Abstract
Transcranial magnetic stimulation (TMS) is increasingly used to modify brain activity noninvasively and to study brain-behavior relations. However, results can be variable and the conditions that affect the functional efficacy of TMS remain unclear. Here we show that online TMS can either facilitate or suppress perceptual functions depending on the baseline level of activity of the targeted brain region. When TMS was applied over the motion selective region V5/MT during a simple motion detection task, subjects’ motion detection ability was impaired. Similarly, suppression of V5/MT activity using offline 1 Hz rTMS disrupted performance in a subsequent motion detection task. However, paradoxically, online V5/MT TMS had a facilitatory effect on motion detection if V5/MT had been suppressed by offline 1 Hz rTMS prior to the motion detection task. These results demonstrate that TMS can have an unexpected facilitatory effect on behavior when the targeted neural population is in a suppressed state. Our findings provide further evidence for the view that the effects of TMS are modulated by the initial activation state of the targeted neural population.
Keywords: Transcranial magnetic stimulation, online TMS, offline 1 Hz TMS, suppression, motion detection, V5/MT
Introduction
Transcranial magnetic stimulation (TMS) allows the study of the neural basis of cognitive functions in neurologically intact subjects (Walsh and Pascual-Leone 2003, Cowey 2005), and may potentially offer therapeutic potential in a number of neurological and psychiatric conditions (e.g., Kobayashi & Pascual-Leone, 2003; Ridding & Rothwell, 2007). Despite its increasingly wide use, the mechanisms through which TMS influences behavior remain poorly understood. Animal studies (Moliazde et al, 2003; Valero-Cabre et al, 2006, 2007) and modelling approaches (Wagner et al 2007) have begun to provide insights into the biological effects of TMS in human subjects, but so far these findings have not been linked to behavioral consequences of TMS. One potentially important factor in understanding the mechanisms of TMS involves the impact of the initial activation state of the stimulated region on the efficacy of stimulation. Studies on the impact of repetitive TMS on cortico-spinal excitability suggest that the baseline level of excitability of the targeted motor cortex may critically influence the results. For example, Siebner et al (2004) showed that when the excitability level of the corticospinal projection is increased using transcranial direct current stimulation (tDCS), a subsequent period of 1 Hz repetitive TMS (rTMS) leads to a lasting reduction in corticospinal excitability. Conversely, when corticospinal excitability is reduced prior to application of rTMS, the same 1 Hz rTMS causes a sustained increase in corticospinal excitability.
The extent to which the initial cortical activation state modulates the effects of online TMS (the neural effects of which are different from those induced by rTMS; cf. Ridding & Rothwell, 2007) is not known. Furthermore, the behavioral consequence of the interaction between the baseline cortical activation state and the application of TMS has so far not been investigated. This is an important issue for the use of TMS in basic research as well as for its efficacy as a therapeutic tool. For example, when offline rTMS is used to determine the necessity of cortical regions in cognitive functions, differences in the initial cortical activation state caused by seemingly irrelevant factors (such as whether the subject is resting or speaking with the experimenter) during the application of rTMS may affect the efficacy of stimulation. For the therapeutic use of TMS this issue is also important as neurological disorders are often associated with abnormal levels of cortical excitability; for example, depression has been linked with hypoactivity in the left dorsolateral prefrontal cortex and hyperactivity in the right DLPFC (Cummings et al 1993). Effective use of TMS thus requires an understanding of how the initial cortical activation state may modify its effects.
In the present study we explored how the baseline activation state of the targeted brain region influences the behavioral impact of TMS. Specifically, we determined whether the application of Offline 1 Hz rTMS to suppress neural activity in the motion area V5/MT prior to performing a motion detection task modulates the effects of online V5/MT TMS applied during that task. Our results show that online TMS over V5/MT can either facilitate or impair motion detection depending on the initial activation state of V5/MT.
Materials and Methods
Subject
Eight subjects (six males and two females, aged 24 to 37), including author J.S., took part in the experiment. Informed consent was obtained from all subjects. The experiment was approved by a local ethics committee and subjects were treated in accordance with the declaration of Helsinki.
Visual stimulus
The stimuli were presented on a 15 inch (800 x 600 pixels) monitor. Viewing distance was 57 cm. The global motion stimulus consisted of 100 white dots (1 pixel each), placed at random positions within an imaginary square subtending 1.5 × 1.5 degrees of visual angle, and moving coherently to either right or the left within the virtual square on a black background. The displacement of the dots on motion trials was one pixel per frame such that they moved with a velocity of 3 degrees per second. On “No motion” trials, the dots were stationary. Each trial began with a fixation point appearing in the middle of the screen for 500 ms, followed by a blank screen for 300 ms, after which the stimulus appeared. Stimulus duration was 40 ms (or 4 frames lasting for 10 ms each). Subjects were required to report whether or not they detected motion in the display. After subjects had given their responses, a blank screen appeared for 1000 ms before the start of the next trial.
In order to obtain a stable level of performance, subjects were given practice blocks prior to testing. If direction discrimination performance was more than 90 percent, one frame was removed from the stimulus, and if performance was below 70 percent, one frame was added to the stimulus. Five of the subjects performed the task with three frames and three subjects with four frames. Each block consisted of 40 trials, of which 20 were motion trials (10 leftward motion, 10 rightward motion) and 20 were no motion trials. All three types of trials were intermixed randomly within a block.
Transcranial magnetic stimulation
TMS was delivered by means of a Magstim Super Rapid machine (Magstim, UK) via a 70 mm figure-of-eight coil. V5/MT was located using the functional method of inducing moving phosphenes (Stewart et al 1999; see Walsh and Pascual-Leone 2003 for detailed discussion), a technique that has been used in a number of studies on V5/MT function (e.g., Pascual-Leone and Walsh 2001; Campana et al 2002; 2006; Silvanto et al 2005). In this procedure, phosphenes are induced from each point in a 3 cm x 3 cm grid around a centralpoint of 3 cm dorsal and 5 cm lateral from the inion, and the site from which the most vivid moving phosphenes are induced is defined as V5/MT. Stimulation of this region has been shown to disrupt motion perception in a large number of studies (e.g., Hotson & Anand, 1999; Matthews et al, 2001). Vertex was used as a control site in the experiment to control for the nonspecific effects of TMS (both the V5/MT TMS and Vertex TMS induce auditory and sensory artefacts such as clicking sounds and tapping sensation on the scalp, so a statistical difference between these conditions cannot be due to these factors). The mean scalp coordinates were 3.5 cm dorsal and 5.67 cm lateral from the inion. V5/MT in the left hemisphere was stimulated in all participants because it has consistently been found to produce phosphenes more reliably than the right hemisphere (Beckers and Hömberg, 1992; Stewart et al, 1999; Antal et al, 2001). In three subjects, double pulses were used (as moving phosphenes could not be induced with single pulses of TMS). Mean phosphene threshold (with single-pulse TMS) measured using the binary search paradigm (Tyrrell & Owens, 1988) was 65.75 of the maximum stimulator output (Standard Deviation: 7.76).
TMS conditions
The objective of this study was to determine whether suppressing V5/MT using Offline 1 Hz TMS prior to performing a motion detection task modulates the behavioral effect of Online V5/MT TMS applied during that task. This was achieved by running two motion detection experiments, with both experiments preceded by one kind of Offline rTMS. In the critical manipulation, the initial activation state of V5/MT was altered with Offline rTMS prior to the motion detection experiment. For the baseline condition, Offline rTMS was applied over the Vertex prior to the motion experiment. Offline rTMS was applied for 10 minutes at a stimulation frequency of 1 Hz; these stimulation parameters have been shown to suppress cortical activity (e.g. Maeda et al 2000; Gerschlager et al 2001; O'Shea et al, 2007) and to induce significant disruptions in various psychophysical paradigms (e.g., Hilgetag et al. 2001; Grossman et al 2005).
To control for nonspecific effects of Online TMS, Vertex was used as the control site against which the behavioral impact of Online V5/MT TMS was compared. There were therefore two Offline rTMS Conditions (with rTMS applied over either V5/MT or the control site, Vertex, prior to the motion detection task) and two Online TMS conditions (with TMS applied over either V5/MT or Vertex during the motion task). The critical question was whether the disruptive behavioral effect of Online V5/MT TMS on motion detection (assessed relative to the control condition, the Online Vertex TMS condition) was affected by the preceding Offline V5/MT rTMS. TMS was applied at an intensity of 60% of maximum stimulator output. A single intensity was used for all subjects (for both online and offline TMS) on the basis of several previous studies (O'Shea et al, 2004; Silvanto et al, 2005, 2007b; Muggleton et al, 2006; Pitcher et al, 2007). No subject reported the impression of phosphenes during the motion detection task.
Each subject completed a total of 4 blocks of trials of the motion detection task following the Offline rTMS. During two of the blocks of the motion detection task, Online TMS was applied to V5/MT, while during the other two blocks Online TMS was applied over the vertex. The order of these four blocks was counterbalanced. Online TMS consisted of a short train of three pulses (pulses applied at 20 Hz; i.e. pulse gap of 50 ms) that began 50 ms after stimulus onset on each trial (this delay was chosen on the basis of estimates that it takes approximately 50 ms for motion information to reach V5/MT; Raiguel et al 1995). In order to rule out the possibility that Vertex TMS had a behavioral effect, two blocks without any TMS were also run, one before the application of Offline rTMS and the other an hour after its completion.
Procedure
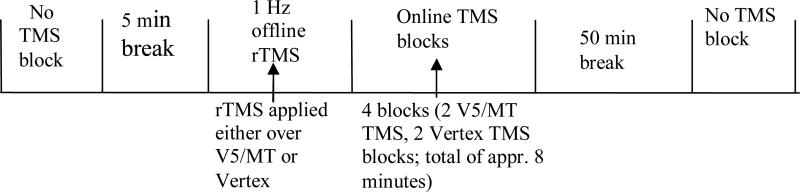
At the beginning of the first session, subjects’ V5/MT region was localized, after which the subjects were thresholded for the behavioral task. Figure 1 shows the timeline of an experimental session. The session began with a No TMS block, followed by application of Offline rTMS either over V5/MT or Vertex for 10 minutes. Immediately after the Offline rTMS the motion detection experiment was run (consisting of 4 blocks of trials; two blocks with Online V5/MT TMS and two blocks with Online Vertex TMS). After a 50 minute break (approximately 1 hour after the completion of the Offline rTMS), a further No TMS block was run. This procedure was then repeated for the second Offline rTMS site on the following day.
Figure 1.
Timeline of the experiment. Each session began with No TMS block. This was followed by the offline 1 Hz rTMS procedure that was applied over either V5/MT or the control site (Vertex). After the offline rTMS procedure, a motion detection experiment was carried out in which online TMS was applied either over V5/MT or the Vertex on each trial. This experiment consisted of a total of four blocks of 40 trials (two blocks with online V5/MT TMS and two blocks with online Vertex TMS), the order of which was counterbalanced. A second No TMS block was run 50 minutes after the completion of the TMS blocks. The V5/MT and the Vertex 1 Hz TMS were carried out on separate days.
Results
We performed a signal-detection analysis (Green & Swets, 1966) on the data to obtain measures of subjects’ sensitivity (d’) and bias (beta) in the motion detection task.
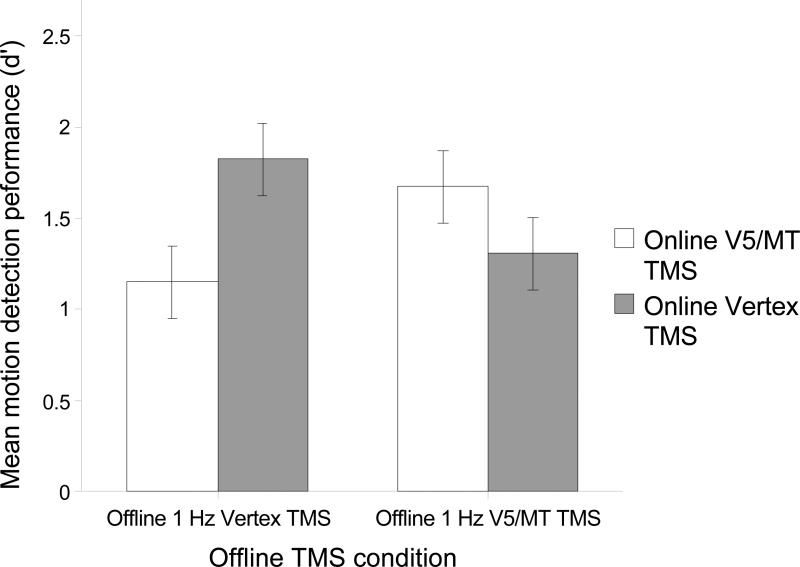
Figure 2 shows subjects’ motion detection sensitivity (d’) in the Online V5/MT and Vertex TMS conditions as a function of whether the preceding Offline rTMS was applied over V5/MT or Vertex. A 2×2 ANOVA with Offline rTMS sites (V5/MT, Vertex) and Online TMS sites (V5/MT, Vertex) as main factors indicated a significant interaction (F(1,7)=51.23; p=0.0002).
Figure 2.
Subjects’ mean (n=8) performance (d’) in the motion detection task. Online V5/MT TMS had the excepted disruptive effect on motion detection (relative to online Vertex TMS that was used as the control condition) when the motion detection task was preceded by the control (Vertex) 1 Hz TMS. In contrast, online V5/MT TMS facilitated motion detection performance relative to online Vertex TMS when the motion detection task was preceded by 1 Hz V5/MT TMS. The error bars indicate +/- 1 Mean Standard Error.
Pairwised comparisons were carried out with the required p-value adjusted with the Bonferroni correction (adjusted p-value = 0.0125). These comparisons revealed that, as expected, Online V5/MT TMS impaired motion detection performance when the initial activation state of V5/MT had not been suppressed prior to the experiment: after Offline Vertex rTMS (i.e. the control Offline rTMS site), Online TMS applied over V5/MT impaired motion detection performance relative to Online TMS applied over the Vertex (t(7)=4.683; p=0.002). Offline rTMS applied over V5/MT also significantly impaired motion detection performance: detection accuracy in the Online Vertex TMS condition (i.e. the control Online TMS condition) was lower after Offline V5/MT rTMS than after Offline Vertex rTMS (t(7)=3.489; p=0.010).
Critically, after Offline V5/MT rTMS, Online TMS applied over V5/MT facilitated motion detection performance relative to Online Vertex TMS (t(7)=5.015; p=0.002). Furthermore, motion detection performance with Online V5/MT TMS was significantly higher after Offline rTMS to V5/MT than after Offline rTMS to Vertex (t(7)=5.248; p=0.003). These comparisons show that Online V5/MT TMS improves motion detection performance if V5/MT is in a suppressed state (i.e. after application of offline V5/MT rTMS).
In order to determine whether the combination of Offline and Online Vertex TMS was a valid control condition, motion detection performance in the No TMS condition was compared to subjects’ performance in the condition in which both offline and online TMS was applied over the Vertex. There was no statistical difference between these conditions (t(7)=0.406; p=0.696). This demonstrates that Vertex TMS had no significant effect on motion detection performance, and that this site was a valid control.
TMS did not significantly influence subjects’ response bias. A 2×2 ANOVA revealed no significant interaction (F(1,7)= 1.37; p = 0.280) nor main effects (F (1,7) = 0.88; p=0.379; F (1,7) = 0.2; p=0.668) were observed. The mean values for each condition are: Offline Vertex TMS/Online V5/MT TMS: 3.91; Offline Vertex TMS/Online Vertex TMS: 5.86; Offline V5/MT TMS/Online V5/MT TMS: 4.57; Offline V5/MT TMS/Online Vertex TMS: 4.37.
Control Experiments
It could be argued that it is the difficulty of the motion task (rather than the initial activation state of V5/MT) that determines whether Online V5/MT impairs or facilitates behavior. This is logically possible given that subjects’ performance in the baseline condition (i.e. Online Vertex TMS condition) was lower after Offline V5/MT rTMS than after offline Vertex rTMS (the mean values for motion-present trials were 69.6% and 81.25%, respectively). To rule out the possibility that Online V5/MT TMS facilitates performance when detection accuracy is low, we determined the effects of Online TMS (without prior 1 Hz rTMS) when the motion task was more difficult. Five subjects (who had all taken part in the main experiment) took part in this control experiment. Subjects were thresholded for a motion detection accuracy of 60-70%, using the procedure described above. The visual stimuli and TMS parameters were identical to those used in the main experiment. Vertex was stimulated as the control site. Six blocks (as described in methods) were run (2 blocks for online V5/MT TMS, 2 blocks from online Vertex TMS and 2 No TMS blocks), the order of which was counterbalanced.
Online V5/MT TMS induced a significant disruption in motion detection relative to the No TMS and the Vertex TMS conditions. Mean motion detection accuracies for the three conditions were: No TMS: 68.5%; Vertex TMS: 68%; V5/MT TMS: 58.3 %; pairwise comparisons: V5/MT TMS vs. No TMS: p = 0.009; V5/MT TMS vs. Vertex TMS: p=0.011; No TMS vs. Vertex TMS: p=0.846). The finding that online V5/MT TMS disrupted motion detection performance even when the motion detection task was made more difficult rules out the possibility that the difficulty of the psychophysical task alone determines whether TMS disrupts or facilitates behavior.
To determine whether 1 Hz TMS as used in the present study was successful in suppressing V5/MT activity, an additional control experiment was carried out. In this control experiment 1 Hz TMS was applied over the V5/MT location that was stimulated in the main experiment, and subjects’ phosphene thresholds were measured before, immediately after, and 1 hour after the 1 Hz TMS. All eight subjects from the main experiment took part. The mean PTs (as percentage of maximum stimulator output) were: Before 1 Hz TMS: 66.13 (Standard Dev: 6.85); Immediately after 1 Hz TMS: 74.75 (SD: 10.47); 1 hour after 1 Hz TMS: 66.3 (SD: 5.6). A paired-sample t-test confirmed that the PT immediately after the 1 Hz TMS was significantly higher than that measured before the 1 Hz TMS (p=0.001), demonstrating that 1 Hz TMS as used here significantly decreases the excitability of V5/MT. The V5/MT PT values measured prior to the main experiment did not statistically differ from the pre 1 Hz PT values in this control experiment (p=0.43).
Discussion
Our results show that online TMS applied over the motion area V5/MT can either suppress or facilitate motion detection depending on the initial activation state of this region. Consistent with a large number of studies (see Walsh and Pascual-Leone 2003; Cowey 2005 for reviews), TMS applied online over V5/MT during a motion detection task impaired subjects’ performance; similarly, suppression of V5/MT activity with offline rTMS impaired subsequent motion detection. Paradoxically, online V5/MT TMS had a facilitatory effect on motion detection when this region had been suppressed with offline rTMS prior to the motion detection task. Effectively, the online V5/MT TMS (that on its own disrupted motion detection performance) removed the behavioral impairment induced by the offline rTMS to V5/MT. The finding that online TMS, which under uncontrolled circumstances impairs behavior, can paradoxically improve performance when the targeted region is in a suppressed state of activity, demonstrates the importance of the initial activation state in determining the behavioral impact of TMS.
The supraliminal motion stimulus in our study was likely to cause excitatory suprathreshold post-synaptic potentials (EPSPs) in a small population of neurons preferring the direction of motion and subthreshold EPSPs in those preferring other directions of motion; this differential activation enables V5/MT to detect the presence of motion. Online TMS as used here is likely to primarily excite neurons (Moliazde et al, 2003) and it was thus likely to turn the subthreshold activity of neurons not strongly driven by the motion stimulus into suprathreshold activation. In contrast, the excitatory influence of online TMS on neurons tuned to the present stimuli is less significant as these neurons are already suprathreshold. As neurons tuned to the motion stimulus are no longer differentially activated relative to the neurons not tuned the presented motion, motion detection is impaired. This explanation is consistent with our recent proposal on how the state-dependency of TMS can explain its behavioral and perceptual effects (cf. Silvanto & Muggleton, 2008).
When TMS is applied offline to suppress neural activity, all neural populations become less excitable causing the activation induced by the motion stimulus to be below perceptual threshold. Although neurons tuned to the presented motion direction are likely to be more active than neurons not tuned to the motion stimulus, their activation is likely to be below the threshold required for motion detection. In this case, with online TMS, the subthreshold activity of neurons tuned to the motion stimulus becomes suprathreshold. The activation of neurons not tuned to the presented motion is also enhanced, but not to a suprathreshold level, as these neurons are initially less strongly activated by the motion stimulus. Therefore, online TMS (when applied over suppressed V5/MT) restores the differential activation of neural populations associated with motion perception. Interestingly, online V5/MT TMS (when applied after offline V5/MT TMS) did not facilitate motion detection beyond the performance found in the control condition when both offline and online TMS were applied over the vertex. This may indicate that online TMS cannot specifically recruit neurons in that network preferring the presented motion direction but push to perceptual threshold the neurons which had become subthreshold due to the offline TMS.
It is possible that 1 Hz rTMS applied over the V5/MT region impaired motion detection by suppressing the reciprocal connections between V1 and V5/MT. Recurrent interactions between these regions, and in particular feedback projections from V5/MT to V1, have been shown to be necessary for conscious perception of visually presented motion (Silvanto et al, 2005). This possibility is supported by a recent study in which fMRI was used to determine the neural correlates of behavioral impairments in a visuospatial task induced by parietal TMS (Sack et al, 2007). Neural activity changes correlating with the impairment were observed in both the directly stimulated parietal as well as remote ipsilateral frontal brain regions that are normally functionally connected during the execution of the behavioral task. This suggests that it may be important to take into account the distal effects of TMS when explaining the neural basis of behavioral impairments induced by this technique. From our behavioral findings it is impossible to infer whether it was the modulation of V5/MT or its connections with V1 that was responsible for the present results.
The role of the baseline activation state in determining the behavioral impact of TMS has been previously studied by using visual adaptation to suppress neural populations of specific tunings within the stimulated region (Silvanto et al, 2007a; Silvanto & Muggleton, 2007). This work showed that TMS interacts with the activity imbalance between distinct neural populations induced by visual adaptation, perceptually facilitating neurons that have been made less active/excitable by adaptation. The present study differed from this work in that the objective was not to selectively suppress specific neural populations but to induce a uniform suppression of all neurons within the stimulated region. This is an important difference, as the finding that a uniform suppression of a cortical region reverses the behavioral effects of online TMS demonstrates that the state-dependency of TMS is not dependent on the presence of an activity imbalance between functionally distinct neural populations. Rather, these findings show that it is the absolute activity state of neurons (rather than their activity state relative to other neurons in the stimulated region) that determines the behavioral outcome of TMS.
In addition to revealing some of the factors that modulate the behavioral efficacy of TMS, these findings have implications for the therapeutic applications of this technique. When designing a treatment protocol, it is important to have an understanding of the activity state of the regions affected by the neurological condition, as this will determine the behavioral impact of TMS. The real-time combination of TMS with electroencephaplography (EEG) may be valuable in this context, as EEG might be used to trigger the TMS and thus assure consistent timing of the TMS stimuli with a given state of cortical activation. This may have implications for the use of TMS in disorders such as epilepsy. Valentin et al (2007) have shown that EEG responses to TMS can identify epileptogenic cortex and may substantially improve the diagnosis of focal epilepsy, particularly, if combined with standard EEG studies. In this study, waveforms resembling interictal epileptiform discharges occurred when stimulating the epileptogenic side of patients but not of normal subjects. Given that the effects of TMS seem to be state-dependent, it may be possible to use TMS to suppress neural activity at the onset of such waveforms and thus prevent epileptic seizures.
The present findings are also related to studies carried out in individual suffering from migraine. In a study by Brighina et al (2003) 1 Hz rTMS over the occipital cortex led to a significantly increased visual cortex excitability expressed as a decrease in PT in subjects affected by migraine with aura. Conversely, after a 1-Hz TMS train, normal subjects showed increased PT values, suggestive of a decrease in visual cortex excitability. Similar findings have been obtained in normal subjects by using light deprivation to modulate the excitability of the visual cortex prior to induction of phosphenes by TMS (Fierro et al, 2005).
The static trials in our psychophysical task consisted of stationary dots (as opposed to non-coherently moving dots). It is therefore possible that even if participants did not detect coherent motion per se, they could detect the presence of motion by the temporal modulation in the visual stimulus. If this is the case TMS in the present study altered motion detection performance by modulating flicker sensitivity. Future studies are required to study the state-dependency of TMS effects on motion perception in more detail.
In conclusion, our results show that the behavioral effects of TMS are dependent on the excitability of neurons in the stimulated region, demonstrating the importance of the initial activity state in modulating the efficacy of TMS.
References
- Antal A, Nitsche MA, Paulus W. External modulation of visual perception in humans. Neuroreport. 2001;12:3553–3555. doi: 10.1097/00001756-200111160-00036. [DOI] [PubMed] [Google Scholar]
- Beckers G, Hömberg V. Cerebral visual motion blindness: transitory akinetopsia induced by transcranial magnetic stimulation of human area V5/MT. Proc R Soc Lond B Biol Sci. 1992;249:173–178. doi: 10.1098/rspb.1992.0100. [DOI] [PubMed] [Google Scholar]
- Brighina F, Piazza A, Daniele O, Fierro B. Modulation of visual cortical excitability in migraine with aura: effects of 1 Hz repetitive transcranial magnetic stimulation. Exp Brain Res. 2002;145(2):177–81. doi: 10.1007/s00221-002-1096-7. [DOI] [PubMed] [Google Scholar]
- Brighina F, Vitello G, Piazza A, Scalia S, Giglia G, Daniele O, Pascual-Leone A. Modulatory effects of low- and high-frequency repetitive transcranial magnetic stimulation on visual cortex of healthy subjects undergoing light deprivation. J Physiol. 2005;565:659–65. doi: 10.1113/jphysiol.2004.080184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana G, Cowey A, Walsh V. Priming of motion perception and area V5/MT: a test of perceptual memory. Cereb Cortex. 2002;12:663–669. doi: 10.1093/cercor/12.6.663. [DOI] [PubMed] [Google Scholar]
- Campana G, Cowey A, Walsh V. Visual area V5/MT remembers “what” but not “where”. Cereb Cortex. 2006;16(12):1766–70. doi: 10.1093/cercor/bhj111. [DOI] [PubMed] [Google Scholar]
- Cowey A. The Ferrier Lecture 2004 what can transcranial magnetic stimulation tell us about how the brain works? Philos Trans R Soc Lond B Biol Sci. 2005;360:1185–205. doi: 10.1098/rstb.2005.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. The neuroanatomy of depression. J Clin Psych. 1993;54:14–20. [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57(3):449–55. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Battelli L, Pascual-Leone A. Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Res. 2005;45:2847–53. doi: 10.1016/j.visres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Harris JA, Clifford CWG, Miniussi C. The functional effect of transcranial magnetic stimulation: Signal suppression or neural noise generation? Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2008.20048. in press. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Theoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nat Neurosci. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Hotson JR, Anand S. The selectivity and timing of motion processing in human temporoparieto-occipital and occipital cortex: a transcranial magnetic stimulation study. Neuropsychologia. 1999;37(2):169–79. doi: 10.1016/s0028-3932(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2(3):145–56. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry. 2004;56(9):634. doi: 10.1016/j.biopsych.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111(5):800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Matthews N, Luber B, Qian N, Lisanby SH. Transcranial magnetic stimulation differentially affects speed and direction judgments. Exp Brain Res. 2001;140:397–406. doi: 10.1007/s002210100837. [DOI] [PubMed] [Google Scholar]
- Mazzocchio R, Rothwell JC, Day BL, Thompson PD. Effect of tonic voluntary activity on the excitability of human motor cortex. J Physiol. 1994;553:665–679. doi: 10.1113/jphysiol.1994.sp020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moliadze V, Zhao Y, Eysel U, Funke K. Effect of transcranial magnetic stimulation on single-unit activity in the cat primary visual cortex. J Physiol. 2003;553:665–79. doi: 10.1113/jphysiol.2003.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muggleton NG, Postma P, Moutsopoulou K, Nimmo-Smith I, Marcel A, Walsh V. TMS over right posterior parietal cortex induces neglect in a scene-based frame of reference. Neuropsychologia. 2006;44(7):1222–9. doi: 10.1016/j.neuropsychologia.2005.10.004. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Muggleton NG, Cowey A, Walsh V. Timing of target discrimination in human frontal eye fields. J Cogn Neurosci. 2004;16(6):1060–7. doi: 10.1162/0898929041502634. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Johansen-Berg H, Trief D, Göbel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54(3):479–90. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–2. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Yovel G, Duchaine B. TMS Evidence for the Involvement of the Right Occipital Face Area in Early Face Processing. Curr Biol. 2007 doi: 10.1016/j.cub.2007.07.063. doi: 10.1016/j.cub.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Raiguel SE, Lagae L, Gulyas B, Orban GA. Response latencies of visual cells in macaque areas V1, V2, and V5. Brain Res. 1989;493(1):155–159. doi: 10.1016/0006-8993(89)91010-x. [DOI] [PubMed] [Google Scholar]
- Rauschecker AM, Bestmann S, Walsh V, Thilo KV. Phosphene threshold as a function of contrast of external visual stimuli. Exp Brain Res. 2004;157:124–7. doi: 10.1007/s00221-004-1910-5. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Is there a future of therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8:559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, Baudewig J. Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous FMRI, TMS, and behavioral studies. Cereb Cortex. 2007;17:2841–52. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–85. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Double Dissociation of V1 and V5/MT activity in Visual Awareness. Cereb Cortex. 2005;15:1736–1741. doi: 10.1093/cercor/bhi050. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG, Cowey A, Walsh V. Neural adapatation reveals state-dependent effects of transcranial magnetic stimulation. Eur J Neurosci. 2007a;25:1874–1881. doi: 10.1111/j.1460-9568.2007.05440.x. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG, Cowey A, Walsh V. Neural activation state determines behavioral susceptibility to transcranial magnetic stimulation. Eur J Neurosci. 2007b;26:523–528. doi: 10.1111/j.1460-9568.2007.05682.x. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Muggleton NG. New light through old windows: moving beyond the virtual lesion approach to transcranial magnetic stimulation. Neuroimage. 2007;39:549–552. doi: 10.1016/j.neuroimage.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Stewart L, Battelli L, Walsh V, Cowey A. Motion perception and perceptual learning studied by magnetic stimulation. Electroenceph Clini Neurophysiol Suppl. 1999;51:334–50. [PubMed] [Google Scholar]
- Tyrell RA, Owens DA. A rapid technique to assess the resting states of the eye and other threshold phenomena: the modified binary search (MOBS). Behav Res Methods Instrum Comput. 1988;20:137–141. [Google Scholar]
- Valentin A, Arunachalam R, Mesquita-Rodrigues A, Garcia Seoane JJ, Richardson MP, Mills KR, Alarcon G. Late EEG responses triggered by transcranial magnetic stimulation (TMS) in the evaluation of focal epilepsy. Epilepsia. 2007 doi: 10.1111/j.1528-1167.2007.01418.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Payne BR, Rushmore J, Lomber SG, Pascual-Leone A. Impact of repetitive transcranial magnetic stimulation of the parietal cortex on metabolic brain activity: a 14C-2DG tracing study in the cat. Exp Brain Res. 2006;163(1):1–12. doi: 10.1007/s00221-004-2140-6. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Payne BR, Pascual-Leone A. Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Exp Brain Res. 2007;176(4):603–15. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre T, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–65, 2007. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial Magnetic Stimulation: A Neurochronometrics of mind. first edition MIT Press; Boston, MA: 2003. [Google Scholar]