Abstract
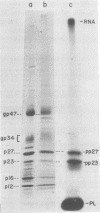
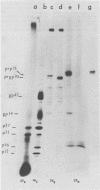
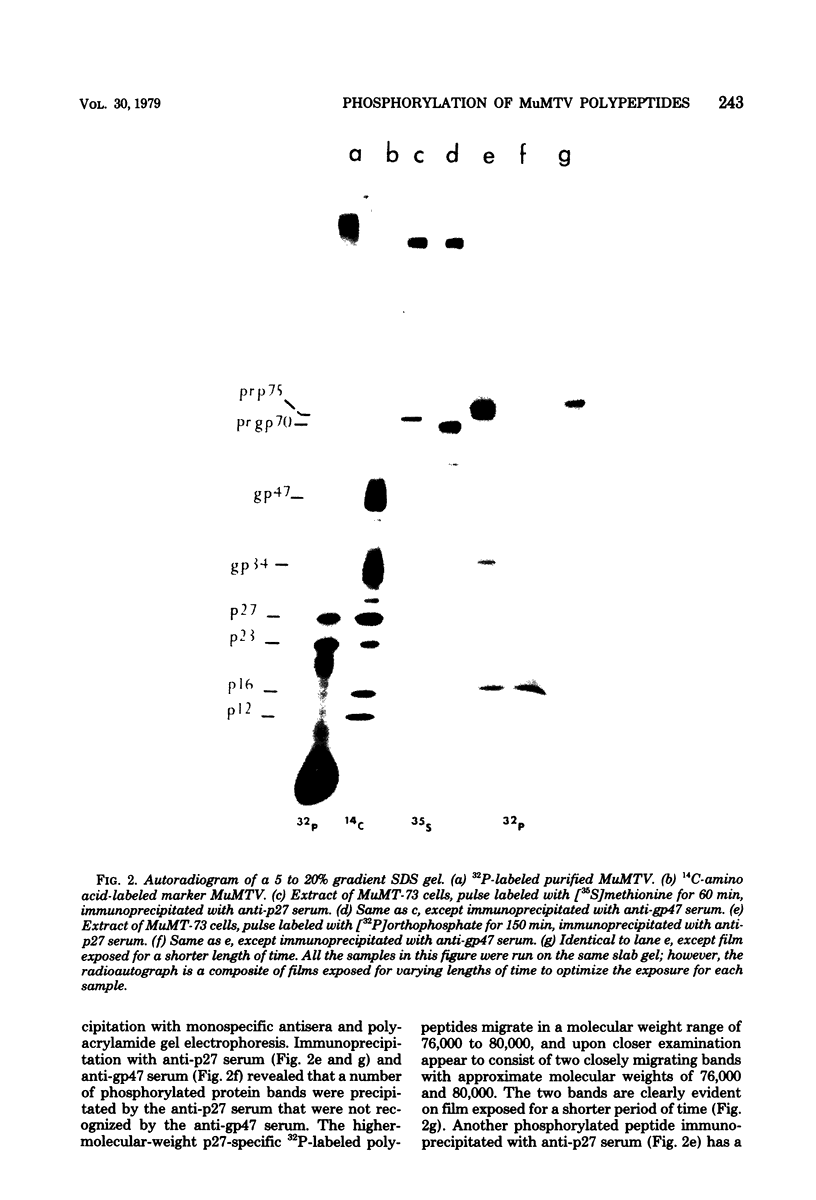
Phosphorylation of the murine mammary tumor virus (MuMTV) structural proteins was studied in an MuMTV-infected epithelial cell line derived from a BALB/cf C3H mouse mammary tumor. Immunoprecipitation of 32P-labeled cell extracts with monospecific anti-p27 serum revealed that phosphorylation occurred at the stage of the core-protein polyprotein precursor prp75. Two forms of phosphorylated prp75 were found: one migrating with an apparent molecular weight of 80,000, and the other with a molecular weight of 76,000. The 80,000-molecular-weight species was found to be the most heavily phosphorylated. In addition, a relatively stable phosphorylated processing intermediate of 34,000 molecular weight was observed as well. Tryptic peptide mapping analysis of the 32P-labeled viral proteins indicated a precursor product relationship between the intracellular phosphorylated, high-molecular-weight peptides and the mature MuMTV phosphoproteins p23 and p27. Phosphopeptide analysis also suggested that phosphorylation of the viral proteins occurred in discrete steps and that the attached phosphate groups were conserved throughout the processing steps.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod N. Phosphoproteins of adenovirus 2. Virology. 1978 Jun 15;87(2):366–383. doi: 10.1016/0042-6822(78)90141-1. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Polyproteins related to the major core protein of mouse mammary tumor virus. J Virol. 1978 Jun;26(3):660–672. doi: 10.1128/jvi.26.3.660-672.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Erikson E., Brugge J. S., Erikson R. L. Phosphorylated and nonphosphorylated forms of avian sarcoma virus polypeptide p19. Virology. 1977 Jul 1;80(1):177–185. doi: 10.1016/0042-6822(77)90390-7. [DOI] [PubMed] [Google Scholar]
- Hayman E. G., Pal B. K., Roy-Burman P. RNA tumour virus phosphoproteins: evidence for virus specificity of phosphorylation. J Gen Virol. 1977 Sep;36(3):459–469. doi: 10.1099/0022-1317-36-3-459. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Lai M. M. Phosphoproteins of Rous sarcoma viruses. Virology. 1976 Oct 15;74(2):287–301. doi: 10.1016/0042-6822(76)90336-6. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Marcus S. L., Smith S. W., Racevskis J., Sarkar N. H. The relative hydrophobicity of oncornaviral structural proteins. Virology. 1978 May 15;86(2):398–412. doi: 10.1016/0042-6822(78)90080-6. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Hackett A. J. Tissue culture studies of mouse mammary tumor cells and associated viruses. J Natl Cancer Inst. 1972 Nov;49(5):1321–1332. [PubMed] [Google Scholar]
- Pal B. K., McAllister R. M., Gardner M. B., Roy-Burman P. Comparative studies on the structural phosphoproteins of mammalian type C viruses. J Virol. 1975 Jul;16(1):123–131. doi: 10.1128/jvi.16.1.123-131.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal B. K., Roy-Burman P. Phosphoproteins: structural components of oncornaviruses. J Virol. 1975 Mar;15(3):540–549. doi: 10.1128/jvi.15.3.540-549.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal B. K., Roy-Burman P. RNA tumor virus phosphoproteins: phosphorylation of precursor and processed polypeptides. Biochem Biophys Res Commun. 1978 Mar 30;81(2):344–350. doi: 10.1016/0006-291x(78)91539-5. [DOI] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. Synthesis and processing of precursor polypeptides to murine mammary tumor virus structural proteins. J Virol. 1978 Jan;25(1):374–383. doi: 10.1128/jvi.25.1.374-383.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Pomenti A. A., Dion A. S. Replication of mouse mammary tumor virus in tissue culture. 1. Establishment of a mouse mammary tumor cell line, virus characterization, and quantitation of virus production. Virology. 1977 Mar;77(1):12–30. doi: 10.1016/0042-6822(77)90402-0. [DOI] [PubMed] [Google Scholar]
- Schochetman G., Long C. W., Oroszlan S., Arthur L., Fine D. L. Isolation of separate precursor polypeptides for the mouse mammary tumor virus glycoproteins and nonglycoproteins. Virology. 1978 Mar;85(1):168–174. doi: 10.1016/0042-6822(78)90421-x. [DOI] [PubMed] [Google Scholar]
- Sen A., Sherr C. J., Todaro G. J. Phosphorylation of murine type C viral p12 proteins regulates their extent of binding to the homologous viral RNA. Cell. 1977 Mar;10(3):489–496. doi: 10.1016/0092-8674(77)90036-8. [DOI] [PubMed] [Google Scholar]
- Strausbauch P., Sulica A., Givol D. General method for the detection of cells producing antibodies against haptens and proteins. Nature. 1970 Jul 4;227(5253):68–69. doi: 10.1038/227068a0. [DOI] [PubMed] [Google Scholar]