Abstract
Hepatitis C virus (HCV) causes chronic systemic infection, primarily affecting the liver. Although HCV mainly causes hepatitis, a significant portion of chronic HCV patients manifests with at least 1 extrahepatic involvement during the course of their illness. Chronic HCV infection can cause various types of renal diseases. The most common renal manifestations of HCV infection are essential mixed cryoglobulinemia leading to membranoproliferative glomerulonephritis (MPGN), MPGN without cryoglobulinemia, and membranous glomerulonephritis. On the other hand, patients with end-stage kidney disease are at an increased risk of acquiring HCV due to their frequent exposure to potentially contaminated devices in dialysis units and their long-term use of vascular access. Among dialysis patients or patients undergoing renal transplantation, the presence of HCV is associated with higher rates of mortality. The optimal antiviral therapy in patients with severe renal insufficiency is not yet well established and, in most cases, is associated with serious adverse effects. Randomized controlled trials looking at treatment options are lacking. This article reviews the pathophysiology of renal manifestations of chronic HCV infection, discusses recent insights into diagnostic and treatment options for HCV-induced glomerulopathies and HCV-infected dialysis patients, and describes the work-up of HCV-positive renal transplant candidates.
Keywords: Hepatitis C virus, renal manifestations, mixed cryoglobulinemia, glomerulonephritis, kidney transplantation
Hepatitis C virus (HCV) causes chronic systemic infection, which primarily affects the liver.1 The existence of HCV was hypothesized in the 1970s, and the virus was eventually isolated in 1989.2 Although HCV mainly causes hepatitis, a significant portion (40%) of chronic HCV patients manifests with at least 1 extrahepatic involvement during the course of their disease.3 It is vital that clinicians recognize, diagnose, and treat those extrahepatic syndromes, as these patients may not have any manifestations of chronic liver disease.4
An estimated 3-4 million people are infected with HCV worldwide annually. Each year, more than 350,000 people die from diseases or syndromes associated with HCV infection. In the United States, about 2% of the population is infected with HCV. The Centers for Disease Control and Prevention (CDC) estimated that approximately 17,000 new HCV infections occur every year, but only 849 cases of confirmed acute HCV infection were reported in 2007. Since acute HCV infection is usually asymptomatic, it is rarely identified. Most HCV infections are diagnosed incidentally via serologic screening tests or when patients develop manifestations of advanced liver disease.5 The World Health Organization predicts that about 3% of the world’s population— 210 million people—have been infected with HCV.6
Hepatitis C Virus and Renal Manifestations
Chronic HCV infection can potentially cause chronic kidney diseases. Both glomerular and tubulointerstitial diseases associated with HCV have been described.7–11 However, the exact mechanism of these diseases is unclear. An association between HCV infection and albuminuria without overt kidney disease has also been described; hence, HCV infection may have a greater influence on renal dysfunction than is presently documented.12,13 The most common renal manifestations of HCV infection are essential mixed cryoglobulinemia (MC) leading to mem-branoproliferative glomerulonephritis (MPGN), MPGN without cryoglobulinemia, and membranous glomerulonephritis.14 On the other hand, patients with end-stage kidney disease are at an increased risk of acquiring HCV infection due to their frequent exposure to potentially contaminated devices in dialysis units and their long-term use of vascular access.15–17
Cryoglobulinemia
Cryoglobulinemia refers to the presence of 1 (monoclonal) or more (mixed or polyclonal) immunoglobulins in the serum, which reversibly precipitate in vitro at low temperatures (<37°C). These immunoglobulins dissolve again when the serum is reheated. Cryoglobulins were first described by Wintrobe and Buell, hema-tologists at Johns Hopkins University, in a patient with multiple myeloma in 1933.18–20 The first report of MC was presented by Meltzer and colleagues in 1966.21
Cryoglobulinemia is classically grouped into 3 types according to the Brouet classification system.22,23 Type 1 cryoglobulinemia is composed of isolated monoclonal immunoglobulin (Ig) M and is most commonly associated with lymphoproliferative disorders. Type 1 cryoglobulinemia represents only 10—15% of cases. Type 2 cryoglobulinemia consists of mixed immune complexes formed by monoclonal IgM and polyclonal IgG. It is seen in viral infections—such as HCV, hepatitis B virus (HBV), and cytomegalovirus—and chronic inflammatory states such as systemic lupus erythematosus, rheumatoid arthritis, and Sjogren syndrome. Type 2 cryoglobulinemia represents 50—60% of reported cases. Type 3 cryoglobulinemia contains mixed immune complexes typically formed by polyclonal IgM, and it represents 25—30% of cases.
Type 2 MC is most commonly associated with chronic HCV infection. Initially, the reported cases of MC suggested its association with HBV.24 However, the role of HCV infection in MC was verified in the 1990s after several cases reported the presence of anti-HCV antibodies and HCV RNA in 70—100% of patients with MC.25 On the other hand, MC is not seen in all HCV patients; only 10—15% of HCV-infected patients develop MC.26 In a recent study, Lidar and colleagues compared patients with HCV-associated and autoimmune disease—associated MC to geographically matched healthy controls.27 Anti-HCV IgG antibodies were detected in all patients with HCV-associated MC but not in any healthy controls, and no anti-HCV antibodies were detected in patients with autoimmune disease—associated MC. This finding supports the idea of a novel association between HCV infection and MC.27
The main features of MC are referred to as Melt-zer’s triad: purpura, arthralgia, and myalgia.28 However, additional features can also be present, such as hepatitis, glomerulonephritis, peripheral neuropathy, skin ulcers, and lymphoproliferative disorders.29
Cryoglobulinemic Glomerulonephritis
The disease burden in patients with cryoglobulinemic glomerulonephritis can range from asymptomatic proteinuria or hematuria to nephrotic or nephritic syndrome, which can progress to chronic renal insufficiency. MC typically causes type 1 MPGN with immune complex depositions in glomeruli. Only 10% of these cases progress to end-stage renal disease (ESRD) and require dialysis.30–32 The diagnosis is often made by low serum concentrations of complement levels (C1q, C4, and C3) and the presence of anti-HCV antibodies and HCV RNA.33
Other Hepatitis C Virus—Related Glomerulopathies
Although MPGN is most commonly associated with HCV infection, other glomerulonephritides are also reportedly associated with HCV, including membranous nephropathy, focal segmental glomerulosclerosis, postinfectious glomerulonephritis, thrombotic micro-angiopathies, IgA nephropathy, and fibrillary or immu-notactoid glomerulopathy.34 While the long-term effects of HCV-associated glomerulopathies are unclear, studies have shown that patients with HCV infection, irrespective of etiology, are 40% more likely to develop ESRD than the general population.35,36 The prognosis of HCV-associated glomerulopathies is poor because of a high incidence of cardiovascular diseases and infections.
Treatment of Hepatitis C Virus—Related Glomerulopathies
Treatment options for HCV-related kidney disease can be simply divided into 3 categories: symptomatic, etiologic, and pathogenetic therapies.
Symptomatic Treatments Renal-protective agents such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, systemic vasodilators, diuretics, and lipid-lowering agents have shown favorable outcomes in symptomatic treatment of HCV-related chronic kidney disease.37–39
Etiologic Therapy or Antiviral Therapy Antiviral therapy against HCV infection, aiming for eradication and reduction of HCV-related antibodies and immune complexes, is the first-line therapy in HCV-related glomerulopathies. Current treatment options that have been well studied in HCV-infected patients with renal insufficiency are interferon α (IFN-α), pegylated IFN α (pegIFN-α), and ribavirin (RBV).40 The efficacy of antiviral therapy is typically measured in terms of sustained virologic response (SVR), which requires that HCV RNA levels be undetectable for at least 6 months after cessation of therapy. Several studies have shown that SVR rates can vary with different genotypes of HCV. SVR can be achieved in up to 65—90% of patients with genotype 2 or 3 HCV who received IFN-a and RBV therapy, compared to only 30—50% of patients with genotype 1 HCV.41–44 A meta-analysis that compared the efficacy and safety of antiviral and immunosuppressive therapies in HCV-associated glomerulonephritis validated the finding that the reduction in proteinuria was significant after IFN-α therapy for at least 6 months.45
Vigani and colleagues recently found that MC is associated with necroinflammatory activity in HCV-infected patients.46 Their study also showed evidence that cryoglobulinemia is associated with high SVR rates in patients with HCV genotypes 1—4 who were treated with pegIFN-α and RBV.46 These findings were later confirmed by another study in which HCV genotype 4 MC patients were treated with peg-INF plus RBV. A complete clinical response was achieved in patients with HCV and cryoglobulinemia but not in patients with HCV alone.47 This study suggests that the presence of cryoglobulinemia may be a positive predictor for treatment response.
Pathogenetic Therapy Since there is a link between HCV infection and immune responses that affect the glomeruli, pathogenetic modalities such as immunosup-pressive agents, high-dose corticosteroids, and plasma exchange have also been used in severe HCV-related glomerulopathies.48,49 It needs to be cautioned that these drugs can potentiate further viral replication.50 Severe active disease is generally defined as involvement of 1 or more of the following manifestations: severe renal disease (nephritic syndrome or nephrotic syndrome with progressive decline in renal function and severe histo-logic lesions on biopsy), severe neuropathy, skin ulcers, and/or widespread vasculitis.51
Corticosteroids High-dose corticosteroids with tapering (oral prednisone 0.5—1.5 mg/kg/day or intravenous pulse-dose methylprednisolone 0.5—1 g/day) for 3 days followed by oral prednisone 1 mg/kg/day are initially used to control the acute phase of the disease.52,53 However, corticosteroids can induce viral replication and exacerbate the underlying hepatic injury.
Cytotoxic Agents Cytotoxic drugs suppress B-lym-phocyte proliferation, thereby inhibiting cryoglobulin production. Cyclophosphamide is the most commonly used agent in this category; it is used along with corti-costeroids to achieve remission in patients with severe MC. Chlorambucil (Leukeran, PBS) and azathioprine are other agents that have been tried.52–54 Mycophenolate mofetil is a selective inhibitor of inosine monophos-phate dehydrogenase (IMPDH), a fundamental enzyme in lymphocyte cell proliferation. Studies have shown that mycophenolate mofetil appears to reduce viral load in HCV-infected renal or heart transplant recipients. This effect is thought to be due to the drug’s ability to inhibit IMPDH, which is also inhibited by RBV.55 Despite the limited amount of supporting data, myco-phenolate mofetil, which has a better safety profile than cyclophosphamide, can be effective in the treatment of cryoglobulinemic glomerulonephritis.56
Plasmapheresis Plasmapheresis (PPH) can also be used successfully, together with other immunosuppressive agents, to induce remission in severe cases.30,57 The therapeutic goals of PPH are removal of cytokines, immune complexes, and pathogenic components; alteration of the antigen-antibody ratio; and stimulation of the endothelium grading system.58–60 As it has no effect on underlying cryoglobulin production, PPH has no benefit in long-term control of the disease. It is essential to begin immunosuppressive therapy along with PPH and to continue immunosuppressive therapy for at least 4—6 weeks to prevent rebound immune reactions.57,61
Double-Filtration Plasmapheresis Recently, a newer modality, double-filtration plasmapheresis (DFPP), has attracted the attention of researchers after Fujiwara and colleagues found that DFPP plus IFN-a therapy achieved a significant reduction in viral load among difficult-to-treat chronic HCV patients with high viral loads and genotype 1b HCV.62 Another recent study from Japan showed that initial therapy with DFPP plus pegIFN-α and RBV followed by additional pegIFN-α and RBV therapy is more effective in relapse patients than in null virologic response patients.63 In contrast to traditional PPH, reinfusion solution (fresh frozen plasma or albumin) is not required in DFPP. In DFPP, plasma is separated by passing it through a plasma component separator with a small pore size. Large molecular weight proteins are discarded, and small molecular weight substances (including valuable albumin) are returned to the patient. Cases of successful treatment of HCV-related cryoglobulinemic glomerulonephritis have been reported. Although no randomized controlled trials for efficacy and safety of DFPP have been published, DFPP may become more favorable than traditional PPH in the future.64,65
Rituximab Rituximab (Rituxan, Genentech; RTX), a human/mouse chimeric monoclonal antibody that selectively targets CD20 antigen on B cells, has been used in patients with HCV-associated MC and glomerulonephritis.66,67 RTX is mainly used in chronic lymphocytic leukemia, non-Hodgkin lymphoma, rheumatoid arthritis, Wegener granulomatosis, and microscopic polyangiitis.68 Many small clinical trials have proven the efficacy and safety of RTX in HCV-related glomerulonephritis.69,70 Common side effects are nausea, vomiting, fever, chills, and bronchospasm; these side effects are usually limited to the infusion period and are mostly well tolerated.
In 1 study, Roccatello and colleagues found that proteinuria and serum creatinine levels were significantly reduced in patients with HCV-related cryoglobulinemia who were treated with RTX.71 Saadoun and colleagues analyzed 13 studies in which RTX was used for HCV-related MC syndromes.72 They found that the best response was for glomerulonephritis (70%), while the responses for skin involvement and arthralgia were 53% and 36%, respectively.72 In a study of 7 renal transplant recipients, RTX was found to be effective for treating de novo cryoglobulinemic MPGN in HCV-positive or HCV-negative patients, although higher rates of infectious complications were identified in the RTX group, possibly due to impairment of B-cell functions.73
In another study, 5 patients with active glomerulo-nephritis in HCV-related type 2 MC were treated with RTX monotherapy, without steroids whenever possible. The study suggested that RTX may provide effective and safe treatment for type 2 MC—related glomerulo-nephritis, possibly as a first-line therapy, thus avoiding the need for steroids and harmful immunosuppressive treatment.74 A recent long-term, prospective, randomized, controlled trial evaluated RTX therapy in 59 patients with severe cryoglobulinemic vasculitis. Fifty-three patients were HCV-positive. In this study, De Vita and colleagues demonstrated that RTX monotherapy is superior to conventional therapies such as corticoste-roids, cyclophosphamide, and plasmapheresis in terms of improvement in target organs, including renal function.75 There were no significant differences between the RTX group and the non-RTX group in terms of serious adverse events or deaths.75
Hepatitis C Virus Infection in Dialysis Patients
Patients with ESRD on hemodialysis (HD) are at greater risk of acquiring HCV because of permanent vascular access and frequent exposure to possibly contaminated medical equipment.76 The prevalence of HCV infection in HD patients varies from 5% to 60% in different parts of the world, likely due to many factors, such as length of time on HD, regional HCV prevalence, contact precaution techniques, and the number of blood transfusions.77 Patient age and the number of transfused blood products are the 2 factors most consistently associated with increased prevalence of HCV infection in dialysis patients, irrespective of geographic location.78
As a result of routine screening, compliance with infection-control precautions, and routine use of recombinant human erythropoietin, the prevalence of HCV infection among HD patients has declined. However, this rate is still significantly higher than the prevalence reported in the non-HD population.79–81 Interestingly, spontaneous disappearance of HCV RNA has been documented in 1% of untreated HD patients.82 Although overall mortality increases with HCV infection in HD patients, disease progression and advancement to liver failure appear to be slower and/or less likely compared to a nonuremic cohort.83
HCV infection usually does not present with acute symptoms, and the progression of disease is a long-term process. Mostly, patients are diagnosed with HCV infection when they are tested after developing nonspecific symptoms such as fatigue, weight loss, jaundice, or elevations of liver enzyme levels. Development of those constitutional symptoms is not uncommon for HD patients. Thus, routinely screening this population is important, as these patients are at risk for acquiring HCV infection.
Natural History of Hepatitis C Virus Infection in Dialysis Patients
The natural course of HCV infection in patients on HD is not well understood, although there is evidence showing that HCV seropositivity can definitely reduce the overall outcomes in these patients. The reason for HCV viral load reduction in HD patients is unclear. HCV infection naturally causes liver injury by immunologic reactions rather than via a direct cytopathic effect on hepatocytes. It has been postulated that HD patients are, in general, immuno-compromised, and this immunocompromised state could be a possible cause of diminished inflammatory reactions and reduced hepatocyte destruction by HCV.
The best case-control study to elucidate the natural course of HCV infection in HD patients was done by Okuda and colleagues.84 These researchers examined 189 patients with chronic HCV infection who were on HD and a control group of twice as many sex- and age-matched controls; the patients were followed for 4—23 years. While chronic HCV infection progressed to cirrhosis in more than 25% of the control group, none of the cases progressed to cirrhosis with high statistical significance (P<.0001).84 In another study, Ishida and colleagues found that the incidences of cirrhosis and hepatocellular carcinoma were significantly lower—8.6% and 1.8%, respectively—in dialysis patients with HCV infection compared to 15—20% and 5—28%, respectively, in normal adults with HCV infection.85 Moreover, the incidence rates of cirrhosis and hepatocellular carcinoma were much lower in patients who had been on dialysis for more than 10 years. This study suggests that the longer patients with HCV remain on dialysis, the less likely it is that their disease will progress.85
Hepatitis C Viral Load and Hemodialysis
Among HCV patients without renal impairment, HCV viral load rises 8-fold or more in immunosuppressed patients such as those with HIV/HCV co-infection or liver and/or kidney transplant recipients. Many studies have found a reduction of HCV viral load in patients on HD compared to nonuremic controls; however, some studies have demonstrated similar findings in both groups.86–88 The discrepancies among studies could result from variations in study methods for detecting HCV RNA (the molecular techniques used to quantify HCV RNA levels are sensitive to heparin, which is commonly used in HD), different types of chemicals used in dialysis membranes, and/or duration of follow-up. Nevertheless, HCV viral load reduction in chronic HCV patients who are not on antiviral treatments is not expected.
The proposed hypotheses for HCV viral load reduction in HD patients involve the passage of viral particles into the dialysate, the trapping of the viral particles on the surface of the dialyzer membrane, and/or an indirect host-mediated immune response.89 The dialysate membrane can be safely reused after high-level disinfection, and this practice might influence the HCV viral load in HD patients. The reuse of dialyzer is practiced in many countries and is considered safe by the CDC.90,91 Reuse of dialyzer is practiced in 60—80% of dialysis centers in the United States.92 Martins and colleagues looked at HCV RNA levels in paired pre-HD and post-HD samples when HD was performed with the reuse of dialyzer.93 They found that HCV viral load did not decrease after the tenth HD session of dialyzer reuse, despite 52.3% of patients showing a decrease in HCV viral load following the first HD session (compared to their pre-HD baseline). The researchers concluded that the dialysis membrane possibly reduces HCV viremia through the trapping of HCV particles on the surface of the membrane over a long-term period. Longitudinal studies are needed to validate this finding.93
Studies have shown that a significant amount of cytokines with antiviral properties—such as interleu-kin-2, interleukin-6, IFN-α, and tumor necrosis factor—are spontaneously released from monocytes and T lymphocytes during HD.94,95 Cellulosic membranes can activate monocytes and lymphocytes due to their bio-incompatibility. However, newer synthetic biocompatible membranes are supposedly not capable of inducing an immune reaction. Badalamenti and colleagues did not find any significant differences in HCV titers among patients undergoing HD with cellulosic versus synthetic membranes, despite a substantial reduction in HCV titers after dialysis.96 They also did not find evidence of a direct cause-effect relationship between the induction of IFN-a and simultaneous reduction in HCV viral load. Additional studies are warranted to assess the timing of and relationship between HCV infection and intracytoplasmic IFN-α in lymphomonocytes before and at different times during HD.96
Hepatitis C Virus Infection in Renal Transplant Recipients
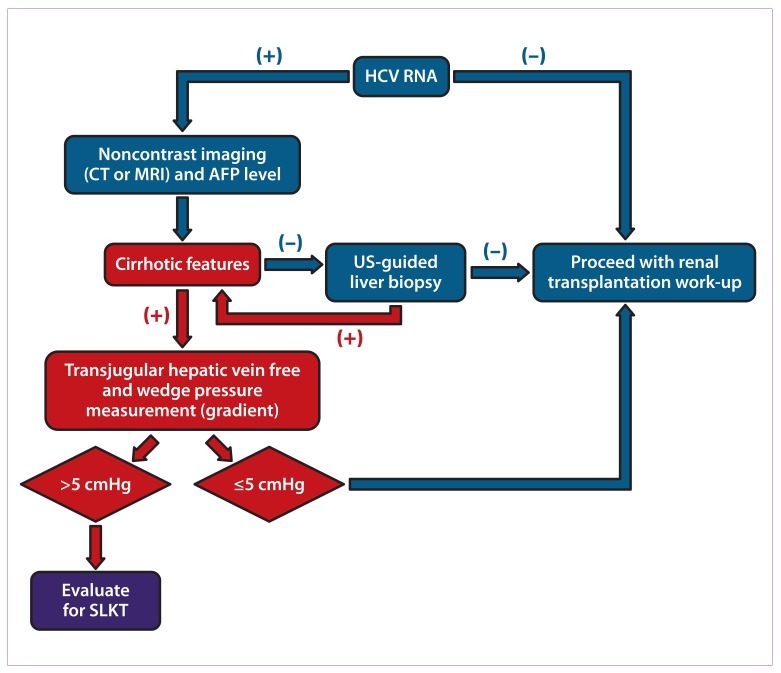
The pretransplantation prevalence of HCV infection is reported to be as high as 40%.97 Studies have revealed that overall survival and graft survival are significantly lower in HCV-seropositive renal transplant recipients compared to their HCV-seronegative counterparts.98–100 A suggested approach for pre—renal transplantation screening for HCV-positive recipients is shown in Figure 1. Use of this algorithm is especially important because it prompts clinicians in evaluating potential renal transplant recipients for evidence of potential portal hypertension, which usually leads to consideration for simultaneous liver-kidney transplantation.
Figure 1.
A suggested algorithm for pre–renal transplantation screening for hepatitis C virus (HCV)-positive candidates.
AFP=α-fetoprotein; CT=computed tomography; MRI=magnetic resonance imaging; SLKT=simultaneous liver-kidney transplantation; US=ultrasound.
The United States Renal Data System Registry reported that all-cause mortality rates in HCV-seropositive and HCV-seronegative renal transplant recipients were 13% and 8.5%, respectively. This variation was accounted for by liver failure and infections.99 However, other studies have found that HCV-positive patients who have biopsy-proven mild liver damage in the pretransplantation period had either no progression or limited progression of fibrosis in the first 5 years after renal transplantation, which is similar to the findings in HCV-infected patients on HD.101 Therefore, the exact role of liver injury in renal transplant recipients is unclear, and further studies are warranted. Other associated risk factors—such as infections, post-transplantation glomerulonephritis, and new-onset diabetes—possibly play a role in increasing mortality in post-transplantation patients with HCV infection.102–104
The HCV status of graft donors also has an impact on patient and graft survival. Some studies have found that transplantation from HCV-positive donors to HCV-negative recipients is associated with poorer outcomes in both graft and patient survival, whereas other studies reported no significant difference.97,105–109 Kasprzyk and colleagues reported no significant differences in graft and patient survival between HCV-positive—positive and HCV-positive—negative donor/recipient groups.110 These data suggest that HCV-positivity in donors and/or recipients is associated with lower survival. However, the mortality in HCV-positive HD patients is still significantly high.111–113 Thus, HCV-positive HD patients should not wait for HCV-negative donors, and transplantation should take place as soon as possible.
Treatment of Hepatitis C Virus Infection in the Setting of Renal Transplantation
HCV infection in renal transplant recipients can lead to post-transplantation de novo glomerulopathies (especially cryoglobulinemic glomerulonephritis and MPGN) and renal impairment. It is recommended that HCV-positive individuals who are candidates for renal transplantation be treated with antiviral therapies to avoid the recurrence of renal disease.114 While there are no definite guidelines for the treatment of HCV infection in renal transplant recipients, IFN-α alone or in combination with RBV can be suggested. Other therapies such as RBV monotherapy, amantadine, and newer agents such as protease inhibitors (boceprevir [Victrelis][Merck] and telaprevir [Incivek][Vertex]) have also been tried.
Interferon-α When the US Food and Drug Administration (FDA) approved IFN-α in 1991, this drug was tested in renal transplant recipients. Many studies showed poor outcomes with very low SVR rates and high numbers of adverse effects. The most serious side effects were acute renal failure and graft losses.115
Fabrizi and colleagues analyzed 12 clinical trials of antiviral therapy with IFN-α alone or IFN-α plus RBV in HCV-positive renal transplant recipients.116 The estimated SVR and discontinuation rates were 18% and 35%, respectively. The most common side effect resulting in discontinuation was graft dysfunction. These authors concluded that IFN-α therapy had a poor safety profile and poor tolerance after renal transplantation.116 IFN-α may trigger graft rejection by producing antibodies and inducing intra-cellular cytokine gene expression and cell surface expression of human leukocyte antigen alloantigen.117 SVR in renal transplant recipients treated with IFN-α was achieved during therapy; however, HCV viral load rebounded to high levels after IFN-α discontinuation. Therefore, it was recommended that use of IFN-α in renal transplant patients be avoided due to its potential of causing graft rejection and recurrence after drug discontinuation.
Interferon-α and Ribavirin In 2004, Shu and colleagues published a study in which ultra-low—dose IFN-α (1 X 106 units subcutaneously 3 times/week) plus RBV (600 mg/day) were given to 11 renal transplant patients with HCV infection for 48 weeks.118 Three patients dropped out, either due to acute graft failure (n=1) or urosepsis (n=2). Five patients (62.5%) cleared HCV after therapy, but only 3 patients (37.5%) achieved SVR. The study concluded that this regimen might be a relatively safe way to achieve SVR in renal transplant recipients.118 In a study by Sharma and colleagues, low-dose IFN-α (1.5 X 106 units 3 times/week) plus RBV were given to 6 patients for 2—18 months.119 Two patients dropped out due to graft dysfunction, and 2 patients (33.3%) achieved SVR, similar to the previous study.119 Interestingly, Schmitz and colleagues later reported that SVR was achieved in 50% of patients, and none developed graft dysfunction or rejection.120 Their studies included 6 combined liver-kidney transplant recipients who were given pegIFN-α2b plus RBV for 48 weeks.120 These studies demonstrate that IFN-α alone or IFN-α plus RBV therapy could be used in individual patients to achieve SVR with little or no adverse effects, although larger randomized controlled trials are needed to validate these findings.
In general, IFN-α alone or IFN-α plus RBV can be given to renal transplant recipients when the benefits of the therapy are greater than the adverse effects. The absolute indications for antiviral therapy among renal transplant recipients are fibrosing cholestatic hepatitis and severe de novo glomerulonephritis. Nevertheless, the risk of chronic graft failure should always be considered before the initiation of IFN-α treatment.
Ribavirin Monotherapy The first study on RBV monotherapy was done by Kamar and colleagues and involved 16 HCV-positive renal transplant recipients who were given RBV (1,000 mg/day) for 1 year.121 A control group of 32 patients were not given RBV. Although liver enzyme levels and serum creatinine levels decreased, there was no significant change in HCV viral load or liver fibrosis in patients who received RBV. Hemolytic anemia was the most serious adverse reaction.121 In another study, Sharma and colleagues gave RBV alone (400—800 mg/day) to 8 HCV-positive renal transplant patients and found similar results.119
Fontaine and colleagues later conducted a study in which 13 renal recipients with high Metavir scores (F3, n=8; F4, n=5) were treated with RBV alone.122 A significant histopathologic improvement was found, with pretreatment and on-treatment biopsy specimens revealing a significant decrease in Metavir scores (2.46±0.78 vs 1.23±1.01; P<.05). Liver enzyme levels were also significantly decreased without reduction in HCV viral load. Hence, RBV monotherapy may improve histopathologic findings and reduce liver enzyme levels with no effect on viral clearance.122 Although RBV monotherapy may be useful after renal transplantation in order to stabilize liver disease, its long-term effects need to be further studied, especially in the presence of serum HCV RNA.
Amantadine Amantadine is an organic compound containing adamantane, which has antiviral and immuno-modulatory effects.123,124 A pilot study of amantadine mono-therapy in HCV patients who had failed IFN-α therapy was published by Smith in 1997.125 After 6 months of therapy, 27% of patients had alanine aminotransferase level normalization, and 18% of patients achieved SVR.125 Numerous later studies have reported similar findings, but patients in these studies were not renal transplant recipients.126,127
Kamar and colleagues tested amantadine mono-therapy in renal transplant subjects and concluded that it could improve liver enzyme levels with no effect on HCV viral load or liver histology.128 A recent prospective, randomized, controlled trial of HCV-positive renal transplant recipients consisted of 3 groups: RBV alone, amantadine plus RBV therapy, or no therapy. The study found no relevant differences among the groups in terms of liver enzyme levels, HCV viremia, hepatic histology, or renal parameters.129
Rituximab in Kidney Transplant Recipients Basse and colleagues reported that RTX could dramatically improve renal parameters and achieve sustained clearance of cryoglobulins in 5 patients with HCV.73 Two patients developed serious disseminated infections with Cryptococcus and herpes simplex virus. In a similar study by Kamar and colleagues, RTX was not associated with HCV flare-ups during or after therapy, which suggests its safety in immunocompromised patients.130 Further randomized controlled trials are essential to confirm these findings.
Protease Inhibitors The FDA recently approved the protease inhibitors telaprevir and boceprevir as an addon to standard therapy with pegIFN-α and RBV, after 2 major trials (SPRINT-2 and ADVANCE) proved the efficacy of both drugs in achieving higher SVR rates in patients with genotype 1 HCV infection.131–133 SVR rates were 75% and 69% for telaprevir and boceprevir, respectively, in treatment-naive white patients with genotype 1 chronic HCV infection. SVR rates were slightly lower—65% and 53% for telaprevir and boceprevir, respectively—in treatment-naive black patients with genotype 1 HCV infection.
Despite their efficacy in achieving SVR in patients with genotype 1 HCV infection, protease inhibitors may have serious adverse effects in transplant patients due to concurrent use of immunosuppressive therapies. Current immunosuppressive therapies in renal transplant recipients consist of calcineurin inhibitors (cyclosporine and tacrolimus), mammalian target of rapamycin inhibitors (sirolimus and everolimus), antimetabolites such as mycophenolate mofetil and azathioprine, and newer monoclonal antibodies.134
Both telaprevir and boceprevir inhibit the cyto-chrome P450 (CYP3A4 substrate) enzyme, which is responsible for the metabolism of cyclosporine and tacrolimus. Garg and colleagues conducted a phase I, open-label, nonrandomized, single-sequence study to evaluate the changes in plasma drug levels of telaprevir when it is coadministered with a single dose of cyclo-sporine (Part A) or tacrolimus (Part B) in 2 separate groups with 10 healthy volunteers in each group.135 They found that coadministration with steady-state telaprevir increased dose-normalized concentrations of cyclosporine and tacrolimus by factors of 4.6 and 70, respectively.135 Since boceprevir and telaprevir share their basic pharmacokinetic properties with calcineurin inhibitors, similar effects can be anticipated with coadministration of cyclosporine or tacrolimus. If immunosuppressive agents are used with protease inhibitors, the risk of drug toxicity is significant. To date, there are no studies of the efficacy and safety of telaprevir or boceprevir in organ transplant recipients, including those who have undergone kidney, liver, or simultaneous liver-kidney transplantation. Randomized, controlled trials are needed in this area to assess the safety of protease inhibitors in transplant patients. At present, use of protease inhibitors in renal transplant recipients to treat HCV infection is not recommended.136
Conclusion
HCV infection is a major medical burden in patients with chronic kidney disease. While HCV infection itself can cause chronic kidney disease, mainly mixed cryoglobulinemic glomerulonephritis and MPGN, patients with chronic kidney disease are also at a higher risk of acquiring HCV infection. Renal transplant recipients or patients on HD who are HCV-positive have higher mortality rates compared to those who are HCV-negative. The optimal antiviral therapy in patients with severe renal insufficiency is not well established and in most cases carries serious adverse effects. The most recent guidelines from the Kidney Disease Improving Global Outcomes group recommend that clinicians screen, prevent, and treat HCV infection in patients with chronic kidney disease who have no contraindications to antiviral therapy (Table 1).81 Treatment of HCV infection prior to renal transplantation is recommended to prevent patients from developing HCV-associated renal diseases in the graft kidney. Therefore, understanding the renal involvements of HCV infection and its complications are very important; trials of antiviral agents with better efficacy and less toxicity are needed to improve the outcomes of chronic kidney disease patients with HCV infection.
Table 1.
Summary of Kidney Disease Improving Global Outcomes Clinical Practice Guidelines for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C Virus (HCV) Infection in Patients with Chronic Kidney Disease (CKD)
| Detection and evaluation of HCV infection in patients with CKD |
| Testing for HCV infection should be performed in patients on maintenance hemodialysis (CKD Stage 5D) and renal transplant candidates. |
| Patients on hemodialysis should be tested when they first start hemodialysis or when they transfer from another hemodialysis facility. |
| Testing for HCV infection with NAT should be performed for hemodialysis patients with unexplained abnormal aminotransferase(s) levels. |
| If a new HCV infection in a hemodialysis unit is suspected to be nosocomial, testing with NAT should be performed in all patients who may have been exposed. |
| Treatment of HCV infection in patients with CKD |
| It is suggested that the decision to treat be based on the potential benefits and risks of therapy, including life expectancy, candidacy for kidney transplantation, and comorbidities. |
| It is suggested that HCV-infected patients accepted for kidney transplantation be treated. |
| It is suggested that treatment of HCV-infected kidney transplant recipients be considered only when the benefits of treatment clearly outweigh the risk of allograft rejection due to IFN-α—based therapy (for example, fibrosing cholestatic hepatitis and life-threatening vasculitis). |
| For HCV-infected patients with CKD Stages 1 and 2, combined antiviral treatment using pegIFN-α and RBV is suggested. |
| For HCV-infected patients with CKD Stages 3, 4, and 5 who are not yet on dialysis, monotherapy with pegIFN-a is suggested, with doses adjusted to the level of kidney function. |
| For HCV-infected patients with CKD Stage 5 who are on maintenance hemodialysis, monotherapy with standard IFN-α that is dose-adjusted for a GFR of 15 mL is suggested. |
| All patients with HCV infection, regardless of treatment or treatment response, should be followed for HCV-associated comorbidities. |
| Prevention of HCV transmission in hemodialysis units |
| Hemodialysis units should ensure implementation of, and adherence to, strict infection-control procedures designed to prevent transmission of blood-borne pathogens, including HCV. |
| Infection-control procedures should include hygienic precautions that effectively prevent the transfer of blood—or fluids contaminated with blood—between patients, either directly or via contaminated equipment or surfaces. |
| Management of HCV-infected patients before and after kidney transplantation |
| All kidney transplant candidates should be evaluated for HCV infection. |
| All kidney donors should be tested for HCV infection. |
| It is suggested that HCV-infected kidney transplant recipients have their liver disease evaluated at least annually once they are more than 6 months post-transplantation. |
| It is suggested that HCV-infected kidney transplant recipients be screened for the development of hyperglycemia. |
| Diagnosis and management of kidney diseases associated with HCV infection |
| It is suggested that HCV-infected patients be tested at least annually for proteinuria, hematuria, and estimated GFR to detect possible HCV-associated kidney disease. |
| It is suggested that a kidney biopsy be performed in HCV-infected patients with clinical evidence of glomerulonephritis. |
| It is suggested that immunosuppressive agents be considered for patients with cryoglobulinemic glomerulonephritis. |
GFR=glomerular filtration rate; IFN-α=interferon α; NAT=nucleic amplification test; pegIFN-α=pegylated interferon α; RBV=ribavirin.
Adapted from Kidney Disease Improving Global Outcomes.81
References
- 1.Ryan KJ, Ray CG, editors. Sherris Medical Microbiology. 4th ed. New York, NY: McGraw-Hill; 2004. pp. 551–552. [Google Scholar]
- 2.Houghton M. The long and winding road leading to the identification of the hepatitis C virus. J Hepatol. 2005;51:939–948. doi: 10.1016/j.jhep.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Cacoub P, Renou C, Rosenthal E, et al. Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. Medicine. 2000;79:47–56. doi: 10.1097/00005792-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ali A, Zein NN. Hepatitis C infection: a systemic disease with extrahepatic manifestations. Cleve Clin J Med. doi: 10.3949/ccjm.72.11.1005. 2005;72:1005-1008, 1010-1014, 1016. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. www.cdc.gov/hepatitis/HCV/HCVfaq.htm#section1
- 6.World Health Organization. Hepatitis C. www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index1.html
- 7.Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE. Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med. 1993;328:465–470. doi: 10.1056/NEJM199302183280703. [DOI] [PubMed] [Google Scholar]
- 8.Altraif IH, Abdulla AS, al Sebayel MI, et al. Hepatitis C associated glomerulonephritis. Am J Nephrol. 1995;5:407–410. doi: 10.1159/000168874. [DOI] [PubMed] [Google Scholar]
- 9.Sabry A, E-Agroudy A, Sheashaa H, et al. HCV associated glomerulopathy in Egyptian patients: clinicopathological analysis. Virology. 2005;334:10–16. doi: 10.1016/j.virol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Kasuno K, Ono T, Matsumori A, Nogaki F, Kusano H. Hepatitis C virus-associated tubulointerstitial injury. Am J Kidney Dis. 2003;41:767–775. doi: 10.1016/s0272-6386(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe H, Ono T, Muso E, Matsumori A, Sasayama S. Hepatitis C virus infection manifesting as tubulointerstitial nephritis, cardiomyopathy, and hepatitis. Am J Med. 2000;109:176–177. doi: 10.1016/s0002-9343(00)00369-7. [DOI] [PubMed] [Google Scholar]
- 12.Liangpunsakul S, Chalasani N. Relationship between hepatitis C and microalbuminuria: results from the NHANES III. Kidney Int. 2005;67:285–290. doi: 10.1111/j.1523-1755.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 13.Tsui JI, Vittinghoff E, Shlipak MG, O’Hare AM. Relationship between hepatitis C and chronic kidney disease: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2006;17:1168–1174. doi: 10.1681/ASN.2005091006. [DOI] [PubMed] [Google Scholar]
- 14.Dammacco F, Lauletta G, Montrone M, Sansonno D. Mixed cryoglobulinemia: a model of virus-related disease in internal medicine. Dig Liver Dis. 2007;39:8–12. doi: 10.1016/s1590-8658(07)80004-1. [DOI] [PubMed] [Google Scholar]
- 15.Hardy NM, Sandroni S, Danielson S, Wilson WJ. Antibody to hepatitis C virus increases with time on hemodialysis. Clin Nephrol. 1992;38:44–48. [PubMed] [Google Scholar]
- 16.Mitwalli A, al-Mohaya S, al-Wakeel J, et al. Hepatitis C in chronic renal failure patients. Am J Nephrol. 1992;12:288–291. doi: 10.1159/000168462. [DOI] [PubMed] [Google Scholar]
- 17.al-Wakeel J, Malik GH, al-Mohaya S, et al. Liver disease in dialysis patients with antibodies to hepatitis C virus. Nephrol Dial Transplant. 1996;11:2265–2268. doi: 10.1093/oxfordjournals.ndt.a027146. [DOI] [PubMed] [Google Scholar]
- 18.Wintrobe M, Buell M. Hyperproteinemia associated with multiple myeloma. With report of a case in which an extraordinary hyperproteinemia was associated with thrombosis of the retinal veins and symptoms suggesting Raynaud’s disease. Bull Johns Hopkins Hosp. 1933;52:156–165. [Google Scholar]
- 19.Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol. 2002;55:4–13. doi: 10.1136/jcp.55.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansonno D, Dammacco F. Hepatitis C virus, cryoglobulinaemia, and vasculitis: immune complex relations. Lancet Infect Dis. 2005;5:227–236. doi: 10.1016/S1473-3099(05)70053-0. [DOI] [PubMed] [Google Scholar]
- 21.Meltzer M, Franklin EC, Elias K, McCluskey RT, Cooper N. Cryoglobulinaemia—a clinical and laboratory study. II. Cryoglobulins with rheumatoid factor activity. Am J Med. 1966;40:837–856. doi: 10.1016/0002-9343(66)90200-2. [DOI] [PubMed] [Google Scholar]
- 22.Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974;57:775–788. doi: 10.1016/0002-9343(74)90852-3. [DOI] [PubMed] [Google Scholar]
- 23.Owlia MB, Sami R, Akhondi M, Salimzadeh A. Cryoglobulinaemia in hepatitis C-positive patients in Iran. Singapore Med J. 2007;48:1136–1139. [PubMed] [Google Scholar]
- 24.Levo Y, Gorevic PD, Kassab HJ, Zucker-Franklin D, Franklin EC. Association between hepatitis B virus and essential mixed cryoglobulinemia. N Engl J Med. 1977;296:1501–1504. doi: 10.1056/NEJM197706302962605. [DOI] [PubMed] [Google Scholar]
- 25.Ferri C, Greco F, Longombardo G, et al. Association between hepatitis C virus and mixed cryoglobulinemia [see comment] Clin Exp Rheumatol. 1991;9:621–624. [PubMed] [Google Scholar]
- 26.Sene D, Ghillani-Dalbin P, Thibault V, et al. Long-term course of mixed cryoglobulinemia in patients infected with hepatitis C virus. J Rheumatol. 2004;31:2199–2206. [PubMed] [Google Scholar]
- 27.Lidar M, Lipschitz N, Agmon-Levin N, et al. Infectious serologies and autoantibodies in hepatitis C and autoimmune disease-associated mixed cryoglobulinemia. Clin Rev Allergy Immunol. 2012;42:238–246. doi: 10.1007/s12016-011-8275-x. [DOI] [PubMed] [Google Scholar]
- 28.Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008;3:25. doi: 10.1186/1750-1172-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trejo O, Ramos-Casals M, García-Carrasco M, et al. Cryoglobulinemia: study of etiologic factors and clinical and immunologic features in 443 patients from a single center. Medicine (Baltimore). 2001;80:252–262. doi: 10.1097/00005792-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 30.D’Amico G. Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int. 1998;54:650–671. doi: 10.1046/j.1523-1755.1998.00028.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith KD, Alpers CE. Pathogenic mechanism in membranoproliferative glo-merulonephritis. Curr Opin Nephrol Hypertens. 2005;14:396–403. doi: 10.1097/01.mnh.0000172729.60122.f9. [DOI] [PubMed] [Google Scholar]
- 32.Tarantino A, Campise M, Banfi G, et al. Long-term predictors of survival in essential mixed cryoglobulinemic glomerulonephritis. Kidney Int. 1995;47:618–623. doi: 10.1038/ki.1995.78. [DOI] [PubMed] [Google Scholar]
- 33.Meyers CM, Seeff LB, Stehman-Breen CO, Hoofnagle JH. Hepatitis C and renal disease: an update. Am J Kidney Dis. 2003;42:631–657. doi: 10.1016/s0272-6386(03)00828-x. [DOI] [PubMed] [Google Scholar]
- 34.Markowitz GS, Cheng JT, Colvin RB, Trebbin WM, D’Agati VD. Hepatitis C viral infection is associated with fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol. 1998;9:2244–2252. doi: 10.1681/ASN.V9122244. [DOI] [PubMed] [Google Scholar]
- 35.Tsui JI, Vittinghoff E, Shlipak MG, et al. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med. 2007;167:1271–1276. doi: 10.1001/archinte.167.12.1271. [DOI] [PubMed] [Google Scholar]
- 36.Dalrymple LS, Koepsell T, Sampson J, et al. Hepatitis C virus infection and the prevalence of renal insufficiency. Clin J Am Soc Nephrol. 2007;2:715–721. doi: 10.2215/CJN.00470107. [DOI] [PubMed] [Google Scholar]
- 37.Ruggenenti P, Schieppati A, Remuzzi G. Progression, remission, regression of chronic renal diseases. Lancet. 2001;357:1601–1608. doi: 10.1016/S0140-6736(00)04728-0. [DOI] [PubMed] [Google Scholar]
- 38.Ruggenenti P, Fassi A, Ilieva AP, et al. Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators. Preventing microalbuminuria in type 2 diabetes. N Engl J Med. 2004;351:1941–1951. doi: 10.1056/NEJMoa042167. [DOI] [PubMed] [Google Scholar]
- 39.Chadban SJ, Atkins RC. Glomerulonephritis. Lancet. 2005;365:1797–1806. doi: 10.1016/S0140-6736(05)66583-X. [DOI] [PubMed] [Google Scholar]
- 40.Kamar N, Rostaing L, Alric L. Treatment of hepatitis C virus-related glomeru-lonephritis. Kidney Int. 2006;69:436–439. doi: 10.1038/sj.ki.5000142. [DOI] [PubMed] [Google Scholar]
- 41.McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 42.Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 43.Fujiwara K, Yokosuka O, Komine F, et al. Tokyo Hepatitis Network. Twenty-four weeks of interferon alpha-2b in combination with ribavirin for Japanese hepatitis C patients: sufficient treatment period for patients with genotype 2 but not for patients with genotype 1. Liver Int. 2006;26:520–528. doi: 10.1111/j.1478-3231.2006.01263.x. [DOI] [PubMed] [Google Scholar]
- 44.Yu ML, Chuang WL, Dai CY, et al. Different viral kinetics between hepatitis C virus genotype 1 and 2 as on-treatment predictors of response to a 24-week course of high-dose interferon-alpha plus ribavirin combination therapy. Translational Res. 2006;148:120–127. doi: 10.1016/j.trsl.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Fabrizi F, Bruchfeld A, Mangano S, et al. Interferon therapy for HCV-associated glomerulonephritis: meta-analysis of controlled trials. Int J Artif Organs. 2007;30:212–219. doi: 10.1177/039139880703000306. [DOI] [PubMed] [Google Scholar]
- 46.Vigani AG, Macedo de Oliveira A, Tozzo R, et al. The association of cryoglobu-linaemia with sustained virological response in patients with chronic hepatitis C. J Viral Hepat. 2011;18:e91–e98. doi: 10.1111/j.1365-2893.2010.01385.x. [DOI] [PubMed] [Google Scholar]
- 47.El Khayat HR, Fouad YM, Ahmad EA, et al. Hepatitis C virus (genotype 4)-associated mixed cryoglobulinemia vasculitis: effects of antiviral treatment. Hepatol Int. 2012;6:606–612. doi: 10.1007/s12072-011-9303-x. [DOI] [PubMed] [Google Scholar]
- 48.Bruchfeld A, Lindahl K, Stahle L, Soderberg M, Schvarcz R. Interferon and ribavirin treatment in patients with hepatitis C-associated renal disease and renal insufficiency. Nephrol Dial Transplant. 2003;18:1573–1580. doi: 10.1093/ndt/gfg209. [DOI] [PubMed] [Google Scholar]
- 49.Fabrizi F, Colucci P, Ponticelli C, Locatelli F. Kidney and liver involvement in cryoglobulinemia. Semin Nephrol. 2002;22:309–318. [PubMed] [Google Scholar]
- 50.Lake JR. The role of immunosuppression in recurrence of hepatitis C. Liver Transpl. 2003;9:S63–S66. doi: 10.1053/jlts.2003.50264. [DOI] [PubMed] [Google Scholar]
- 51.Iannuzzella F, Vaglio A, Garini G. Management of hepatitis C virus-related mixed cryoglobulinemia. Am J Med. 2010;123:400–408. doi: 10.1016/j.amjmed.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 52.Lamprecht P, Gause A, Gross WL. Cryoglobulinemic vasculitis. Arthritis Rheum. 1999;42:2507–2516. doi: 10.1002/1529-0131(199912)42:12<2507::AID-ANR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 53.Vassilopoulos D, Calabrese LH. Hepatitis C virus infection and vasculitis: implications of antiviral and immunosuppressive therapies. Arthritis Rheum. 2002;46:585–597. doi: 10.1002/art.10107. [DOI] [PubMed] [Google Scholar]
- 54.Campise M, Tarantino A. Glomerulonephritis in mixed cryoglobulinemia: what treatment? Nephrol Dial Transplant. 1999;14:281–283. doi: 10.1093/ndt/14.2.281. [DOI] [PubMed] [Google Scholar]
- 55.Ramos-Casals M, Font J. Mycophenolate mofetil in patients with hepatitis C virus infection. Lupus. 2005;14(suppl 1):S64–S72. doi: 10.1191/0961203305lu2122oa. [DOI] [PubMed] [Google Scholar]
- 56.Reed MJ, Alexander GJ, Thiru S, Smith KG. Hepatitis C-associated glomerulonephritis—a novel therapeutic approach. Nephrol Dial Transplant. 2001;16:869–871. doi: 10.1093/ndt/16.4.869-a. [DOI] [PubMed] [Google Scholar]
- 57.Bombardieri S, Ferri C, Paleologo G, et al. Prolonged plasma exchange in the treatment of renal involvement in essential mixed cryoglobulinemia. Int J Artif Organs. 1983;6(suppl 1):S47–S50. [PubMed] [Google Scholar]
- 58.Madore F. Plasmapheresis: technical aspects and indications. Crit Care Clin. 2002;18:375–392. doi: 10.1016/s0749-0704(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 59.Guillevin L, Pagnoux C. Indications of plasma exchanges for systemic vasculitides. TherApher Dial. 2003;7:155–160. doi: 10.1046/j.1526-0968.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 60.Drew MJ. Plasmapheresis in the dysproteinemias. TherApher. 2002;6:45–52. doi: 10.1046/j.1526-0968.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 61.Scarpato S, Tirri E, Naclerio C, Moscato P, Salvati G. Plasmapheresis in cryoglobulinemic neuropathy: a clinical study. Dig Liver Dis. 2007;39(suppl 1):S136–S137. doi: 10.1016/s1590-8658(07)80027-2. [DOI] [PubMed] [Google Scholar]
- 62.Fujiwara K, Kaneko S, Kakumu S, Sata M, Hige S. Double filtration plasma-pheresis and interferon combination therapy for chronic hepatitis C patients with genotype 1 and high viral load. Hepatol Res. 2007;37:701–710. doi: 10.1111/j.1872-034X.2007.00117.x. [DOI] [PubMed] [Google Scholar]
- 63.Kim SR, Saito J, Imoto S, et al. Double-filtration plasmapheresis plus interferon-β for HCV-1b patients with non-sustained virological response to previous combination therapy. Digestion. 2011;84(suppl 1):10–16. doi: 10.1159/000333209. [DOI] [PubMed] [Google Scholar]
- 64.Ramunni A, Lauletta G, Brescia P, et al. Double-filtration plasmapheresis in the treatment of leg ulcers in cryoglobulinemia. J Clin Apher. 2008;23:118–122. doi: 10.1002/jca.20166. [DOI] [PubMed] [Google Scholar]
- 65.Namba T, Shiba R, Yamamoto T, et al. Successful treatment of HCV-related cryoglobulinemic glomerulonephritis with double-filtration plasmapheresis and interferon combination therapy. Clin Exp Nephrol. 2010;14:372–376. doi: 10.1007/s10157-010-0282-3. [DOI] [PubMed] [Google Scholar]
- 66.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by chi-meric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 67.Sansonno D, De Re V, Lauletta G, Tucci FA, Boiocchi M, Dammacco F. Monoclonal antibody treatment of mixed cryoglobulinemia resistant to interferon alpha with an anti-CD20. Blood. 2003;101:3818–3826. doi: 10.1182/blood-2002-10-3162. [DOI] [PubMed] [Google Scholar]
- 68.Kazkaz H, Isenberg D. Anti B cell therapy (rituximab) in the treatment of autoimmune diseases. Curr Opin Pharmacol. 2004;4:398–402. doi: 10.1016/j.coph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Zaja F, De Vita S, Mazzaro C, et al. Efficacy and safety of rituximab in type II mixed cryoglobulinemia. Blood. 2003;101:3827–3834. doi: 10.1182/blood-2002-09-2856. [DOI] [PubMed] [Google Scholar]
- 70.De Vita S, Quartuccio L, Fabris M. Rituximab in mixed cryoglobulinemia: increased experience and perspectives. Dig Liver Dis. 2007;39(suppl 1):S122–S128. doi: 10.1016/s1590-8658(07)80024-7. [DOI] [PubMed] [Google Scholar]
- 71.Roccatello D, Baldovino S, Rossi D, et al. Long-term effects of anti-CD20 monoclonal antibody treatment of cryoglobulinaemic glomerulonephritis. Nephrol Dial Transplant. 2004;19:30–54. doi: 10.1093/ndt/gfh469. [DOI] [PubMed] [Google Scholar]
- 72.Saadoun D, Delluc A, Piette JC, Cacoub P. Treatment of hepatitis C-associated mixed cryoglobulinemia vasculitis. Curr Opin Rheumatol. 2008;20:23–28. doi: 10.1097/BOR.0b013e3282f1330c. [DOI] [PubMed] [Google Scholar]
- 73.Basse G, Ribes D, Kamar N, Mehrenberger M, Sallusto F. Rituximab therapy for mixed cryoglobulinemia in seven renal transplant patients. Transplant Proc. 2006;38:2308–2310. doi: 10.1016/j.transproceed.2006.06.131. [DOI] [PubMed] [Google Scholar]
- 74.Quartuccio L, Soardo G, Romano G, et al. Rituximab treatment for glomeru-lonephritis in HCV-associated mixed cryoglobulinaemia: efficacy and safety in the absence of steroids. Rheumatology (Oxford). 2006;45:842–846. doi: 10.1093/rheumatology/kel004. [DOI] [PubMed] [Google Scholar]
- 75.De Vita S, Quartuccio L, Isola M, et al. A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum. 2012;64:843–853. doi: 10.1002/art.34331. [DOI] [PubMed] [Google Scholar]
- 76.Hayashi J, Nakashima K, Kajiyama W, et al. Prevalence of antibody to hepatitis C virus in hemodialysis patients. Am J Epidemiol. 1991;134:651–657. doi: 10.1093/oxfordjournals.aje.a116137. [DOI] [PubMed] [Google Scholar]
- 77.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: effect of hepatitis C virus infection on mortality in dialysis. Aliment Pharmacol Ther. 2004;20:1271–1277. doi: 10.1111/j.1365-2036.2004.02290.x. [DOI] [PubMed] [Google Scholar]
- 78.Tang S, Lai KN. Chronic viral hepatitis in hemodialysis patients. Hemodial Int. 2005;9:169–179. doi: 10.1111/j.1492-7535.2005.01129.x. [DOI] [PubMed] [Google Scholar]
- 79.Tokars JI, Frank M, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States. Semin Dial. 2002;15:162–171. doi: 10.1046/j.1525-139x.2002.00051.x. [DOI] [PubMed] [Google Scholar]
- 80.Carneiro MA, Teles SA, Dias MA, et al. Decline of hepatitis C infection in hemodialysis patients in central Brazil: ten years of surveillance. Mem Inst Oswaldo Cruz. 2005;100:345–349. doi: 10.1590/s0074-02762005000400002. [DOI] [PubMed] [Google Scholar]
- 81.Kidney Disease Improving Global Outcomes. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation and treatment of hepatitis C in chronic kidney disease. Kidney Int. 2008;73:S1–S99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 82.Gordon CE, Uhlig K, Lau J, et al. Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection: a systematic review of the literature and meta-analysis of treatment efficacy and harms. Am J Kidney Dis. 2008;51:263–277. doi: 10.1053/j.ajkd.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 83.Trevizoli JE, de Paula Menezes R, Ribeiro Velasco LF, et al. Hepatitis C is less aggressive in hemodialysis patients than in nonuremic patients. Clin J Am Soc Nephrol. 2008;3:1385–1390. doi: 10.2215/CJN.01330308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okuda K, Yokosuka O. Natural history of chronic hepatitis C in patients on haemodialysis: case control study with 4—23 years of follow-up. World J Gastroenterol. 2004;10:2209–2212. doi: 10.3748/wjg.v10.i15.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ishida H, Agishi T, Koyama I, et al. Hemodialysis paradox: survey on the incidence rate of hepatocellular carcinoma in anti-hepatitis virus C-antibody positive chronic haemodialysis patients. Artif Organs. 2001;25:58–71. doi: 10.1046/j.1525-1594.2001.025001058.x. [DOI] [PubMed] [Google Scholar]
- 86.Furusyo N, Hayashi J, Ariyama I, et al. Maintenance hemodialysis decreases serum hepatitis C virus (HCV) RNA levels in hemodialysis patients with chronic HCV infection. Am J Gastroenterol. 2000;95:490–496. doi: 10.1111/j.1572-0241.2000.01773.x. [DOI] [PubMed] [Google Scholar]
- 87.Sterling RK, Sanyal AJ, Luketic VA, et al. Chronic hepatitis C infection in patients with end-stage renal disease: characterization of liver histology and viral load in patients awaiting renal transplantation. Am J Gastroenterol. 1999;94:3576–3582. doi: 10.1111/j.1572-0241.1999.01649.x. [DOI] [PubMed] [Google Scholar]
- 88.Azevedo HA, Villela-Nogueira CA, Perez RM, et al. Similar HCV viral load levels and genotype distribution among end-stage renal disease patients on hemodialysis and HCV-infected patients with normal renal function. J Nephrol. 2007;20:609–616. [PubMed] [Google Scholar]
- 89.Fabrizi F, Messa P, Martin P. Impact of hemodialysis therapy on hepatitis C virus infection: a deeper insight. Int J Artif Organs. 2009;32:1–11. doi: 10.1177/039139880903200101. [DOI] [PubMed] [Google Scholar]
- 90.National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis. 2006;48(suppl 1):S1–S322. [Google Scholar]
- 91.Centers for Disease Control and Prevention. Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep. 2001;50:1–43. [PubMed] [Google Scholar]
- 92.Upadhyay A, Sosa MA, Jaber BL. Single-use versus reusable dialysers: the known unknowns. Clin J Am Soc Nephrol. 2007;2:1079–1086. doi: 10.2215/CJN.01040207. [DOI] [PubMed] [Google Scholar]
- 93.Martins RS, Filho OA, Gonçales NS, et al. Kinetics of hepatitis C virus load and hemodialysis: is there any influence of the reuse of dialysis membrane on HCV viremia? Scand J Infect Dis. 2012;44:190–196. doi: 10.3109/00365548.2011.627377. [DOI] [PubMed] [Google Scholar]
- 94.Rostaing L, Peres C, Tkaczuk J, et al. Ex vivo flow cytometry determination of intracytoplasmic expression of IL-2, IL6, IFN-gamma and TNF alpha in monocytes and T-lymphocytes in chronic hemodialysis patients. Am J Nephrol. 2000;20:18–26. doi: 10.1159/000013550. [DOI] [PubMed] [Google Scholar]
- 95.Malaponte G, Bevelacqua V, Fatuzzo P. IL-1, TNF and IL-6 release from monocytes in haemodialysis patients in relation to dialytic age. Nephrol Dial Transplant. 2002;17:1964–1970. doi: 10.1093/ndt/17.11.1964. [DOI] [PubMed] [Google Scholar]
- 96.Badalamenti S, Catania A, Lunghi G, et al. Changes in viremia and circulating interferon-alpha during hemodialysis in hepatitis C virus-positive patients: only coincidental phenomena? Am J Kidney Dis. 2003;42:143–150. doi: 10.1016/s0272-6386(03)00417-7. [DOI] [PubMed] [Google Scholar]
- 97.Abbott KC, Bucci JR, Matsumoto CS, et al. Hepatitis C and renal transplantation in the era of modern immunosuppression. J Am Soc Nephrol. 2003;14:2908–2918. doi: 10.1097/01.asn.0000090743.43034.72. [DOI] [PubMed] [Google Scholar]
- 98.Bloom RD, Lake JR. Emerging issues in hepatitis C virus positive liver and kidney transplant recipients. Am J Transplant. 2006;6:2232–2237. doi: 10.1111/j.1600-6143.2006.01457.x. [DOI] [PubMed] [Google Scholar]
- 99.Batty DSJ, Swanson SJ, Kirk AD, et al. Hepatitis C virus seropositivity at the time of renal transplantation in the United States: associated factors and patient survival. Am J Transplant. 2001;1:179–184. [PubMed] [Google Scholar]
- 100.Mitwalli AH, Alam A, Al-Wakeel J, et al. Effects of chronic viral hepatitis on graft survival in Saudi renal transplant patients. Nephron Clin Pract. 2006;102:72–80. doi: 10.1159/000089090. [DOI] [PubMed] [Google Scholar]
- 101.Roth D, Reddy R, Kupin W. Long-term impact of HCV on clinical outcomes and liver histology in kidney recipients. Am J Transplant. 2004;4:289–293. [Google Scholar]
- 102.Gursoy M, Guvener N, Koksal R, et al. Impact of HCV infection on development of post transplantation diabetes mellitus in renal allograft recipients. Transplant Proc. 2000;32:561–562. doi: 10.1016/s0041-1345(00)00890-3. [DOI] [PubMed] [Google Scholar]
- 103.Cruzado J, Carrera M, Torras J, Grinyo J. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant. 2001;1:171–178. [PubMed] [Google Scholar]
- 104.Bouthot BA, Murthy BV, Schmid CH, Levey AS, Pereira BJ. Long-term follow-up of hepatitis C virus infection among organ transplant recipients: implications for policies on organ procurement. Transplantation. 1997;63:849–853. doi: 10.1097/00007890-199703270-00010. [DOI] [PubMed] [Google Scholar]
- 105.Pedroso S, Martins L, Fonseca I, et al. Impact of hepatitis C virus on renal transplantation: association with poor survival. Transplant Proc. 2006;38:1890–1894. doi: 10.1016/j.transproceed.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 106.Maluf DG, Fisher RA, King AL, et al. Hepatitis C virus infection and kidney transplantation: predictors of patient and graft survival. Am J Transplant. 2007;83:853–857. doi: 10.1097/01.tp.0000259725.96694.0a. [DOI] [PubMed] [Google Scholar]
- 107.Behzad-Behbahani A, Mojiri A, Tabei SZ, et al. Outcome of hepatitis B and C virus infection on graft function after renal transplantation. Transplant Proc. 2005;37:3045–3047. doi: 10.1016/j.transproceed.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 108.Veroux P, Veroux M, Sparacino V, et al. Kidney transplantation from donors with viral B and C hepatitis. Transplant Proc. 2006;38:996–998. doi: 10.1016/j.transproceed.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 109.Manga Sahin G, Sahin S, Kantarci G, Ergin H. Impact of hepatitis C virus infection on patient and graft survival in kidney transplantation. Transplant Proc. 2006;38:499–501. doi: 10.1016/j.transproceed.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 110.Kasprzyk T, Kwiatkowski A, Wszola M, et al. Long-term results of kidney transplantation from HCV-positive donors. Transplant Proc. 2007;39:2701–2703. doi: 10.1016/j.transproceed.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 111.Dattolo P, Lombardi M, Ferro G, et al. Natural history of HCV infection and risk of death in a cohort of patients on long-term hemodialysis. G Ital Nefrol. 2006;23:585–590. [PubMed] [Google Scholar]
- 112.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol. 2007;18:1584–1593. doi: 10.1681/ASN.2006070736. [DOI] [PubMed] [Google Scholar]
- 113.Kamar N, Ribes D, Izopet J, et al. Treatment of hepatitis C virus infection (HCV) after renal transplantation: implications for HCV-positive dialysis patients awaiting a kidney transplant. Transplantation. 2006;82:853–856. doi: 10.1097/01.tp.0000238898.14393.c9. [DOI] [PubMed] [Google Scholar]
- 114.Rostaing L, Weclawiaka H, Izopetb J, Kamara N. Treatment of hepatitis C virus infection after kidney transplantation. Contrib Nephrol. 2012;176:87–96. doi: 10.1159/000333775. [DOI] [PubMed] [Google Scholar]
- 115.Rostaing L, Modesto A, Baron E, Cisterne JM, Chabannier MH, Durand D. Acute renal failure in kidney transplant patients treated with interferon alpha 2b for chronic hepatitis C. Nephron. 1996;74:512–516. doi: 10.1159/000189444. [DOI] [PubMed] [Google Scholar]
- 116.Fabrizi F, Lunghi G, Dixit V, Martin P. Meta-analysis: anti-viral therapy of hepatitis C virus-related liver disease in renal transplant patients. Aliment Pharmacol Ther. 2006;24:1413–1422. doi: 10.1111/j.1365-2036.2006.03151.x. [DOI] [PubMed] [Google Scholar]
- 117.Baid S, Tolkoff-Rubin N, Saidman S, et al. Acute humoral rejection in hepatitis C infected renal transplant recipients receiving antiviral therapy. Am J Transplant. 2003;3:74–78. doi: 10.1034/j.1600-6143.2003.30113.x. [DOI] [PubMed] [Google Scholar]
- 118.Shu KH, Lan JL, Wu MJ, et al. Ultralow-dose alpha-interferon plus ribavirin for the treatment of active hepatitis C in renal transplant recipients. Transplantation. 2004;77:1894–1896. doi: 10.1097/01.tp.0000131151.07818.d7. [DOI] [PubMed] [Google Scholar]
- 119.Sharma RK, Bansal SB, Gupta A, Gulati S, Kumar A, Prasad N. Chronic hepatitis C virus infection in renal transplant: treatment and outcome. Clin Transplant. 2006;20:677–683. doi: 10.1111/j.1399-0012.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 120.Schmitz V, Kiessling A, Bahra M, et al. Peginterferon alfa-2b plus ribavirin for the treatment of hepatitis C recurrence following combined liver and kidney transplantation. Ann Transplant. 2007;12:22–27. [PubMed] [Google Scholar]
- 121.Kamar N, Sandres-Saune K, Selves J, et al. Long-term ribavirin therapy in hepatitis C virus—positive renal transplant patients: effects on renal function and liver histology. Am J Kidney Dis. 2003;42:184–192. doi: 10.1016/s0272-6386(03)00422-0. [DOI] [PubMed] [Google Scholar]
- 122.Fontaine H, Vallet-Pichard A, Equi-Andrade C, et al. Histopathologic efficacy of ribavirin monotherapy in kidney allograft recipients with chronic hepatitis C. Transplantation. 2004;78:853–857. doi: 10.1097/01.tp.0000128911.87538.aa. [DOI] [PubMed] [Google Scholar]
- 123.Martin J, Navas S, Fernandez M, et al. In vitro effect of amantadine and interferon alpha-2a on hepatitis C virus markers in cultured peripheral blood mononuclear cells from hepatitis C virus-infected patients. Antiviral Res. 1999;42:59–70. doi: 10.1016/s0166-3542(99)00017-0. [DOI] [PubMed] [Google Scholar]
- 124.De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 125.Smith JP. Treatment of chronic hepatitis C with amantadine. Dig Dis Sci. 1997;42:1681–1687. doi: 10.1023/a:1018857314351. [DOI] [PubMed] [Google Scholar]
- 126.Brillanti S, Levantesi F, Masi L, Foli M, Bolondi L. Triple antiviral therapy as a new option for patients with interferon nonresponsive chronic hepatitis C. Hepatology. 2000;32:630–634. doi: 10.1053/jhep.2000.16235. [DOI] [PubMed] [Google Scholar]
- 127.Mangia A, Minerva N, Annese M, et al. A randomized trial of amantadine and interferon versus interferon alone as initial treatment for chronic hepatitis C. Hepatology. 2001;33:989–993. doi: 10.1053/jhep.2001.23537. [DOI] [PubMed] [Google Scholar]
- 128.Kamar N, Rostaing L, Sandres-Saune K, Ribes D, Durand D, Izopet J. Amantadine therapy in renal transplant patients with hepatitis C virus infection. J Clin Virol. 2004;30:110–114. doi: 10.1016/j.jcv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 129.Calanca LN, Fehr T, Jochum W, et al. Combination therapy with ribavirin and amantadine in renal transplant patients with chronic hepatitis C virus infection is not superior to ribavirin alone. J Clin Virol. 2007;39:54–58. doi: 10.1016/j.jcv.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 130.Kamar N, Sandres-Sauné K, Rostaing L. Influence of rituximab therapy on hepatitis C virus RNA concentration in kidney-transplant patients. Am J Transplant. 2007;7:2440. doi: 10.1111/j.1600-6143.2007.01943.x. [DOI] [PubMed] [Google Scholar]
- 131.Poordad F, McCone J, Jr, Bacon BR, et al. SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jacobson IM, McHutchison JG, Dusheiko G. ADVANCE Study Team. Tela-previr for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 133.Jensen DM. A new era of hepatitis C therapy begins. N Engl J Med. 2011;364:1272–1274. doi: 10.1056/NEJMe1100829. [DOI] [PubMed] [Google Scholar]
- 134.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 135.Garg V, van Heeswijk R, Lee JE, Alves K, Nadkarni P, Luo X. The effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimus. Hepatology. 2011;54:20–27. doi: 10.1002/hep.24443. [DOI] [PubMed] [Google Scholar]
- 136.Charlton M. Telaprevir, boceprevir, cytochrome P450 and immunosuppres-sive agents—a potentially lethal cocktail. Hepatology. 2011;54:3–5. doi: 10.1002/hep.24470. [DOI] [PubMed] [Google Scholar]