., Bisswanger H. Multifunctional proteins. Annu Rev Biochem. 1976;45:143–166. doi: 10.1146/annurev.bi.45.070176.001043. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Anderson C. W. Block to multiplication of adenovirus serotype 2 in monkey cells. J Virol. 1975 Dec;16(6):1650–1668. doi: 10.1128/jvi.16.6.1650-1668.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lebowitz P., Kelly T. J., Jr, Nathans D., Lee T. N., Lewis A. M., Jr A colinear map relating the simian virus 40 (SV40) DNA segments of six adenovirus-SV40 hybrids to the DNA fragments produced by restriction endonuclease cleavage of SV40 DNA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):441–445. doi: 10.1073/pnas.71.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Baum S. G., Prigge K. O., Rowe W. P. Occurrence of adenovirus-SV40 hybrids among monkey kidney cell adapted strains of adenovirus. Proc Soc Exp Biol Med. 1966 May;122(1):214–218. doi: 10.3181/00379727-122-31093. [DOI] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Levin M. J., Wiese W. H., Crumpacker C. S., Henry P. H. A nondefective (competent) adenovirus-SV40 hybrid isolated from the AD.2-SV40 hybrid population. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1128–1135. doi: 10.1073/pnas.63.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Levine A. S., Crumpacker C. S., Levin M. J., Samaha R. J., Henry P. H. Studies of nondefective adenovirus 2-simian virus 40 hybrid viruses. V. Isolation of additional hybrids which differ in their simian virus 40-specific biological properties. J Virol. 1973 May;11(5):655–664. doi: 10.1128/jvi.11.5.655-664.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Prigge K. O., Rowe W. P. Studies of adenovirus-SV40 hybrid viruses. IV. An adenovirus type 2 strain carrying the infectious SV40 genome. Proc Natl Acad Sci U S A. 1966 Mar;55(3):526–531. doi: 10.1073/pnas.55.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rabson A. S., Levine A. S. Studies of nondefective adenovirus 2-simian virus 40 hybrid viruses. Transformation of hamster kidney cells by adenovirus 2 and the nondefective hybrid viruses. J Virol. 1974 Jun;13(6):1291–1301. doi: 10.1128/jvi.13.6.1291-1301.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rowe W. P. Studies of nondefective Adenovirus 2-Simian virus 40 hybrid viruses. 8. Association of Simian virus 40 transplantation antigen with a specific region of the early viral genome. J Virol. 1973 Oct;12(4):836–840. doi: 10.1128/jvi.12.4.836-840.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Revilla R., Walter G. Polypeptide specific for cells with adenovirus 2-SV40 hybrid Ad2+ND1. Nat New Biol. 1973 Aug 8;244(136):165–167. doi: 10.1038/newbio244165a0. [DOI] [PubMed] [Google Scholar]
- Mann K., Hunter T., Walter G., Linke H. Evidence for simian virus 40 (SV40) coding of SV40 T-antigen and the SV40-specific proteins in HeLa cells infected with nondefective adenovirus type 2-SV40 hybrid viruses. J Virol. 1977 Oct;24(1):151–169. doi: 10.1128/jvi.24.1.151-169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., Maizel J. V., Salzman N. P. Mapping of transcription sites of simian virus 40-specific late 16S and 19S mRNA by electron microscopy. Proc Natl Acad Sci U S A. 1977 Feb;74(2):496–500. doi: 10.1073/pnas.74.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Berg P., Kelly T. J., Jr, Lewis A. M., Jr Mapping of simian virus 40 early functions on the viral chromosome. J Virol. 1973 Sep;12(3):653–658. doi: 10.1128/jvi.12.3.653-658.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C., Graessmann A., Graessmann M. Mapping of early SV40-specific functions by microinjection of different early viral DNA fragments. Cell. 1978 Oct;15(2):579–585. doi: 10.1016/0092-8674(78)90026-0. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Kania J. Lac repressor can be fused to beta-galactosidase. Nature. 1974 Jun 7;249(457):561–563. doi: 10.1038/249561a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON A. S., O'CONOR G. T., BEREZESKY I. K., PAUL F. J. ENHANCEMENT OF ADENOVIRUS GROWTH IN AFRICAN GREEN MONKEY KIDNEY CELL CULTURES BY SV40. Proc Soc Exp Biol Med. 1964 May;116:187–190. doi: 10.3181/00379727-116-29197. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Rundell K., Collins J. K., Tegtmeyer P., Ozer H. L., Lai C. J., Nathans D. Identification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):636–646. doi: 10.1128/jvi.21.2.636-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Blair G. E. Polypeptide phosphorylation in adenovirus-infected cells. J Gen Virol. 1977 Jan;34(1):19–35. doi: 10.1099/0022-1317-34-1-19. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Williams J., Sharp P. A., Grodzicker T. Physical mapping of temperature-sensitive mutations of adenoviruses. J Mol Biol. 1975 Sep 25;97(3):369–390. doi: 10.1016/s0022-2836(75)80046-5. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Casadaban M. J., Shuman H. A., Beckwith J. R. Conversion of beta-galactosidase to a membrane-bound state by gene fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3423–3427. doi: 10.1073/pnas.73.10.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Walter G., Martin H. Simian virus 40-specific proteins in HeLa cells infected with nondefective adenovirus 2-simian virus 40 hybrid viruses. J Virol. 1975 Nov;16(5):1236–1247. doi: 10.1128/jvi.16.5.1236-1247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]