Abstract
Maximal exercise vasodilatation results from the balance between vasoconstricting and vasodilating signals combined with the vascular reactivity to these signals. During maximal exercise with a small muscle mass the skeletal muscle vascular bed is fully vasodilated. During maximal whole body exercise, however, vasodilatation is restrained by the sympathetic system. This is necessary to avoid hypotension since the maximal vascular conductance of the musculature exceeds the maximal pumping capacity of the heart. Endurance training and high-intensity intermittent knee extension training increase the capacity for maximal exercise vasodilatation by 20–30%, mainly due to an enhanced vasodilatory capacity, as maximal exercise perfusion pressure changes little with training. The increase in maximal exercise vascular conductance is to a large extent explained by skeletal muscle hypertrophy and vascular remodelling. The vasodilatory capacity during maximal exercise is reduced or blunted with ageing, as well as in chronic heart failure patients and chronically hypoxic humans; reduced vasodilatory responsiveness and increased sympathetic activity (and probably, altered sympatholysis) are potential mechanisms accounting for this effect. Pharmacological counteraction of the sympathetic restraint may result in lower perfusion pressure and reduced oxygen extraction by the exercising muscles. However, at the same time fast inhibition of the chemoreflex in maximally exercising humans may result in increased vasodilatation, further confirming a restraining role of the sympathetic nervous system on exercise-induced vasodilatation. This is likely to be critical for the maintenance of blood pressure in exercising patients with a limited heart pump capacity.
 |
Jose A. L. Calbet (left) is a professor on Exercise Physiology working in the Department of Physical Education at the University of Las Palmas de Gran Canaria (Spain). His main research interests are on oxygen transport and skeletal muscle signaling. He is an MD with great experience on invasive human experiments addressing cardiovascular regulation mechanisms in humans. Carsten Lundby (right) is leading the ‘O2 transport and utilization’ group at the Institute of Physiology, University of Zürich. His main interests include physiological responses/adaptations to exercise and hypoxia.
Introduction
During low intensity exercise muscle blood flow increases proportionally to the oxygen demand and experiments manipulating arterial oxygen concentration (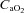 ) have demonstrated that, in general, muscle blood flow is regulated to match O2 delivery with O2 demand (Saltin et al. 1998; Roach et al. 1999; Gonzalez-Alonso et al. 2006). This is achieved by increasing vasodilatation when
) have demonstrated that, in general, muscle blood flow is regulated to match O2 delivery with O2 demand (Saltin et al. 1998; Roach et al. 1999; Gonzalez-Alonso et al. 2006). This is achieved by increasing vasodilatation when 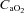 is reduced by hypoxia (Koskolou et al. 1997a; Calbet et al. 2003), isovolaemic anaemia (Koskolou et al. 1997b), hypervolaemic haemodilution (Calbet et al. 2004b) or carbon monoxide breathing (Gonzalez-Alonso et al. 2001) while at the same time essentially maintaining perfusion pressure with increasing cardiac outputs. Conversely, when
is reduced by hypoxia (Koskolou et al. 1997a; Calbet et al. 2003), isovolaemic anaemia (Koskolou et al. 1997b), hypervolaemic haemodilution (Calbet et al. 2004b) or carbon monoxide breathing (Gonzalez-Alonso et al. 2001) while at the same time essentially maintaining perfusion pressure with increasing cardiac outputs. Conversely, when 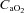 is increased by erythrocytic autologous transfusion (Gonzalez-Alonso et al. 2006) or hyperbaric hyperoxia (Casey et al. 2011), muscle blood flow is reduced during submaximal exercise and thus leaves convective O2 delivery unaffected. In some circumstances, the blood flow response to exercise may be influenced by O2 diffusive limitations, which have been extensively treated elsewhere (Wagner, 1988; Calbet & Lundby, 2009; Calbet et al. 2009). When during submaximal exercise both acute isovolaemic anaemia and hypoxia are combined to reduce arterial
is increased by erythrocytic autologous transfusion (Gonzalez-Alonso et al. 2006) or hyperbaric hyperoxia (Casey et al. 2011), muscle blood flow is reduced during submaximal exercise and thus leaves convective O2 delivery unaffected. In some circumstances, the blood flow response to exercise may be influenced by O2 diffusive limitations, which have been extensively treated elsewhere (Wagner, 1988; Calbet & Lundby, 2009; Calbet et al. 2009). When during submaximal exercise both acute isovolaemic anaemia and hypoxia are combined to reduce arterial 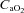 by ∼60%, muscle blood flow is increased by almost ∼45% and the muscular
by ∼60%, muscle blood flow is increased by almost ∼45% and the muscular 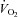 is maintained by increasing O2 extraction (Roach et al. 1999). However, in humans with a limited heart pumping capacity, as for example in patients with heart failure, vasodilatation may be restrained by a sympathetically mediated mechanism to avoid hypotension (Piepoli et al. 1996; Crisafulli et al. 2007), which may be mediated by muscle mechanoreceptors (Middlekauff & Chiu, 2004; Amann et al. 2010). Another exception to this mechanism is observed in patients with mitochondrial or metabolic myopathies. In these patients
is maintained by increasing O2 extraction (Roach et al. 1999). However, in humans with a limited heart pumping capacity, as for example in patients with heart failure, vasodilatation may be restrained by a sympathetically mediated mechanism to avoid hypotension (Piepoli et al. 1996; Crisafulli et al. 2007), which may be mediated by muscle mechanoreceptors (Middlekauff & Chiu, 2004; Amann et al. 2010). Another exception to this mechanism is observed in patients with mitochondrial or metabolic myopathies. In these patients 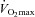 is reduced and the anaerobic contribution to energy production is high already with low intensity exercise, and muscle blood flow and cardiac output are increased disproportionally to the oxygen demand (Taivassalo et al. 2003; Jeppesen et al. 2012). Consequently the degree of vasodilatation per work load is greater in patients with mitochondrial myopathies than in healthy controls. However, maximal exercise vasodilatation is also restrained in these patients (Jeppesen et al. 2012). Therefore, the magnitude of muscular vasodilatation during submaximal exercise results from the interplay between oxygen delivery, oxygen demand, muscle metabolism, the pumping capacity of heart, and sympathetic activity. The same factors combined with the maximal conductance permitted by the vascular structure when fully vasodilated may contribute to limit maximal muscular hyperaemia in humans.
is reduced and the anaerobic contribution to energy production is high already with low intensity exercise, and muscle blood flow and cardiac output are increased disproportionally to the oxygen demand (Taivassalo et al. 2003; Jeppesen et al. 2012). Consequently the degree of vasodilatation per work load is greater in patients with mitochondrial myopathies than in healthy controls. However, maximal exercise vasodilatation is also restrained in these patients (Jeppesen et al. 2012). Therefore, the magnitude of muscular vasodilatation during submaximal exercise results from the interplay between oxygen delivery, oxygen demand, muscle metabolism, the pumping capacity of heart, and sympathetic activity. The same factors combined with the maximal conductance permitted by the vascular structure when fully vasodilated may contribute to limit maximal muscular hyperaemia in humans.
Maximal muscle hyperaemia
The first measures of muscle blood flow during exercise yielding peak values between 50 and 60 ml (100 g)−1 min−1 were based on xenon clearance or venous occlusion plethysmography (Grimby et al. 1967), which both underestimate the actual values (Saltin, 2007). In the 1980s, the development of the knee extension model by Andersen and Saltin allowed the measurement of quadriceps muscle blood flow in humans in response to incremental exercise (Andersen & Saltin, 1985). The observed values of 250 ml (100 g tissue)−1 min−1 were later confirmed using the ultrasound Doppler methods to measure the arterial inflow (Rådegran et al. 1999). Even higher levels of muscle hyperaemia were reported in elite cyclists with values up to ∼400 ml (100 g tissue)−1 min−1 (Richardson et al. 1993). Thus, human skeletal muscle may reach peak levels of perfusion similar to those reported in other mammals including athletic species (Parks & Manohar, 1983; Armstrong & Laughlin, 1985; Manohar, 1986; Musch et al. 1987; Poole & Erickson, 2011).
The real levels of peak hyperaemia for the human quadriceps muscle during one leg knee extension exercise may, however, be slightly lower (80–90% of the previously reported values) than initially claimed, once the perfusion of the inactive muscle mass of the leg (about 5.5 kg) during knee extension exercise (∼5–7 ml (100 g tissue)−1 min−1 (Heinonen et al. 2012), the skin, and other leg tissues are discounted. Since each leg of a healthy young male has a muscle mass of ∼8 kg (Wang et al. 1999), if all leg muscles were to accommodate as much blood flow as the quadriceps muscle during maximal knee extension exercise, the maximal theoretical blood flows would range between 32 and 50–60 l min−1 in physically active men and elite athletes, respectively (Andersen & Saltin, 1985). This level of perfusion is impossible since the maximal cardiac output in physically active men ranges between 20 and 25 l min−1 (Asmussen & Nielsen, 1955; Åstrand et al. 1964; Calbet et al. 2007) and the maximal values reported for elite athletes are just above 40 l min−1 (Ekblom & Hermansen, 1968).
However, the previous calculation did not account for the fact that blood flow is proportional to the perfusing pressure, and the latter is about 20–30% greater during knee extension than during upright exercise (Hermansen et al. 1970; Andersen & Saltin, 1985; Calbet et al. 2004a). With intra-arterial infusion of adenosine or ATP at doses that cause maximal vasodilatation, peak leg blood flow in the supine position ranges between 6 and 8 l min−1 (0.75–1.00 l min−1 kg−1), with a mean arterial pressure close to 80 mmHg (Rådegran & Calbet, 2001; Rosenmeier et al. 2004; Calbet et al. 2006). Thus, increasing the mean arterial pressure to 120–130 mmHg and assuming a similar pressure gradient between the femoral artery and the femoral vein, the maximal leg blood flow attainable with a mean arterial pressure (MAP) of 120 mmHg, as often observed at peak exercise while bicycling, should lie in between 9 and 12 l min−1 (1.13–1.50 l min−1 kg−1), for untrained men. Then, with a perfusion pressure similar to that observed during knee extension exercise, the mean muscle blood flow for the full leg should be below 150 ml (100 g tissue)−1 min−1 in physically active men (12 l min−1 per leg, assuming 8 kg of muscle mass and that muscle vasodilatation is maximal and similar during peak knee extension and bicycling).
How does this lower value reconcile with the much higher perfusions observed during maximal knee extension exercise? The main explanation is that there are regional differences in hyperaemia between muscle fibres, as reported in rodents (Armstrong & Laughlin, 1985) and foxhounds (Musch et al. 1987) with microspheres, and in humans with positron emission tomography (Kalliokoski et al. 2000) (Fig. 1). In fact, peak values in deep regions of the quadriceps muscle of up to 400 ml (100 g tissue)−1 min−1 have been reported during isometric intermittent knee extensions using positron emission tomography in physically active men (Heinonen et al. 2010). However, the observed mean values for the whole quadriceps were ∼40 ml (100 g tissue)−1 min−1, reflecting marked heterogeneity in the regional distribution of blood flow in the exercising quadriceps muscle in humans (Heinonen et al. 2010).
Figure 1. High heterogeneity of skeletal muscle blood flow in humans.
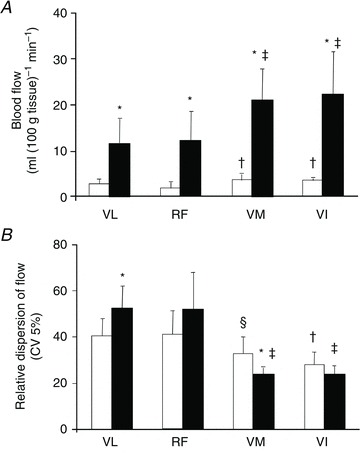
Mean blood flow (A) and relative dispersion of blood flow (B) in the different portions of the quadriceps femoris muscle measured with positron emission tomography and H215O during intermittent leg extension isometric contractions (Kalliokoski et al. 2000). *P < 0.01 rest versus exercise, †P < 0.001versus resting RF and VL, ‡P < 0.001 versus exercising RF and VL, §P < 0.001 versus resting VL. VL: vastus lateralis; RF: rectus femoris, VM: vastus medialis; VI: vastus intermedious.
Another mechanism that has been suggested to contribute to enhance muscle hyperaemia is ‘the muscle pump’ (Laughlin, 1987). The most prominent explanation for how this theoretical construct may facilitate blood flow is by reducing the venous hydrostatic pressure in dependent limbs (for review see Laughlin, 1987; Tschakovsky & Sheriff, 2004). In addition to this, muscle contractions generate a small arterial retrograde flow, whilst at the same time facilitating venous blood to be propelled towards the heart. In the drained venous segments negative pressures may be generated when the muscles relax and return to their original length, facilitating the inflow of blood from arteries, since retrograde flow from more proximal venous segments is impeded by venous valves. It has also been suggested that the muscle pump may increase the kinetic energy of blood, though the mechanism is unclear. According to Tschakovsky & Sheriff (2004), contraction frequency constitutes a major determinant of muscle pump efficacy, but this contrasts with the observation of no effect of contraction frequency on muscle perfusion at a given exercise intensity in humans (Hoelting et al. 2001; Osada & Radegran, 2002). Moreover, the effect of a muscle pump should be proportional to the mechanical work of the pump, i.e. to exercise intensity. However, once peak leg blood flow has reached its maximum value, increasing exercise intensity (which must enhance the action of the pump) does not result in higher perfusion. Similar conclusions have been achieved by others (Gonzalez-Alonso et al. 2008).
Is there a functional reserve in muscle vasodilatory capacity during maximal exercise in humans?
In general, during maximal dynamic exercise with either a small muscle mass (one- or two-legged knee extension exercise) similar levels of muscle hyperaemia are reached regardless of 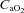 ,
, 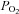 , venous muscle pH, femoral vein blood temperature, haemoglobin desaturation or acclimatization to altitude in healthy humans (Koskolou et al. 1997b; Roach et al. 1999; Mourtzakis et al. 2004; Calbet & Lundby, 2009). This could indicate that during exercise engaging a small muscle mass vasodilatation is already maximal in the active regions of the muscle, implying that to even further increase muscle blood flow perfusion pressure must be increased. To test if there is some residual vasodilatory capacity during small muscle mass exercise, Barden et al. (2007) infused adenosine intra-arterially during maximal knee extension exercise performed with hyperoxia (
, venous muscle pH, femoral vein blood temperature, haemoglobin desaturation or acclimatization to altitude in healthy humans (Koskolou et al. 1997b; Roach et al. 1999; Mourtzakis et al. 2004; Calbet & Lundby, 2009). This could indicate that during exercise engaging a small muscle mass vasodilatation is already maximal in the active regions of the muscle, implying that to even further increase muscle blood flow perfusion pressure must be increased. To test if there is some residual vasodilatory capacity during small muscle mass exercise, Barden et al. (2007) infused adenosine intra-arterially during maximal knee extension exercise performed with hyperoxia (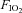 = 1). In the four subjects from whom maximal data were obtained, peak vascular conductance was not increased by adenosine (Barden et al. 2007). Although this study is in agreement with a lack of vasodilatory reserve at maximal exercise in humans, there are two limitations that preclude any definitive conclusion. First, only the quadriceps muscle was recruited during the exercise but the adenosine was infused into the common femoral artery, and this causes massive vasodilatation in the hamstrings and other leg tissues (Heinonen et al. 2010). Second, adenosine increases muscle sympathetic activity when infused at rest and is not as potent as for example ATP as a sympatholytic agent, leaving open the possibility of some vasodilator reserve in the active muscles not revealed by the infusion of adenosine, due to some sympathetic vasoconstriction still remaining during the adenosine infusion (Rosenmeier et al. 2004).
= 1). In the four subjects from whom maximal data were obtained, peak vascular conductance was not increased by adenosine (Barden et al. 2007). Although this study is in agreement with a lack of vasodilatory reserve at maximal exercise in humans, there are two limitations that preclude any definitive conclusion. First, only the quadriceps muscle was recruited during the exercise but the adenosine was infused into the common femoral artery, and this causes massive vasodilatation in the hamstrings and other leg tissues (Heinonen et al. 2010). Second, adenosine increases muscle sympathetic activity when infused at rest and is not as potent as for example ATP as a sympatholytic agent, leaving open the possibility of some vasodilator reserve in the active muscles not revealed by the infusion of adenosine, due to some sympathetic vasoconstriction still remaining during the adenosine infusion (Rosenmeier et al. 2004).
Theoretical calculations by Saltin (1985) indicated that the combined maximal vasodilatory capacity of the arm and leg muscles would be of such a magnitude, that in order to maintain MAP during maximal whole body exercise, cardiac output would have to be increased 2- to 3-fold higher than that achievable at peak exercise. This also implies that to prevent hypotension during whole body exercise and to avoid insufficient oxygen delivery to the heart, brain and respiratory muscles, which may require between 3 and 5 l min−1 of blood flow, vasodilatation in the active muscles must be restrained and precisely controlled. The regulating mechanism(s) should respond to changes in arterial pressure and must integrate signals from the other vascular beds also in order to ensure sufficient oxygen supply. There is strong evidence suggesting that the sympathetic nervous system plays this role by restraining muscle vasodilatation during whole body exercise, or in exercise conditions for which there is risk of hypotension (Marshall et al. 1961; Secher et al. 1977; Lang et al. 1997; Dela et al. 2003; Schrage et al. 2004). For example, Marshall et al. (1961) reported that blood pressure was reduced during supine exercise in a man who had undergone surgical thoracolumbar sympathectomy for essential hypertension.
Similarly, MAP drops during electrostimulation-induced exercise on the cycle ergometer in paraplegics who lack functional sympathetic innervation in the legs (Dela et al. 2003). In chronic heart failure (CHF) patients sympathetic inhibition with clonidine results in greater leg vascular conductance during peak exercise on the cycle ergometer (Lang et al. 1997). Several exercise models during which blood flow demand beyond the main active muscles was increased by superimposing intense arm exercise on leg exercise (Secher et al. 1977) or vice versa (Volianitis & Secher, 2002), or by increasing the work of the respiratory muscles (Harms et al. 1997), have reported a subsequent vasoconstriction on the main active muscles, which is likely to be mediated by the sympathetic nervous system. Conversely, unloading the respiratory muscles during exercise resulted in greater quadriceps muscle blood flow in chronic obstructive pulmonary disease (COPD) patients (Vogiatzis et al. 2011).
Thus, indirect evidence suggests that during whole body exercise muscle vasodilatation must be restrained to maintain MAP. More definitive evidence for such a mechanism was obtained by studying a group of elite cross-country skiers in which maximal cardiac output, and leg and arm blood flows were assessed while skiing using different techniques involving various combinations of arm and leg exercise (Calbet et al. 2004a). It was observed that during maximal diagonal skiing, where arms and legs are strongly engaged simultaneously, that the vasodilatatory response was restricted to values below the maximal attainable to maintain MAP (Fig. 2).
Figure 2. The combined vasodilatory capacity of the arm and leg muscles exceeds the pumping capacity of the heart (Calbet et al. 2004a).
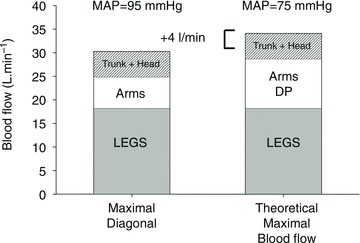
Cross-country skiers were studied during submaximal (76% 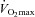 ) skiing while using arm and legs (diagonal technique), only arm (double poling technique) and leg skiing (like skating). They were also studied during maximal exercise with the diagonal technique. Trunk and head perfusion at maximal diagonal was calculated by subtracting peak leg and arm blood flows from peak cardiac output. The maximal theoretical cardiac output was calculated by adding the maximal values that were observed for leg blood flow (during maximal diagonal), the peak arm blood flow (observed during double poling) and the 5 l min−1 of blood flow necessary to perfuse the head and trunk. The latter gave 4 l min1 more cardiac output than actually measured, implying that in humans with well trained arm and leg muscles the combined peak perfusion of the head trunk and arm muscle exceeds the pumping capacity of the heart. This also implies that during maximal upright arm and leg combined exercise, muscle vasodilatation must be restrained to avoid hypotension. (Figure from Calbet & Joyner, 2010.)
) skiing while using arm and legs (diagonal technique), only arm (double poling technique) and leg skiing (like skating). They were also studied during maximal exercise with the diagonal technique. Trunk and head perfusion at maximal diagonal was calculated by subtracting peak leg and arm blood flows from peak cardiac output. The maximal theoretical cardiac output was calculated by adding the maximal values that were observed for leg blood flow (during maximal diagonal), the peak arm blood flow (observed during double poling) and the 5 l min−1 of blood flow necessary to perfuse the head and trunk. The latter gave 4 l min1 more cardiac output than actually measured, implying that in humans with well trained arm and leg muscles the combined peak perfusion of the head trunk and arm muscle exceeds the pumping capacity of the heart. This also implies that during maximal upright arm and leg combined exercise, muscle vasodilatation must be restrained to avoid hypotension. (Figure from Calbet & Joyner, 2010.)
To determine if counteracting ongoing sympathetic vasoconstriction during whole body cycle exercise would enhance muscle blood flow and oxygen delivery, and thereby exercise performance, ATP was infused intra-arterially at near maximal and maximal exercise under control conditions (at sea level) (Calbet et al. 2006) and after 8–12 days at high altitude (4559 m above sea level) (Lundby et al. 2008). At sea level, ATP increased maximal exercise leg vascular conductance by 17% (Calbet et al. 2006). MAP was not affected by the infusion of ATP due to a small (6%) but significant elevation of cardiac output. About half of the increase in cardiac output was directed to the ATP-infused leg, which showed a trend (P = 0.08) for 0.8 l min−1 higher LBF (8% more than under control conditions). However, the infusion of ATP reduced the a-vO2 difference, likely to be due to a deviation of some blood flow to less active muscle fibres and other tissues of the leg. After 8–12 days of residence at altitude the resting MAP was elevated by ∼20 mmHg, likely to be due to increased sympathetic activity mediated in part an increased chemoreflex response in chronic hypoxia (Calbet, 2003; Hansen & Sander, 2003). At the same time maximal cardiac output and peak leg blood flow were reduced by ∼20%, indicating that at maximal exercise in hypoxia there was a functional reserve (about 5 l min−1) to increase cardiac output and, hence leg blood flow, in response to the ATP infusion. Similar to at sea level, maximal exercise leg vascular conductance was increased, whereas leg blood flow was not. The latter was likely to be the consequence of a reduction of maximal exercise MAP with ATP. O2 extraction across the leg was reduced with ATP at altitude confirming that unselective vasodilatation at maximal exercise may cause 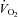 /
/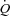 mismatch in normoxic conditions or when sympathetic activity is increased, as for example in chronic hypoxia.
mismatch in normoxic conditions or when sympathetic activity is increased, as for example in chronic hypoxia.
Muscle fibre type, training and reduced physical activity
Regional differences in peak exercise hyperaemia have been attributed to differences in fibre type composition and training. In rodents, peak muscle hyperaemia may be 2- to 4-folds greater in red (type I) than in predominantly white (type II) muscles (Armstrong & Laughlin, 1985). Cross-sectional studies in humans indicate that endurance trained muscles may have a 30–60% higher peak hyperaemic responses to exercise than untrained muscles (Andersen & Saltin, 1985; Snell et al. 1987; Richardson et al. 1993). There are few longitudinal studies in humans where the effect of training on peak exercise muscle hyperaemia has been examined (Juel et al. 2004; Mourtzakis et al. 2004; Blomstrand et al. 2011). Blomstrand et al. (2011) studied 14 recreationally active subjects who performed one-legged knee extension exercise for 5–7 weeks, 3–5 days per week (∼1 h per session), while the other leg remained untrained (Fig. 3). Five of the subjects trained at approximately 70% of their pre-determined one-legged peak work rate (5 days week−1), four others performed 40 bouts each consisting of 1 min exercise at approximately 100% of maximal single leg oxygen uptake, separated by 30 s rest periods (aerobic intermittent training; 3 days week−1), and five subjects performed 15 × 1 min bouts of exercise at 150% of the maximal single leg oxygen uptake, separated by 3 min rest periods (anaerobic intermittent training; 4 days week−1). The three groups all improved their peak leg 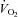 by ∼32%, and this improvement was related to a 30% increase in leg blood flow, i.e. oxygen delivery. Since the muscle mass was increased by 5.8%, the normalized peak quadriceps muscle hyperaemia was increased by 22%, from 216 to 265 ml (100 g)−1 min−1 (Blomstrand et al. 2011). In six subjects, Juel et al. (2004) reported an increase of peak quadriceps muscle blood flow of 25% after 7–8 week of one-legged knee extension high-intensity interval training with (15 × 1 min bouts at 150% of
by ∼32%, and this improvement was related to a 30% increase in leg blood flow, i.e. oxygen delivery. Since the muscle mass was increased by 5.8%, the normalized peak quadriceps muscle hyperaemia was increased by 22%, from 216 to 265 ml (100 g)−1 min−1 (Blomstrand et al. 2011). In six subjects, Juel et al. (2004) reported an increase of peak quadriceps muscle blood flow of 25% after 7–8 week of one-legged knee extension high-intensity interval training with (15 × 1 min bouts at 150% of 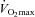 , with 3 min rest periods). Aerobic one-legged knee extension training (1 h day−1 at 70% maximum workload for 5 days week−1) in six subjects resulted in a ∼27% greater leg blood flow and vascular conductance (Mourtzakis et al. 2004). Interestingly, the level of maximal hyperaemia after training was almost identical when tested in normoxia and hyperoxia (
, with 3 min rest periods). Aerobic one-legged knee extension training (1 h day−1 at 70% maximum workload for 5 days week−1) in six subjects resulted in a ∼27% greater leg blood flow and vascular conductance (Mourtzakis et al. 2004). Interestingly, the level of maximal hyperaemia after training was almost identical when tested in normoxia and hyperoxia (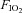 : 0.6), indicating that during exercise engaging only a small muscle mass peak muscle blood flow reaches the same value regardless of
: 0.6), indicating that during exercise engaging only a small muscle mass peak muscle blood flow reaches the same value regardless of 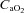 (Mourtzakis et al. 2004).
(Mourtzakis et al. 2004).
Figure 3. Changes in peak leg blood flow after three different knee extension training programs: anaerobic intervalic (Ana I), aerobic intervalic (Aer I), and submaximal aerobic (Sub A).
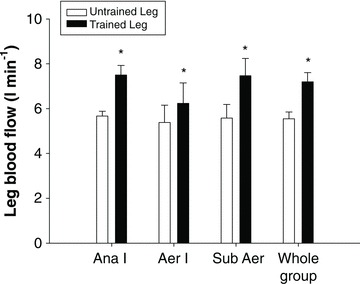
The three groups improved similarly their peak leg 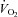 by 32%, and this improvement was due to a 30% increase in leg blood flow (Whole group) (Blomstrand et al. 2011).
by 32%, and this improvement was due to a 30% increase in leg blood flow (Whole group) (Blomstrand et al. 2011).
The effect of training on maximal exercise hyperaemia during whole body exercise, as for example during bicycling in the semi-recumbent position, was studied by Roca et al. (1992). In nine non-active men, 9 weeks of endurance training increased peak leg blood flow by ∼27% in normoxia and hypoxia (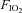 : 0.12) (Roca et al. 1992). The effect of training on the leg blood flow response to submaximal exercise at the same absolute work load remains controversial, with some studies reporting a small reduction after training (Kiens et al. 1993; Proctor et al. 2001; Nyberg et al. 2012b) or no change at low intensities but increases at near-maximal exercise intensity (Krustrup et al. 2004). Consequently, submaximal haemodynamic responses to exercise cannot be used to infer the response at maximal exercise.
: 0.12) (Roca et al. 1992). The effect of training on the leg blood flow response to submaximal exercise at the same absolute work load remains controversial, with some studies reporting a small reduction after training (Kiens et al. 1993; Proctor et al. 2001; Nyberg et al. 2012b) or no change at low intensities but increases at near-maximal exercise intensity (Krustrup et al. 2004). Consequently, submaximal haemodynamic responses to exercise cannot be used to infer the response at maximal exercise.
Overall, these studies demonstrate that in a relatively short time maximal muscle hyperaemia during small or large muscle mass exercise may be increased by 20–30%, without concomitant changes in MAP. A more prolonged training stimulus may cause even greater increases of peak vascular conductance, as suggested by the study of Sinoway et al. (1986), who showed that post-ischaemic forearm maximal vascular conductance was 42% higher in the dominant compared to the contralateral arm of tennis players (Fig. 4). The increase in perfusion may be explained by two main mechanisms, (a) increased vasodilatation (functional cross-sectional area) or (b) increased anatomical vascular cross-sectional area, or a combination of both. The first mechanism would imply that before training there is a functional vasodilator reserve not utilized even at peak exercise, which is made available with training. The second mechanism requires vascular remodelling leading to widening of conduit arteries and arteriologenesis in response to training (increasing the capillary bed alone does not enhance the cross-sectional area of the resistance vessels).
Figure 4. Maximal post-ischaemic vasodilatation in the dominant and non-dominant arms of tennis players and control subjects with similar 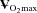 , measured with plethysmography after 5 min of arterial occlusion coupled with 1 min of exercise.
, measured with plethysmography after 5 min of arterial occlusion coupled with 1 min of exercise.
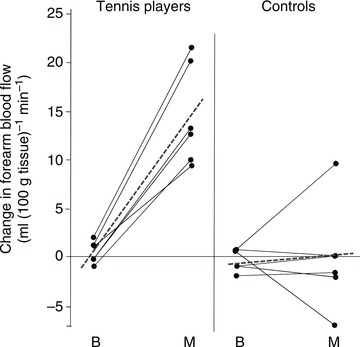
In the tennis players maximal forearm hyperaemia was 42% higher in the dominant than in the non-dominant forearm (Sinoway et al. 1986).
Vascular remodelling increases the cross-sectional area of the capillary bed (Andersen & Henriksson, 1977; Duscha et al. 1999; Blomstrand et al. 2011; Esposito et al. 2011) and conduit arteries (Schmidt-Trucksass et al. 2000; Naylor et al. 2006), by a mechanism involving shear stress and NO (Tinken et al. 2010; Green et al. 2012). For example, the internal diameter of the femoral artery is wider in cyclists, middle-distance runners and triathletes, while that of the carotid artery is similar in athletes and controls (Schmidt-Trucksass et al. 2000). Moreover, increases in brachial artery diameter have been shown following training resumption in elite rowers (Naylor et al. 2006).
Interestingly, if shear stress is attenuated in one arm during bilateral handgrip training by cuff inflation the training-induced improvement of peak reactive hyperaemia is completely blunted in the cuffed arm (Tinken et al. 2010) (Fig. 5). In this study, no increase was observed in the cuffed arm brachial artery resting diameter after training, while in the non-cuffed arm the brachial artery diameter was increased by 0.2 mm. Although this change was non-statistically significant it accounted for one-third of the increase in peak reactive hyperaemia after training in the non-cuffed arm. This is due to the great impact that the radius of the vessels have on the vascular conductance (conductance = πr4/8ηL, where η represents the viscosity and L the length of the vessel). Consequently, a small error of only 0.1–0.2 mm in the assessment of the diameter of the brachial artery has a major impact on the calculations of blood flow and vascular conductance.
Figure 5. Relative change in brachial artery flow mediated dilatation from baseline in response to ischaemic exercise across the 8-week handgrip exercise training in healthy young men.
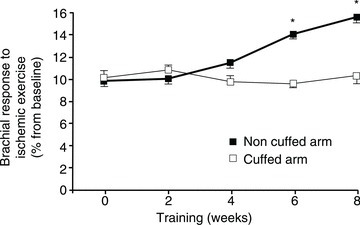
One arm was trained with a cuff around the arm inflated at 60 mmHg to prevent shear stress. Error bars represent SEM. *P < 0.05 between the cuffed and non-cuffed arm (Tinken et al. 2010).
Maximal exercise vascular conductance is reduced with ageing by unknown mechanism(s) (Proctor et al. 2003, 2004). Animal studies have reported arteriolar rarefaction (reduced number of arterioles) in rodent muscle with ageing (Behnke et al. 2006). This may be facilitated by a combination of factors including reduced physical activity (Sugawara et al. 2004; Rakobowchuk et al. 2011), endothelial dysfunction (Taddei et al. 2001) and lower nitric oxide bioavailability (Nyberg et al. 2012a), leading to remodelling of the vascular tree (rarefaction). Other mechanisms, like altered sympatholysis, may also play a role (see below).
Sympathetic overactivity may limit exercise vasodilatation in patients
Excessive sympathetic activation is involved in both initiation and progression of chronic heart failure (CHF) (Hasking et al. 1986; Brunner-La Rocca et al. 2001). This sympathetic overactivity has been attributed to an impairment of baroreflex control of sympathetic activity (Grassi et al. 1995) and increased peripheral chemoreflex sensitivity (Despas et al. 2012). Although forearm vascular resistance is increased in patients with sympathetic overactivity (Roveda et al. 2003) and intense sympathoexcitation during exercise contributes to a reduced exercise capacity in CHF (Piepoli et al. 1996; Notarius et al. 2001; Crisafulli et al. 2007), it remains unknown whether the increased sympathetic overactivity may play a role in limiting maximal exercise induced vasodilatation. In chronic heart failure maximal cardiac output is reduced and hence the capacity to perfuse the active muscles (Magnusson et al. 1997). These patients have a rather limited capacity to perform exercise on the cycle ergometer in part due to the reduced oxygen delivery; however, they can reach a peak skeletal muscle perfusion and a leg oxygen uptake similar to that of healthy individuals when only a sufficiently small muscle mass is activated (Magnusson et al. 1997).
Increasing the active muscle mass in heart failure patients results in lower peak leg blood flow and leg vascular conductance accompanied by increased noradrenaline spillover (an indirect measure of sympathetic activation). This sympathoactivation is likely to be necessary to limit the exercise-induced skeletal muscle vasodilatation and thereby preserve a minimal degree of perfusion levels for the brain, heart and respiratory muscles (Poole et al. 2012).
Exercise training decreases muscle sympathetic nerve activity (MSNA) in CHF (Roveda et al. 2003) and hypertensive patients (Laterza et al. 2007). The reduction in sympathetic overactivity has been associated with improved baroreflex control of MSNA and heart rate (HR) during increases and decreases in MAP (Laterza et al. 2007). Moreover, in CHF exercise training elicits a marked increase of maximal exercise hyperaemia, although this does not seem related to a reduced exercise sympathetic activation (Esposito et al. 2010). However, exercise capacity and quality of life of these patients is improved with cycle training by several mechanisms which may include enhanced maximal cardiac output and O2 extraction capacity (independent or in combination) (Cattadori et al. 2011).
It has been reported that training one leg at a time with knee extensions (8 weeks of endurance training, 3 times a week, 50 min session−1 leg−1) resulted in 38% increase in peak leg blood flow (+1.2 l min−1) during single knee extension exercise and 19% (+1.8 l min−1) during exercise on the cycle ergometer (Esposito et al. 2011), despite the fact that maximal cardiac output (measured during cycling) was increased only by 5% (not statistically significant). The corresponding improvements for leg 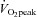 during bike and single leg exercise were 40 and 54%, respectively. The increase in peak muscle perfusion was explained by a 40 and 53% greater peak vascular conductance. Interestingly, this increase in vascular conductance was not associated with lower calculated noradrenaline spillover values, but was accompanied by increased muscle fibre cross-sectional area, capillary-to-fibre ratio, number of capillaries around each fibre, and mitochondrial volume (Esposito et al. 2011).
during bike and single leg exercise were 40 and 54%, respectively. The increase in peak muscle perfusion was explained by a 40 and 53% greater peak vascular conductance. Interestingly, this increase in vascular conductance was not associated with lower calculated noradrenaline spillover values, but was accompanied by increased muscle fibre cross-sectional area, capillary-to-fibre ratio, number of capillaries around each fibre, and mitochondrial volume (Esposito et al. 2011).
In heart failure patients with an haematocrit below 35%, erythropoietin treatment increasing [Hb] from 11 to 14 g dl−1 increased 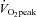 during cycle ergometer exercise by 15% (from 11 ± 0.8 to 12.7 ± 2.8 ml kg−1 min−1) and the 6 min walking distance by 12%, without significant changes in post-ischaemic forearm vascular conductance (Mancini et al. 2003). Altogether, these studies indicate that in heart failure patients peak exercise hyperaemia is limited to a great extent by the reduced leg flow conductance due to structural factors (a smaller vascular tree).
during cycle ergometer exercise by 15% (from 11 ± 0.8 to 12.7 ± 2.8 ml kg−1 min−1) and the 6 min walking distance by 12%, without significant changes in post-ischaemic forearm vascular conductance (Mancini et al. 2003). Altogether, these studies indicate that in heart failure patients peak exercise hyperaemia is limited to a great extent by the reduced leg flow conductance due to structural factors (a smaller vascular tree).
Altered sympatholysis could contribute to reduce maximal exercise vasodilatation
Sympathetic nerve activity increases with central command and exercise intensity (Victor et al. 1995), and may be enhanced by muscle afferent feedback from mechanoreceptors, venous distension (Cui et al. 2011) and the peripheral chemoreceptors (Stickland et al. 2011). The increase of sympathetic nerve activity restricts muscle blood flow (O’Leary et al. 1997) through α-adrenoreceptor stimulation at the feed arteries (VanTeeffelen & Segal, 2003). Muscle contractions cause the release of vasodilator substances that initiate a conducted vasodilatation signal that travels along the vessel wall retrogradely up to the feed arteries, counteracting the vasoconstrictor action of the sympathetic nerves (VanTeeffelen & Segal, 2006). This process is named functional sympatholysis (Remensnyder et al. 1962).
Moore et al. (2010) have demonstrated that the conducted vasodilatation is increased by phentolamine (which blocks the α-adrenergic receptors) even in response to an isolated muscle contraction lasting 0.1 s. This implies that some constitutive sympathetic tone at rest is necessary to avoid unwanted conducted vasodilatation spreading to neighbouring non-contracting muscle regions. Insufficient sympatholysis due to either increased sympathetic tone or reduced sympatholytic activity could explain the reduced maximal exercise vasodilatation observed with ageing (Jackson et al. 2010) and in conditions accompanied by increased sympathetic activity such as CHF, chronic kidney failure, preeclampsia and COPD. In this regard, enhanced activation of α-adrenoreceptors restricting conducted vasodilatation has been shown in ageing mice (Jackson et al. 2010) and combined with the exercise induced enhancement of sympathetic neural activity, this could explain the reported limited maximal exercise vasodilatation observed in old men and women (Koch et al. 2003; Proctor et al. 2004).
Interestingly, Kirby et al. (2012) have reported that old compared to young men have blunted increase of plasma venous ATP concentration and reduced effluent ATP values during forearm exercise due to impaired ATP release from the erythrocytes in response to haemoglobin deoxygenation (see Ellsworth & Sprague (2012) for review). Impaired functional sympatholysis has been observed after 2 weeks of muscle immobilization (Mortensen et al. 2012) and in hypertensive patients (Vongpatanasin et al. 2011). Nitric oxide (NO) bioavailability is also reduced with ageing in humans, however improving NO bioavailability with an intra-arterial infusion of the antioxidant N-acetylcysteine did not increase leg blood flow in exercising men (Nyberg et al. 2012a). The influence that changes in circulating or interstitial putative sympatholytic signals (K+, ATP, NO, etc.) and responsiveness to these sympatholytic agents in health and disease on peak exercise vasodilatation deserves future study.
Conclusions
Human skeletal muscle may reach similar levels of peak hyperaemia during exercise to those observed in other mammals, including athletic species. Exercise elicits heterogeneous muscle hyperaemia, reflecting differences in vascular structure and motor unit recruitment. Maximal muscle vasodilatation in exercising humans depends on the active muscle mass, is likely to be restrained by sympathetic nervous system during whole body exercise, increases with training, whilst it is reduced with ageing and in diseases accompanied by increased sympathetic overactivity or reduced pumping capacity of the heart. It remains unknown if a reduction of the sympathetically mediated vasoconstriction during maximal exercise plays a role in the training-induced increase of peak muscle vasodilatation in healthy humans. In patients with chronic heart failure, hypertension and probably other conditions accompanied by sympathetic overactivity, or limited maximal cardiac output, peak skeletal muscle vasodilatation may be limited by the sympathetic system, even during exercise with a small muscle mass. Exercise training may normalize sympathetic overactivity and enhance maximal exercise vascular conductance and thereby increase the quality of life and prognosis of these patients.
Acknowledgments
This work was supported by a Grant from Ministerio de Educación y Ciencia (DEP2009-11638).
References
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol. 2010;109:966–976. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270:677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RB, Laughlin MH. Rat muscle blood flows during high-speed locomotion. J Appl Physiol. 1985;59:1322–1328. doi: 10.1152/jappl.1985.59.4.1322. [DOI] [PubMed] [Google Scholar]
- Asmussen E, Nielsen M. The cardiac output in rest and work at low and high oxygen pressures. Acta Physiol Scand. 1955;35:73–83. doi: 10.1111/j.1748-1716.1955.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Åstrand PO, Cuddy TE, Saltin B, Stenberg J. Cardiac output during submaximal work and maximal work. J Appl Physiol. 1964;19:268–274. doi: 10.1152/jappl.1964.19.2.268. [DOI] [PubMed] [Google Scholar]
- Barden J, Lawrenson L, Poole JG, Kim J, Wray DW, Bailey DM, Richardson RS. Limitations to vasodilatory capacity and VO2max in trained human skeletal muscle. Am J Physiol Heart Circ Physiol. 2007;292:H2491–2497. doi: 10.1152/ajpheart.01396.2006. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Prisby RD, Lesniewski LA, Donato AJ, Olin HM, Delp MD. Influence of ageing and physical activity on vascular morphology in rat skeletal muscle. J Physiol. 2006;575:617–626. doi: 10.1113/jphysiol.2006.108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstrand E, Krustrup P, Sondergaard H, Radegran G, Calbet JA, Saltin B. Exercise training induces similar elevations in the activity of oxoglutarate dehydrogenase and peak oxygen uptake in the human quadriceps muscle. Pflugers Arch. 2011;462:257–265. doi: 10.1007/s00424-011-0978-6. [DOI] [PubMed] [Google Scholar]
- Brunner-La Rocca HP, Esler MD, Jennings GL, Kaye DM. Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur Heart J. 2001;22:1136–1143. doi: 10.1053/euhj.2000.2407. [DOI] [PubMed] [Google Scholar]
- Calbet JA. Chronic hypoxia increases blood pressure and noradrenaline spillover in healthy humans. J Physiol. 2003;551:379–386. doi: 10.1113/jphysiol.2003.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, Saltin B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R291–R303. doi: 10.1152/ajpregu.00155.2002. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Gonzalez-Alonso J, Helge JW, Sondergaard H, Munch-Andersen T, Boushel R, Saltin B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J Appl Physiol. 2007;103:969–978. doi: 10.1152/japplphysiol.01281.2006. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Jensen-Urstad M, Van Hall G, Holmberg HC, Rosdahl H, Saltin B. Maximal muscular vascular conductances during whole body upright exercise in humans. J Physiol. 2004a;558:319–331. doi: 10.1113/jphysiol.2003.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Joyner MJ. Disparity in regional and systemic circulatory capacities: do they affect the regulation of the circulation? Acta Physiol (Oxf) 2010;199:393–406. doi: 10.1111/j.1748-1716.2010.02125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Lundby C. Air to muscle O2 delivery during exercise at altitude. High Alt Med Biol. 2009;10:123–134. doi: 10.1089/ham.2008.1099. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Lundby C, Sander M, Robach P, Saltin B, Boushel R. Effects of ATP-induced leg vasodilation on VO2peak and leg O2 extraction during maximal exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R447–453. doi: 10.1152/ajpregu.00746.2005. [DOI] [PubMed] [Google Scholar]
- Calbet JA, Radegran G, Boushel R, Saltin B. On the mechanisms that limit oxygen uptake during exercise in acute and chronic hypoxia: role of muscle mass. J Physiol. 2009;587:477–490. doi: 10.1113/jphysiol.2008.162271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calbet JA, Radegran G, Boushel R, Sondergaard H, Saltin B, Wagner PD. Plasma volume expansion does not increase maximal cardiac output or VO2max in lowlanders acclimatized to altitude. Am J Physiol Heart Circ Physiol. 2004b;287:H1214–1224. doi: 10.1152/ajpheart.00840.2003. [DOI] [PubMed] [Google Scholar]
- Casey DP, Joyner MJ, Claus PL, Curry TB. Hyperbaric hyperoxia reduces exercising forearm blood flow in humans. Am J Physiol Heart Circ Physiol. 2011;300:H1892–1897. doi: 10.1152/ajpheart.00165.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattadori G, Schmid JP, Brugger N, Gondoni E, Palermo P, Agostoni P. Hemodynamic effects of exercise training in heart failure. J Card Fail. 2011;17:916–922. doi: 10.1016/j.cardfail.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol. 2007;292:H2988–2996. doi: 10.1152/ajpheart.00008.2007. [DOI] [PubMed] [Google Scholar]
- Cui J, Leuenberger UA, Gao Z, Sinoway LI. Sympathetic and cardiovascular responses to venous distension in an occluded limb. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1831–1837. doi: 10.1152/ajpregu.00170.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela F, Mohr T, Jensen CM, Haahr HL, Secher NH, Biering-Sorensen F, Kjaer M. Cardiovascular control during exercise: insights from spinal cord-injured humans. Circulation. 2003;107:2127–2133. doi: 10.1161/01.CIR.0000065225.18093.E4. [DOI] [PubMed] [Google Scholar]
- Despas F, Lambert E, Vaccaro A, Labrunee M, Franchitto N, Lebrin M, Galinier M, Senard JM, Lambert G, Esler M, Pathak A. Peripheral chemoreflex activation contributes to sympathetic baroreflex impairment in chronic heart failure. J Hypertens. 2012;30:753–760. doi: 10.1097/HJH.0b013e328350136c. [DOI] [PubMed] [Google Scholar]
- Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II–III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33:1956–1963. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Ekblom B, Hermansen L. Cardiac output in athletes. J Appl Physiol. 1968;25:619–625. doi: 10.1152/jappl.1968.25.5.619. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Sprague RS. Regulation of blood flow distribution in skeletal muscle: role of erythrocyte-released ATP. J Physiol. 2012;590:4985–4991. doi: 10.1113/jphysiol.2012.233106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55:1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol. 2011;58:1353–1362. doi: 10.1016/j.jacc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol. 2008;586:2405–2417. doi: 10.1113/jphysiol.2008.152058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation. 1995;92:3206–3211. doi: 10.1161/01.cir.92.11.3206. [DOI] [PubMed] [Google Scholar]
- Green DJ, Spence A, Rowley N, Thijssen DH, Naylor LH. Vascular adaptation in athletes: is there an ‘athlete's artery’? Exp Physiol. 2012;97:295–304. doi: 10.1113/expphysiol.2011.058826. [DOI] [PubMed] [Google Scholar]
- Grimby G, Haggendal E, Saltin B. Local xenon 133 clearance from the quadriceps muscle during exercise in man. J Appl Physiol. 1967;22:305–310. doi: 10.1152/jappl.1967.22.2.305. [DOI] [PubMed] [Google Scholar]
- Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol. 2003;546:921–929. doi: 10.1113/jphysiol.2002.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–621. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Duncker DJ, Knuuti J, Kalliokoski KK. The effect of acute exercise with increasing workloads on inactive muscle blood flow and its heterogeneity in humans. Eur J Appl Physiol. 2012 doi: 10.1007/s00421-012-2329-5. (in press) [DOI] [PubMed] [Google Scholar]
- Heinonen I, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos MM, Oikonen V, Nuutila P, Knuuti J, Hellsten Y, Boushel R, Kalliokoski KK. Comparison of exogenous adenosine and voluntary exercise on human skeletal muscle perfusion and perfusion heterogeneity. J Appl Physiol. 2010;108:378–386. doi: 10.1152/japplphysiol.00745.2009. [DOI] [PubMed] [Google Scholar]
- Hermansen L, Ekblom B, Saltin B. Cardiac output during submaximal and maximal treadmill and bicycle exercise. J Appl Physiol. 1970;29:82–86. doi: 10.1152/jappl.1970.29.1.82. [DOI] [PubMed] [Google Scholar]
- Hoelting BD, Scheuermann BW, Barstow TJ. Effect of contraction frequency on leg blood flow during knee extension exercise in humans. J Appl Physiol. 2001;91:671–679. doi: 10.1152/jappl.2001.91.2.671. [DOI] [PubMed] [Google Scholar]
- Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol. 2010;588:2269–2282. doi: 10.1113/jphysiol.2010.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen TD, Vissing J, Gonzalez-Alonso J. Influence of erythrocyte oxygenation and intravascular ATP on resting and exercising skeletal muscle blood flow in humans with mitochondrial myopathy. Mitochondrion. 2012;12:414–422. doi: 10.1016/j.mito.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Juel C, Klarskov C, Nielsen JJ, Krustrup P, Mohr M, Bangsbo J. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E245–251. doi: 10.1152/ajpendo.00303.2003. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Kemppainen J, Larmola K, Takala TO, Peltoniemi P, Oksanen A, Ruotsalainen U, Cobelli C, Knuuti J, Nuutila P. Muscle blood flow and flow heterogeneity during exercise studied with positron emission tomography in humans. Eur J Appl Physiol. 2000;83:395–401. doi: 10.1007/s004210000267. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res. 2012;111:220–230. doi: 10.1161/CIRCRESAHA.112.269571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol. 2003;551:337–344. doi: 10.1113/jphysiol.2003.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskolou MD, Calbet JA, Radegran G, Roach RC. Hypoxia and the cardiovascular response to dynamic knee-extensor exercise. Am J Physiol Heart Circ Physiol. 1997a;272:H2655–2663. doi: 10.1152/ajpheart.1997.272.6.H2655. [DOI] [PubMed] [Google Scholar]
- Koskolou MD, Roach RC, Calbet JA, Radegran G, Saltin B. Cardiovascular responses to dynamic exercise with acute anemia in humans. Am J Physiol Heart Circ Physiol. 1997b;273:H1787–1793. doi: 10.1152/ajpheart.1997.273.4.H1787. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Hellsten Y, Bangsbo J. Intense interval training enhances human skeletal muscle oxygen uptake in the initial phase of dynamic exercise at high but not at low intensities. J Physiol. 2004;559:335–345. doi: 10.1113/jphysiol.2004.062232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CC, Rayos GH, Chomsky DB, Wood AJ, Wilson JR. Effect of sympathoinhibition on exercise performance in patients with heart failure. Circulation. 1997;96:238–245. doi: 10.1161/01.cir.96.1.238. [DOI] [PubMed] [Google Scholar]
- Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrao CE, Rondon MU. Exercise training restores baroreflex sensitivity in never-treated hypertensive patients. Hypertension. 2007;49:1298–1306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol Heart Circ Physiol. 1987;253:H993–1004. doi: 10.1152/ajpheart.1987.253.5.H993. [DOI] [PubMed] [Google Scholar]
- Lundby C, Boushel R, Robach P, Moller K, Saltin B, Calbet JA. During hypoxic exercise some vasoconstriction is needed to match O2 delivery with O2 demand at the microcirculatory level. J Physiol. 2008;586:123–130. doi: 10.1113/jphysiol.2007.146035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G, Kaijser L, Sylven C, Karlberg KE, Isberg B, Saltin B. Peak skeletal muscle perfusion is maintained in patients with chronic heart failure when only a small muscle mass is exercised. Cardiovasc Res. 1997;33:297–306. doi: 10.1016/s0008-6363(96)00249-0. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294–299. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- Manohar M. Blood flow to the respiratory and limb muscles and to abdominal organs during maximal exertion in ponies. J Physiol. 1986;377:25–35. doi: 10.1113/jphysiol.1986.sp016174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RJ, Schirger A, Shepherd JT. Blood pressure during supine exercise in idiopathic orthostatic hypotension. Circulation. 1961;24:76–81. doi: 10.1161/01.cir.24.1.76. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J. Cyclooxygenase products sensitize muscle mechanoreceptors in healthy humans. Am J Physiol Heart Circ Physiol. 2004;287:H1944–1949. doi: 10.1152/ajpheart.00329.2004. [DOI] [PubMed] [Google Scholar]
- Moore AW, Bearden SE, Segal SS. Regional activation of rapid onset vasodilatation in mouse skeletal muscle: regulation through α-adrenoreceptors. J Physiol. 2010;588:3321–3331. doi: 10.1113/jphysiol.2010.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Morkeberg J, Thaning P, Hellsten Y, Saltin B. Two weeks of muscle immobilization impairs functional sympatholysis but increases exercise hyperemia and the vasodilatory responsiveness to infused ATP. Am J Physiol Heart Circ Physiol. 2012;302:H2074–2082. doi: 10.1152/ajpheart.01204.2011. [DOI] [PubMed] [Google Scholar]
- Mourtzakis M, Gonzalez-Alonso J, Graham TE, Saltin B. Hemodynamics and O2 uptake during maximal knee extensor exercise in untrained and trained human quadriceps muscle: effects of hyperoxia. J Appl Physiol. 2004;97:1796–1802. doi: 10.1152/japplphysiol.00169.2004. [DOI] [PubMed] [Google Scholar]
- Musch TI, Friedman DB, Pitetti KH, Haidet GC, Stray-Gundersen J, Mitchell JH, Ordway GA. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol. 1987;63:2269–2277. doi: 10.1152/jappl.1987.63.6.2269. [DOI] [PubMed] [Google Scholar]
- Naylor LH, O’Driscoll G, Fitzsimons M, Arnolda LF, Green DJ. Effects of training resumption on conduit arterial diameter in elite rowers. Med Sci Sports Exerc. 2006;38:86–92. doi: 10.1249/01.mss.0000181220.03855.1c. [DOI] [PubMed] [Google Scholar]
- Notarius CF, Atchison DJ, Floras JS. Impact of heart failure and exercise capacity on sympathetic response to handgrip exercise. Am J Physiol Heart Circ Physiol. 2001;280:H969–976. doi: 10.1152/ajpheart.2001.280.3.H969. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol. 2012a doi: 10.1113/jphysiol.2012.239053. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg M, Jensen LG, Thaning P, Hellsten Y, Mortensen SP. Role of nitric oxide and prostanoids in the regulation of leg blood flow and blood pressure in humans with essential hypertension: effect of high-intensity aerobic training. J Physiol. 2012b;590:1481–1494. doi: 10.1113/jphysiol.2011.225136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DS, Robinson ED, Butler JL. Is active skeletal muscle functionally vasoconstricted during dynamic exercise in conscious dogs? Am J Physiol Regul Integr Comp Physiol. 1997;272:R386–391. doi: 10.1152/ajpregu.1997.272.1.R386. [DOI] [PubMed] [Google Scholar]
- Osada T, Radegran G. Femoral artery inflow in relation to external and total work rate at different knee extensor contraction rates. J Appl Physiol. 2002;92:1325–1330. doi: 10.1152/japplphysiol.00848.2001. [DOI] [PubMed] [Google Scholar]
- Parks CM, Manohar M. Distribution of blood flow during moderate and strenuous exercise in ponies (Equus caballus) Am J Vet Res. 1983;44:1861–1866. [PubMed] [Google Scholar]
- Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93:940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- Poole DC, Erickson HH. Highly athletic terrestrial mammals: Horses and dogs. Compr Physiol. 2011;1:1–37. doi: 10.1002/cphy.c091001. [DOI] [PubMed] [Google Scholar]
- Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol. 2012;302:H1050–1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol. 2003;95:1963–1970. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Smithmyer SL, Leuenberger UA. Leg blood flow and VO2 during peak cycle exercise in younger and older women. Med Sci Sports Exerc. 2004;36:623–631. doi: 10.1249/01.mss.0000121951.10417.b5. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Miller JD, Dietz NM, Minson CT, Joyner MJ. Reduced submaximal leg blood flow after high-intensity aerobic training. J Appl Physiol. 2001;91:2619–2627. doi: 10.1152/jappl.2001.91.6.2619. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Blomstrand E, Saltin B. Peak muscle perfusion and oxygen uptake in humans: importance of precise estimates of muscle mass. J Appl Physiol. 1999;87:2375–2380. doi: 10.1152/jappl.1999.87.6.2375. [DOI] [PubMed] [Google Scholar]
- Rådegran G, Calbet JA. Role of adenosine in exercise-induced human skeletal muscle vasodilatation. Acta Physiol Scand. 2001;171:177–185. doi: 10.1046/j.1365-201x.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- Rakobowchuk M, Crozier J, Glover EI, Yasuda N, Phillips SM, Tarnopolsky MA, MacDonald MJ. Short-term unilateral leg immobilization alters peripheral but not central arterial structure and function in healthy young humans. Eur J Appl Physiol. 2011;111:203–210. doi: 10.1007/s00421-010-1636-y. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK, Wagner PD. High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol. 1993;75:1911–1916. doi: 10.1152/jappl.1993.75.4.1911. [DOI] [PubMed] [Google Scholar]
- Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol Heart Circ Physiol. 1999;276:H438–445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at VO2max. J Appl Physiol. 1992;73:1067–1076. doi: 10.1152/jappl.1992.73.3.1067. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrao CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol. 2003;42:854–860. doi: 10.1016/s0735-1097(03)00831-3. [DOI] [PubMed] [Google Scholar]
- Saltin B. Hemodynamic adaptations to exercise. Am J Cardiol. 1985;55:42D–47D. doi: 10.1016/0002-9149(85)91054-9. [DOI] [PubMed] [Google Scholar]
- Saltin B. Exercise hyperaemia: magnitude and aspects on regulation in humans. J Physiol. 2007;583:819–823. doi: 10.1113/jphysiol.2007.136309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Trucksass A, Schmid A, Brunner C, Scherer N, Zach G, Keul J, Huonker M. Arterial properties of the carotid and femoral artery in endurance-trained and paraplegic subjects. J Appl Physiol. 2000;89:1956–1963. doi: 10.1152/jappl.2000.89.5.1956. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Eisenach JH, Dinenno FA, Roberts SK, Johnson CP, Sandroni P, Low PA, Joyner MJ. Effects of midodrine on exercise-induced hypotension and blood pressure recovery in autonomic failure. J Appl Physiol. 2004;97:1978–1984. doi: 10.1152/japplphysiol.00547.2004. [DOI] [PubMed] [Google Scholar]
- Secher NH, Clausen JP, Klausen K, Noer I, Trap-Jensen J. Central and regional circulatory effects of adding arm exercise to leg exercise. Acta Physiol Scand. 1977;100:288–297. doi: 10.1111/j.1748-1716.1977.tb05952.x. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Musch TI, Minotti JR, Zelis R. Enhanced maximal metabolic vasodilatation in the dominant forearms of tennis players. J Appl Physiol. 1986;61:673–678. doi: 10.1152/jappl.1986.61.2.673. [DOI] [PubMed] [Google Scholar]
- Snell PG, Martin WH, Buckey JC, Blomqvist CG. Maximal vascular leg conductance in trained and untrained men. J Appl Physiol. 1987;62:606–610. doi: 10.1152/jappl.1987.62.2.606. [DOI] [PubMed] [Google Scholar]
- Stickland MK, Fuhr DP, Haykowsky MJ, Jones KE, Paterson DI, Ezekowitz JA, McMurtry MS. Carotid chemoreceptor modulation of blood flow during exercise in healthy humans. J Physiol. 2011;589:6219–6230. doi: 10.1113/jphysiol.2011.218099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara J, Hayashi K, Kaneko F, Yamada H, Kizuka T, Tanaka H. Reductions in basal limb blood flow and lumen diameter after short-term leg casting. Med Sci Sports Exerc. 2004;36:1689–1694. doi: 10.1249/01.mss.0000142410.45142.28. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, Haller RG. The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain. 2003;126:413–423. doi: 10.1093/brain/awg028. [DOI] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55:312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol. 2004;97:739–747. doi: 10.1152/japplphysiol.00185.2004. [DOI] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. doi: 10.1113/jphysiol.2003.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTeeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol. 2006;290:H119–127. doi: 10.1152/ajpheart.00197.2005. [DOI] [PubMed] [Google Scholar]
- Victor RG, Secher NH, Lyson T, Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ Res. 1995;76:127–131. doi: 10.1161/01.res.76.1.127. [DOI] [PubMed] [Google Scholar]
- Vogiatzis I, Habazettl H, Aliverti A, Athanasopoulos D, Louvaris Z, Lomauro A, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Effect of helium breathing on intercostal and quadriceps muscle blood flow during exercise in COPD patients. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1549–1559. doi: 10.1152/ajpregu.00671.2010. [DOI] [PubMed] [Google Scholar]
- Volianitis S, Secher NH. Arm blood flow and metabolism during arm and combined arm and leg exercise in humans. J Physiol. 2002;544:977–984. doi: 10.1113/jphysiol.2002.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol. 2011;589:1209–1220. doi: 10.1113/jphysiol.2010.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner PD. An integrated view of the determinants of maximum oxygen uptake. Adv Exp Med Biol. 1988;227:245–256. doi: 10.1007/978-1-4684-5481-9_22. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang Z, Faith MS, Kotler D, Shih R, Heymsfield SB. Regional skeletal muscle measurement: evaluation of new dual-energy X-ray absorptiometry model. J Appl Physiol. 1999;87:1163–1171. doi: 10.1152/jappl.1999.87.3.1163. [DOI] [PubMed] [Google Scholar]


