Abstract
Boceprevir or telaprevir plus ribavirin (RBV) and pegylated interferon-α (pegIFN-α) is the new standard-of-care therapy for patients who are chronically infected with genotype 1 hepatitis C virus (HCV). The addition of these protease inhibitors to the RBV/pegIFN-α combination regimen has significantly improved rates of sustained virologic response (SVR); however, the incidence of anemia has also increased significantly. Anemia can interfere with patients’ quality of life, work productivity, and treatment adherence. Severe anemia can cause morbidity and even mortality. For the management of anemia during triple combination therapy, RBV dose reduction is recommended as an initial course of action. Retrospective analyses of carefully selected patient cohorts suggest that RBV dose reduction does not reduce SVR rates. However, this observation needs to be confirmed in prospective trials with cohorts that more accurately reflect the challenging patients treated in real-world practice. Adequate doses of RBV should be maintained during triple combination therapy, as phase II trials have demonstrated that RBV is essential for attaining optimal SVR rates and preventing viral breakthrough, relapse, and emergence of resistant variants. This roundtable addresses key points related to the management of anemia in the era of triple combination therapy, including the increasing problem of anemia, strategies for anemia management, and the importance of maintaining adequate RBV exposure as part of the HCV treatment regimen.
Increased Problem of Anemia in the Era of Triple Combination Therapy
According to practice guidelines published by the American Association for the Study of Liver Diseases (AASLD) in 2011, the optimal treatment for chronic genotype 1 hepatitis C virus (HCV) infection is triple therapy consisting of ribavirin (RBV) and pegylated interferon-α (pegIFN-α) plus either telaprevir or boceprevir.1 Telaprevir and boceprevir are HCV NS3/4A serine protease inhibitors (PIs) with demonstrated efficacy against genotype 1 HCV. Triple combination therapy that includes either boceprevir or telaprevir significantly improves sustained virologic response (SVR) rates. The presence of adequate levels of pegIFN-α and RBV limits the emergence of resistant viral variants—a new clinical issue that has emerged with the advent of the PIs.2–6 PI-based therapy is also associated with new or compounded side effects—including rash, anemia, and other hematologic disturbances—compared to the side effects previously observed with RBV/pegIFN-α therapy.1
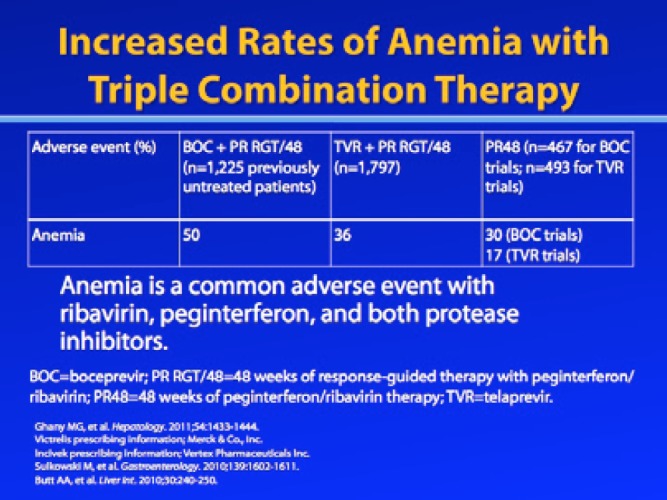
In particular, anemia rates have increased dramatically with triple combination therapy.1 Anemia was a prominent adverse event in both treatment-nai’ve and treatment-experienced patients in the phase III trials that evaluated boceprevir or telaprevir plus RBV/pegIFN-α for treatment of chronic genotype 1 HCV infection.2–5 In treatment-nai’ve patients, an approximately 20% increase in anemia was observed among patients treated with a PI-containing regimen compared to patients treated with RBV/pegIFN-α.2,4 In treatment-experienced patients, an additional 15% of telaprevir-treated patients and an additional 23—26% of boceprevir-treated patients experienced anemia compared to patients in the RBV/pegIFN-α control arms.3,5 Aspects of PI-induced anemia that have surprised clinicians include the speed with which it can occur (within 2—4 weeks) and its severity. The rate of hemoglobin decline with RBV/pegIFN-α dual therapy is more gradual and occurs between 4 and 12 weeks of treatment, whereas the drop in hemoglobin level during the first 12 weeks of PI-based therapy is more rapid.4
In my practice, I have observed significantly higher rates of anemia with PI-based HCV therapy. An important caveat is that I have a specialized, tertiary-care referral practice and many of my patients who have early-stage disease are enrolled in clinical trials; therefore, patients who receive standard-of-care therapy tend to have more advanced disease, often marked by cirrhosis and/or hepatocellular carcinoma. In addition to observing severe anemia with HCV triple combination therapy, I have also observed that erythropoietin (EPO) is not as effective for managing anemia in this setting, at least in patients with advanced disease. As a result, some patients require blood transfusions. Overall, anemia in patients with advanced disease appears to be more severe, more difficult to manage, and less responsive to EPO when patients are receiving triple combination therapy.
Mechanism of Anemia in Patients Receiving Triple Combination Therapy
The PI registration trials used modified World Health Organization guidelines to define toxicity grades for anemia based on hemoglobin levels: Grade 0 anemia was defined as a hemoglobin level of 11 g/dL or above; grade 1 anemia was defined as a hemoglobin level between 9.5 g/dL and 11 g/dL; grade 2 anemia was defined as a hemoglobin level of 8.0—9.5 g/dL; grade 3 anemia was defined as a hemoglobin level of 6.5—8.0 g/dL; and grade 4 anemia was defined as a hemoglobin level below 6.5 g/dL.2–5 The package insert for RBV states that dose reduction is appropriate if hemoglobin levels fall below 10 g/dL; if hemoglobin levels fall below 8.5 g/dL, discontinuation of RBV is recommended.7 These were also the guidelines used in the PI registration trials to manage anemia.
Anemia in patients treated with triple combination therapy is caused by all 3 drugs and likely occurs due to multiple mechanisms. While these mechanisms have not been completely elucidated, evidence suggests that RBV-induced hemolytic anemia is partly responsible.8 A significant proportion of RBV from the circulation is transported into erythrocytes where it is metabolized into active phos-phorylated derivatives. The relative lack of phosphatase activity in these erythrocytes leads to hemolytic anemia. In addition, both PIs and pegIFN-α are thought to cause anemia through direct bone marrow suppression.9–11 The use of PIs and pegIFN-α thus appears to inhibit the compensatory increase in natural erythropoiesis-stimulating growth factor that is seen when RBV is used alone. Finally, any analysis of the mechanism of anemia in HCV-infected patients should consider the presence of cirrhosis, which could cause spur cell anemia (a type of acquired hemolytic anemia) or other types of low-grade hemolysis that may be independent of HCV therapy.
Predictors of Anemia
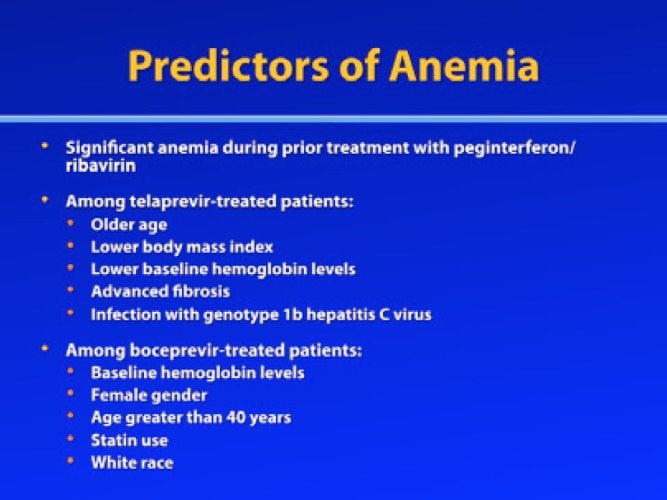
Several factors are associated with an increased risk for anemia. Patients are likely to develop anemia during triple combination therapy if they experienced significant anemia during prior treatment with RBV/pegIFN-α, and the addition of boceprevir or telaprevir to the treatment regimen may make this anemia more severe. Additionally, a post—hoc analysis of the phase III telaprevir registration trials identified older age, lower body mass index (BMI), lower baseline hemoglobin levels, advanced fibrosis, and infection with genotype 1b HCV as predictors of anemia.12 A multivariate analysis of the phase III trials of boceprevir (SPRINT-2 and RESPOND-2) identified baseline hemoglobin levels, female gender, age greater than 40 years, statin use, and white race as predictive factors associated with anemia.13 Polymorphisms in the inosine triphosphate (ITPA) genes may also predict a patient’s risk for anemia.14,15 Research is underway to identify whether certain polymorphisms in these genes drive hemolysis and, possibly, RBV-induced response.
One population that has a particularly increased risk of anemia during triple combination therapy consists of patients with cirrhosis. Experience from clinical practice has shown that patients with cirrhosis suffer from greater degrees of hemoglobin decline during PI-based therapy, and this observation is supported by clinical trial data. In pooled data from the ADVANCE and ILLUMINATE trials, grade 3 anemia was more common in cirrhotic patients than in noncirrhotic patients.16 In addition, preliminary safety data from a large cohort of patients with compensated cirrhosis who were treated with either telaprevir-based or boceprevir-based triple combination therapy revealed higher rates of severe adverse events than the rates observed in the phase III trials of telaprevir and boceprevir.17 These cirrhotic patients experienced higher rates of anemia and had a poor response to EPO, which was administered in 52% of boceprevir-treated patients and 55% of telaprevir-treated patients; furthermore, 6% of boceprevir-treated patients and 18% of telaprevir-treated patients required blood transfusions.
Impact of Anemia on Patient Outcomes
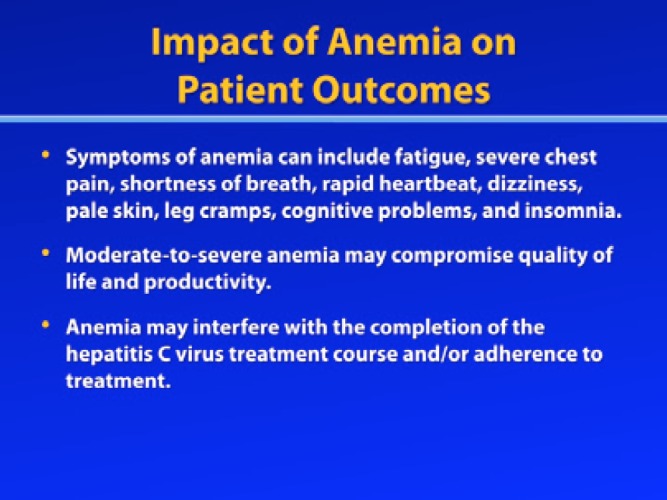
While patients with mild anemia may experience few or no symptoms, patients with more severe anemia may experience fatigue, severe chest pain, shortness of breath, rapid heartbeat, dizziness, pale skin, leg cramps, cognitive problems, and insomnia. Thus, moderate-to-severe anemia may compromise quality of life and productivity, and anemia may also interfere with the completion of the HCV treatment course and/or adherence to treatment. In fact, an analysis of a large national cohort of HCV-infected veterans identified a negative association between treatment completion and anemia.18 Failure to complete treatment or failure to take medications as prescribed is particularly problematic in the setting of PI-based HCV therapy, due to the risk of viral resistance. Both boceprevir and telaprevir have low genetic barriers to resistance. Thus, treatment nonadherence can increase the risk for the emergence of resistant viral variants and virologic rebound.
While anemia clearly affects quality of life, it does not appear to compromise SVR rates, at least based on retrospective data. Prior to the era of direct-acting antiviral (DAA) agents, anemia was associated with improved SVR rates, and the same trend appears to hold true among at least some PI-treated patients. In the IDEAL study, which pro-spectively assessed RBV plus pegIFN-α 2a or pegIFN-α 2b for the treatment of genotype 1 HCV infection, patients with anemia who required a RBV dose reduction had higher SVR rates than patients who had hemoglobin levels of 10 g/dL or higher and did not require RBV dose reduction (48.8% vs 36.7%, respectively; P<.001).19 The IDEAL study investigators suggested that the magnitude of anemia may be a pharmacodynamic marker of increased RBV exposure and, therefore, greater RBV efficacy. A similar effect was also observed in a second study by Sulkowski and associates.20 In this retrospective analysis of data from a large multicenter study, SVR was associated with decreased hemoglobin levels; patients with a greater—than—3 g/dL decline in hemoglobin levels had an SVR rate of 43.7% compared to an SVR rate of only 29.9% among patients with a hemoglobin decline of 3 g/dL or less (P<.001).
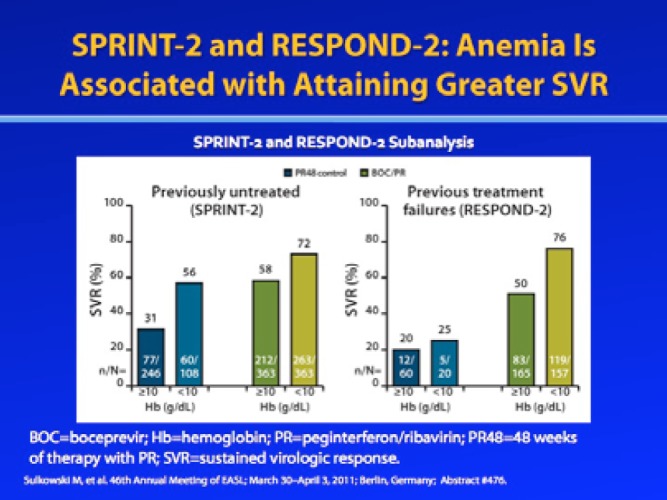
Similar to these observations from the pre-DAA era, a retrospective, post—hoc analysis from the SPRINT-2 and RESPOND-2 trials of boceprevir demonstrated that patients with anemia exhibited higher SVR rates compared to those who did not develop anemia.13 This analysis included 1,097 treatment-na’ve patients and 403 treatment-experienced patients with genotype 1 HCV infection who were randomized to receive either boceprevir plus RBV/pegIFN-α or RBV/pegIFN-α alone. Among treat-ment-na’ve patients, treatment-related anemia (hemoglobin level <10 g/dL) occurred in 50% of boceprevir-treated patients and 30% of control patients. Among patients who had failed prior treatment with RBV/pegIFN-α, anemia occurred in 49% of boceprevir-treated patients and 25% of control patients. In both treatment-na’ve and treatment-experienced patients, SVR occurred more frequently in patients who developed anemia compared to those who did not; this trend was observed in both the boceprevir and control arms. Among patients with a hemoglobin level less than 10 g/dL, SVR rates in the boceprevir and control arms were 73—76% and 25—56%, respectively. These data further support the hypothesis that treatment-related anemia may be a marker of exposure to RBV.
In contrast, retrospective analyses of the phase III trials of telaprevir—REALIZE, ADVANCE, and ILLUMINATE— found that patients with and without anemia achieved similar SVR rates.12,16 Among treatment-naive patients, anemia (hemoglobin level <10 g/dL) developed in 41% of the patients treated with telaprevir plus RBV/pegIFN-α and in 26% of the patients treated with RBV/pegIFN-α alone. Overall, SVR was achieved by 70—77% of the telaprevir-treated patients and 41—50% of the control patients, regardless of their anemia status.16 The investigators of the study concluded that anemia appeared to have no effect on SVR rates when patients received a telaprevir-based regimen. However, readers should note that the majority of patients enrolled in the ADVANCE and ILLUMINATE studies had minimal fibrosis, were mostly white and/or European, and had lower BMIs than patients in actual clinical practice.4,21 Thus, further research is needed to determine whether this finding will hold true in a patient cohort that better reflects the diversity of patients seen in real-world clinical practice.
Similar to the findings observed in treatment-naive patients, a post—hoc analysis of 662 treatment-experienced patients found that the presence of anemia did not appear to influence SVR rates during telaprevir treatment.12 In this analysis, anemia was observed in 41% of patients in the telaprevir plus RBV/pegIFN-α arm and in 22% of patients in the RBV/pegIFN-α control arm. A multivariate analysis indicated that anemia was a significant predictor of SVR for patients treated with RBV/pegIFN-α; however, no difference was seen in the proportion of patients with or without anemia who achieved SVR. The study authors therefore concluded that, among patients who had failed prior treatment with RBV/pegIFN-α, anemia did not significantly affect SVR rates during re-treatment with telaprevir plus RBV/pegIFN-α.
Again, further prospective trials are needed to confirm the relationships between anemia and SVR rates and between RBV dose reduction and SVR rates.
Conclusion
The introduction of triple combination therapy as the standard-of-care therapy for genotype 1 HCV infection has resulted in higher SVR rates but also more side effects. Increasingly, anemia has become a challenge for clinicians, particularly as cohorts of HCV-infected patients age and exhibit more advanced liver disease. Although anemia does not appear to compromise SVR rates with triple combination therapy, this finding was based on retrospective data. Additional prospective studies are needed to confirm this finding. Anemia is a side effect that must be addressed, as it can potentially have a negative impact on patients’ quality of life, work productivity, and medication adherence—the latter of which can potentially impact overall outcomes.
Acknowledgement
Kris V. Kowdley, MD, has received consulting fees (payable to his institution) from Abbott, Gilead, Novartis, and Vertex; he has also received research funding from Abbott, Boeringer Ingelheim, Bristol-Myers Squibb, Conatus, Gilead, Intercept, Merck, and Mochida.
References
- 1.Ghany MG, Nelson DR, Strader DB, et al. Genotype 1 HCV treatment guidelines. Hepatology. 2011;54:1433–1444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poordad F, McCone J, Bacon BR, et al. SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacon BR, Gordon SC, Lawitz E, et al. HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson IM, McHutchison JG, Dusheiko G, et al. ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 5.Zeuzem S, Andreone P, Pol S, et al. REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 6.Hezode C, Forestier N, Dusheiko G, et al. PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1827–1838. [Google Scholar]
- 7. Ribasphere (ribavirin USP) [package insert]. Warrendale, PA: Kadmon Corporation, LLC; 2012.
- 8.De Franceschi L, Fattovich G, Turrini F, et al. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997–1004. doi: 10.1053/he.2000.5789. [DOI] [PubMed] [Google Scholar]
- 9.Peck-Radosavljevic M, Wichlas M, Homoncik-Kraml M, et al. Rapid suppression of hematopoiesis by standard or pegylated interferon-alpha. Gastroenterology. 2002;123:141–151. doi: 10.1053/gast.2002.34175. [DOI] [PubMed] [Google Scholar]
- 10. Victrelis (boceprevir) [package insert]. Whitehouse Station, PA: Merck & Co., Inc; 2012.
- 11. Incivek (telaprevir) [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2012.
- 12.Roberts S, Andreone P, Pol S, et al. Impact of anemia and ribavirin dose reduction on SVR in a telaprevir-based regimen in patients with HCV genotype 1 and prior peginterferon/ribavirin treatment failure in the phase III REALIZE study. Presented at the Annual Meeting of the American Association for the Study of Liver Diseases; November 4—8, 2011; San Francisco, California. Abstract 1366. [Google Scholar]
- 13.Sulkowski M, Poordad F, Manns MP, et al. Anemia during treatment with peginterferon alfa-2b/ribavirin with or without boceprevir is associated with higher SVR rates: analysis of previously untreated and previous-treatment-failure patients. J Hepatol. 2011;54(suppl 1):S195–S196. [Google Scholar]
- 14.Naggie S, Rallon NI, Benito JM, et al. Variants in the ITPA gene protect against ribavirin-induced hemolytic anemia in HIV/HCV-coinfected patients with all HCV genotypes. J Infect Dis. 2012;205:376–383. doi: 10.1093/infdis/jir754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura T, Osaki R, Shioya M, et al. Polymorphism of the inosine triphosphate pyrophosphatase gene predicts ribavirin-induced anemia in chronic hepatitis C patients. Mol Med Report. 2012;5:517–520. doi: 10.3892/mmr.2011.659. [DOI] [PubMed] [Google Scholar]
- 16.Sulkowski MS, Reddy R, Afdhal NH, et al. Anemia had no effect on efficacy outcomes in treatment-naive patients who received telaprevir-based regimens in the ADVANCE and ILLUMINATE phase 3 studies. J Hepatol. 2011;54(suppl 1):S195. [Google Scholar]
- 17.Hézode C, Dorival C, Zoulim F, et al. Safety of telaprevir or boceprevir in combination with peginterferon alfa/ribavirin, in cirrhotic non responders. First Results of the French Early Access Program (ANRS CO20-CUPIC) in real-life setting. Presented at HepDART 2011; December 4—8, 2011; Kauai, Hawaii. Abstract 46. [Google Scholar]
- 18.Butt AA, McGinnis KA, Skanderson M, Juctice AC. Hepatitis C treatment completion rates in routine clinical care. Liver Int. 2010;30:240–250. doi: 10.1111/j.1478-3231.2009.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McHutchison JG, Lawitz EJ, Shiffman ML, et al. IDEAL Study Team. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;36:580–589. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 20.Sulkowski MS, Shiffman ML, Afdhal NH, et al. Hepatitis C virus treatment-related anemia is associated with higher sustained virologic response rate. Gastroenterology. 2010;139:1602–1611. doi: 10.1053/j.gastro.2010.07.059. [DOI] [PubMed] [Google Scholar]
- 21.Sherman KE, Flamm SL, Afdhal NH, et al. ILLUMINATE Study Team. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–1024. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strategies for Management of Anemia During Triple Combination Therapy
Even in the pre-DAA era, the strategy that guided anemia management was RBV dose reduction. Following a landmark study by Shiffman and colleagues, the idea was proposed that RBV dose reduction in 200-mg increments (or 400-mg increments if the initial RBV dose was 1,400 mg per day) could be beneficial for patients who developed anemia while on RBV/pegIFN-α therapy.1 Indeed, this modest dose reduction seemed to preserve SVR rates and allowed patients to continue therapy without the use of hematopoietic growth factors.
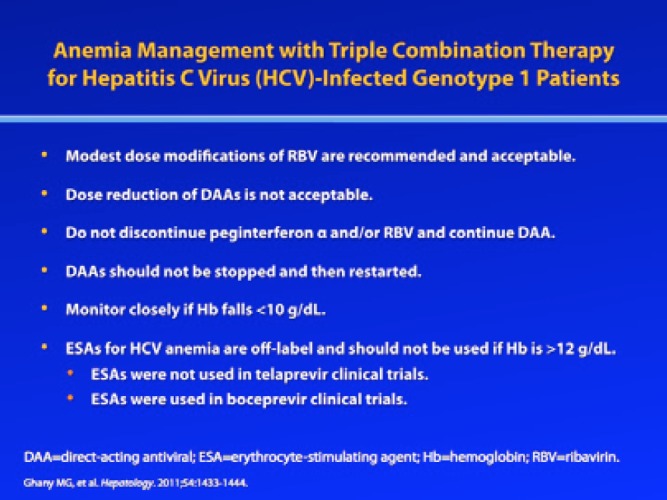
However, the addition of boceprevir and telaprevir to RBV/pegIFN-α has somewhat altered this paradigm. In particular, it is thought that boceprevir and telaprevir suppress the bone marrow, thus causing anemia via a different mechanism than that of RBV-induced hemolytic anemia. Since telaprevir and boceprevir cannot be administered without RBV/pegIFN-α and the dose of the PI cannot be reduced without a significant risk for the emergence of resistant viral variants, RBV dose adjustment remains a key strategy for the management of anemia in the era of DAA therapy.2 Indeed, RBV dose reduction is recommended by the AASLD as the first-line treatment for anemia. Another strategy some clinicians have considered is using EPO to boost hemoglobin levels. In 2009, the AASLD noted that erythrocyte-stimulating agents (ESAs), such as EPO, were not indicated for treatment of anemia in this setting, and the AASLD upheld this recommendation in its 2011 treatment guidelines, which noted that RBV dose reduction should be the primary strategy for addressing anemia.2,3 Now that telaprevir and boceprevir have entered clinical use, however, some clinicians have found that use of EPO may be necessary if a patient’s hemoglobin falls below 10 g/dL, in order to maintain adequate RBV exposure and prevent treatment discontinuation due to anemia. However, ESAs should not be used if the patient’s hemoglobin level is greater than 12 g/dL, due to an increased risk for serious adverse events. Also, clinicians should keep in mind that use of EPO to address RBV-induced anemia is off-label.
Protocols for Ribavirin Dose Reduction
In the phase III registration trials of boceprevir and telaprevir, RBV dose reduction was employed to address anemia, which was defined as a hemoglobin level less than 10 g/dL; EPO was also allowed in the boceprevir trials.4–7 Specifically, patients in the boceprevir trials who developed anemia were managed at the investigator’s discretion with either EPO or RBV dose reductions—in 200-mg increments for patients treated with 1,000 mg or 1,200 mg of weight-based RBV per day or 400-mg increments in patients treated with 1,400 mg of weight-based RBV per day.4,5 The short-acting formulations of EPO were allowed at a dose of 40,000 IU weekly, administered subcutaneously; this dose was adjusted according to hemoglobin levels, and EPO was discontinued if hemoglobin levels rebounded above 12 g/dL. For patients receiving telaprevir-based triple therapy, anemia was managed by RBV dose reduction only; the use of EPO was prohibited in the telaprevir studies. Specifically, RBV was reduced to a dose of 600 mg per day according to the prescribing information for RBV tablets.6–8 In both studies, if patients required discontinuation of RBV during treatment, then the PI and pegIFN-α were discontinued as well. It is important to note that hemoglobin levels should be monitored frequently in clinical practice (eg, every week or at least every 2 weeks during the first month of therapy), since hemoglobin levels can fall precipitously during triple combination therapy. At my institution, in those with advanced liver disease and high-normal renal function, we check the hemoglobin level at Week 1, regardless of which DAA agent is being used.
Ribavirin Dose Reductions and Sustained Virologic Response in Telaprevir Trials
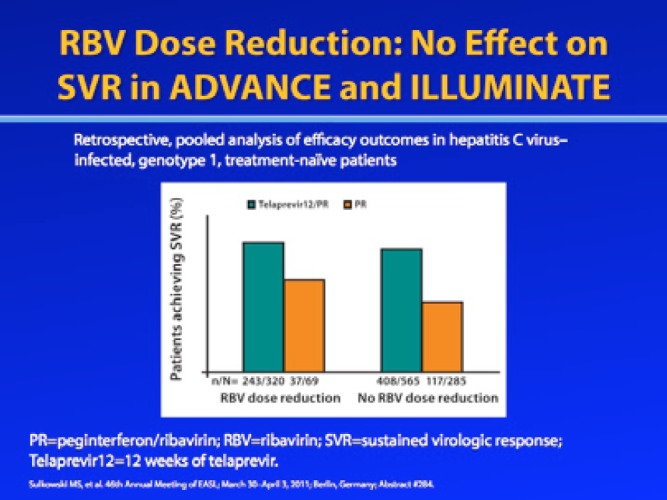
Retrospective analyses of data from the ADVANCE, ILLUMINATE, and REALIZE phase III trials of telaprevir found that RBV dose reduction due to anemia did not appear to affect SVR rates.9–11 In a pooled analysis of treatment-naive patients in the ADVANCE and ILLUMINATE trials, the SVR rate was 76% among tela-previr-treated patients who underwent RBV dose reduction and 72% among telaprevir-treated patients without RBV dose reduction.9 Among patients in the RBV/pegIFN-α control group, SVR rates were 54% among patients who required RBV dose reduction and 41% among those without RBV dose reduction, which is actually counterintuitive to what was seen historically with RBV dose reduction in dual therapy with pegIFN-α.
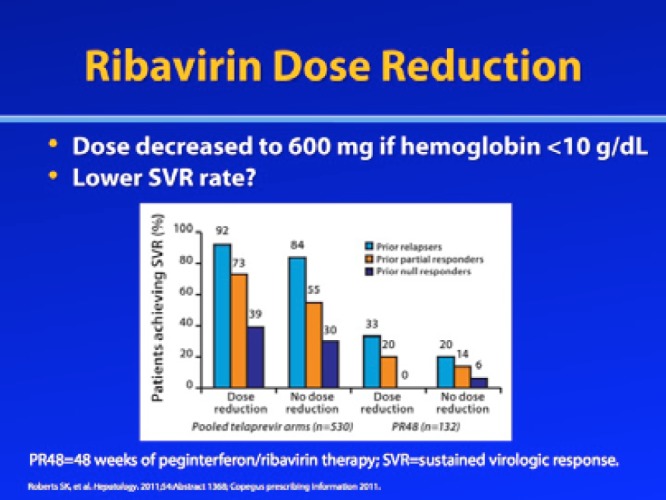
Similarly, a post—hoc analysis of the REALIZE trial, which enrolled patients who had failed prior treatment with RBV/pegIFN-α, found that reducing the RBV dose due to anemia (hemoglobin <10 g/dL) did not appear to significantly impact SVR rates, although the SVR rates were slightly higher among patients with RBV dose reduction.11 In the telaprevir treatment arms, SVR rates were 92% versus 84% for prior relapsers, 73% versus 55% for partial responders, and 39% versus 30% for prior null responders (RBV dose reduction vs no dose reduction, respectively). In the RBV/pegIFN-α control arm, SVR rates were 33% versus 20% for prior relapsers, 20% versus 14% for prior partial responders, and 39% versus 6% for prior null responders (RBV dose reduction vs no RBV dose reduction, respectively). However, a logistic regression analysis revealed that RBV dose was not significantly associated with SVR in these studies.
Ribavirin Dose Reductions and Sustained Virologic Response in Boceprevir Trials
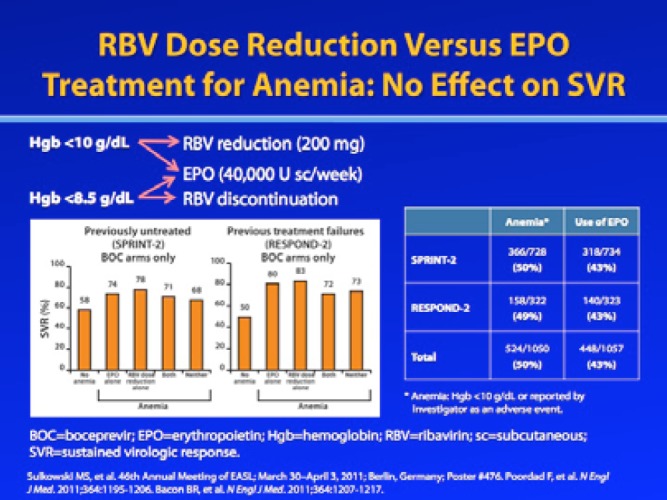
Similar to the telaprevir studies discussed above, a retrospective analysis of the phase III boceprevir trials SPRINT-2 (of treatment-naive patients) and RESPOND-2 (of prior treatment failures) demonstrated that SVR rates were preserved when anemia was managed with RBV dose reduction, EPO administration, or both.12 Among the anemic patients treated with boceprevir plus RBV/pegIFN-α, 4—10% were managed by RBV dose reduction, 36—38% were managed with EPO, and 42—43% received RBV dose reduction plus EPO; the use of RBV dose reduction and/or EPO was at the discretion of the investigator. Among previously untreated patients who developed anemia during boceprevir-based therapy, SVR rates were 74% for patients managed with EPO alone, 78% for patients managed with RBV dose reduction alone, 71% for patients managed with both EPO and RBV dose reduction, and 68% for patients with no anemia management. Among prior treatment failures who developed anemia during boceprevir-based therapy, SVR rates were 80% (EPO alone), 83% (RBV dose reduction alone), 72% (both), and 73% (neither).
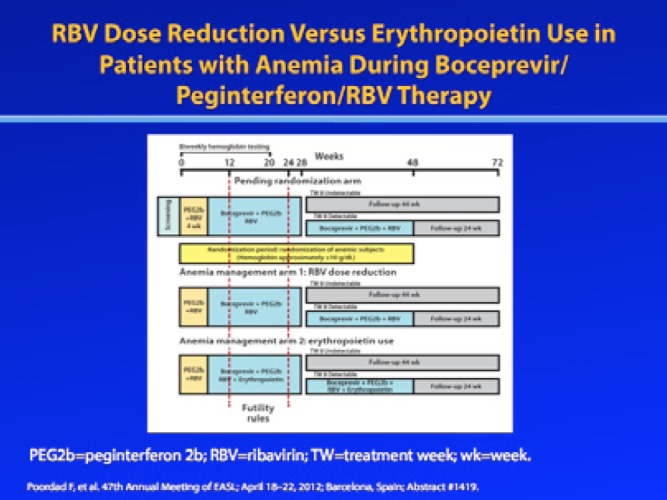
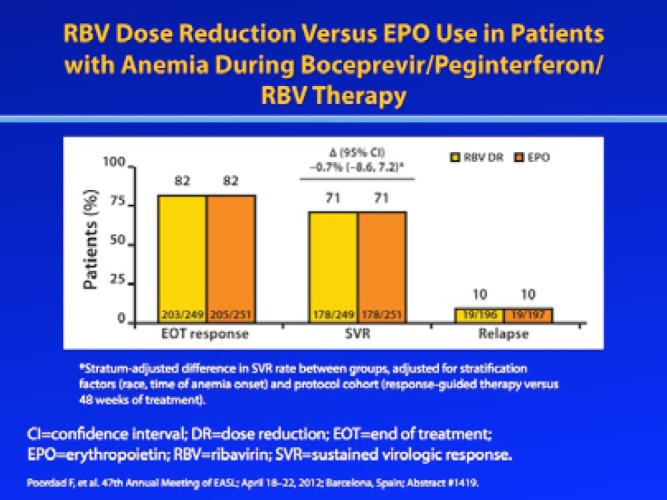
Because the aforementioned study allowed investigators to manage anemia in a nonrandomized, nonblinded manner, it cannot directly compare different anemia management strategies. However, a prospective randomized trial is currently underway to evaluate whether RBV dose modification or use of EPO is preferred for the management of anemia during boceprevir-containing triple combination therapy. At the 2012 Annual Meeting of the European Association for the Study of the Liver (EASL), Poordad and associates reported the initial results of this study.13 In this study, patients whose hemoglobin level was 10 g/dL or lower were randomized to receive either RBV dose reduction (dose reduced by 200—400 mg of the original RBV dosage) or EPO (40,000 IU/week). If the hemoglobin level fell below 8.5 g/dL during first-line anemia management, a second anemia management strategy was employed. Treatment was discontinued if hemoglobin levels fell to 7.5 g/dL or below. The overall SVR rate among patients who met the protocol-defined anemia criteria was 71%. End-of-treatment response rates (82%), relapse rates (10%), and SVR rates (71%) were identical in both the RBV dose reduction arm and the EPO arm. However, fewer patients managed with RBV dose reduction required secondary anemia intervention: The primary anemia intervention was successful in 82% of the RBV dose reduction arm and 62% of the EPO arm. The results of this study provide assurance to clinicians that either anemia management strategy can be used without adversely affecting SVR rates.
While SVR rates are preserved with both RBV dose reduction and EPO, clinicians should consider factors beyond SVR when selecting an anemia management strategy. Currently, the use of EPO is off-label for management of anemia in patients receiving triple combination therapy, and clinicians lack guidelines regarding the use of EPO in this setting; thus, its use is up to the judgment of the individual clinician. Given the potential side effects of the drug, clinicians should select patients carefully before administering EPO, particularly when treating older HCV-infected patients who may have underlying cardiac risk factors. Also, while hemoglobin levels above 12 g/dL are less common during triple combination therapy, clinicians should not use EPO in these patients due to concerns of thrombosis or other complications.
Timing of Ribavirin Dose Modification and Sustained Virologic Response
While studies suggest that RBV dose reduction does not impact SVR rates, the timing of RBV dose modification may impact SVR. A retrospective pooled analysis of ADVANCE and ILLUMINATE, phase III studies of telaprevir plus RBV/pegIFN-α in treatment-naïve patients, suggests that SVR rates are lower when earlier RBV dose reduction is implemented in the presence of anemia.9 SVR rates among patients treated with telaprevir plus RBV/pegIFN-α, when grouped according to the time of first RBV dose reduction, were 68% (0—4 weeks following the first dose), 74% (>4—12 weeks following the first dose), 86% (>12—24 weeks following the first dose), and 100% (>24—48 weeks following the first dose).
Ribavirin Discontinuation
RBV discontinuation is recommended if hemoglobin levels fall below 8.5 g/dL, although this recommendation is not always followed.8 In some cases, clinicians prefer to perform a blood transfusion and/or use other anemia management strategies, such as EPO, rather than discontinue RBV, due to its importance in clinical outcomes.14–16 In the interest of giving patients every opportunity to achieve SVR, my center’s practice is to accept hemoglobin levels down to 7.5 g/dL, while using a secondary anemia management strategy similar to that used in the aforementioned study by Poordad and colleagues.13 If a patient’s hemoglobin level falls below 7.5 g/dL and he or she is responding, the patient is transfused, and EPO is then administered; we make every effort to give at least 400 mg of RBV daily.
If RBV therapy must be suspended, the break in therapy should be kept to a minimum (2—3 days, after which RBV should be restarted at a lower dose), since a prolonged break in RBV could allow the emergence of resistant viral variants.14,15 If RBV is stopped, then the PI must also be permanently discontinued, as the rates of viral breakthrough and relapse are significantly increased in the absence of RBV.
Monitoring for Anemia
According to the EASL clinical practice guidelines for RBV/ pegIFN-α treatment, hematologic monitoring should be performed at Weeks 1, 2, and 4 of therapy and then at 4—8 week intervals.17 The RBV package insert indicates that anemia due to RBV usually occurs within the first 2 weeks of therapy; as such, it recommends hemoglobin measurements prior to treatment, at Weeks 2 and 4, and as deemed clinically necessary.8 When monitoring for anemia in patients receiving triple therapy, clinicians typically follow the guidelines provided in the PI package inserts; however, these guidelines are not consistent.8,18,19 The boceprevir package insert suggests performing complete blood counts (CBC) prior to treatment; at Treatment Weeks 4, 8, and 12; and at other time points as necessary.18 The telaprevir package insert was recently updated to indicate that hemoglobin levels should be measured at baseline and at least at Treatment Weeks 2, 4, 8, and 12.19
At a minimum, I recommend that a CBC be obtained every 2 weeks until Treatment Week 8 and monthly thereafter. Patients who are at increased risk for anemia— including patients with cirrhosis, HIV co-infection, a liver transplant, a history of gastrointestinal bleeding, or advanced fibrosis—should be monitored more frequently at the start of therapy. In addition, patients with a history of cardiac disease should be closely monitored, as this condition could be worsened by drug-induced anemia.8 When treating patients with a PI, particularly telaprevir, my practice is to check patients’ CBC at Week 1 post-treatment. A very steep early decline in hemoglobin level can be difficult to recover from and may result in clinically severe anemia requiring blood transfusions. Thus, I have a very low threshold for adding EPO in sicker patients who experience early and substantial anemia (<10 g/dL).
Acknowledgement
Paul Y. Kwo, MD, has received consulting fees from Abbott, Bristol-Myers Squibb, Gilead, Johnson & Johnson, Merck, Novartis, and Vertex; fees for non-CME/CE services from Gilead, Merck, and Vertex; and contracted research funding from Abbott, Anadys, Bayer, Bristol-Myers Squibb, Conatus, Gilead, GlaxoSmithKline, Merck, Novartis, Roche, and Vertex.
References
- 1.Shiffman ML, Ghany MG, Morgan TR, et al. Impact of reducing peginterferon alfa-2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology. 2007;132:103–112. doi: 10.1053/j.gastro.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Ghany MG, Nelson DR, Strader DB, et al. Genotype 1 HCV treatment guidelines. Hepatology. 2011;54:1433–1444. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poordad F, McCone J, Bacon BR, et al. SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacon BR, Gordon SC, Lawitz E, et al. HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson IM, McHutchison JG, Dusheiko G, et al. ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 7.Zeuzem S, Andreone P, Pol S, et al. REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 8. Ribasphere (ribavirin, USP) Tablets [package insert]. Warrendale, PA: Kadmon Corporation, LLC; 2012.
- 9.Sulkowski MS, Reddy R, Afdhal NH, et al. Anemia had no effect on efficacy outcomes in treatment-naive patients who received telaprevir-based regimens in the ADVANCE and ILLUMINATE phase 3 studies. J Hepatol. 2011;54(suppl 1):S195. [Google Scholar]
- 10.Sulkowski MS, Roberts S, Afdhal N, et al. Ribavirin dose modification in treatment-naive and previously treated patients who received telaprevir combination treatment: no impact on sustained virologic response in phase 3 studies. J Hepatol. 2012;56(suppl 2):S459–S460. [Google Scholar]
- 11.Roberts S, Andreone P, Pol S, et al. Impact of anemia and ribavirin dose reduction on SVR in a telaprevir-based regimen in patients with HCV genotype 1 and prior peginterferon/ribavirin treatment failure in the phase III REALIZE study. Presented at the Annual Meeting of the American Association for the Study of Liver Diseases; November 4—8, 2011; San Francisco, CA. Abstract 1366. [Google Scholar]
- 12.Sulkowski M, Poordad F, Manns MP, et al. Anemia during treatment with peginterferon alfa-2b/ribavirin with or without boceprevir is associated with higher SVR rates: analysis of previously untreated and previous-treatment-failure patients. J Hepatol. 2011;54(suppl 1):S195–S196. [Google Scholar]
- 13.Poordad F, Lawitz E, Reddy R, et al. A randomized trial comparing ribavi-rin dose reduction versus erythropoietin for anemia management in previously untreated patients with chronic hepatitis C receiving boceprevir plus peginterferon/ ribavirin. J Hepatol. 2012;56(suppl 2):S559. [Google Scholar]
- 14.Hézode C, Forestier N, Dusheiko G, et al. PROVE2 Study Team. Telaprevir and peg-interferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1827–1838. [Google Scholar]
- 15.Kwo P, Lawitz E, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomized, multicenter phase 2 trial. Lancet. 2010;376:705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 16.McHutchison JG, Manns MP, Muir AJ, et al. PROVE3 Study Team. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–1303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 18. Victrelis (boceprevir) [package insert]. Whitehouse Station, PA: Merck & Co., Inc; 2012.
- 19. Incivek (telaprevir) [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2012.
Importance of Maintaining Adequate Ribavirin Exposure with Dose Modification for Anemia
A patient’s overall RBV exposure is determined not only by RBV dose modification to address adverse effects such as anemia but also by the patient’s adher- ence to therapy.1 Patient adherence is particularly important for achieving SVR and preventing the emergence of viral variants in triple combination therapy, since PIs have a low genetic barrier to resistance.2 Thus, if RBV dose modification is necessary, clinicians should reinforce the importance of adhering to the revised dosing regimen in order to ensure sufficient drug exposure. Patients should also be reminded that failing to adhere to the revised dosing regimen of RBV, skipping doses, and/or taking prolonged breaks between doses not only reduces the efficacy of RBV but also reduces the efficacy of the PI and engenders a significantly increased risk for the emergence of resistant viral variants.
Importance of Ribavirin in Hepatitis C Virus Therapy
Even with the introduction of more potent PI-containing therapies, RBV remains an important component of current regimens.2 Indeed, PI therapy cannot be continued in the absence of RBV; as the protocol for the ADVANCE trial and the published trial results very clearly state, “[i]f RBV was discontinued owing to anemia, discontinuation of telaprevir (or placebo) was required.”3
The importance of RBV to the overall treatment success of PI-based therapy was validated in phase II trials of boceprevir and telaprevir, which showed that RBV is critical for achieving optimal SVR rates with both drugs. The phase II trial of boceprevir in treatment-naive patients (SPRINT-1) included a low-dose RBV arm, in which patients received boceprevir plus pegIFN-α and low-dose RBV (400—1,000 mg/day).4 The SVR rate in this low-dose RBV arm was only 36% compared to 50—67% in the standard-dose RBV (800—1,400 mg/day) arms. Similarly, the phase II telaprevir trials of treatment-naive patients (PROVE-2) and treatment-experienced patients (PROVE-3) included arms in which RBV was omitted completely from the treatment regimen.5,6 Similar to the boceprevir study, the on-treatment response rates were significantly compromised when patients did not receive RBV. In the PROVE-2 study, the SVR rates were 36% in the group that received 12 weeks of telaprevir plus pegIFN-α (without RBV) and 60% in the group that received 12 weeks of telaprevir plus RBV/pegIFN-α. Similarly, in the PROVE-3 study, the SVR rate was only 24% in the group that received 24 weeks of telaprevir plus pegIFN-α (without RBV) compared to 51—53% in the group that received telaprevir plus RBV/pegIFN-α. While these results are derived from only a small cohort of patients, they clearly illustrate that patients need to receive RBV at the initially recommended, weight-based dose in order to achieve optimal SVR rates.
Intrinsic to the requirement for RBV to achieve optimal SVR rates, RBV helps to prevent viral breakthrough, relapse, and emergence of resistant viral variants. In the SPRINT-1 and RESPOND-1 studies of boceprevir, use of low-dose RBV or omission of RBV was associated with high rates of viral breakthrough and relapse.4,7 Specifically, low-dose RBV resulted in a 27% rate of viral breakthrough and a 22% rate of relapse.4 The same trend holds true for telaprevir-based therapy. In treatment-naïve patients, 24% of the patients who received telaprevir plus pegIFN-α (without RBV) experienced viral breakthrough by Week 12, compared to 1% of patients who received RBV/pegIFN-α and 1—5% of patients who received telaprevir plus RBV/pegIFN-α.5 Interestingly, viral decline was comparable with and without RBV during the first 2 weeks of therapy; however, the group that did not receive RBV as part of the treatment regimen began to show evidence of viral breakthrough around 2 weeks.5,8 Among patients who showed viral breakthrough, the majority of the viruses were telaprevir-resistant, which is consistent with the low genetic barrier to resistance of PIs.
Viral breakthrough also occurred in patients who had failed prior RBV/pegIFN-α treatment. The rate of viral breakthrough at Week 24 was higher in the group treated with telaprevir plus pegIFN-α alone (32%) than in the group treated with telaprevir plus RBV/pegIFN-α (12—13%).6 Additionally, the relapse rate for both untreated and previously treated patients was higher in patients who did not receive RBV (telaprevir plus pegIFN-α, 48—53%; telaprevir plus RBV/pegIFN-α, 13—30%).5,6
While IFN-α—free regimens are on the horizon, RBV will likely remain part of HCV treatment for the foreseeable future. In the majority of IFN-α–free regimens tried thus far, RBV is still required to achieve optimal results.8 For example, the uridine nucleotide analog HCV polymerase inhibitor PSI-7977 works better when administered with RBV than when given as monotherapy.9 In the ELECTRON study, treatment-naive patients without cirrhosis who were infected with genotype 2 or 3 HCV were treated with PSI-7977 plus RBV in the presence or absence of pegIFN-α. Interestingly, all of the patients who received PSI-7977 plus RBV attained SVR at both 12 and 24 weeks post-treatment, regardless of pegIFN-α status. In contrast, relapse occurred by 4 weeks post-treatment in 4 of the 10 patients who were treated with PSI-7977 alone.
Several other studies have clearly shown that adjunctive use of RBV is necessary to improve response rates to various DAA-based therapies. In the ZENITH study, the nonnucleo-side inhibitor VX-222 was administered in conjunction with telaprevir. When VX-222 and telaprevir were administered in the absence of RBV and pegIFN-α, patients experienced on-treatment viral breakthrough, and further studies of this dual combination therapy were halted.10 However, very preliminary results suggest that the addition of RBV to VX-222 and telaprevir may prevent viral breakthrough; additional data from this study are awaited.11
Similarly, RBV was required to achieve optimal treatment efficacy in IFN-α—free studies of mericitabine (an HCV polymerase inhibitor) and ritonavir-boosted dano-previr (the latter of which is an HCV PI).12 In preliminary results from this study, the group that did not receive RBV had higher rates of viral breakthrough (20.9% vs 9.6%). All patients with viral breakthrough were positive for danoprevir-resistant variants.
The need for RBV in IFN-α—free regimens appears to depend on the regimen, the HCV subtype, and the specific patient population. Genotype 1 HCV subtype has a substantial impact on a patient’s likelihood of achieving SVR with certain regimens and, as a corollary, on the need to include RBV in IFN-α—free regimens. Even when treated with telaprevir or boceprevir plus RBV/pegIFN-α regimens, patients infected with genotype 1a HCV have a somewhat lower SVR rate than those infected with HCV genotype 1b. Another group of patients who may require RBV with IFN-α—free regimens includes those individuals who are intrinsically poor responders (ie, prior null responders, nonresponders, or patients with less favorable interleukin-28 β genotypes).13,14 Contrary to clinicians’ expectations, response to an IFN-α—free regimen still appears to depend on IFN-α responsiveness or host pathways in general. This responsiveness is inextricably linked with the greater need for RBV, at least as suggested thus far, in some patients than others.
The apparent importance of RBV for optimal clinical results prompts the question of how RBV works. Despite over a decade of research, however, RBV’s exact mechanism of action remains elusive, and, indeed, it may have multiple mechanisms; mutagenesis and “error catastrophe,” along with immunologic effects and augmentation of IFN response, are among the several explanations that have been advanced.15 As noted previously, the PROVE-2 study, which included a telaprevir treatment arm that omitted RBV, showed that initial viral decline is similar with or without RBV.8,16 After 2 weeks of therapy, however, patients in the RBV-free treatment arm began to show viral breakthrough with emergence of resistance variants. This finding suggests that RBV has an effect on the second phase of viral decline, which likely helps to prevent viral breakthrough and relapse.
Strategies for Adherence to Ribavirin and Triple Combination Therapy
The foregoing considerations raise the question of what constitutes an appropriate degree of RBV dose reduction in response to anemia. Our best guides in this regard are the data from the phase III trials of telaprevir and boceprevir. In these trials, the protocol-mandated dose reductions of RBV were from 1,000—1,200 mg to 600 mg in the telaprevir studies and a reduction of 200—400 mg in daily RBV dose from a starting dose of600—1,400 mg in the boceprevir studies. In both sets of studies, patients who had reduced RBV doses had similar rates of SVR compared to those who did not, providing reassurance to clinicians about these levels of RBV dose reductions and constituting the basis for the dose reduction recommendations in the package inserts.17,18 Some clinicians may still seek to minimize the degree of RBV dose reductions, but careful observation is required in this setting to determine whether additional RBV dose reductions are necessary. In addition, although off-label, the adjunctive use of ESAs is applied by many clinicians if RBV dose reduction is not sufficient to control anemia or if hemoglobin reductions are very substantial. Notably, a recent randomized trial of dose reductions versus ESAs as the initial intervention for anemia, with secondary interventions subsequently allowed, showed no difference in SVR rates.19
Management of other side effects, depression, and substance abuse is an important part of effective HCV therapy.1 While the increase in unpleasant side effects associated with triple combination therapy—which can include not just anemia but also rash, diarrhea, and nausea—may compromise patient adherence, successful management of these side effects should help to alleviate this issue. In fact, a substudy of the IDEAL trial found that the use of ESAs for treatment of anemia reduced patient dropout.20
As part of the effort to deal with side effects of treatment and maintain patient adherence, clinicians may need to treat patients’ depression. Depression is a significant side effect of RBV/pegIFN-α treatment, with 20—50% of patients experiencing symptoms.4,21,22 Not only does depression have a significant negative impact on health-related quality of life during treatment, it has been associated with nonadherence and/or noncompletion of therapy.1,23 While the presence of depression before the start of antiviral therapy is associated with lower treatment completion rates, patients without preexisting depression who receive on-demand antidepressant therapy have high rates of treatment completion (92%).24 Antidepressant medications, such as those in the selective serotonin reuptake inhibitor class (eg, citalopram), should therefore be prescribed as needed in patients who are undergoing HCV therapy, since such therapy has been shown to reduce the incidence of depression without affecting SVR rates.24
Substance abuse is another factor that can limit adherence with HCV therapy. While determining cause versus effect in this area is challenging, one possibility is that patients who are actively engaged in substance abuse prior to therapy might be those already particularly prone to nonadherence with the treatment regimen. Clinicians should therefore attempt to identify patients actively engaged in substance abuse before treatment and, ideally, collaborate with a psychiatrist and/or addiction medicine specialist to address this problem before HCV treatment is initiated.
Finally, good communication between the physician and the patient is essential for maintaining patient adherence. Patients who are discouraged during therapy or who are having difficulty with tolerability benefit from being reminded why they are undergoing HCV treatment. Attaining SVR is a very important goal, and informing patients that they are achieving an excellent virologic response may help them overcome whatever tolerability issues they are experiencing. Thus, ongoing communication with the patient about the benefits of the treatment regimen, in language tailored to the individual patient’s level of understanding, can motivate the patient to adhere to and complete his or her treatment regimen.
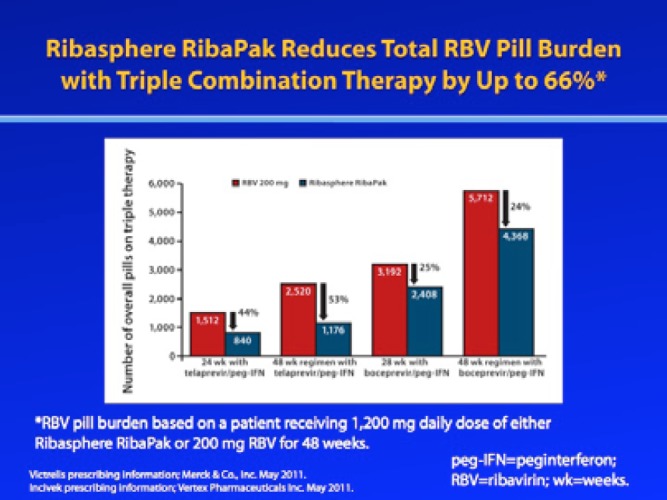
In addition to underscoring the importance of continued adherence to the prescribed dosing regimen, clinicians can also improve adherence by simplifying the treatment regimen whenever possible. Specifically, reducing the patient’s pill burden or using calendarized blister packaging may help to increase patient adherence.1,25 An analysis of the ADHERE registry found that HCV patients with a higher RBV pill burden were more likely to prematurely discontinue treatment or take less than 80% of their prescribed doses compared to patients who were taking a high-dose formulation (eg, 400 mg or 600 mg) tablet in calendarized blister packaging that reduced their RBV pill burden; patients with a higher pill burden also missed more mean milligrams of RBV per day.26
Acknowledgement
Ira M. Jacobson, MD, has received grant/research support from Achillion, Anadys, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlobeImmune, Novartis, Pfizer, Pharmasset, Roche/Genentech, Schering/Merck, Tibotec/Janssen, Vertex, and Zymogenetics; he is also a consultant/advisor for Abbott, Achillion, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, GlobeImmune, Idenix, Inhibitex, Kadmon, Novartis, Pharmasset, Presidio, Roche/Genentech, Schering/ Merck, Tibotec/Janssen, and Vertex; and he serves on the speakers’ bureau for Bristol-Myers Squibb, Gilead, Roche/Genentech, Schering/Merck, and Vertex.
References
- 1.Weiss J, Brau N, Stivala S, Swan T, Fishbein D. Adherence to medication for chronic hepatitis C—building on the model of human immunodeficiency virus antiretroviral adherence research. Aliment Pharmacol Ther. 2009;30:14–27. doi: 10.1111/j.1365-2036.2009.04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pawlotsky J. Treatment failure and resistance with direct-acting antiviral drugs against hepatitis C virus. Hepatology. 2011;53:1742–1751. doi: 10.1002/hep.24262. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 4.Kwo P, Lawitz E, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomized, multicenter phase 2 trial. Lancet. 2010;376:705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 5.Hézode C, Forestier N, Dusheiko G, et al. Telaprevir and peg-interferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1827–1838. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- 6.McHutchison J, Manns M, Muir A, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292–1303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- 7.Schiff E, Poordad FF, Jacobson IM, et al. Role of interferon response during retreatment of null responders with boceprevir combination therapy: results of phase II trial. Gastroenterology. 2008;134:A755. [Google Scholar]
- 8.Feld J. Is there a role for ribavirin in the era of hepatitis C virus direct-acting antivirals? Gastroenterology. 2012;142:1356–1359. doi: 10.1053/j.gastro.2011.12.064. [DOI] [PubMed] [Google Scholar]
- 9.Gane EJ, Hyland RH, Sorensen RD, et al. Once daily PSI-7977 plus RBV: pegylated interferon-alfa not required for complete rapid viral response in treatment-naive patients with HCV GT2 or GT3 [abstract] Hepatology. 2011;54:34. [Google Scholar]
- 10.Di Bisceglie AM, Nelson DR, Gane E, et al. VX-222 with TELAPREVIR alone or in combination with peginterferon alfa-2a and ribavirin in treatment-naive patients with chronic hepatitis C: ZENITH study interim results. J Hepatol. 2011;54(suppl 1):S540. [Google Scholar]
- 11. Vertex announces 12-week on-treatment data and SVR4 from phase 2 study of interferon-free (all-oral) treatment regimen of INCIVEK VX-222 and ribavirin in people with genotype 1 hepatitis C [press release]. Cambridge, MA: Vertex Pharmaceuticals; February 23, 2012.
- 12.Gane EJ, Pockros P, Zeuzem S, et al. Interferon-free treatment with a combination of mericitabine and danoprevir/r with or without ribavirin in treatment-naive HCV genotype 1-infected patients. J Hepatol. 2012;56(suppl 2):S555–S556. [Google Scholar]
- 13.Zeuzem S, Soriano V, Asselah T, et al. SVR4 and SVR12 with an interferon-free regimen of BI201335 and BI207127, +/- ribavirin, in treatment-naive patients with chronic genotype-1 HCV infection: interim results of SOUND-C2. Presented at the 47th Annual Meeting of the European Association for the Study of the Liver; April 18—22, 2012; Barcelona, Spain. Abstract 101. [Google Scholar]
- 14.Lok A, Gardiner D, Hezode C, et al. Confirmation that quadruple therapy with daclatasvir (NS5A inhibitor), asunaprevir (NS3 inhibitor) and peginterferon/ ribavirin results in high rate of SVR4 in HCV genotype 1 null respond. Presented at the 47th Annual Meeting of the European Association for the Study of the Liver; April 18—22, 2012; Barcelona, Spain. Abstract 1415. [Google Scholar]
- 15.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 16.Hézode C, Forestier N, Dusheiko G, et al. PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839–1850. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- 17. Victrelis (boceprevir) [package insert]. Whitehouse Station, PA: Merck & Co., Inc; 2012.
- 18. Incivek (telaprevir) [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2012.
- 19.Poordad FF, Lawitz EJ, Reddy KR, et al. A randomized trial comparing riba-virin dose reduction versus erythropoietin for anemia management in previously untreated patients with chronic hepatitis C receiving boceprevir plus peginterferon/ riba. Presented at the 47th Annual Meeting of the European Association for the Study of the Liver; April 18—22, 2012; Barcelona, Spain. Abstract 1419. [Google Scholar]
- 20.Sulkowski MS, Shiffman ML, Afdhal NH, et al. Hepatitis C virus treatment-related anemia is associated with higher sustained virologic response rate. Gastro-enterology. 2010;139:1602–1611. doi: 10.1053/j.gastro.2010.07.059. [DOI] [PubMed] [Google Scholar]
- 21.Schafer A, Wittchen HU, Seufert J, Kraus MR. Methodological approaches in the assessment of interferon-alfa-induced depression in patients with chronic hepatitis C—a critical review. Int J Methods Psychiatr Res. 2007;16:186–201. doi: 10.1002/mpr.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raison CL, Borisov AS, Broadwell SD, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butt AA, McGinnis KA, Skanderson M, Juctice AC. Hepatitis C treatment completion rates in routine clinical care. Liver Int. 2010;30:240–250. doi: 10.1111/j.1478-3231.2009.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu SS, Schneekloth TD, Talwalkar TD, et al. Impact of depressive symptoms and their treatment on completing antiviral treatment in patients with chronic hepatitis C. J Clin Gastroenterol. 2010;44:e178–e185. doi: 10.1097/MCG.0b013e3181dc250f. [DOI] [PubMed] [Google Scholar]
- 25.Zedler B, Kakad P, Colilla S, Murelle L, Shah NR. Does packaging with a calendar feature improve adherence to self-administered medication for long-term use? A systematic review. Clin Ther. 2011;33:62–73. doi: 10.1016/j.clinthera.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Alam I, Stainbrook T, Cecil B, Kistler KD. Enhanced adherence to HCV therapy with high dose ribavirin formulation: final analyses from ADHERE registry. Aliment Pharmacol Ther. 2010;32:535–542. doi: 10.1111/j.1365-2036.2010.04381.x. [DOI] [PubMed] [Google Scholar]
Conclusion
Triple combination therapy undeniably improves HCV treatment outcomes, which is why such regimens have become the standard of care for treatment of chronic genotype 1 HCV infection. However, these regimens are associated with an increase in certain side effects. In particular, anemia rates have significantly increased with triple combination therapy, especially in cir-rhotic patients. Anemia can have several adverse effects on patient outcomes, including compromised quality of life, work productivity, and medication adherence. In addition, profound anemia can be dangerous; patients with severe anemia are at an increased risk for hospitalization, transfusions, and even ischemic events. Thus, all patients receiving triple combination therapy should be closely monitored for anemia, particularly those patients who already have an increased risk for drug-induced anemia.
The first-line management strategy for anemia is RBV dose reduction. Post—hoc analyses of pivotal PI trials have demonstrated that RBV dose reduction does not appear to adversely affect SVR rates. Another alternative for anemia management is use of EPO; however, the black-box warnings with EPO are concerning, and the use of EPO for management of anemia during HCV therapy remains off-label. Thus, it is with some reluctance that many clinicians have begun using EPO more frequently to address the anemia caused by triple combination therapy.
When considering strategies for anemia management, clinicians should keep in mind the importance of maintaining RBV exposure, even with dose reduction, due to the critical role RBV plays in triple combination therapy. If the clinician must reduce the RBV dose to manage anemia, it is important to ensure that patients are especially adherent to this reduced RBV regimen.
Over the next 1—2 years, clinicians hopefully will be able to optimize SVR rates for HCV-infected patients by using triple combination therapy with telaprevir and boceprevir. Clinicians will therefore need to become proficient in managing treatment-induced anemia, as this side effect will remain a barrier to success with HCV therapy. With careful attention to anemia management, however, high cure rates will likely be attainable even for difficult-to-treat patients who just a few years ago were unable to achieve SVR.
Acknowledgement
Ira M. Jacobson, MD, has received grant/research support from Achillion, Anadys, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Globelmmune, Novartis, Pfizer, Pharmasset, Roche/Genentech, Schering/Merck, Tibotec/Janssen, Vertex, and Zymogenetics; he is also a consultant/advisor for Abbott, Achillion, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Globelmmune, Idenix, Inhibitex, Kadmon, Novartis, Pharmasset, Presidio, Roche/Genentech, Schering/Merck, Tibotec/Janssen, and Vertex; and he serves on the speakers’ bureau for Bristol-Myers Squibb, Gilead, Roche/Genentech, Schering/Merck, and Vertex.
Question-and-Answer Forum
G&H Is preemptive RBV dose reduction an option for preventing anemia?
Kris V. Kowdley, MD, FACP, FACG, AGAF Preemptive RBV dose reduction has not been tested; therefore, clinicians should be cautioned against this approach. Also, analyses of the phase III trials of telaprevir and boceprevir demonstrate that anemia is not a universal phenomenon. Approximately 30—50% of treatment-naive patients and three quarters of treatment-experienced patients who received telaprevir plus RBV/pegIFN-α in the phase III trials did not require RBV dose reductions. Thus, clinicians should begin triple combination therapy with weight-based dosing of RBV and only reduce the dose if needed.
Ira M. Jacobson, MD Selective RBV dose reduction in patients who develop anemia—which for treatment purposes is often defined as a hemoglobin level below 10 g/ dL—differs from reducing the RBV dose a priori for all patients. It has been postulated that patients who require RBV dose reductions due to early-onset anemia are more likely to have a high tissue exposure to RBV; therefore, these patients will presumably continue to have adequate tissue exposure to RBV even if the dose is reduced. In contrast, patients who do not have intrinsically good tissue exposure to RBV with the standard dose would be deprived of sufficient RBV if early RBV dose reduction were universally implemented.
G&H Is there a marker for sensitivity to RBV and subsequent anemia?
KVK The degree of anemia might be a marker for the individual’s sensitivity to RBV, but this sensitivity cannot be ascertained before starting the patient on combination therapy. Until we have better genetic tests that can predict RBV responsiveness, close monitoring of patients is the only way to ascertain whether a patient is likely to develop anemia.
IMJ Initial data regarding the association between ITPA gene polymorphisms and RBV-associated anemia are intriguing, and clinicians could speculate that an ITPA genotype test might be used as a basis for prescribing a reduced dose of RBV at the onset of treatment. If such a test becomes available, careful studies would be needed before this test could be implemented in clinical practice.
Paul Y. Kwo, MD In a perfect world, clinicians would be able to measure RBV plasma levels in a manner similar to how we currently measure trough cyclosporine or tacrolimus levels in liver transplant recipients. Until such measurements become available, however, clinicians must depend on anemia as a pharmacodynamic measure of plasma RBV levels.
G&H Is the use of lead-in therapy with RBV/pegIFN-α useful in patients at high risk for anemia?
IMJ Starting therapy with a RBV/pegIFN-α lead-in is standard practice when administering boceprevir. Use of a RBV/pegIFN-α lead-in period is off-label for telaprevir-based therapy, but it is nonetheless being employed with increasing frequency. Use of RBV/pegIFN-α lead-in allows clinicians to test the hematologic safety of the regimen before the addition of a PI, which is likely to further decrease hemoglobin levels, and possibly adjust the treatment plan accordingly. Patients who are most likely to benefit from a RBV/pegIFN-α lead-in are those who already have borderline levels of hemoglobin or are anemic, patients with a history of cardiac disease, or patients with advanced cirrhosis.
KVK I agree. Patients may be selectively chosen for an off-label RBV/pegIFN-α lead-in prior to treatment with telaprevir, and this approach may be especially beneficial for treatment-experienced patients or patients with borderline hematologic parameters. If hemoglobin levels fall precipitously during the lead-in period, interventions such as EPO can be administered to preserve the bone marrow responses before the introduction of the PI.
PYK The use of a RBV/pegIFN-α lead-in is an option for assessing sensitivity to RBV before the introduction of the DAA agent. In particular, individuals who had difficulty with anemia during prior treatment or patients with risk factors for anemia—such as low baseline hemoglobin levels or renal function at the low end of normal—would benefit from a RBV/pegIFN-α lead-in. If the lead-in therapy results in a significant hemoglobin decline, early administration of EPO is a useful strategy for prevention of anemia prior to the addition of the DAA agent.
Acknowledgements
Ira M. Jacobson, MD, has received grant/research support from Achillion, Anadys, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlobeImmune, Novartis, Pfizer, Pharmasset, Roche/Genentech, Schering/Merck, Tibotec/Janssen, Vertex, and Zymogenetics; he is also a consultant/advisor for Abbott, Achillion, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, GlobeImmune, Idenix, Inhibitex, Kadmon, Novartis, Pharmasset, Presidio, Roche/Genentech, Schering/Merck, Tibotec/Janssen, and Vertex; and he serves on the speakers’ bureau for Bristol-Myers Squibb, Gilead, Roche/Genentech, Schering/Merck, and Vertex.
Kris V. Kowdley, MD, has received consultingfees (payable to his institution) from Abbott, Gilead, Novartis, and Vertex; he has also received research funding from Abbott, Boeringer Ingelheim, Bristol-Myers Squibb, Conatus, Gilead, Intercept, Merck, and Mochida.
Paul Y. Kwo, MD, has received consultingfees from Abbott, Bristol-Myers Squibb, Gilead, Johnson & Johnson, Merck, Novartis, and Vertex; fees for non-CME/CE services from Gilead, Merck, and Vertex; and contracted research funding from Abbott, Anadys, Bayer, Bristol-Myers Squibb, Conatus, Gilead, GlaxoSmithKline, Merck, Novartis, Roche, and Vertex.
Biographies



Slide Library

For a free electronic download of these slides, please direct your browser to the following web address: http://www.clinicaladvances.com/index.php/our_publications/gastro-hep-issue/gh_september_2012/
Footnotes
Disclaimer: Every effort has been made to ensure that drug usage and other information are presented accurately; however, the ultimate responsibility rests with the prescribing physician. Gastro-Hep Communications, Inc., the supporter, and the participants shall not be held responsible for errors or for any consequences arising from the use of information contained herein. Readers are strongly urged to consult any relevant primary literature. No claims or endorsements are made for any drug or compound at present under clinical investigation.
Contributor Information
Ira M. Jacobson, Chief of the Division of Gastroenterology and Hepatology Weill Cornell Medical College New York, New York.
Kris V. Kowdley, Director of the Liver Center of Excellence Director of Research Digestive Disease Institute Virginia Mason Medical Center Seattle, Washington.
Paul Y. Kwo, Medical Director of Liver Transplantation Indiana University School of Medicine Indianapolis, Indiana.


