Abstract
Despite growing evidence of autonomic nervous system involvement in the regulation of cerebral blood flow, the specific contribution of cholinergic vasodilatation to cerebral autoregulation remains unknown. We examined cerebral and forearm blood flow responses to augmented arterial pressure oscillations with and without cholinergic blockade. Oscillatory lower body negative pressure was applied at six frequencies from 0.03 to 0.08 Hz in nine healthy subjects with and without cholinergic blockade via glycopyrrolate. Cholinergic blockade increased cross-spectral coherence between arterial pressure and cerebral flow at all frequencies except 0.03 Hz and increased the transfer function gain at frequencies above 0.05 Hz. In contrast, gain between pressure and forearm flow increased only at frequencies below 0.06 Hz. These data demonstrate that the cholinergic system plays an active and unique role in cerebral autoregulation. The frequency region and magnitude of effect is very similar to what has been seen with sympathetic blockade, indicating a possible balance between the two reflexes to most effectively respond to rising and falling pressure. These findings might have implications for the role of dysfunction in autonomic control of the vasculature in cerebrovascular disease states.
Key points
Cerebral autoregulation maintains cerebral perfusion relatively constant in the face of slow changes in arterial pressure, but is less effective against more rapid changes (i.e. functions as a ‘high-pass’ filter).
While thought to be maintained mainly through myogenic adjustments to changes in transmural pressure, recent work has highlighted a possibility of active autonomic involvement in cerebral autoregulation.
In this study we examined the cerebrovascular effects of cholinergic blockade on nine healthy volunteers during the application of oscillatory lower body pressure at six frequencies from 0.03 to 0.08 Hz.
Cholinergic blockade impaired autoregulation at frequencies above 0.04 Hz, suggesting a role for active cholinergic vasodilatation in the maintenance of cerebral perfusion.
Introduction
Cerebral autoregulation is the ability of the cerebrovasculature to maintain cerebral perfusion relatively constant despite changes in arterial pressure (Paulson et al. 1990; Panerai et al. 1998). This reflex functions as a ‘high-pass’ filter in that it is most effective against slow changes in arterial pressure (i.e. 10 s or slower), but faster changes, such as respiratory fluctuations, pass through unaltered (Zhang et al. 1998; Hamner et al. 2004). The physiological basis for this mechanism has long been thought to be primarily myogenic in nature (Paulson et al. 1990), but recent studies have suggested an important role for the autonomic nervous system in the maintenance of cerebral perfusion. Zhang et al. (2002) provided initial evidence of autonomic involvement in cereberal autoregulation via full ganglionic blockade, and we extended that finding by quantifying the specific sympathetic contribution to cerebral autoregulation in humans (Hamner et al. 2010). However, sympathetic blockade did not affect control of cerebral blood flow at the slowest frequencies (less than 0.05 Hz), suggesting the presence of additional compensatory mechanisms for the maintenance of perfusion that may operate on their own characteristic time scales.
It has long been known that the cerebrovascular bed is well innervated with both adrenergic and cholinergic fibres (Edvinsson, 1975), but whether the cholinergic system plays a role in cerebral autoregulation is far from clear. Unfortunately, there is evidence from animals both for (D’Alecy & Rose, 1977) and against (Busija & Heistad, 1981) a cholinergic vasodilator reflex in the cerebral circulation, but the differences in haemodynamic challenges faced by bipeds compared with quadrupeds suggest that the question of cholinergic involvement in human cerebral autoregulation must be addressed with data from humans. Clinical use of acetylcholinesterase inhibitors for symptoms of Alzheimer's disease (Birks & Harvey, 2006; Howard et al. 2012) and vascular dementia (Roman et al. 2010), which compromise cerebral vascular function (Brown & Thore, 2011), suggests that cholinergic fibres may play some role in cerebral flow regulation. In addition, recent work by Seifert et al. (2010) has shown an effect of cholinergic blockade on cerebral blood flow regulation in humans during exercise. However, it is important to note that exercise involves profound changes in haemodynamic state, brain metabolism and arterial gas concentrations, all of which are important determinants of cerebral blood flow (Querido & Sheel, 2007). Furthermore, Ogoh et al. (2005) found that exercise itself impairs cerebral autoregulation. Therefore, while the findings of Seifert et al. are intriguing, they do not substantially inform the role of cholinergic vasodilatation in cerebral autoregulation at rest. Moreover, our previous work showed that sympathetic blockade ‘impaired’ cerebral autoregulation by not simply shifting the relationship but changing its shape (Hamner et al. 2010). Thus, interrogation of cerebral autoregulation across a wide range of frequencies is necessary to fully characterize the effect of an intervention.
An active cholinergic vasodilatory reflex in the cerebrovasculature has significant clinical implications for the use of acetylcholinesterase inhibitors in the treatment of low flow conditions such as postural tachycardia syndrome (Ocon et al. 2009) and ischaemic stroke (Barrett et al. 2011). Given that the sympathetic nervous system appears to play no role in the in the effectiveness of cerebral autoregulation below 0.05 Hz, we hypothesized that cholinergic control of the cerebral vasculature might be active at these lower frequencies and that cholinergic blockade would significantly alter its frequency response. To test this hypothesis we determined the effect of cholinergic blockade on cerebral autoregulation across a wide range of frequencies via application of oscillatory lower body negative pressure (OLBNP) from 0.03 to 0.08 Hz in healthy volunteers and examined the frequency response of both forearm and cerebral vasculatures before and after blockade.
Methods
Ethical approval
This protocol was approved by the Institutional Review Board of Spaulding Rehabilitation Hospital and conformed to the Declaration of Helsinki. All volunteers gave their informed consent for this study.
Subjects
Nine healthy volunteers (4 females) aged 21–30 years (27.1 ± 0.77 years) with a BMI of 20–30 (24.4 ± 0.97) participated in this study. Volunteers were non-smokers free from cardiovascular and neurological disorders and cardioactive medications. Participants were normotensive and refrained from alcohol, caffeine and rigorous exercise at least 24 h prior to study.
Instrumentation
For each subject, a 20-gauge catheter was inserted into an antecubital vein for drug infusion. Subsequently, they were instrumented for electrocardiogram lead II (Dash 2000, General Electric), beat-by-beat photoplethysmographic arterial pressures (Portapres, Finapres Medical Systems) and oscillometric brachial pressures (DASH 2000, General Electric). Brachial pressures were taken as a check for photoplethysmographic finger pressures throughout the study session. In addition, subjects were instrumented for measurement of blood flow velocities in the middle cerebral and brachial arteries (2 and 4 MHz probes; Multidop T2, DWL). The transcranial Doppler ultrasonograph probe was positioned to measure cerebral flow velocity at the M1 segment of the middle cerebral artery at a depth of 50–65 mm. A custom probe fixation device held the probe in place. The brachial Doppler ultrasonograph probe was placed to measure brachial artery flow velocity at the antecubital fossa ispalateral to the infusion site. Expired CO2 was monitored via an infrared carbon dioxide analyser (CO2 Analyzer Model 17515, Vacumed) connected to a nasal cannula. All signals were digitized and stored at 1000 Hz (PowerLab, ADInstruments).
Protocols
Oscillatory lower body negative pressure (OLBNP)
To create controlled blood pressure oscillations of varying frequencies, OLBNP was applied similar to that previously described (Hamner et al. 2010). The subject's lower body was sealed in a tank and a vacuum pump connected to a timing mechanism controlled suction intervals. During the control portion of the protocol, OLBNP was applied at 30 mmHg across six frequencies (0.03, 0.04, 0.05, 0.06, 0.07 and 0.08 Hz) in pseudo-random order. The duration at each frequency provided 15 oscillations so that the range of frequencies encompassing the previously observed cerebral autoregulation (Hamner et al. 2004) could be studied reliably over a relatively short period of time.
Cholinergic blockade
To block muscarinic receptors on the cerebrovascular endothelium, stepwise intravenous injections of 0.2 mg glycopyrrolate were given over a period of 20–30 min to reach a stable mean heart rate >100 beats min−1. This target is comparable to heart rates achieved during cholinergic block in other published work (Ogoh et al. 2010; Seifert et al. 2010). Glycopyrrolate was chosen as the cholinergic blocking agent because it does not readily cross the blood–brain barrier (Proakis & Harris, 1978) and thus can block muscarinic receptors present on the endothelium of the pial arteries (Elhusseiny et al. 1999) without introducing the confound of central cholinergic impairment. After blockade was achieved, OLBNP was applied as above but at an amplitude of only 15 mmHg to mitigate against the augmented blood pressure oscillations resulting from blockade. During OLBNP, maintenance doses of 0.05 mg of glycopyrrolate were given to sustain blockade.
Data analysis
Data were analysed using custom software written in Matlab (Version 7.1, Mathworks). The 1000 Hz waveforms of arterial pressure and cerebral and brachial blood flow were decimated to 5 Hz and low-pass filtered with a cut-off of 0.4 Hz to provide mean values. These mean waveforms, as well as breath-by-breath CO2, R–R interval and cerebrovascular resistance (arterial pressure/cerebral blood flow), were subsequently averaged within each frequency of OLBNP to provide overall means. Power spectral density estimates were calculated via Welch's average modified periodogram method (Welch, 1967). For each frequency of OLBNP, the filtered time series was divided into five segments of equal length that overlapped by 50%. This windowing was chosen to provide equal confidence in coherence across the range of OLBNP frequencies, and so that an estimated squared coherence of >0.49 indicated a significant spectral relation existed. The signals in each segment were linearly detrended, smoothed via a Hamming window and then fast-Fourier transformed. Spectral power estimates were averaged across all windows. The product of the pressure signal with the complex conjugate of the cerebral or brachial flow velocity signals provided the cross spectrum from which coherence and transfer functions were derived. Confidence intervals and precision of estimate for the transfer function were derived based on the level of coherence from standard random process theory (Koopmans, 1995). We examined coherence and gain between arterial pressure and brachial flow, and arterial pressure and cerebral flow at each OLBNP frequency. Gain was weighted by its precision to obtain the most accurate means for statistical analysis. In this way, unreliable estimates received appropriately small weights when group averages and statistics were computed (Searle, 1987).
Statistics
Log transformations were applied to spectral powers and the r-to-z transform to coherence to provide estimates with asymptotically standard distributions (Fisher, 1958; Koopmans, 1995). The Box–Cox transformation was applied to all other data to ensure normality (Box & Cox, 1964). However, for ease of interpretation, values and confidence intervals presented herein are standard units. To account for the precision of the transfer function estimates, a weighted repeated-measures two-way analysis of variance was used to determine the effects of frequency and cholinergic blockade on gain, and a standard repeated-measures two-way analysis of variance was used to determine the effects for all other variables. If a significant interaction between frequency and condition was observed, paired t tests (weighted t tests for gain) were performed to determine at which frequency a significant effect of cholinergic blockade occurred. Differences were considered significant when P < 0.05. Values are reported as mean ± standard error.
Results
As expected, cholinergic blockade resulted in a tachycardia that averaged ∼35 beats min−1. This resulted in an increase in arterial pressure and a (presumably, baroreflex-mediated) reduction in forearm vascular resistance. In contrast, cerebral blood flow was unaltered, due to an increase in cerebrovascular resistance (Table 1). Cholinergic blockade had no effect on arterial CO2. Consistent with prior work, there was no effect of OLBNP frequency on mean values of any variable. (Hamner et al. 2004, 2010)
Table 1.
Mean values across frequency of OLBNP before and after cholinergic blockade
| Control | Cholinergic blockade | |
|---|---|---|
| R–R interval (ms) | 955 ± 16 | 601±7* |
| Mean arterial pressure (mmHg) | 87.8 ± 0.9 | 94.6±1.1* |
| Cerebral blood flow (cm s−1) | 65.6 ± 1.0 | 65.4 ± 1.1 |
| Forearm blood flow (cm s−1) | 3.51 ± 1.7 | 4.65±0.17* |
| Cerebrovascular resistance (mmHg cm−1 s) | 1.36 ± 0.03 | 1.47±0.03* |
| Forearm vascular resistance (mmHg cm−1 s) | 29.5 ± 2.0 | 21.7±0.8* |
| CO2 (mmHg) | 37.0 ± 0.7 | 36.9 ± 0.5 |
Statistics from Frequency × Condition ANOVA: * indicates condition effect (P < 0.001). There was no frequency or interaction effect in any variable.
Arterial pressure and cerebral flow oscillations in response to OLBNP were increased with cholinergic blockade despite the lower OLBNP amplitude (30 mmHg vs. 15 mmHg). Arterial pressure oscillations were increased three- to four-fold across all frequencies after blockade (Fig. 1). However, cerebral blood flow oscillations increased only at the higher frequencies and were, in fact, more than five times as large at 0.08 Hz (2.72 ± 1.89 vs. 14.1 ± 4.95 cm2 s−2 Hz−1, P < 0.001), but were decreased by nearly 20% at 0.03 Hz (3.63 ± 0.8 vs. 2.96 ± 1.6 cm2 s−2 Hz−1, P < 0.05) after cholinergic blockade. Transfer function analysis of cerebral blood flow responses revealed that cholinergic blockade increased cross-spectral coherence at all frequencies except 0.03 Hz (0.49 ± 0.07 vs. 0.44 ± 0.09, P= 0.074; Fig. 2). Greater coherence between pressure and flow at higher frequencies after blockade corresponded to gains that were higher by ∼40% at 0.06, 0.07 and 0.08 Hz. Although estimates of phase can be quite unreliable when coherence is low (Hamner et al. 2004), we did observe a consistent and significant decrease at frequencies greater than 0.04 Hz (average decrease: 24.0 ± 6.3 deg). In contrast to the response at higher frequencies, the cerebrovascular response to blockade was markedly different at 0.03 Hz. Gain decreased from 0.33 ± 0.05 to 0.11 ± 0.03 and neither coherence nor phase was significantly altered.
Figure 1. Spectral density of arterial blood pressure and cerebral blood flow at each frequency of OLBNP with and without cholinergic blockade.

Freq, frequency; Cond, condition; Freq × Cond, frequency and condition interaction term. *P < 0.05 vs. control at the OLBNP frequency.
Figure 2. Transfer function coherence and gain between arterial blood pressure and cerebral blood flow with and without cholinergic blockade.

Freq, frequency; Cond, condition; Freq × Cond, frequency and condition interaction term. *P < 0.05 vs. control at the OLBNP frequency.
In the forearm, the amplitude of blood flow oscillations demonstrated a frequency dependence similar to arterial pressure (P < 0.05), but this was unaffected by blockade (0.153 ± 0.035 vs. 0.166 ± 0.025 cm2 s−2 Hz−1, P= 0.87). Coherence between arterial pressure and forearm blood flow was increased in a small but significant manner across frequencies by cholinergic blockade (0.67 ± 0.033 vs. 0.77 ± 0.023, P < 0.01) with decreased gain at 0.03, 0.04 and 0.05 Hz. This was a nearly opposite pattern of change in comparison with the cerebral circulation (decreased gain at frequencies below 0.06 Hz vs. increased gain at frequencies greater than 0.05 Hz; Fig. 3). The exception was at 0.03 Hz, where the decrease in gain with blockade appeared qualitatively similar in both vascular beds.
Figure 3. Change in transfer function gain with cholinergic blockade.
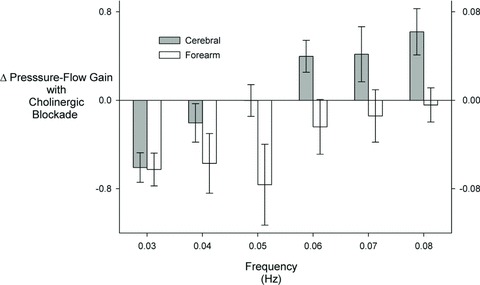
Left axis is cerebral and right axis is forearm.
As an additional check against cholinergic blockade causing a generalized change in cerebral vascular responsiveness instead of having a direct effect on cerebral autoregulation, we examined breath-by-breath CO2 reactivity. We performed an analysis of 5 min of unpaced breathing during supine rest before and after cholinergic blockade for each subject and explored the transfer function gain between end-tidal CO2 and cerebrovascular conductance in a 0.1 Hz band around each subject's dominant respiratory frequency. There was no change in this index of breath-by-breath CO2 reactivity with cholinergic blockade (P= 0.23).
Discussion
Our data are the first to characterize the frequency-dependent role of the cholinergic system in cerebrovascular regulation in humans. Surprisingly, the frequency range and the magnitude of effect were very similar to that of the sympathetic system. This suggests that a balance between these two regulatory systems may be important for appropriate autoregulation. Furthermore, it may be important to explore the possible role that disruptions in this balance have in the vascular pathology present in numerous cerebrovascular disease states.
Despite mounting evidence, the role of the autonomic nervous system in cerebral autoregulation remains controversial (van Lieshout & Secher, 2008). In 2002, Zhang et al. (2002) showed an >80% increase in transfer function gain between arterial pressure and cerebral blood flow in the frequency range 0.02–0.09 Hz after full ganglionic blockade, but cross-spectral coherence was diminished (probably due to the decrease in spontaneous pressure fluctuations after blockade) to such an extent that clear interpretation of gain changes was problematic. Ogoh et al. (2008) examined the response to bilateral ischaemic thigh cuffs after alpha adrenergic blockade and found reduced cerebral flow responsiveness to this brief acute stimulus, but the role the sympathetic system across the broad range of frequency responses remained ill-defined. Our previous work showed that the ability of the cerebrovasculature to buffer against augmented blood pressure fluctuations was impaired after sympathetic blockade, but only at frequencies greater than 0.05 Hz (Hamner et al. 2010). Thus, while the sympathetic nervous system clearly plays a prominent role in the regulation of cerebral perfusion at faster frequencies, it is not sufficient to explain the buffering of slower oscillations.
Considering this together with the findings of Zhang et al. (2002), we hypothesized that at least a portion of the unexplained cerebral autoregulation at slower frequencies is due to an active cholinergic vasodilatory system. Although it is commonly believed that slower oscillations are buffered by myogenic mechanisms, recent work has suggested that calcium channel blockade has no effect on the transfer function gain between pressure and flow (Tzeng et al. 2011). Thus, other mechanisms must be active in this region, and so we expected to find the cholinergic system most active in the frequencies below 0.05 Hz, where neither the sympathetic nervous system nor myogenic mechanisms appear to play a role. To our surprise, cholinergic blockade increased coherence across all frequencies except 0.03 Hz and increased gain only at frequencies greater than 0.05 Hz. This latter effect was similar to sympathetic blockade. Unlike sympathetic blockade, however, gain decreased with cholinergic blockade at 0.03 Hz. Regardless, the correspondence between cholinergic and sympathetic cerebrovascular regulation above 0.05 Hz is striking. This might indicate that the cerebral circulation engages different mechanisms to protect against rising vs. falling pressures. Vasodilatory or vasoconstrictor mechanisms alone may be less effective in regulating flow responses to pressure changes in both directions. Potential evidence for this is shown by the signal averaged response to 0.08 Hz OLBP for both arterial pressure and cerebrovascular resistance in Fig. 4. In the unblocked state, resistance appropriately decreases during the decrease in pressure to maintain perfusion, but after cholinergic blockade, resistance paradoxically increases. This might be consistent with a sympathetic activation due to falling pressure that is not counteracted by cholinergic vasodilatation. Thus, in the healthy cerebral autoregulation, cholinergic vasodilatation may ‘balance’ sympathetic vasoconstriction.
Figure 4. Signal averaged arterial blood pressure and cerebrovascular resistance for one cycle of 0.08 Hz OLBNP.
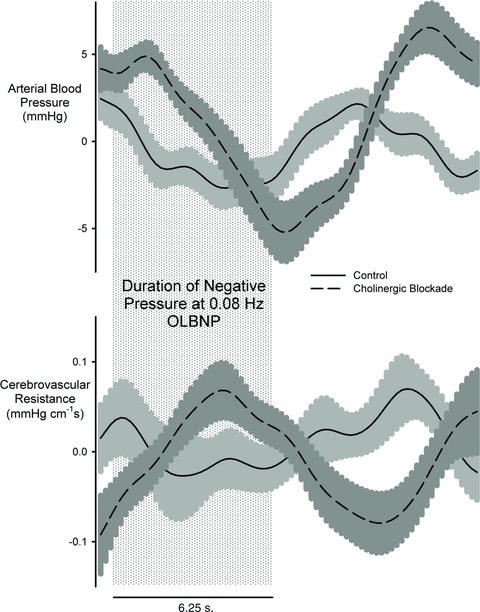
The shaded area indicates the application of negative pressure for 6.25 s.
Our data also suggest that the cholinergic control in the cerebral vasculature is unique to the brain and that blockade did not exert non-specific changes in cerebrovascular regulation. Data from the forearm demonstrate a nearly opposite effect of cholinergic blockade, suggesting differential control of vasodilatation between the two vascular beds. While gain between pressure and flow increased with cholinergic blockade at higher frequencies (>0.05 Hz) in the cerebral circulation, it decreased at lower frequencies (<0.06 Hz) in the forearm, with the only similarity being that a decrease in gain at 0.03 Hz occurred in both. Although it seems that cholinergically mediated vasodilatation should operate on a similar time scale regardless of end organ, the distribution muscarinic receptor subtypes may play a key role in how vasodilatation is effected. In skeletal muscle, M3 receptors are dominant and are almost solely responsible for the vasodilatory response to acetylcholine (Gericke et al. 2011), whereas in the cerebral vasculature, M5 receptors are most prevalent and mediate vasodilatation (Elhusseiny & Hamel, 2000; Yamada et al. 2001). Moreover, cholinergic blockade in the periphery would inhibit nitric oxide release from the endothelium, and consistent with our findings in the forearm, nitric oxide appears to buffer blood pressure oscillations at frequencies between ∼0.02 and 0.05 Hz (50–20 s fluctuations) (Cordero et al. 1994) and (Stauss et al. 1999). We also found that the effect of cholinergic blockade did not impact the gain between end-tidal CO2 and cerebrovascular conductance. This result, coupled with the fact that end-tidal CO2 was unaffected by cholinergic blockade, suggests that non-specific changes in vascular responsiveness due to blockade cannot explain our findings.
Implications
Given that glycopyrrolate does not readily cross the blood–brain barrier (Proakis & Harris, 1978), our data provide evidence for a vascular role of cholinergic control of cerebral perfusion that is independent of possible interactions within the central nervous system. An active cholinergic vasodilatory reflex in balance with sympathetic vasoconstriction in the cerebral circulation has numerous implications for our understanding of cerebrovascular disorders. Acetylcholinesterase inhibitors are not typically used for their vascular effects, but disruption of cholinergic vasodilatation seems likely given the cholinergic deficits (Ogawa et al. 1996) and neuronal damage (Sakuma et al. 2008) observed after brain ischaemia. In the case of ischaemic stroke, The Mayo Clinic recently completed phase IIA of a clinical trial exploring the therapeutic effects of donepezil in enhancing recovery (Barrett et al. 2011). While their hypothesis relates to cognitive mechanisms, increasing cholinergic activity may also improve cerebral perfusion and autoregulation in these patients. Indeed, there is evidence of an association between decreased cerebral control of perfusion and cognitive impairment (Ghogawala et al. 2012; Gommer et al. 2012), wherein insufficient blood flow supply to meet metabolic demand may underlie these deficits. Similarly, rivastigmine's beneficial effect on cognitive function after traumatic brain injury (Silver et al. 2009), might relate to improvements in cerebral vascular function. In Alzheimer's disease patients, acetylcholinesterase inhibitors are also used primarily for their central neural effects, but it is plausible that some portion of their symptoms results from hypoperfusion (Brown & Thore, 2011). Indeed, there is already evidence that treatment improves or preserves regional cerebral blood flow (Ceravolo et al. 2004; Venneri et al. 2002), but our data suggest a possible improvement in global cerebral autoregulation as well. In vascular dementia, where a vascular role in symptomatology is not debated, acetylcholinesterase inhibitors have also been successful in symptom treatment (Roman et al. 2010). Therefore, while our study involved only healthy young subjects, our results may have import for the understanding and treatment of numerous cerebral vascular disease states.
Limitations
One potential issue with our study is that we did not test the completeness of cholinergic blockade. However, our mean R–R interval of roughly 600 ms is comparable to that seen in complete cholinergic blockade with atropine (Taylor et al. 1998). Another possible limitation is that Doppler ultrasonography measures blood flow velocity, not blood flow itself, and thus can be confounded if the diameter of the artery being insonated changes. There is strong evidence that LBNP does not alter the diameter of the middle cerebral (Serrador et al. 2000) nor the brachial arteries (Padilla et al. 2010), but given that muscarinic receptors are present in both, it is possible that cholinergic blockade could have resulted in a constriction of either. Though atropine appears to have no effect on brachial diameter in humans (Shoemaker et al. 1997), there are no comparable data to address this question in the middle cerebral artery in humans. Cerebral artery diameters in M5 knockout mice are not different from wild type (Yamada et al. 2001), but it is unclear if this would hold true with pharmacological blockade in humans. If there is a cholinergic component to middle cerebral artery vasomotor tone, then blockade would cause a constriction of the middle cerebral artery, leading to an overall decrease in flow in response to higher arterial pressure. This could indicate a sympathetic counter regulatory response that might fit with the improvement in gain seen at the slowest frequency of OLBNP. Regardless of any possible changes in the effect of diameter on steady-state measures of flow, it is important to note that transfer function analysis utilizes data with linear trends removed and means subtracted. Therefore, if cholinergic blockade altered mean flow it would not affect frequency domain analysis.
Summary
The cholinergic system plays a prominent role in the regulation of brain blood flow. It appears to tonically balance sympathetic vasoconstriction with active vasodilatation, while also dynamically responding to falls in pressure while sympathetic activation counteracts rises. Much like the sympathetic system, cholinergic vasodilatation appears to be most effective regulating against pressure changes faster than 0.05 Hz (∼20 s). In contrast, cholinergic vasodilatation in the forearm appears most effective at slower frequencies (<0.06 Hz). This suggests differential cholinergic control of vasodilatation between the cerebral and peripheral vasculatures. These findings might have implications for the role of dysfunction in autonomic control of the vasculature in cerebrovascular disease states.
Acknowledgments
This research was supported by grant HL093113 from the National Heart, Lung and Blood Institute. We also thank the subjects for their generous participation. The authors have no conflicts of interest to disclosure or declare.
Glossary
- OLBNP
oscillatory lower body negative pressure
References
- Barrett KM, Brott TG, Brown RD, Jr, Carter RE, Geske JR, Graff-Radford NR, McNeil RB, Meschia JF. Enhancing recovery after acute ischemic stroke with donepezil as an adjuvant therapy to standard medical care: results of a phase IIA clinical trial. J Stroke Cerebrovasc Dis. 2011;20:177–182. doi: 10.1016/j.jstrokecerebrovasdis.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev. 2006:CD001190. doi: 10.1002/14651858.CD001190.pub2. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An Analysis of transformations (with discussion) J Roy Statist Soc Ser B. 1964;26:211–252. [Google Scholar]
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busija DW, Heistad DD. Effects of cholinergic nerves on cerebral blood flow in cats. Circ Res. 1981;48:62–69. doi: 10.1161/01.res.48.1.62. [DOI] [PubMed] [Google Scholar]
- Ceravolo R, Volterrani D, Tognoni G, Dell’Agnello G, Manca G, Kiferle L, Rossi C, Logi C, Strauss HW, Mariani G, Murri L. Cerebral perfusional effects of cholinesterase inhibitors in Alzheimer disease. Clin Neuropharmacol. 2004;27:166–170. doi: 10.1097/01.wnf.0000138636.42121.45. [DOI] [PubMed] [Google Scholar]
- Cordero JJ, Gonzalez J, Feria M. Effects of N omega-monomethyl-L-arginine on short-term RR interval and systolic blood pressure oscillations. J Cardiovasc Pharmacol. 1994;24:323–327. [PubMed] [Google Scholar]
- D’Alecy LG, Rose CJ. Parasympathetic cholinergic control of cerebral blood flow in dogs. Circ Res. 1977;41:324–331. doi: 10.1161/01.res.41.3.324. [DOI] [PubMed] [Google Scholar]
- Edvinsson L. Neurogenic mechanisms in the cerebrovascular bed. Autonomic nerves, amine receptors and their effects on cerebral blood flow. Acta Physiol Scand Suppl. 1975;427:1–35. [PubMed] [Google Scholar]
- Elhusseiny A, Cohen Z, Olivier A, Stanimirovic DB, Hamel E. Functional acetylcholine muscarinic receptor subtypes in human brain microcirculation: identification and cellular localization. J Cereb Blood Flow Metab. 1999;19:794–802. doi: 10.1097/00004647-199907000-00010. [DOI] [PubMed] [Google Scholar]
- Elhusseiny A, Hamel E. Muscarinic – but not nicotinic – acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: a possible role for the M5 receptor subtype. J Cereb Blood Flow Metab. 2000;20:298–305. doi: 10.1097/00004647-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. Edinburgh: Originally published by Oliver and Boyd; 1958. [Google Scholar]
- Gericke A, Sniatecki JJ, Mayer VG, Goloborodko E, Patzak A, Wess J, Pfeiffer N. Role of M1, M3, and M5 muscarinic acetylcholine receptors in cholinergic dilation of small arteries studied with gene-targeted mice. Am J Physiol Heart Circ Physiol. 2011;300:H1602–H1608. doi: 10.1152/ajpheart.00982.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghogawala Z, min-Hanjani S, Curran J, Ciarleglio M, Berenstein A, Stabile L, Westerveld M. The effect of carotid endarterectomy on cerebral blood flow and cognitive function. J Stroke Cerebrovasc Dis. 2012 doi: 10.1016/j.jstrokecerebrovasdis.2012.03.016. (published ahead of print) [DOI] [PubMed] [Google Scholar]
- Gommer ED, Martens EG, Aalten P, Shijaku E, Verhey FR, Mess WH, Ramakers IH, Reulen JP. Dynamic cerebral autoregulation in subjects with Alzheimer's disease, mild cognitive impairment, and controls: evidence for increased peripheral vascular resistance with possible predictive value. J Alzheimers Dis. 2012;30:805–813. doi: 10.3233/JAD-2012-111628. [DOI] [PubMed] [Google Scholar]
- Hamner JW, Cohen MA, Mukai S, Lipsitz LA, Taylor JA. Spectral indices of human cerebral blood flow control: responses to augmented blood pressure oscillations. J Physiol. 2004;559:965–73. doi: 10.1113/jphysiol.2004.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner JW, Tan CO, Lee K, Cohen MA, Taylor JA. Sympathetic control of the cerebral vasculature in humans. Stroke. 2010;41:102–109. doi: 10.1161/STROKEAHA.109.557132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Donepezil and memantine for moderate-to-severe Alzheimer's disease. N Engl J Med. 2012;366:893–903. doi: 10.1056/NEJMoa1106668. [DOI] [PubMed] [Google Scholar]
- Koopmans LH. The Spectral Analysis of Time Series. 2nd ed. New York: Academic Press; 1995. p. 366. [Google Scholar]
- Ocon AJ, Medow MS, Taneja I, Clarke D, Stewart JM. Decreased upright cerebral blood flow and cerebral autoregulation in normocapnic postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H664–H673. doi: 10.1152/ajpheart.00138.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N, Asanuma M, Tanaka K, Hirata H, Kondo Y, Goto M, Kawauchi M, Ogura T. Long-term time course of regional changes in cholinergic indices following transient ischemia in the spontaneously hypertensive rat brain. Brain Res. 1996;712:60–68. doi: 10.1016/0006-8993(95)01446-2. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Eubank WL, Raven PB. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke. 2008;39:1979–1987. doi: 10.1161/STROKEAHA.107.510008. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, Raven PB, Secher NH. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol. 2005;288:H1461–H1467. doi: 10.1152/ajpheart.00948.2004. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Tzeng YC, Lucas SJ, Galvin SD, Ainslie PN. Influence of baroreflex-mediated tachycardia on the regulation of dynamic cerebral perfusion during acute hypotension in humans. J Physiol. 2010;588:365–371. doi: 10.1113/jphysiol.2009.180844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol. 2010;298:H1128–H1135. doi: 10.1152/ajpheart.01133.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panerai RB, Rennie JM, Kelsall AW, Evans DH. Frequency-domain analysis of cerebral autoregulation from spontaneous fluctuations in arterial blood pressure. Med Biol Eng Comput. 1998;36:315–322. doi: 10.1007/BF02522477. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Proakis AG, Harris GB. Comparative penetration of glycopyrrolate and atropine across the blood–brain and placental barriers in anaesthetized dogs. Anesthesiology. 1978;48:339–344. doi: 10.1097/00000542-197805000-00007. [DOI] [PubMed] [Google Scholar]
- Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Med. 2007;37:765–782. doi: 10.2165/00007256-200737090-00002. [DOI] [PubMed] [Google Scholar]
- Roman GC, Salloway S, Black SE, Royall DR, Decarli C, Weiner MW, Moline M, Kumar D, Schindler R, Posner H. Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke. 2010;41:1213–1221. doi: 10.1161/STROKEAHA.109.570077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma M, Hyakawa N, Kato H, Araki T. Time dependent changes of striatal interneurons after focal cerebral ischemia in rats. J Neural Transm. 2008;115:413–422. doi: 10.1007/s00702-007-0860-z. [DOI] [PubMed] [Google Scholar]
- Searle SR. Linear Models for Unbalanced Data. New York: Wiley; 1987. [Google Scholar]
- Seifert T, Fisher JP, Young CN, Hartwich D, Ogoh S, Raven PB, Fadel PJ, Secher NH. Glycopyrrolate abolishes the exercise-induced increase in cerebral perfusion in humans. Exp Physiol. 2010;95:1016–1025. doi: 10.1113/expphysiol.2010.054346. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol Heart Circ Physiol. 1997;273:H2388–H2395. doi: 10.1152/ajpheart.1997.273.5.H2388. [DOI] [PubMed] [Google Scholar]
- Silver JM, Koumaras B, Meng X, Potkin SG, Reyes PF, Harvey PD, Katz DI, Gunay I, Arciniegas DB. Long-term effects of rivastigmine capsules in patients with traumatic brain injury. Brain Inj. 2009;23:123–132. doi: 10.1080/02699050802649696. [DOI] [PubMed] [Google Scholar]
- Stauss HM, Godecke A, Mrowka R, Schrader J, Persson PB. Enhanced blood pressure variability in eNOS knockout mice. Hypertension. 1999;33:1359–1363. doi: 10.1161/01.hyp.33.6.1359. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- Tzeng YC, Chan GS, Willie CK, Ainslie PN. Determinants of human cerebral pressure-flow velocity relationships: new insights from vascular modelling and Ca2+ channel blockade. J Physiol. 2011;589:3263–3274. doi: 10.1113/jphysiol.2011.206953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lieshout JJ, Secher NH. Point:Counterpoint: Sympathetic activity does/does not influence cerebral blood flow. Point: Sympathetic activity does influence cerebral blood flow. J Appl Physiol. 2008;105:1364–1366. doi: 10.1152/japplphysiol.90597.2008. [DOI] [PubMed] [Google Scholar]
- Venneri A, Shanks MF, Staff RT, Pestell SJ, Forbes KE, Gemmell HG, Murray AD. Cerebral blood flow and cognitive responses to rivastigmine treatment in Alzheimer's disease. Neuroreport. 2002;13:83–87. doi: 10.1097/00001756-200201210-00020. [DOI] [PubMed] [Google Scholar]
- Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroaccoust. 1967;15:70–73. [Google Scholar]
- Yamada M, Lamping KG, Duttaroy A, Zhang W, Cui Y, Bymaster FP, McKinzie DL, Felder CC, Deng CX, Faraci FM, Wess J. Cholinergic dilation of cerebral blood vessels is abolished in M(5) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A. 2001;98:14096–14101. doi: 10.1073/pnas.251542998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–1820. doi: 10.1161/01.cir.0000031798.07790.fe. [DOI] [PubMed] [Google Scholar]


