Abstract
p120-Catenin is the prototypic member of a subfamily of armadillo repeat domain proteins. Like its structural homologues, β- and γ-catenin, p120-catenin is an essential component of adherens junctions in endothelial cells and other polarized adherent cells. p120-Catenin binds directly to the cytoplasmic domain of cadherin and contributes to the regulation of cell–cell junctional integrity. Studies have demonstrated that p120-catenin plays important roles in cell–cell adhesion, embryonic development, cell proliferation and polarity, tumor cell migration, and cancer progression. However, recent insights have generated an entirely new perspective, suggesting that p120-catenin is implicated in the anti-inflammatory responses in the absence and presence of infection. This review summarizes the present knowledge and recent progress toward elucidating the novel role of p120-catenin in the regulation of innate immunity and inflammation.
Keywords: adherens junctions, Toll-like receptor, neutrophil, nuclear factor-κB, adhesion, cytokines
I. INTRODUCTION
Members of the p120-catenin subfamily include p120-catenin, armadillo repeat protein deleted in Velo-Cardio-Facial syndrome (ARVCF)-catenin, δ-catenin/neural plakophilin-related armadillo repeat protein (NPRAP), and p0071, as well as the more distantly related plakophilins 1–3.1,2 p120-Catenin (also known as catenin delta-1), a member of the armadillo supergene family, was initially discovered as a substrate for Src kinase in 1989.3 Three years later, a nearly full-length complementary DNA (cDNA) encoding murine p120-catenin was isolated.4 In 1994, p120-catenin was shown to interact with epithelial (E)-cadherin in NIH 3T3 (murine fibroblast), Madin Darby canine kidney epithelial cells,5 and HT29 cells.6 Armadillo repeats mediate specific protein–protein interactions, analogous to the role of Src Homology 2 (SH2) or ankyrin domains.4,7 p120-Catenin can be tyrosine phosphorylated in response to platelet-derived growth factor, epidermal growth factor, and colony-stimulating factor 1.8 p120-Catenin binds the E-cadherin or vascular endothelial (VE)-cadherin cytoplasmic tail at a highly conserved octapeptide sequence (YDEEGGGE)9 within the juxtamembrane domain.10,11–18 Mutations of the cadherin juxtamembrane domain have shown that this domain is both necessary and sufficient for recruitment of p120-catenin to adherens junctions,15 indicating an important role in the regulation of cell–cell adhesion and cell motility.19 In addition to the regulatory role of cadherin stability, p120-catenin is physically or functionally linked to a wide variety of proteins, including receptor tyrosine kinases (Src, Yes, Fer, and Fyn),20 receptor-like protein tyrosine phosphatase μ,21 protein-tyrosine phosphatase Src homology phosphatase-1,22,23 tumor-suppressor adenomatous polyposis coli,7,14 Rho GTPases,24–26 transcription regulator Kaiso,27–31 and Wnt signaling proteins glycogen synthase kinase 3β (GSK-3β) and axin.32 These findings unambiguously demonstrated the important roles of p120-catenin in the regulation of cell–cell adhesion, embryonic development, cell proliferation and polarity, tumor cell migration, and cancer progression.33,34
Although p120-catenin is best known for its role in cell adhesion, there is growing evidence that p120-catenin functions as an endogenous anti-inflammatory mediator in many tissues and organs.35,36 Conditional knockout mice for tissue-specific ablation of p120-catenin in the absence of infection exhibited increased inflammatory cell infiltration and production of proinflammatory cytokines.35,36 p120-Catenin also has a function in regulating innate immunity and sepsis-related inflammation. p120-Catenin expressed in endothelial cells modulates endotoxin-induced lung inflammation through its ability to interfere with Toll-like receptor (TLR) 4 signaling.37 The focus of this review is to elucidate the role of p120-catenin in the regulation of innate immunity and inflammatory response.
II. STRUCTURAL AND FUNCTIONAL PROPERTIES OF P120-CATENIN
A. The Basic Structure and Isoforms of p120-Catenin
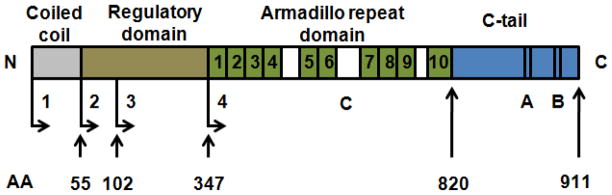
p120-Catenin is an armadillo repeat-containing protein composed of four distinct functional regions (Figure 1). From the N- to C-terminus, these regions include coiled coil, regulatory domain, and armadillo domain containing 10 armadillo repeats and a short C-terminal tail. Owing to alternative splicing and multiple translation initiation codons, multiple p120-catenin isoforms can be expressed from a single gene.38 In the N-terminal regulatory domains, alternative splicing events leads to the use of four different start codons, resulting in the expression of four main p120-catenin isoforms type 1, 2, 3 or 4, according to the respective ATG used as the translation start site. The relative abundance of each p120-catenin isoform varies among cell types. p120-Catenin isoform 1 is the full-length protein containing a 347-amino-acid N-terminal domain, whereas p120-catenin isoforms 2 and 3 start at amino acids 55 and 102, respectively. In contrast, p120-catenin isoform 4 lacks the N-terminal domain, with only the central armadillo domain, the 10 tandem 42-amino-acid repeats, and the C-terminal domain.39,40 Alternative splicing events also occur in the C-terminal end, leading to the use of exons A, B, or neither of them. Exon C is rarely inserted within armadillo repeat 6 of p120-catenin. Therefore, various combinations of these N- and C-terminal alternative splicing events result in approximately 32 isoforms of p120-catenin in human.38,41 In addition, differential posttranslational modifications could further increase the molecular variety of these proteins because they could be phosphorylated on serine/threonine and tyrosine residues by several protein kinases.42
FIGURE 1.
The structure of p120-catenin.
p120-Catenin is widely expressed in all cells capable of adhering to other cells, including endothelial and epithelial cells, fibroblasts, macrophages, cardiomyocytes, and neurons,43 but it is weakly expressed or absent in B and T lymphocytes.43 The most commonly expressed isoforms are isoforms 1 and 3. Isoform 1 is predominantly expressed in motile cells such as fibroblasts and in epithelial tumors, while isoform 3 is the most abundant isoform in sessile epithelial cells.40
B. Interaction Between p120-Catenin and Other Proteins
Association of proteins to p120-catenin takes place through different elements. Two domains of p120-catenin are likely to mediate protein–protein interactions, either directly or indirectly following p120 phosphorylation.44 Kaiso and cadherin interact with p120-catenin through the central armadillo domain,45 whereas other cofactors, such as the Fer or Fyn tyrosine kinases46 and RhoA GTPase,44,47 bind sequences in the regulatory domain. p120-Catenin phosphorylation occurs not only on tyrosine but also on serine and threonine residues, which are predominantly in the N-terminal domain of the protein.48,49 p120-Catenin acts as guanine nucleotide dissociation inhibitor for RhoA24,50 and also binds Vav2, an guanine nucleotide exchange factor that specifically activate Cdc42 and Rac1,25 regulating cell–cell adhesion.
p120-Catenin exists in three pools: a membrane-associated cadherin-bound pool,33,34,44 a soluble, cytoplasmic pool that affects Rho GTPases,6,15,16,51–53 and a nuclear pool that is thought to associate with the methylation-relevant transcriptional repressor Kaiso.54 Cadherin-bound p120-catenin on the cell membrane is unable to affect the structure of the actin cytoskeleton, whereas cadherin-unbound p120-catenin in a cytoplasmic pool can interact with Vav2 and possibly other regulators of Rho family activity.25 Cytosolic p120-catenin inhibits the activity of RhoA by acting as a guanine nucleotide dissociation inhibitor and sequestering RhoA in its inactive form.24,55 Overexpression of exogenous p120-catenin mainly increases the cytosolic pool of p120-catenin relative to the fraction associated with the membrane.25 The distribution of p120-catenin between cadherin-bound and cytoplasmic pools may provide a crosstalk mechanism for regulating cadherin-mediated cell–cell junctions and the motile machinery of cells.25
C. Regulation of p120-Catenin Expression
p120-Catenin expression is downregulated in human cancers of diverse etiologies, and it correlates closely with poor patient prognosis.56 Following lipopolysaccharide (LPS) challenge, p120-catenin expression level in the mouse lungs was rapidly decreased. The p120-catenin protein expression was correlated inversely with severity of inflammation.37 The molecular mechanisms by which p120-catenin is degraded are not exactly clear. Calpain 1 has been shown to mediate the degradation of p120-catenin in ischemic human neuroblastoma SH-SY5Y cells57 and endothelial cells.58 Pretreatment of epithelial cells with calpain inhibitor or knockdown of calpain 1 with a specific small interfering RNA (siRNA) prevented mechanical stretch-induced loss of p120-catenin.59 These results support the notion that calpain-1 may mediate p120-catenin degradation. A recent study indicated that p120-catenin ubiquitination and proteasomal degradation is phosphorylation dependent. The N-terminal region of p120-catenin can interact with destruction-complex proteins (e.g., casein kinase-1α and GSK3β). Like β-catenin, p120-catenin is phosphorylated by casein kinase-1α and GSK3β and degraded through the ubiquitin-proteasome pathway.32 p120-Catenin was stabilized by the proteasome inhibitor MG132 (carbobenzoxy-Leu-Leu-leucinal) in a dose-dependent manner. Furthermore, p120-catenin ubiquitination was dramatically increased upon its coexpression with HA–ubiquitin in HeLa cells, and it dropped when cells were incubated in the presence of LiCl.32 These findings suggest the important role of canonical Wnt signaling in the regulation of endogenous p120-catenin expression.
D. The Physiological Functions of p120-Catenin
The physiological functions of p120-catenin are not completely understood. Evidence has accumulated over the past decade that p120 acts as a critical regulator of cell–cell adhesion by stabilizing E-cadherin hemophilic interactions and by maintaining the total level of E-cadherin expression in epithelial cells.33 p120-Catenin binding to the cytoplasmic domain in juxtamembrane regions via its central armadillo domain prevents the endocytosis and degradation of E-cadherin. Knockout of p120-catenin in mice causes early embryonic lethality, despite the presence of the genome of several potentially redundant p120-catenin family members.33 Tissue-specific deletion of p120-catenin in mice results in a variety of phenotypes with differing degrees of severity. Ablation of p120-catenin specifically restricted to the gastrointestinal tract is lethal within a few weeks after birth.39 Conditional knockout mice for endothelial p120-catenin died embryonically beginning at embryonic day 11.5.60 Mice lacking endothelial p120-catenin exhibit a reduced VE-cadherin and neural cadherin levels, as well as hemorrhages, decreased microvascular density, reduced pericyte coverage, and disorganized vascular networks in both embryonic and extraembryonic tissues, indicating a crucial role for p120-catenin in vascular development and endothelial function.60 The conditional knockout of p120-catenin in the salivary gland resulted in disorganized ducts, reductions in E-cadherin levels, and the formation of epithelial masses that followed a cancer-like growth progression.61 Depletion of p120-catenin specifically in forebrain neuroepithelia resulted in reduced density of neuronal spines and synapses, an effect owing more to the misregulation of Rho GTPases than changes in neural cadherin levels.62 Epidermal conditional p120-catenin knockout mice displayed a chronic inflammatory response,35,36 suggesting the intrinsic anti-inflammatory role of p120-catenin.
III. ROLE OF P120-CATENIN IN THE REGULATION OF INNATE IMMUNITY AND INFLAMMATION
The innate immune system is the main, first line of host defense against invading microorganisms in a non-specific manner and relies on a large family of pattern recognition receptors (PRRs), which detect distinct evolutionarily conserved structures on pathogens (known as pathogen-associated molecular patterns) and endogenous stress signals (known as danger-associated molecular patterns). PRRs are expressed not only on a variety of immune cells including macrophages, dendritic cells and B cells but also on nonimmune cells including endothelial and epithelial cells. These PRRs include cell surface- or intracellular compartment-expressed receptors such as the TLRs, intracytosolic receptors such as nucleotide binding domain/leucine-rich repeat receptors (NLRs), C-type lectin receptors (CLRs), scavenger receptors, and a variety of other receptor molecules that recognize danger-associated molecular patterns.63,64 Activation of PRRs results in initiation of several extracellular activating cascades as well as various intracellular signaling pathways that cause inflammatory responses. Among the PRRs, the TLRs have been studied most extensively. TLRs can recognize diverse pathogens and can generate inflammatory signals to activate innate immune responses.63
A. Loss of p120-Catenin Causes Intrinsic Inflammation
Depletion of p120-catenin in conditional knockout mice exhibits an inflammatory response in intestines65–67 and epidermis35,36 evidenced by tissue immune cell infiltration and proinflammatory cytokine release. Ultrastructural study indicated that the conditional knockout underlying dermis was infiltrated with immune cells, including lymphocytes, mast cells, granulocytes (neutrophils and eosinophils), and macrophages.35 There was a significant correlation between p120-catenin loss and increased incidence of inflammatory bowel disease.65–67
p120-Catenin regulates inflammatory responses in the skin through regulation of NF-κB activation and immune cell migration in the absence of inflammatory stimuli.53 p120-catenin null epidermal cells have elevated levels of nuclear NF-κB activity, triggering a cascade of proinflammatory NF-κB targets both in vivo and in vitro.47,55 Loss of p120-catenin causes activation of RhoA/Rho-kinase pathway, which may result in NF-κB activation and cytokine production.47 In human endothelial cells, depletion of p120-catenin expression with a specific siRNA increased transcription-factor promoter reporter activities, adhesion molecule expression, neutrophil adhesion, and extracellular signal-regulated kinase 1/2 signaling.68 Overexpression of a p120-catenin fusion protein in human umbilical vein and dermal microvascular endothelial cells inhibited neutrophil transendothelial migration.69 These studies clearly demonstrate that the inflammatory response following p120-catenin loss is cell-autonomous at initial stages, mediated by an intrinsic mechanism that does not appear to rely upon noxious stimuli.
B. Innate Immune Function of p120-Catenin in Response to Endotoxin
Our recent findings have indicated the central role of p120-catenin expression in the regulation of sepsis-induced lung injury.37 In the mouse lung, p120 was rapidly degraded in response to LPS challenge and the levels of p120 protein expression correlated inversely with the severity of lung inflammation. Depletion of p120 in pulmonary vasculature with a specific siRNA rendered the mice highly sensitive to endotoxin, induced a robust inflammatory response evidenced by neutrophil infiltration into the lung, increased proinflammatory cytokine production (tissue necrosis factor-α and Interleukin-6), pulmonary vascular hyperpermeability, and edema formation. 37 Consistently, deletion of p120-catenin with specific siRNA enhanced LPS-induced an increase in neutrophil adhesion to cultured pulmonary endothelial cells and neutrophil transendothelial migration. In contrast, overexpression of p120-catenin blocked LPS-induced effects.37 Taken together, these results support the novel concept that endothelial p120 degradation that occurs after endotoxemia is required for the amplification of pulmonary inflammation. Therefore, endothelial p120-catenin may be a critical molecule for endothelial homeostasis, and it acts as a crucial negative regulator of sepsis-induced lung inflammatory injury.
C. Regulatory Role of p120-Catenin in TLR4 Signaling Pathway
1. TLR4 Signaling Pathway
TLR4 is the main LPS receptor, although co-receptors including TLR2 are most likely involved.70–72 LPS binding to TLR4 initiates myeloid differentiation factor (MyD88)-dependent and -independent signaling pathways to activate inflammatory gene expression (Figure 2).73–76 Recruitment of the adaptor protein MyD88 initiates the early activation of NF-κB and mitogen-activated protein kinase. In parallel, the MyD88-independent pathway leads to rapid activation of interferon regulatory factor 3 (IRF 3)77,78 but delayed NF-κB activation. 79 MyD88 promotes association with the IL-1 receptor-associated kinase 4 (IRAK4) and IRAK-1. TNF-associated factor 6 (TRAF6) is recruited to IRAK-1.73,80–82 The complex IRAK-4/IRAK-1/TRAF6 dissociates from the receptor and then interacts with transforming growth factor-β-activated kinase (TAK1) complex. TAK1 activates an inhibitor of NF-κB (IκB) kinase (IKK), leading to phosphorylation and degradation of IκB, and consequent release of NF-κB.83,84 Once translocated into the nucleus, NF-κB induces the expression of inflammatory chemokines and cytokines. Mitogen-activated protein kinase-mediated activation of transcription factors activator protein 1 and cAMP response element-binding protein also coordinates the induction of many genes encoding adhesion molecules and inflammatory mediators. In addition to MyD88, TRIF (TIR-domain-containing adaptor protein inducing interferon-β)-mediated MyD88-independent induction of interferon-β activates the expression of interferon-inducible genes such as C-X-C motif chemokine 10.83
FIGURE 2.
TLR4 signaling pathways regulating inflammation. Abbreviations: AP-1, activator protein 1; cAMP response element-binding protein; ERK, extracellular-signal-regulated kinases; IκB, inhibitor of NF-κB; IKK, IκB kinase; IRF 3, interferon regulatory factor 3; IRAK-1 and IRAK-4, interleukin-1 receptor-associated kinase 1 and 4; JNK, Jun N-terminal protein kinase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MyD88, myeloid differentiation factor; NF-κB, nuclear factor-κB; TLR, Toll-like receptor; TAK1, transforming growth factor-β-activated kinase 1; TRAF6, TNF receptor-associated factor 6; TRIF, TIR-domain-containing adaptor protein inducing interferon-β.
2. Regulation of TLR4 Signaling by p120-Catenin
Using a genetic approach, we demonstrated that p120-catenin significantly inhibited IκB-α degradation and subsequent activation of NF-κB induced by LPS in lungs and pulmonary microvascular endothelial cells.37 NF-κB activation occurred in p120 null epidermal cells through stimulation of RhoA in the absence of inflammatory stimuli.35 However, p120 knockdown alone in mouse pulmonary endothelial cells did not affect NF-κB activation but instead significantly enhanced LPS-induced NF-κB activation,37 suggesting the important role of endothelial p120 in the modulation of LPS/TLR4-dependent NF-κB signaling.
Following LPS-induced TLR4 activation, MyD88 is recruited to the membrane by interaction of its TIR domain with the analogous domain in TLR4. MyD88 binds to IRAK-4 and promotes activation (phosphorylation) of critical IRAK-1 residues by IRAK-4.85 Upon activation and modification, IRAK-1 dissociates from the receptor complex and associates with TRAF6 to trigger downstream signaling pathways, including the activation of NF-κB and the induction of inflammatory cytokines.86 The kinase activity of IRAK-4 is required for the recruitment of IRAK-1 to the receptor complex and for the activation and subsequent degradation of IRAK-1 protein. 87 In pulmonary endothelial cells transfected with a scrambled siRNA, we demonstrated that LPS induced association of MyD88 and TLR4, downstream activation of IRAK4, and subsequent degradation of IRAK-1. Importantly, the increase in TLR4 signaling was further augmented by p120-catenin depletion with a specific siRNA, whereas overexpression of p120-catenin inhibited TLR4 signaling.37 Although the molecular mechanism(s) by which p120 physically regulates the interaction between MyD88 and TLR4 remains unknown, our study clearly demonstrates that p120 functions as a “negative regulator” of LPS/TLR4-mediated NF-κB activation.37
IV. CONCLUDING REMARKS
Studies have greatly expanded our understanding of the functions of p120-catenin during the past two decades. p120-Catenin is primarily an adherens junction-associated protein that regulates cell-cell adhesion via controlling cadherin function and stability. It is now known that p120-catenin participate in a plethora of functions in cells, including cell–cell adhesion, embryonic development, cell proliferation and polarity, tumor cell migration, cancer progression, and anti-inflammatory effects. Despite significant progress in understanding the role of p120-catenin in the development of inflammation, our knowledge is far from complete with regard to the cellular and molecular mechanisms of p120-catenin in the regulation of innate immunity. Studies from conditional knockout mice and cells show that ablation of p120-catain induces inflammatory responses evidenced by immune-cell infiltration, proinflammatory cytokine release, and disruption of cell–cell barrier in the absence of noxious stimuli. It seems likely that p120-catenin is an endogenous anti-inflammatory molecule. We do not yet have a full understanding of whether the inhibitory effect of p120-catenin on inflammation is mainly due to its direct action on cell homeostasis or is secondary to its regulatory role in cell–cell barrier function. Especially in the presence of bacterial infection such as sepsis, how p120-catenin regulates different inflammatory signaling pathways needs further investigation. Finally, we must be aware that most of the data on the role of p120-catenin in inflammation discussed herein, though fascinating, are based on studies commonly using conditional knockout or germ-free mice. It will be important to explore their relevance in human biology.
Acknowledgments
This work was supported by NIH NHLBI grants to Guochang Hu. The project is titled “Role of p120-catenin in sepsis-induced lung injury” (ID No. 5R01HL104092). This research was support by the American Heart Association (Scientist Development Grant No. 0730331N). The author thanks Zhibo Yan and Yu Sun for drafting Figures.
ABBREVIATIONS
- E-cadherin
epithelial cadherin
- GSK-3β
glycogen synthase kinase 3β
- IκB
inhibitor of NF-κB
- IKK
IκB kinase
- IRF 3
interferon regulatory factor 3
- IRAK-1 and IRAK-4
interleukin-1 receptor-associated kinase 1 and 4
- LPS
lipopolysaccharide
- MyD88
myeloid differentiation factor
- NF-κB
nuclear factor-κB
- PRRs
pattern recognition receptors
- siRNA
small interfering RNA
- TLR
Toll-like receptor
- TAK1
transforming growth factor-β-activated kinase 1
- TRAF6
TNF receptor-associated factor 6
- TRIF
TIR-domain-containing adaptor protein inducing interferon-β
- VE
vascular endothelial
References
- 1.Carnahan RH, Rokas A, Gaucher EA, Reynolds AB. The molecular evolution of the p120-catenin subfamily and its functional associations. PLoS One. 2010;5:e15747. doi: 10.1371/journal.pone.0015747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCrea PD, Park JI. Developmental functions of the p120-catenin subfamily. Biochim Biophys Acta. 2007;1773:17–33. doi: 10.1016/j.bbamcr.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds AB, Roesel DJ, Kanner SB, Parsons JT. Transformation specific tyrosine phosphorylation of a novel cellular protein in chicken cells expressing oncogenic variants of the avian cellular src gene. Mol Cell Biol. 1989;9:629–38. doi: 10.1128/mcb.9.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reynolds AB, Herbert L, Cleveland JL, Berg ST, Gaut JR. p120, a novel substrate of protein tyrosine kinase receptors and of p60v-src, is related to cadherin-binding factors beta-catenin, plakoglobin and armadillo. Oncogene. 1992;7:2439–45. [PubMed] [Google Scholar]
- 5.Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu JJ, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–42. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Miyazawa K, Kitamura N, Johnson KR, Wheelock MJ, Matsuyoshi N, Takeichi M, Ito F. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J Cell Biol. 1995;128:949–57. doi: 10.1083/jcb.128.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peifer M, Berg S, Reynolds AB. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994;76:789–91. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 8.Downing JR, Reynolds AB. PDGF, CSF-1, and EGF induce tyrosine phosphorylation of p120, a pp60src transformation-associated substrate. Oncogene. 1991;6:607–13. [PubMed] [Google Scholar]
- 9.Ferber A, Yaen C, Sarmiento E, Martinez J. An octapeptide in the juxtamembrane domain of VE-cadherin is important for p120ctn binding and cell proliferation. Exp Cell Res. 2002;274:35–44. doi: 10.1006/excr.2001.5436. [DOI] [PubMed] [Google Scholar]
- 10.Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, Ikura M. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell–cell adhesion. Cell. 2010;141:117–28. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–76. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–34. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–45. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel JM, Reynolds AB. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or α-catenin. Mol Cell Biol. 1995;15:4819–24. doi: 10.1128/mcb.15.9.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120ctn from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–201. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozawa M, Kemler R. The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity. J Cell Biol. 1998;142:1605–13. doi: 10.1083/jcb.142.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulson AF, Mooney E, Fang X, Ji H, McCrea PD. Xarvcf, Xenopus member of the p120 catenin subfamily associating with cadherin juxtamembrane region. J Biol Chem. 2000;275:30124–31. doi: 10.1074/jbc.M003048200. [DOI] [PubMed] [Google Scholar]
- 18.Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening and interaction with p120ctn. J Cell Biol. 1997;14:779–89. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piedra J, Miravet S, Castaño J, Pálmer HG, Heisterkamp N, García de Herreros A, Duñach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate β-catenin Tyr-142 phosphorylation and β-catenin-α-catenin interaction. Mol Cell Biol. 2003;23:2287–97. doi: 10.1128/MCB.23.7.2287-2297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zondag GC, Reynolds AB, Moolenaar WH. Receptor protein-tyrosine phosphatase RPTPμ binds to and dephosphorylates the catenin p120ctn. J Biol Chem. 2000;275:11264–9. doi: 10.1074/jbc.275.15.11264. [DOI] [PubMed] [Google Scholar]
- 22.Keilhack H, Hellman U, van Hengel J, van Roy F, Godovac-Zimmermann J, Böhmer FD. The protein-tyrosine phosphatase SHP-1 binds to and dephosphorylates p120 catenin. J Biol Chem. 2000;275:26376–84. doi: 10.1074/jbc.M001315200. [DOI] [PubMed] [Google Scholar]
- 23.Keilhack H, Müller M, Böhmer SA, Frank C, Weidner KM, Birchmeier W, Ligensa T, Berndt A, Kosmehl H, Günther B, Müller T, Birchmeier C, Böhmer FD. Negative regulation of Ros receptor tyrosine kinase signaling. An epithelial function of the SH2 domain protein tyrosine phosphatase SHP-1. J Cell Biol. 2001;152:325–34. doi: 10.1083/jcb.152.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2:637–44. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- 25.Noren NK, Liu BP, Burridge K, Kreft B. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol. 2000;150:567–80. doi: 10.1083/jcb.150.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell–cell contact formation and regulation of cell locomotion. J Cell Sci. 2001;114:695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- 27.Daniel JM, Reynolds AB. The catenin p120ctn interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614–23. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spring CM, Kelly KF, O’Kelly I, Graham M, Crawford HC, Daniel JM. The catenin p120ctn inhibits Kaiso-mediated transcriptional repression of the beta-catenin/TCF target gene matrilysin. Exp Cell Res. 2005;305:253–65. doi: 10.1016/j.yexcr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Kim SW, Park JI, Spring CM, Sater AK, Ji H, Otchere AA, Daniel JM, McCrea PD. Non-canonical Wnt signals are modulated by the Kaiso transcriptional repressor and p120-catenin. Nat Cell Biol. 2004;6:1212–20. doi: 10.1038/ncb1191. [DOI] [PubMed] [Google Scholar]
- 30.Park JI, Kim SW, Lyons JP, Ji H, Nguyen TT, Cho K, Barton MC, Deroo T, Vleminckx K, Moon RT, McCrea PD. Kaiso/p120-catenin and TCF/β-catenin complexes coordinately regulate canonical Wnt gene targets. Dev Cell. 2005;8:843–54. doi: 10.1016/j.devcel.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Prokhortchouk A, Hendrich B, Jorgensen H, Ruzov A, Wilm M, Georgiev G, Bird A, Prokhortchouk E. The p120 catenin partner Kaiso is a DNA methylation-dependent transcriptional repressor. Genes Dev. 2001;15:1613–8. doi: 10.1101/gad.198501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong JY, Park JI, Cho K, Gu D, Ji H, Artandi SE, McCrea PD. Shared molecular mechanisms regulate multiple catenin proteins: canonical Wnt signals and components modulate p120-catenin isoform-1 and additional p120 subfamily members. J Cell Sci. 2010;123:4351–65. doi: 10.1242/jcs.067199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds AB. p120-catenin: Past and present. Biochim Biophys Acta. 2007;1773:2–7. doi: 10.1016/j.bbamcr.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–56. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–44. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez-Moreno M, Song W, Pasolli HA, Williams SE, Fuchs E. Loss of p120 catenin and links to mitotic alterations, inflammation, and skin cancer. Proc Natl Acad Sci U S A. 2008;7;105:15399–404. doi: 10.1073/pnas.0807301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YL, Malik AB, Sun Y, Hu S, Reynolds AB, Minshall RD, Hu G. Innate immune function of the adherens junction protein p120-catenin in endothelial response to endotoxin. J Immunol. 2011;186:3180–7. doi: 10.4049/jimmunol.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keirsebilck A, Bonne S, Staes K, van Hengel J, Nollet F, Reynolds A, van Roy F. Molecular cloning of the human p120ctn catenin gene (CTNND1): expression of multiple alternatively spliced isoforms. Genomics. 1998;50:129–46. doi: 10.1006/geno.1998.5325. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–56. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- 40.Del Valle-Pérez B, Casagolda D, Lugilde E, Valls G, Codina M, Dave N, de Herreros AG, Duñach M. Wnt controls the transcriptional activity of Kaiso through CK1ε-dependent phosphorylation of p120-catenin. J Cell Sci. 2011;124:2298–309. doi: 10.1242/jcs.082693. [DOI] [PubMed] [Google Scholar]
- 41.Paredes J, Correia AL, Ribeiro AS, Schmitt F. Expression of p120-catenin isoforms correlates with genomic and transcriptional phenotype of breast cancer cell lines. Cell Oncol. 2007;29:467–76. doi: 10.1155/2007/395913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alema S, Salvatore AM. p120 catenin and phosphorylation: mechanisms and traits of an unresolved issue. Biochim Biophys Acta. 2007;1773:47–58. doi: 10.1016/j.bbamcr.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Mo YY, Reynolds AB. Identification of murine p120-catenin isoforms and heterogeneous expression of p120cas isoforms in human tumor cell lines. Cancer Res. 1996;56:2633–40. [PubMed] [Google Scholar]
- 44.Yanagisawa M, Huveldt D, Kreinest P, Lohse CM, Cheville JC, Parker AS, Copland JA, Anastasiadis PZ. A p120-catenin isoform switch affects Rho activity, induces tumor cell invasion and predicts metastasis disease. J Biol Chem. 2008;283:18344–54. doi: 10.1074/jbc.M801192200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds AB, Daniel JM, Mo YY, Wu J, Zhang Z. The novel catenin p120cas binds classical cadherins and induces an unusual morphological phenotype in NIH3T3 fibroblasts. Exp Cell Res. 1996;225:328–37. doi: 10.1006/excr.1996.0183. [DOI] [PubMed] [Google Scholar]
- 46.Kim L, Wong TW. The cytoplasmic tyrosine kinase Fer is associated with the catenin substrate pp120-catenin and is activated by growth factors. Mol Cell Biol. 1995;15:4553–61. doi: 10.1128/mcb.15.8.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castaño J, Solanas G, Casagolda D, Raurell I, Villagrasa P, Bustelo XR, García de Herreros A, Duñach M. Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA. Mol Cell Biol. 2007;27:1745–57. doi: 10.1128/MCB.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mariner DJ, Anastasiadis P, Keilhack H, Böhmer FD, Wang J, Reynolds AB. Identification of Src phosphorylation sites in the catenin p120ctn. J Biol Chem. 2001;276:28006–13. doi: 10.1074/jbc.M102443200. [DOI] [PubMed] [Google Scholar]
- 49.Xia X, Mariner DJ, Reynolds AB. Adhesion-associated and PKC-modulated changes in serine/threonine phosphorylation of p120-catenin. Biochemistry. 2003;42:9195–204. doi: 10.1021/bi034597h. [DOI] [PubMed] [Google Scholar]
- 50.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–39. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 51.Staddon JM, Smales C, Schulze C, Esch FS, Rubin LL. p120, a p120-related protein (p100), and the cadherin/catenin complex. J Cell Biol. 1995;130:369–81. doi: 10.1083/jcb.130.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papkoff J. Regulation of complexed and free catenin pools by distinct mechanisms. Differential effects of Wnt-1 and v-Src. J Biol Chem. 1997;272:4536–43. [PubMed] [Google Scholar]
- 53.Kinch MS, Clark J, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of Ras-transformed breast epithelial. J Cell Biol. 1995;130:461–71. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Hengel J, Vanhoenacker P, Staes K, van Roy F. Nuclear localization of the p120ctn Armadillo-like catenin is counteracted by a nuclear export signal and by E-cadherin expression. Proc Natl Acad Sci U S A. 1999;96:7980–5. doi: 10.1073/pnas.96.14.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anastasiadis PZ. p120-ctn: a nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta. 2007;1773:34–46. doi: 10.1016/j.bbamcr.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 56.van Hengel J, van Roy F. Diverse functions of p120ctn in tumors. Biochim Biophys Acta. 2007;1773:78–88. doi: 10.1016/j.bbamcr.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 57.Ohno H, Uemura K, Shintani-Ishida K, Nakamura M, Inomata M, Yoshida K. Ischemia promotes calpain-mediated degradation of p120-catenin in SH-SY5Y cells. Biochem Biophys Res Commun. 2007;353:547–52. doi: 10.1016/j.bbrc.2006.12.061. [DOI] [PubMed] [Google Scholar]
- 58.Kusaba T, Okigaki M, Matui A, Murakami M, Ishikawa K, Kimura T, Sonomura K, Adachi Y, Shibuya M, Shirayama T, Tanda S, Hatta T, Sasaki S, Mori Y, Matsubara H. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+channel to maintain endothelial integrity. Proc Natl Acad Sci USA. 2010;107:19308–13. doi: 10.1073/pnas.1008544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Minshall RD, Schwartz DE, Hu G. Cyclic stretch induces alveolar epithelial barrier dysfunction via calpain-mediated degradation of p120-catenin. Am J Physiol Lung Cell Mol Physiol. 2011;301:L197–206. doi: 10.1152/ajplung.00048.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oas RG, Xiao K, Summers S, Wittich KB, Chiasson CM, Martin WD, Grossniklaus HE, Vincent PA, Reynolds AB, Kowalczyk AP. p120-Catenin is required for mouse vascular development. Circ Res. 2010;106:941–51. doi: 10.1161/CIRCRESAHA.109.207753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis MA, Reynolds AB. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev Cell. 2006;10:21–31. doi: 10.1016/j.devcel.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Elia LP, Yamamoto M, Zang K, Reichardt LF. p120 Catenin regulates dendritic spine and synapse development through rho-family GTPases and cadherins. Neuron. 2006;51:43–56. doi: 10.1016/j.neuron.2006.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minnicozzi M, Sawyer RT, Fenton MJ. Innate immunity in allergic disease. Immunol Rev. 2011;242:106–27. doi: 10.1111/j.1600-065X.2011.01025.x. [DOI] [PubMed] [Google Scholar]
- 64.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 65.Smalley-Freed WG, Efimov A, Burnett PE, Short SP, Davis MA, Gumucio DL, Washington MK, Coffey RJ, Reynolds AB. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest. 2010;120:1824–35. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karayiannakis AJ, Syrigos KN, Efstathiou J, Valizadeh A, Noda M, Playford RJ, Kmiot W, Pignatelli M. Expression of catenins and E-cadherin during epithelial restitution in inflammatory bowel disease. J Pathol. 1998;185:413–8. doi: 10.1002/(SICI)1096-9896(199808)185:4<413::AID-PATH125>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 67.Smalley-Freed WG, Efimov A, Short SP, Jia P, Zhao Z, Washington MK, Robine S, Coffey RJ, Reynolds AB. Adenoma formation following limited ablation of p120-catenin in the mouse intestine. PLoS One. 2011;6:e19880. doi: 10.1371/journal.pone.0019880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Donnell JJ, 3rd, Zhuge Y, Holian O, Cheng F, Thomas LL, Forsyth CB, Lum H. Loss of p120 catenin upregulates transcription of pro-inflammatory adhesion molecules in human endothelial cells. Microvasc Res. 2011;82:105–12. doi: 10.1016/j.mvr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, Yacono P, Vincent P, Kowalczyk A, Luscinskas FW. p120-catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood. 2008;112:2770–9. doi: 10.1182/blood-2008-03-147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589:1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 71.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogata H, Su I, Miyake K, Nagai Y, Akashi S, Mecklenbräuker I, Rajewsky K, Kimoto M, Tarakhovsky A. The Toll-like receptor protein RP105 regulates lipopolysaccharide signaling in B cells. J Exp Med. 2000;192:23–9. doi: 10.1084/jem.192.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–6. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 74.Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, Mantovani A, Rothe M, Muzio M, Arditi M. Bacterial lipopolysaccharide activates nuclear factor-κB through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274:7611–4. doi: 10.1074/jbc.274.12.7611. [DOI] [PubMed] [Google Scholar]
- 75.Lomaga MA, Yeh WC, Sarosi I, Duncan GS, Furlonger C, Ho A, Morony S, Capparelli C, Van G, Kaufman S, van der Heiden A, Itie A, Wakeham A, Khoo W, Sasaki T, Cao Z, Penninger JM, Paige CJ, Lacey DL, Dunstan CR, Boyle WJ, Goeddel DV, Mak TW. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signaling. Genes Dev. 1999;13:1015–24. doi: 10.1101/gad.13.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- 77.Kawai T, Takeuchi O, Fujita T, Inoue J, Mühlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–94. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 78.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-κB activation. Science. 2005;309:1854–7. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 79.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–8. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 80.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–8. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 81.Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–5. doi: 10.1126/science.278.5343.1612. [DOI] [PubMed] [Google Scholar]
- 82.Wesche H, Gao X, Li X, Kirschning CJ, Stark GR, Cao Z. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J Biol Chem. 1999;274:19403–10. doi: 10.1074/jbc.274.27.19403. [DOI] [PubMed] [Google Scholar]
- 83.Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16:693–701. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- 84.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 85.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 86.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–60. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 87.Lye E, Mirtsos C, Suzuki N, Suzuki S, Yeh WC. The role of interleukin 1 receptor-associated kinase-4 (IRAK-4) kinase activity in IRAK-4-mediated signaling. J Biol Chem. 2004;279:40653–58. doi: 10.1074/jbc.M402666200. [DOI] [PubMed] [Google Scholar]