Abstract
Heating skin is believed to activate vanilloid type III and IV transient receptor potential ion channels (TRPV3, TRPV4, respectively), resulting in the release of ATP into the interstitial fluid. We examined the hypothesis that local skin heating would result in an accumulation of ATP in the interstitial fluid that would be related with a rise in skin blood flow (SkBF) and temperature sensation. Two microdialysis probes were inserted into the dermis on the dorsal aspect of the forearm in 15 young, healthy subjects. The probed skin was maintained at 31°C, 35°C, 39°C and 43°C for 8 min periods, during which SkBF was monitored as cutaneous vascular conductance (CVC). Dialysate was collected and analysed for ATP ([ATP]d) using a luciferase-based assay, and ratings of perceived warmth were taken at each temperature. At a skin temperature of 31°C, [ATP]d averaged 18.93 ± 4.06 nm and CVC averaged 12.57 ± 1.59% peak. Heating skin to 35°C resulted in an increase in CVC (17.63 ± 1.27% peak; P < 0.05), but no change in [ATP]d. Heating skin to 39°C and 43°C resulted in a decreased [ATP]d (5.88 ± 1.68 nm and 8.75 ± 3.44 nm, respectively; P < 0.05), which was accompanied by significant elevations in CVC (38.90 ± 1.37% peak and 60.32 ± 1.95% peak, respectively; P < 0.05). Ratings of perceived warmth increased in proportion to the increase in skin temperature (r2 = 0.75, P < 0.05). In conclusion, our data indicate that an accumulation of interstitial ATP does not occur during local heating, and therefore does not have a role in temperature sensation or the dilator response in human skin. Nevertheless, the low threshold of dilatation (35°C) indicates a possible role for the TRPV3, TRPV4 channels or the sensitization of other ion channels in mediating the dilator response.
Key points
Heating human skin results in a localized increase in blood flow (hyperaemia) to the skin.
Experiments in mouse skin and cultured human cells suggest that skin cells known as keratinocytes release the chemical ATP, a known cutaneous vasodilator, and that the release of this ATP increases when temperature-sensing vanilloid type III and IV transient receptor potential ion channels (TRPV3, TRPV4 respectively) are activated by heating. Studies also suggest that this ATP release is necessary for temperature sensation.
We hypothesized that, like mouse skin locally heating human skin to temperatures that activate heat-sensing TRPV3 and TRPV4 channels would be associated with an accumulation of ATP in the interstitial space that would be related to temperature sensation.
We also hypothesized that the accumulation of ATP would be associated with the magnitude of heat-induced hyperaemia to the area.
We report that, unlike mouse skin, such local heating does not result in an accumulation of ATP in the interstitial space of human skin, and therefore such an accumulation is not necessary for cutaneous temperature sensation and local reactive hyperaemia.
We also report that warming skin from 31°C results in dilatation at temperatures as low as 35°C, which is several degrees lower than previously reported. This suggests that TRPV3 and/or TRPV4 channels have a role in heat-induced hyperaemia or that such heating sensitizes TRPV1 channels to respond to temperatures below their typical threshold.
Introduction
Skin blood flow (SkBF) plays a major role in maintaining thermal homeostasis when the body is under heat stress (Rowell, 1977; Kellogg, 2006; Johnson & Kellogg, 2010). Adjustments in SkBF in response to thermal stimuli are mediated by local and reflex neural mechanisms (Charkoudian, 2003; Kellogg, 2006). While changes in whole-body temperature typically result in a whole-body response, the application of a heat source to a localized area of skin results in a localized increase in blood flow to the heated skin.
Under normal circumstances, a localized, biphasic increase in SkBF is observed when human skin is heated to innocuous temperatures (between 35°C and 43°C; (Minson et al. 2001; Johnson & Kellogg, 2010). The initial phase of the SkBF response is a prompt dilatation around the heated area that peaks within 3–5 min followed by a rapid, yet transient decrease in blood flow. Subsequently a gradual increase in blood flow around the heated area results in a long-lasting dilatation that comes to a plateau between 20 and 40 min (Minson et al. 2001; Charkoudian, 2003; Kellogg, 2006). Greater understanding of the mechanisms at play in the SkBF response to local heating may lead to increased utility of measures that attempt to use this response to assess microvascular function (Minson, 2010).
A significant amount of research has been directed towards understanding the mechanisms behind the two phases of the response, but parts of the response remain enigmatic. Much, but not all, of the initial phase appears to be the result of a rapid neural event known as an axon reflex (Minson et al. 2001). Many researchers (Minson et al. 2001; Charkoudian, 2003; Wong & Minson, 2011) suggest that the initiation and termination of the axon reflex is a function of the release of calcitonin gene-related peptide and Substance P from local nerve endings in the skin. Part of the dilatation induced by the axon reflex appears to be mediated by the enzyme nitric oxide synthase (NOS) and its product NO as blockade of NOS results in an attenuated initial peak in SkBF (Minson et al. 2001).
The axon reflex and NOS do not account for all of the dilatation observed during the first phase, as blockade of both results in a blunted, yet persistent, response (Minson et al. 2001). This indicates that there are additional mechanisms involved in the first phase of dilatation besides the axon reflex. One other factor in this response appears to be the nucleoside adenosine, which has recently been shown to account for about 16% of the initial peak, upstream of NOS (Fieger & Wong, 2010). The source of the adenosine involved in this dilatation is not clear at this point.
Temperature-sensitive ion channels from the vanilloid family of transient receptor potential ion channels (TRPV) appear to also have a role in mediating the initial peak of the SkBF response to local heating. Type I TRPV channels (TRPV1), abundantly expressed in the free nerve endings of sensory fibres innervating the skin, are sensitive to temperatures above ∼43°C (Caterina et al. 1997; Tominaga et al. 1998; Schepers & Ringkamp, 2010). The TRPV1 channel has recently been found to play a substantial role in the initial peak of the SkBF response to local heating. Inhibition of the TRPV1 channel results in a substantial attenuation of the initial peak, such that TRPV1 appears to account for sensing and mediating 50% of the initial response, part of which appears to be independent of NOS (Wong & Fieger, 2010).
Type III (TRPV3) and type IV (TRPV4) TRPV ion channels are activated by temperatures above ∼33°C and ∼27°C, respectively, and are increasingly activated as the temperatures increase above these lower thresholds (Guler et al. 2002; Schepers & Ringkamp, 2010). Unlike the TRPV1 channels, the TRPV3 and TRPV4 channels are abundantly found in the keratinocytes of the skin (Peier et al. 2002; Chung et al. 2004). Mandadi et al. (2009) showed that upon heating the skin of a mouse, the TRPV3 and TRPV4 ion channels appear to evoke the release of ATP from the keratinocytes into the interstitial fluid, which subsequently binds to purinergic receptors on nearby nerves, thereby possibly relaying thermal information from the keratinocytes to the nervous system. The magnitude of the release of ATP in this model appears to be proportional to the degree of heat-induced activation of the TRPV3 and TRPV4 ion channels (Mandadi et al. 2009). While human keratinocytes in culture have been shown to release ATP (Dixon et al. 1999; Burrell et al. 2005), it is unclear if ATP accumulates in the dermal interstitial space in response to heating in human skin in vivo.
The role of the TRPV3 and TRPV4 ion channels in the initial peak of the SkBF response to local heating is unknown at this time, but considering that heat-activation of these channels may induce the release of ATP, a putative cutaneous vasodilator (Wingo et al. 2010), it seems feasible that these ion channels are involved in mediating the hyperemic response to local heating. Furthermore, if ATP is released upon heating human skin, it seems like a possible source of the adenosine that has been shown to play a role in the response (Fieger & Wong, 2010), as ATP can be broken down extracellularly to adenosine (Zimmerman, 1997; Burnstock et al. 2012).
The purpose of this study was to investigate the possibility that heating human skin to temperatures believed to activate the TRPV3 and TRPV4 ion channels results in the accumulation of ATP in the dermal interstitial fluid. In addition we set out to determine if the concentration of ATP in the interstitial fluid has any relationship with the initial peak of the SkBF response to local heating or with the magnitude of perceived warmth.
The most direct way to determine if ATP has a role in the SkBF response to local heating and in temperature sensation in humans would be to heat the skin with and without ATP receptor antagonists on board. This method remains impossible at the current time because, to our knowledge, no antagonist of ATP receptors is safe for human use. As such, this study was designed to test the role of ATP during the first phase of the SkBF response to local heating by heating the skin to temperatures that vary in the magnitude of activation of TRPV3 and TRPV4 ion channels while measuring changes in SkBF, perceived warmth and the concentration of ATP in dermal interstitial fluid at each temperature. We hypothesized that the concentration of ATP present in the interstitial fluid of the skin during the first phase of the SkBF response to local heating would increase as the temperature of the skin was warmed to temperatures known to increasingly activate the TRPV3 and TRPV4 ion channels in the skin. We also hypothesized that the concentration of ATP in the interstitial fluid of the skin would correlate to SkBF and perceived magnitude of thermal sensation at each temperature.
Methods
Ethical approval
All methods and procedures for this study were approved by the Institutional Review Board at Brigham Young University. Prior to participating in the study, the procedures and risks of the study were explained to the subjects, and all subjects provided written informed consent. All protocols used in this study conformed to the criteria described by the Declaration of Helsinki.
Subjects
Fifteen healthy, non-smokers (eight males and seven females) between 19 and 31 years old participated in this study. Subjects had no history of cardiovascular disease or diabetes. Subjects refrained from exercise and the consumption of alcohol and caffeine for 12 h immediately before participating in the study. Subjects were also free from any prescription medication at the time of the study. Female subjects were tested during the first week after completion of the menstrual cycle to minimize the variation in body temperature and SkBF associated with fluctuations in hormone concentrations (Charkoudian, 2003).
Procedures
After giving written consent, subjects were seated in a dental chair inside an environmental chamber with the temperature set to 28 ± 1°C. Sterile, 27-gage needles were used to insert, in parallel (within 1–2 mm of each other), two microdialysis probes with hollow fibres of a molecular weight cutoff of 18 kDa (Spectrum Laboratories Inc., Rancho Dominguez, CA, USA). The two microdialysis probes were inserted ≍1 mm deep into the skin at a site on the dorsal aspect of the forearm free of superficial veins. After the insertion of the probes, 0.9% sterile saline was perfused through the probes at a rate of 10 μl min−1 using a Harvard model PHD 2000 pump (Harvard Apparatus, Holliston, MA, USA) until the SkBF stabilized (minimum of 60 min). Blood pressure and heart rate were monitored in 4 min intervals throughout the experiment on the arm free of probes using a non-invasive automated brachial artery cuff and three-lead EKG (Model 2120, Tango+ Stress BP; Suntech Medical, Morrisville, NC, USA).
Immediately after placing the microdialysis probes in the skin, a peltier module (3 × 3 cm) was placed on the skin above the probes to maintain the skin at a temperature of 31°C. A laser-Doppler probe (Model A25059; Moor Instruments, Devon, England) inserted through the middle of the peltier module was also placed on the skin at this time.
Once the SkBF stabilized, at least 60 min after needle insertion, the perfusion rate of sterile saline in the probes was slowed to 2 μl min−1. Pilot studies indicated that in vitro recovery of ATP from a saline bath at a perfusion rate of 2 μl min−1 with our microdialysis probes averaged 36%. At this point, the experimental protocol commenced. Local skin temperatures included in the experimental protocol (31°C, 35°C, 39°C and 43°C) were chosen specifically to manipulate the activation of the TRPV3 and TRPV4 ion channels from minimal activation (31°C) to near-maximal activation (43°C; Guler et al. 2002; Peier et al. 2002; Chung et al. 2004). During the heating protocol, skin temperature was increased above baseline (31°C) at a rate of 0.2°C per second, held at the stimulus temperature for 8 min and returned to baseline at a rate of 0.2°C per second. Following each 8 min heating stimulus, the skin temperature was maintained at 31°C until SkBF stabilized at baseline levels. This required 16 min following heating to 35°C and 32 min following heating to 39°C. The order of the skin temperature changes was similar in all trials, with the hottest temperatures being performed last (31°C, 35°C, 39°C and then 43°C) to minimize the possibility of temperature sensitization of the TRPV channels (Schepers & Ringkamp, 2010) and to avoid the long-lasting elevations of SkBF associated with local heating to warmer temperatures (39°C and 43°C). The dialysate from both microdialysis probes was collected into a single 1.5 ml Eppendorf tube during each 8 min collection phase, which corresponded with the 8 min intervals of the heating protocol. As previously described by others (Mortensen et al. 2011), the small pore size of the microdialysis probes (18 kDa) enhances the stability of the ATP concentration in the dialysate by filtering out larger enzymes like ectonucleotidases. Nevertheless, once a dialysate sample was collected it was immediately frozen on dry ice and stored in a freezer at −80°C. Following the last 8 min heating phase (43°C), skin temperature was maintained at 43°C for an additional 24 min to elicit a peak SkBF.
To determine if subjects could sense the heat and differentiate between different thermal stimuli, subjects provided a rating of perceived warmth for each heating phase using a labelled magnitude scale developed by Green et al. (1993).
Analysis of concentration of ATP
The concentration of ATP in each dialysate sample ([ATP]d) was measured using a chemiluminescence assay (Enliten ATP Assay System; Promega, Madison, WI, USA). In brief, 10 μl of each dialysate sample was placed in a well of a 96 well optiplate (Optiplate 96; Perkin Elmer, Waltham, MA, USA) with 100 μl of luciferase reagent. Each well was exposed to light for 10 s, and counts per second (CPS) for this complex were measured using a luminometer (Perkin Elmer, 1420 Multilabel Counter, Victor3, Finland). Using a standard curve of known concentrations of ATP, the CPS for each dialysate sample were converted to a concentration of ATP (mol l−1). The assay was effective in discriminating concentrations of ATP between 0.1 nm and 1000 nm. The concentration of ATP in each dialysate sample was measured in duplicate, while the standard curve was measured in triplicate. Samples were stored (frozen at −80°C) for no more than 7 days before they were used in the ATP assay. About 25 min passed between sample thawing and the completion of the assay.
Statistical analysis
Major variables that were measured include local skin temperature (°C), the concentration of ATP in the dialysate (mol l−1), heart rate (beats min−1), blood pressure (mmHg) and laser-Doppler flux (LDF; volts). Ratings of warmth were reported as percentages of the strongest imaginable heat sensation by dividing the distance of the rating from zero by the distance of the strongest imaginable label from zero. Vasomotor responses to heating were expressed as cutaneous vascular conductance (CVC) by dividing LDF by mean arterial pressure (MAP) (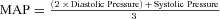 ). CVC was normalized by dividing by the peak CVC observed during minutes 24–32 of the 43°C heating phase and expressed as a percentage of peak CVC.
). CVC was normalized by dividing by the peak CVC observed during minutes 24–32 of the 43°C heating phase and expressed as a percentage of peak CVC.
The differences between means of ratings of perceived warmth, MAP and [ATP]d for each temperature were determined using one-way, repeated measures ANOVA followed by a Holm–Sidak post hoc test. In order to test for significant differences between CVC (% peak) at baseline (average of 30 s immediately prior to each heating phase) and CVC (% peak) at each 30 s interval of the heating phase, a one-way, repeated measures ANOVA was performed, followed by a Holm–Sidak post hoc test. The functional relationship between our outcome variables was evaluated with linear and non-linear, least squares regression analysis. Statistical significance was declared when the P-values were less than 0.05. All statistical analyses were performed on Sigma Plot 11 (Systat Software, Chicago, IL, USA), while the graphs were created on PRISM 4 (Graphpad Software, Inc., La Jolla, CA, USA).
Results
Figure 1 illustrates the average thermal rating, CVC and [ATP]d response for each heating phase (31°C, 35°C, 39°C and 43°C). Baseline [ATP]d obtained during the first 8 min when skin temperature was controlled at 31°C averaged 18.93 ± 4.06 nm. Duing local heating to 35°C the [ATP]d tended to be lower than the baseline (13.71 ± 3.38 nm), but was not found to be significantly different than the baseline (P > 0.05). The values of [ATP]d when the skin was heated to 39°C (5.88 ± 1.68 nm) and 43°C (8.75 ± 3.44 nm) were both found to be significantly less than the baseline [ATP]d (P < 0.05), but not significantly different from each other.
Figure 1. The effect of locally heating skin to different temperatures on rating of perceived warmth, cutaneous vascular conductance (CVC) and the concentration of ATP in dialysate [ATP]d.
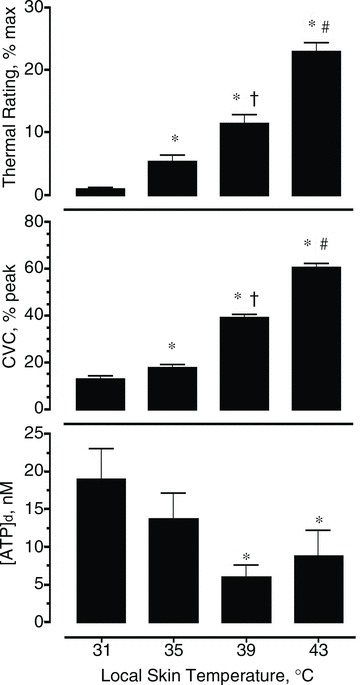
Top, thermal ratings increased significantly as skin temperature increased. Middle, CVC increased significantly with each increase in skin temperature. Bottom, [ATP]d significantly decreased when skin was heated to 39°C and 43°C. Values are mean ± SEM of n = 15 subjects for steady-state readings. *P < 0.05 different from 31°C; †P < 0.05 different from 35°C; #P < 0.05 different from 39°C.
The [ATP]d demonstrated a very weak, negative, linear correlation with peltier module temperature (r2 = 0.11, P < 0.05). While these data indicate that as skin temperature increased the concentration of ATP in the dialysate decreased, no correlation, linear or non-linear, was found between the magnitude of change (from baseline) in temperature and [ATP]d for any heating phase (r2 = 0.04, P = 0.19).
CVC responded to heating in a dose–response fashion, with the hotter temperatures eliciting a greater increase in CVC. The average CVC (% peak) for each 8 min heating period is shown in Fig. 1. CVC (% peak) significantly increased above baseline conductance at 31°C (12.58 ± 1.60% peak), and above each previous temperature, such that the response to each increase in temperature was significantly greater than the response to cooler temperatures. Warming the heater to 35°C resulted in a small yet significant increase in conductance (17.63 ± 1.23% peak; P < 0.05), while heating to 39°C and 43°C resulted in larger, significant increases in CVC (38.89 ± 1.34% peak and 60.32 ± 1.95% peak respectively; P < 0.05).
CVC and [ATP]d were found to be weakly, yet significantly, negatively correlated (r2 = 0.07, P < 0.05). The ratings of perceived warmth showed a weak negative linear correlation with [ATP]d (r2 = 0.07, P < 0.05). While statistically significant, the very low correlation coefficients for [ATP]d with CVC and ratings of perceived warmth may indicate that these relationships are not very meaningful.
Each increase in temperature resulted in a significant increase in the rating of perceived warmth, such that each temperature elicited ratings that were unique and significantly different from the other temperatures. Ratings of perceived warmth were strongly correlated with CVC (% peak; r2 = 0.75, P < 0.05; Fig. 2).
Figure 2. Relationship between the rating of perceived warmth and cutaneous vascular conductance (CVC) expressed as a percentage of the peak CVC following 40 min of heating at 43°C.
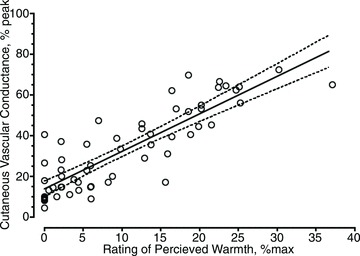
Best fit least squares regression line with 95% confidence intervals. r2 = 0.75, P < 0.05.
When examining the kinetics of the onset of dilatation in more detail, significant dilatation at 35°C occurred within 1 min of the onset of heating (P < 0.05), significant dilatation at 39°C and 43°C occurred within 1.5 min after the onset of heating (P < 0.05; Fig. 3). The delay in dilatation at 39°C and 43°C compared with 31°C appears to be a factor of greater variability in CVC at the onset of heating to warmer temperatures. It is important to note that MAP was constant throughout the entire experiment (79.3 ± 0.3 mmHg), indicating that any change in CVC came as a result of local factors and not as a consequence of a systemic response.
Figure 3. Thirty second averages of cutaneous vascular conductance (CVC% peak) for three different heating temperatures.
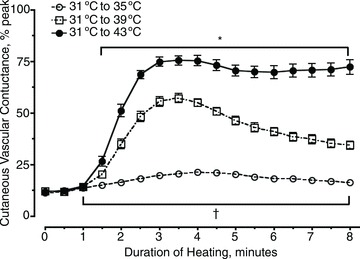
Zero minutes represents baseline CVC (% peak) at 31°C immediately prior to each heating phase. *P < 0.05 different from baseline (0 min) for 39°C and 43°C heating. †P < 0.05 different from baseline (0 min) for 35°C heating.
Discussion
This study yielded two major findings. First, in vivo innocuous local heating is not associated with an accumulation of dermal interstitial [ATP] during the first phase of the biphasic SkBF response in human skin. In fact, intradermal dialysate levels of ATP fell with heating, signifying that a rise in interstitial ATP may not play a role in the axon reflex-induced hyperaemia or in conveying temperature information from the keratinocytes to the nervous system in human skin. Second, significant increases in SkBF are observed when locally heating the skin from 31°C to 35°C, which is several degrees lower than previously reported thresholds (Magerl & Treede, 1996). These two major findings each have several implications for the mechanism of dilatation to local heating and for methods that should be considered in future studies.
As mentioned previously, heating mouse keratinocytes of the skin and of the oesophagus from ∼25°C to 38.5–42.0°C has been shown to result in the release of ATP from the keratinocytes (Mandadi et al. 2009; Mihara et al. 2011). Mandadi et al. (2009) heated excised mouse scrotal skin from 25°C to 42°C, and observed a dose–response increase in the concentration of ATP in the tissue bath that was dependent on the expression of TRPV3 and TRPV4 ion channels in the keratinocytes. Contrary to our hypothesis, we observed a decrease in the concentration of interstitial ATP when heating to temperatures that activate the TRPV3 and TRPV4 ion channels (Fig. 1). As such, locally heating intact human skin does not result in the same increase in [ATP] as it does in excised animal skin. Furthermore, a rise in interstitial ATP does not appear to play a role in the initial peak in SkBF during local heating, as no rise in interstitial ATP was observed. In fact, our data indicate that [ATP] in the interstitial fluid bears no meaningful relationship with CVC during the first phase of local heating (r2 = 0.03, P < 0.05).
While it is impossible to declare with certainty that our protocol did indeed manipulate the activation of the TRPV3 and TRPV4 ion channels, evidence from other studies shows that it is likely that our protocol did so. The TRPV4 ion channels in keratinocytes reportedly have a threshold temperature about 32°C (Chung et al. 2004), and are increasingly activated by further heating up to 42°C (Guler et al. 2002). Similarly, the TRPV3 ion channels are increasingly activated by temperatures from ∼33°C to well above the 43°C employed in our study (Peier et al. 2002). As such it seems likely that our protocol did manipulate the magnitude of activation of the ion channels. Therefore, it appears that heat-induced activation of the TRPV3 and TRPV4 ion channels does not result in an accumulation of ATP in the interstitial fluid of intact human skin during heating like it does in isolated mouse scrotal skin (Mandadi et al. 2009).
As the concentration of any substance in the interstitial fluid can be dramatically affected by many factors, we cannot rule out any role of ATP in the first phase of local heating, but we can safely say that an accumulation of ATP in the dermal interstitial fluid does not appear to have a role in the first phase of local hyperaemia. Testing the effects of the inhibition of the purinergic receptors could provide a more definitive test of the role of ATP in local hyperaemia than collecting interstitial fluid but, to our knowledge, no such antagonist has been approved for human use. Interestingly, inhibition of receptors for adenosine, a component of ATP, results in a blunted initial peak in CVC during the first phase of the SkBF response to local heating in human skin. (Fieger & Wong, 2010). As adenosine can be derived from extracellular ATP (Zimmerman, 1997; Burnstock et al. 2012), it would seem likely that ATP could be a source of adenosine in this response. Nevertheless, we observed that [ATP]d demonstrated no meaningful relationship with CVC or rating of perceived warmth. Therefore, these observations do not support the hypothesis that an accumulation of ATP or its metabolites are necessary for dilatation or thermal sensation in human skin.
The fact that we observed a decrease in interstitial ATP with heating also suggests that different mechanisms may be used in mice and human skin to transmit thermal information from the keratinocytes to nearby nerves. Heating mouse scrotal skin in the presence of TRPV1-deficient nerves results in an increase in cytosolic Ca2+ of the nerve cell, which can be entirely prevented by removing the keratinocyte from the bath or by inhibiting purinergic receptors. This indicates that ATP from keratinocytes plays a significant role in temperature sensation in mouse skin (Mandadi et al. 2009). Given that our human subjects could sense and discriminate between the different temperatures (Fig. 1) without accompanying increases in [ATP]d, interstitial ATP may not play the same role in human skin as it does in mouse skin.
While [ATP]d was found to be a weak predictor of CVC, the subjective ratings of perceived warmth were found to be very strongly correlated with CVC (Fig. 2). The observation that the magnitude of sensation is strongly related to the degree of hyperaemia induced by heating is not unique to this study. Augmenting the sensation of a given heat with the application of capsaicin has been shown to result in an increased hyperemic response (Stephens et al. 2001). Together these data suggest that local hyperaemia due to heating is tightly linked with the mechanisms employed in sensing the heat. It is possible that temperature sensation may have been altered due to probe insertion, but the likelihood of this being a major factor in our study was reduced by allowing the skin 60–90 min to recover before proceeding with any temperature sensation measurements.
The differences in the response of interstitial ATP to heating between our study and those of others (Mandadi et al. 2009; Mihara et al. 2011) may be due to differences between species and the anatomical location of the keratinocytes. However, differences in methodology are also likely to play a role in the results, and should be carefully considered when interpreting the results of this and other studies. The greatest difference between the model that we employed and the model used by others (Dixon et al. 1999; Mandadi et al. 2009) is that our model was in vivo and included natural, physiological fluctuations in SkBF associated with skin heating.
Fluctuations in SkBF may influence the concentration of ATP in the dermal interstitial fluid as [ATP]d showed a negative correlation with CVC (r2 = −0.27, P < 0.05). Furthermore, in our study, the greatest [ATP]d (18.9 ± 4.1 nm) was observed at baseline (31°C) when CVC was at its lowest (Fig. 1.). Assuming that our measure of ATP represents about 36% of the total ATP in the interstitial fluid, as our pilot studies indicate, the baseline concentration of ATP in the interstitial fluid would be about 56 nm, which is consistent with previous research that has reported extracellular ATP concentrations from human keratinocytes in culture without flow to range from 17 nm (Dixon et al. 1999) to 100 nm (Burrell et al. 2005) depending on the number of cells in culture. As we heated the skin to temperatures comparable to those employed by Mandadi et al., there was an expected increase in CVC accompanied by consistent decreases in [ATP]d below our baseline values. It is important to note that significant decreases in [ATP]d occurred only when CVC was markedly elevated above baseline (Fig. 1), and that as the CVC recovered towards baseline during the recovery phase, the [ATP]d tended to recover to baseline levels (Fig. 4).
Figure 4. Concentration of ATP in the dialysate ([ATP]d) throughout the entirety of the protocol, including recovery phases.
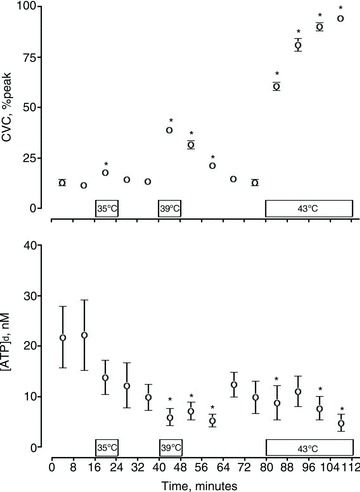
Unless otherwise indicated, skin temperature was set to 31°C. *P < 0.05 different from baseline ATP (31°C sample between 8 and 16 min). CVC, cutaneous vascular conductance.
While our data indicate a decrease in the [ATP] in interstitial fluid during heating, the possibility of increased ATP release cannot be completely ruled out by these methods, as any ATP released due to heating would probably be diluted by the increased flow that accompanies skin heating. As such, if ATP was released by the keratinocytes upon heating, any effect that it could have on SkBF or on temperature perception must come by binding to receptors that are very proximal to the site of ATP release. For example, Mandadi et al. (2009) found that heat-induced release of ATP from keratinocytes had little effect on axon terminals that were further than 10 μm away from the keratinocytes. In intact skin, this distance would probably be shorter due to increased flow through the area.
As mentioned earlier, the order of heating phases in this study was held constant such that the hottest temperatures were always performed last in order to avoid sensitization (Schepers & Ringkamp, 2009) and prolonged elevations in blood flow associated with the hotter temperatures. As such the study is limited in that the possibility that the observed [ATP]d may have been influenced by the order of the temperatures cannot be ruled out.
In this study we heated the skin from 31°C to 35°C, 39°C and 43°C. We observed significant increases in CVC during each heating phase. Previous research has indicated that significant dilatation occurs between 37°C and 38.5°C when heating from a similar baseline temperature (Magerl & Treede, 1996; Houghton et al. 2006). The mechanism behind the increase in blood flow in our study is unclear, but probably includes a mix of the removal of sympathetic vasoconstriction and active vasodilatation as our baseline was slightly below the typical skin temperature of 33–34°C (Alvarez et al. 2006). It should be noted that the amount of sympathetic tone present in the skin during our experiment was probably minimized by performing the experiment in an ambient temperature of 28°C, given that we have previously shown these conditions are thermoneutral for a semi-nude man resulting in a mean skin temperature of 34°C (Lee & Mack, 2006). Whatever the mechanism, the fact that we and others (Magerl & Treede, 1996; Houghton et al. 2006) have observed dilatation below the reported threshold for the TRPV1 ion channel (∼43°C; Caterina et al. 1997; Tominaga et al. 1998; Schepers & Ringkamp, 2010) suggests that temperature-sensitive mechanisms other than TRPV1 channels may be involved or that the TRPV1 channels are potentiated to sense lower temperatures than normally reported.
TRPV3 and TRPV4 ion channels are sensitive to temperatures between 27°C and 42°C (Schepers & Ringkamp, 2010), and as such are possible candidates for contributors to the lower temperature threshold for dilatation. This possibility is supported by the observation that the initial hyperemic peak, while significantly blunted, still occurs when TRPV1 ion channels are inhibited with capsazepine (Wong & Fieger, 2010). This suggests that TRPV1-independent mechanisms are most likely at play during local hyperaemia. Existing data are not definitive as far as how much of a role TRPV3 and TRPV4 ion channels play in warmth sensation (Chung et al. 2004; Huang et al. 2011), and leaves the role of these in SkBF largely unexplored.
The dilatation that we and others have observed below the standard temperature threshold of ∼43°C for the TRPV1 ion channels might also be accounted for by a sensitization of the TRPV1 channels that some suggest takes place with local heating (Zhang et al. 2007; Wong & Fieger, 2010). Chemicals like Substance P, a chemical released during an axon reflex, have been shown to decrease the temperature threshold of the TRPV1 channels from ∼43°C to 36 ± 1.5°C (Zhang et al. 2007). Furthermore, the TRPV3 channels, which are often collocated on free nerve endings with TRPV1 ion channels, appear to enhance the sensitivity of the TRPV1 channels (Smith et al. 2002). A decrease in pH is also known to enhance the sensitivity of the TRPV1 channels (Tominaga et al. 1998). Therefore, it is not unreasonable to hypothesize that the TRPV1 ion channels played a role in the dilatation that we observed at skin temperatures of 35°C or 39°C in our study. It seems likely that both sensitization of the TRPV1 channels and the TRPV3 and TRPV4 ion channels play a role in this response. Future research investigating the potential roles for each of these ion channels will be important in detailing the mechanism of the SkBF response to local heating.
In summary, we observed that presumably innocuous local heating to temperatures known to activate the TRPV3 and TRPV4 ion channels is not associated with an increase in interstitial ATP, but rather with a significant decrease in the concentration of ATP in the interstitial fluid of the skin in humans. The decrease in the concentration of ATP in the interstitial fluid is probably accounted for, at least in part, by fluctuations in SkBF. We interpret this to mean that an accumulation of ATP in the interstitial fluid of the dermis is not necessary for temperature sensation or the SkBF response to local heating. We also observed that significant dilatation occurs at a skin temperature as low as 35°C. We suggest that the TRPV3 and TRPV4 ion channels, as well as the sensitization of TRPV1 ion channels, may contribute to this dilatation.
Acknowledgments
The authors acknowledge and thank Pat Vehrs, Allen Parcell and Casey Gifford for their insight in designing and writing this study. The authors also thank the subjects in this study for their committed participation.
Glossary
- [ATP]
concentration of ATP
- [ATP]d
concentration of ATP in dialysate
- CPS
counts per second
- CVC
cutaneous vascular conductance
- LDF
laser-Doppler flux
- MAP
mean arterial pressure
- NOS
nitric oxide synthase
- SkBF
skin blood flow
- TRPV
vanilloid family of transient receptor potential ion channels
- TRPV1
type I of the TRPV ion channels
- TRPV3
type III of the TRPV ion channels
- TRPV4
type IV of the TRPV ion channels
Author contributions
All data collection was performed in the Health and Human Performance Research Laboratory at Brigham Young University. Conception and Design of Experiments: JG, CH, SG, GM. Collection, Analysis and Interpretation of Data: JG, CH, JB, GM. Drafting or Revising Article: JG, SG, JB, GM. All authors have approved of this manuscript.
References
- Alvarez GE, Zhao K, Kosiba WA, Johnson JM. Relative roles of local and reflex components in cutaneous vasoconstriction during skin cooling in humans. J Appl Physiol. 2006;100:2083–2088. doi: 10.1152/japplphysiol.01265.2005. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight GE, Greig AVH. Purinergic signaling in healthy and diseased skin. J Investig Dermatol. 2012;132:526–546. doi: 10.1038/jid.2011.344. [DOI] [PubMed] [Google Scholar]
- Burrell HE, Wlodarski B, Foster BJ, Buckley KA, Sharpe GR, Quayle JM, Simpson AWM, Gallagher JA. Human keratinocytes release ATP and utilize three mechanisms for nucleotide interconversion at the cell surface. J Biol Chem. 2005;280:29667–29676. doi: 10.1074/jbc.M505381200. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Charkoudian N. Skin blood flow in adult human thermoregulation: How it works, when it does not, and why. Mayo Clinic Proc. 2003;78:603–612. doi: 10.4065/78.5.603. [DOI] [PubMed] [Google Scholar]
- Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- Dixon CJ, Bowler WB, Littlewood-Evans A, Dillon JP, Bilbe G, Sharpe GR, Gallagher JA. Regulation of epidermal homeostasis through P2Y(2) receptors. Br J Pharmacol. 1999;127:1680–1686. doi: 10.1038/sj.bjp.0702653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieger S, Wong B. Adenosine receptor inhibition with theophylline attenuates the skin blood flow response to local heating in humans. Exp Physiol. 2010;95:946–954. doi: 10.1113/expphysiol.2010.053538. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993;18:683–702. [Google Scholar]
- Guler AD, Lee HS, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol. 2006;572:811–820. doi: 10.1113/jphysiol.2005.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Li XX, Yu YY, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain. 2011;7:37. doi: 10.1186/1744-8069-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Kellogg DL. Local thermal control of the human cutaneous circulation. J Appl Physiol. 2010;109:1229–1238. doi: 10.1152/japplphysiol.00407.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DL. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006;100:1709–1718. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- Lee K, Mack GW. Role of nitric oxide in methacholine-induced sweating and vasodilation in human skin. J Appl Physiol. 2006;100:1355–1360. doi: 10.1152/japplphysiol.00122.2005. [DOI] [PubMed] [Google Scholar]
- Magerl W, Treede RD. Heat-evoked vasodilatation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol. 1996;497:837–848. doi: 10.1113/jphysiol.1996.sp021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, Patapoutian A, Fukumi-Tominaga T, Mizumura K, Tominaga M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458:1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol. 2011;589:3471–3482. doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol. 2010;109:1239–1246. doi: 10.1152/japplphysiol.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Thaning P, Nyberg M, Saltin B, Hellsten Y. Local release of ATP into the arterial inflow and venous drainage of human skeletal muscle: insight from ATP determination with the intravascular microdialysis technique. J Physiol. 2011;589:1847–1857. doi: 10.1113/jphysiol.2010.203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Reflex control of cutaneous vasculature. J Invest Dermatol. 1977;69:154–166. doi: 10.1111/1523-1747.ep12497938. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Ringkamp M. Thermoreceptors and thermosensitive afferents. Neurosci Biobehav Rev. 2009;33:205–212. doi: 10.1016/j.neubiorev.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Ringkamp M. Thermoreceptors and thermosensitive afferents. Neurosci Biobehav Rev. 2010;34:177–184. doi: 10.1016/j.neubiorev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe J, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Stephens DP, Charkoudian N, Benevento JM, Johnson JM, Saumet JL. The influence of topical capsaicin on the local thermal control of skin blood flow in humans. Am J Physiol Regul Integr Comp Physiol. 2001;281:R894–R901. doi: 10.1152/ajpregu.2001.281.3.R894. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Wingo JE, Brothers RM, Del Coso J, Crandall CG. Intradermal administration of ATP does not mitigate tyramine-stimulated vasoconstriction in human skin. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1417–R1420. doi: 10.1152/ajpregu.00846.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol. 2010;588:4317–4326. doi: 10.1113/jphysiol.2010.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BJ, Minson CT. Altered thermal hyperaemia in human skin by prior desensitization of neurokinin-1 receptors. Exp Physiol. 2011;96:599–609. doi: 10.1113/expphysiol.2011.057356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Cang C-L, Kawasaki Y, Liang L-L, Zhang Y-Q, Ji R-R, Zhao Z-Q. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKC epsilon: a novel pathway for heat hyperalgesia. J Neurosci. 2007;27:12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman H. Extracellular purine metabolism. Drug Dev Res. 1997;39:337–352. [Google Scholar]


