Abstract
Background
Due to effective antiretroviral therapy HIV patients are living longer, and their risk of cardiovascular disease (CVD) is a growing concern. It is unknown whether co-infection with hepatitis C (HCV) changes an HIV person’s CVD risk, and how the risks compare to the general population. The objective of this study was to compare the Framingham Risk Score (FRS) and vascular age differences in persons with HIV, HCV or HIV/HCV disease to the general population.
Methods
HIV, HCV and HIV/HCV patients with clinic visits between 2004–2009 were sampled from medical clinics in Rochester, NY. Uninfected persons were randomly selected from the National Health and Nutrition Examination Survey (NHANES) and individually matched on sex, race, and age. We stratified by infection group and conducted separate multivariable linear regression between each infection group and the sex, race, and age matched participants from NHANES.
Results
Rochester patients (HIV=239, HCV=167, HIV/HCV=182) were compared 3:1 to the NHANES participants. After controlling for weight, marital status, current pharmacotherapies, and the matching variables of sex, race and age, HIV/HCV patients had a 2% higher general FRS compared to the general population (p=0.03) and vascular age differences that were 4.1 years greater (p=.01). HCV patients had a 2.4% higher general FRS than the general population (p<.001), and vascular age differences that were 4.4 years greater (p<.001). CVD risk was elevated, but not significantly different between HIV patients and the general population.
Conclusion
CVD risk is elevated among HIV/HCV and HCV infected persons compared to the general population.
Keywords: HIV, Hepatitis C, HIV/HCV co-infection, cardiovascular disease risk, Framingham Risk Score
INTRODUCTION
With the availability of HAART (Highly Active Antiretroviral Therapy), non-AIDS related causes of death in people with HIV have increased from 7% in the pre-HAART era to 32–42% [1, 2] and HIV infection is associated with an increased risk of CVD surrogate markers (such as carotid intima-media thickness, arterial stiffness, endothelial dysfunction), and cardiovascular events [3–6]. Despite the documented increased risk of CVD among HIV mono-infected patients, limited research [7, 8] has been conducted to assess the risk of CVD among the approximately 2–4 million persons co-infected with HIV/Hepatitis C (HCV), or the 150 million mono-infected with HCV [9, 10]. Risk algorithms such as the Framingham Risk Score (FRS), or vascular age are oftentimes used to predict CVD in the general population [11, 12]. The FRS has been shown to reasonably predict CVD surrogate markers among persons mono-infected with HIV [13, 14]. Thus, the objective of this cross-sectional study was to compare the FRS and vascular ages among persons mono-infected with HIV, co-infected with HIV/HCV and mono-infected with HCV to a population-based comparison sample of sex-, race- and age-matched, uninfected US residents.
MATERIALS AND METHODS
Definitions of outcome variables
The primary outcomes of interest were the general CVD FRS and vascular age for patients with HIV, HCV and co-infected with HIV/HCV compared to a sex-, race- and age-matched uninfected group [11]. The general CVD FRS and vascular age algorithms were developed from the Framingham Heart Study cohort, and have been well described and validated elsewhere [11]. The general CVD FRS predicts the 10-year absolute risk of CVD events (defined as: coronary death, myocardial infarction, coronary insufficiency, angina, ischemic stroke, hemorrhagic stroke, transient ischemic attack, peripheral artery disease and heart failure) by incorporating into an algorithm a person’s age, sex, total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), systolic blood pressure (SBP), smoking status, presence of diabetes, and current pharmacotherapy for hypertension. Based on the 10-year general CVD FRSs, Framingham investigators also developed an algorithm to estimate the age of the person based on their heart’s fitness, referred to as their “vascular age”. For instance, regardless of chronologic age, a person with a vascular age of 50 would have the heart disease risk of a 50 year old with normal levels of all general CVD FRS risk factors [11]. For the purposes of this manuscript, this estimate of premature aging (subtracting the chronologic age from the vascular age) will be referred to as the “vascular age difference” and the general CVD FRS will be referred to as general FRS.
Participants
In the Rochester community, approximately 85% of all patients with known HIV obtain their care at one of three clinic sites (University of Rochester Medical Center’s Infectious Disease Clinic, AIDS Community Health Center, and Unity Health Infectious Diseases Clinic), and 70% of patients with known HCV obtain their care at the University of Rochester Medical Center’s Gastrointestinal Clinic. Subjects with HIV, HCV, or HIV/HCV infection with a clinic visit at one of the four sites between 2004–2009 were entered into a database and random samples were selected using SAS 9.2 (SAS Institute, Cary, North Carolina) and assessed for study eligibility. Data were abstracted through paper and electronic medical record review and included information on demographic characteristics, blood pressure, anthropometric measurements, metabolic and lipid profiles, disease specific lab measurements (CD4 cell counts, HIV and HCV viral load, degree of fibrosis), history of drug use, and smoking status. Comparison groups for each infection group were randomly selected from the 2003–2006 National Health and Nutrition Examination Surveys (NHANES) and were individually matched 3:1 on sex, race, and age using stratified random sampling.
Eligibility criteria and definitions
FRSs were developed in populations without CVD at baseline among subjects between the ages of 30 and 74, thus subjects outside of this age range, or reporting a history of CVD were excluded from our study. Subjects missing data on any of the components of the FRSs were also excluded. Due to reported variation in lipids during HCV treatment, and differences in lipids between acute and chronic HCV infections [15, 16], only patients with chronic HCV infection (detectable HCV viral load and positive HCV antibodies with normal bilirubin and transaminases less than 5× ULN) were eligible, and virally infected patients currently receiving HCV treatment were excluded. NHANES participants that tested positive for either HIV or HCV were excluded (<1%).
The fasting status of the Rochester subjects was not always known. Thus, diabetes was conservatively defined as blood sugar ≥ 200 mg/dL, or self-report of current or former diagnosis of diabetes or pharmacotherapy. Hypertension was defined as a single measurement of SBP ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, self-report of current or former diagnosis of hypertension, or pharmacotherapy for hypertension. Non-HDL cholesterol was defined as TC – HDL-C.
Statistical Analyses
All analyses were stratified by infection group. Chi-square and t-tests were used for descriptive analyses involving categorical and continuous variables, respectively. Multivariable linear regression analyses tested for significant differences in general FRS and vascular age differences between each infection group and their sex-, race-, and age- matched NHANES counterparts after adjusting for weight status (overweight or obese compared to normal- or under-weight), marital status, and current use of lipid-lowering medications. For analyses specific to The HIV and HIV/HCV groups, adjustment for use of HIV medications was built into the models. We did not statistically adjust for the lifestyle behaviors that are part of the general FRS (such as smoking, lipids and blood pressure) in order to avoid over-adjustment, and to most accurately estimate general CVD risk based on the true prevalence of these risk factors in our clinic sample. All analyses were conducted with SAS 9.2. The study was approved by the Institutional Review Board at the University of Rochester, AIDS Community Health Center, and Unity Health Infectious Diseases Clinic.
RESULTS
A total of 1,399 charts (HIV: 338, HIV/HCV: 356, HCV: 705) were reviewed. After excluding ineligible participants, our sample included 588 infected subjects (HIV: n=239, HIV/HCV: n=182, HCV: n=167) and 1,764 uninfected subjects from NHANES (Figure 1). To assess the generalizability of our HIV and HIV/HCV subjects, we compared the age, sex, and ethnicity of our study sample with the full clinic sample, as well as those subjects screened and deemed ineligible for our study. These data were available for all HIV and HIV/HCV patients with a clinic visit at the University of Rochester Medical Center during our study period. The demographic characteristics of our HIV study sample did not significantly differ (p>0.05) from the excluded HIV participants, or the entire HIV clinic sample in age (45.0 vs 47.6 and 45.8, respectively), sex (69% male vs 75% and 66%, respectively), or ethnicity (36% Black vs 52% and 42%, respectively). The HIV/HCV study participants did not significantly differ from the excluded HIV/HCV participants or the entire HIV/HCV study sample in age (50.1 vs 50.2 and 50.0, respectively), sex (64% male vs 61% male and 66%, respectively) or ethnicity (52% Black vs 48% and 54%, respectively). These basic demographic data were unavailable for the HCV clinic sample; however, the demographic characteristics of our HCV subjects are similar to the New York State HCV virally infected population [17]. Because there was no evidence for demographic heterogeneity across Rochester clinics, we did not control for clinic.
Figure 1.
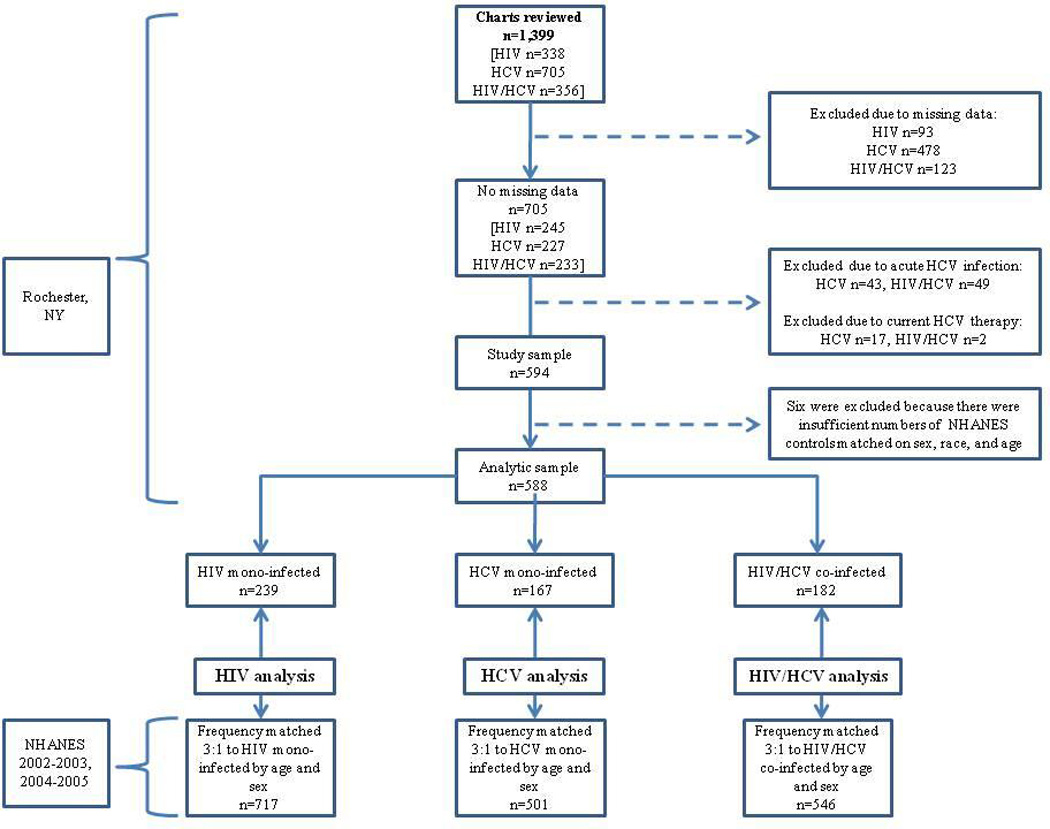
Flowchart of study population
Other than sex, race, and age on which matching was conducted, the NHANES subjects differed (p <0.05) from the virally infected patients in nearly all demographic and health characteristics of interest (Table 1). The Rochester participants had more favorable non-HDL-C and TC lipid profiles than their uninfected NHANES counterparts, but also had lower HDL-C, and higher proportions were smokers and hypertensive. Individuals with HCV were more likely to be diabetic at their clinic visit, or were more likely to be treated with pharmacotherapy for either hypertension or diabetes than their uninfected counterparts.
Table 1.
Characteristics of the study sample
| HIV comparison | HIV/HCV comparison | HCV comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HIV (n=239) |
NHANES (n=717) |
p | HIV/ HCV (n=182) |
NHANES (n=546) |
p | HCV (n=167) |
NHANES (n=501) |
p | |
| Age, mean (SD) | 44.5 (9.5) | 44.5 (9.5) | >.99 | 49.5 (7.1) | 49.5 (7.1) | >.99 | 51.2 (7.7) | 51.2 (7.7) | >.99 |
| Male | 165 (69%) | 495 (69%) | >.99 | 116 (64%) | 348 (64%) | >.99 | 101 (60%) | 303 (60%) | >.99 |
| Body Mass Index, mean (SD) | 28.2 (5.8) | 29.1 (5.9) | 0.03 | 25.9 (5.5) | 30.0 (6.4) | <.001 | 29.7 (6.9) | 30.1 (7.0) | 0.38 |
| Ethnicity | |||||||||
| White/Other | 125 (52%) | 375 (52%) | >.99 | 46 (25%) | 138 (25%) | >.99 | 77 (46%) | 231 (46%) | >.99 |
| Black | 88 (37%) | 264 (37%) | 94 (52%) | 282 (52%) | 60 (36%) | 180 (36%) | |||
| Hispanic | 26 (11%) | 78 (11%) | 42 (23%) | 126 (23%) | 30 (18%) | 90 (18%) | |||
| Marital status | |||||||||
| Single | 154 (64%) | 153 (21%) | <.001 | 104 (57%) | 102 (19%) | <.001 | 82 (48%) | 80 (16%) | <.001 |
| Married | 46 (19%) | 439 (61%) | 34 (19%) | 330 (60%) | 52 (31%) | 304 (61%) | |||
| Divorced/widowed | 39 (16%) | 125 (18%) | 44 (24%) | 114 (21%) | 33 (20%) | 117 (23%) | |||
| Current smoker | 104 (43%) | 195 (27%) | <.001 | 118 (65%) | 151 (28%) | <.001 | 85 (51%) | 129 (26%) | <.001 |
| Current or former cocaine or heroin use | 81 (34%) | 85 (19%) | <.001 | 148 (82%) | 75 (22%) | <.001 | 105 (63%) | 67 (20%) | <.001 |
| Hypertensiveb | 91 (38%) | 136 (19%) | <.001 | 88 (48%) | 136 (25%) | <.0001 | 108 (65%) | 134 (27%) | <.001 |
| Diabeticc | 18 (7%) | 55 (8%) | 0.94 | 25 (14%) | 85 (16%) | 0.55 | 47 (28%) | 89 (18%) | 0.004 |
| On lipid medications | 57 (24%) | 69 (10%) | <.001 | 9 (5%) | 82 (15%) | <.0001 | 23 (14%) | 86 (17%) | 0.30 |
| On blood pressure medications | 45 (19%) | 114 (16%) | 0.29 | 45 (25%) | 138 (25%) | 0.88 | 71 (43%) | 135 (27%) | <.001 |
| On glucose medications | 9 (4%) | 21 (3%) | 0.52 | 10 (5%) | 35 (6%) | 0.66 | 33 (20%) | 42 (8%) | <.001 |
| Lipid Profile (mg/dL), mean (SD) | |||||||||
| Total cholesterol (TC) | 192.6 (39.1) | 201.5 (40.3) | 0.003 | 164.1 (41.1) | 204.8 (37.4) | <.001 | 168.9 (43.4) | 205.8 (41.4) | <.001 |
| High density lipoprotein cholesterol (HDL) | 45.2 (14.2) | 51.1 (16.0) | <.001 | 44.9 (16.6) | 52.7 (15.7) | <.001 | 46.6 (16.6) | 52.1 (15.1) | <.001 |
| Non-HDL | 147.5 (37.2) | 150.4 (43.0) | 0.30 | 119.2 (38.5) | 152.1 (40.5) | <.001 | 122.3 (37.7) | 153.6 (42.8) | <.001 |
| 10-yr General Framingham Risk Score % | 8.9 (7.5) | 7.9 (7.4) | 0.08 | 12.0 (8.7) | 11.1 (9.0) | 0.23 | 14.2 (8.9) | 11.6 (8.4) | <.001 |
| Vascular age difference | 7.2 (10.0) | 4.5 (10.0) | <.001 | 9.6 (12.1) | 7.0 (12.1) | 0.01 | 12.5 (11.6) | 7.5 (12.0) | <.001 |
SD: Standard deviation.
Hypertension: systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or self-report of current or former diagnosis of hypertension or pharmacotherapy for hypertension;
Diabetes: fasting glucose ≥ 126 mg/dL, random or oral glucose tolerance ≥ 200 mg/dL, or self-report of current or former diagnosis of diabetes or pharmacotherapy for diabetes
Approximately half of the HCV and HIV/HCV patients never had a liver biopsy performed, and of those who did, six percent had cirrhosis (Table 2). Excluding those with known cirrhosis did not affect our general CVD risk or vascular age differences (data not shown). Although this study was not a comparison of CVD risk between infection groups, it is important to note that the HIV patients had a significantly higher CD4 cell count than the HIV/HCV patients (518 cells/uL and 410 cells/uL, respectively, p<.05), and a higher proportion had HIV viral loads < 50 copies/mL (63% and 50%, respectively, p<.05). None of the HAART exposure variables (duration of HAART, components of current HAART, or current adherence to HAART) differed by infection group (p>0.05), although a higher proportion of HIV patients were on antiretrovirals (79% vs 69%, p<0.05).
Table 2.
Disease characteristics of the virally infected sample
| Disease characteristics | HIV only (n=239) |
HCV only (n=167) |
HIV/ HCV (n=182) |
P |
|---|---|---|---|---|
| HCV disease characteristics | ||||
| Degree of fibrosis (liver biopsy) | ||||
| None | 11 (6%) | 15 (8%) | 0.008 | |
| Low (score of 1 or 2) | 65 (34%) | 23 (12%) | ||
| Mild (score of 3 or 4) | 35 (18%) | 20 (10%) | ||
| Cirrhosis (score of 5 or 6) | 10 (5%) | 11 (6%) | ||
| No biopsy performed | 60 (33%) | 127 (65%) | ||
| HCV genotype 11 | 133 (81%) | 133 (69%) | 0.06 | |
| HCV viral load2 | 6.62 (6.9) | 6.84 (7.2) | 0.11 | |
| HCV treatment | 83 (46%) | 42 (21%) | <.0001 | |
| HIV disease characteristics | ||||
| HIV risk behavior3 | ||||
| Heterosexual contact | 114 (48%) | 69 (40%) | <.0001 | |
| MSM | 103 (43%) | 6 (3%) | ||
| IDU | 8 (3%) | 74 (43%) | ||
| MSM and IDU | 3 (1%) | 10 (6%) | ||
| Other | 11 (5%) | 11 (6%) | ||
| Duration of HIV (mean years) | 8.11 (6.4) | 11.66 (6.4) | <.0001 | |
| CD4 count (cells/μL) (mean) | 518 (330) | 410 (311) | 0.001 | |
| Nadir CD4 count (cells/μL) (mean) | 224 (211) | 194 (196) | 0.13 | |
| HIV viral load, mean | 4.24 (4.7) | 4.41 (4.9) | 0.25 | |
| HIV viral load < 50 copies/mL | 150 (64%) | 89 (49%) | 0.003 | |
| HIV antiretroviral characteristics | ||||
| Currently on antiretrovirals | 189 (79%) | 125 (69%) | 0.01 | |
| Cumulative HAART exposure in years (SD) | 4.36 (3.6) | 3.86 (3.5) | 0.15 | |
| Current HAART includes a PI | 99 (52%) | 68 (54%) | 0.72 | |
| Current HAART includes a NRTI | 152 (80%) | 111 (89%) | 0.05 | |
| Current HAART includes an NNRTI | 95 (50%) | 61 (49%) | 0.80 | |
| HIV viral load < 400 copies/mL | 163 (86%) | 98 (78%) | 0.07 | |
| At least 95% treatment adherence to HAART4 | 143 (93%) | 87 (90%) | 0.28 |
N=163 (HCV), n=125 (HIV/HCV);
N=165 (HCV), n=142 (HIV/HCV);
n=170 (HIV/HCV);
N=153 (HIV), n=97 (HIV/HCV).
After controlling for covariates participants with HIV and HCV had a 10-year risk of general CVD that was 2% higher (Table 3) compared to their NHANES counterparts (p=0.03). HCV patients had a general FRSs that was 2.4% higher compared to their NHANES counterparts in adjusted analyses (p<.0001). Although HIV subjects had an absolute 10-year risk of general CVD events that was 0.88% higher than the risk in their NHANES counterpart, it was not statistically significant (p=0.24). The use of HIV therapy was not associated with the general FRS.
Table 3.
Beta estimates from multivariable linear regression analyses of infection group and Framingham Risk Score after adjusting for covariates
| HIVa (n=956) |
HIV and HCVa (n=728) |
HCVa (n=668) |
||||
|---|---|---|---|---|---|---|
| β Coefficients for Gen CVD Risk % (95% CI) |
p | β Coefficients for Gen CVD Risk % (95% CI) |
p | β Coefficients for Gen CVD Risk % (95% CI) |
p | |
| Infection versus NHANES | 0.88 (−0.58, 2.33) | 0.24 | 2.04 (0.20, 3.89) | 0.03 | 2.37 (1.15, 3.60) | <.001 |
| Body Mass Index (BMI) | ||||||
| Underweight versus Normal | 1.13 (−2.31, 4.57) | 0.52 | 0.45 (−2.56, 3.45) | 0.77 | −0.01 (−5.00, 4.99) | 0.99 |
| Overweight versus Normal | 0.83 (0.02, 1.63) | 0.04 | 0.45 (−0.54, 1.96) | 0.27 | −0.20 (−1.60, 1.21) | 0.78 |
| Obese versus Normal | 2.20 (1.40, 3.00) | <.001 | 2.75 (1.45, 4.05) | <.001 | 2.49 (1.14, 3.85) | <.001 |
| Marital status | ||||||
| Single versus married | 0.83 (0.04, 1.63) | 0.04 | 0.84 (−0.39, 2.08) | 0.18 | 1.47 (0.15, 2.79) | 0.03 |
| Formerly married versus married | 0.21 (−0.68, 1.11) | 0.63 | 0.67 (−0.58, 1.91) | 0.29 | 2.22 (0.92, 3.51) | <.001 |
| Lipid lowering medications | 1.56 (0.57, 2.55) | 0.002 | 0.92 (−0.55, 2.40) | 0.22 | 1.42 (0.01, 2.83) | 0.05 |
| On HIV medications | −0.45 (−1.97, 1.08) | 0.56 | −1.14 (−3.18, 0.91) | 0.28 | ||
CI: Confidence interval
Gen CVD: General Cardiovascular Disease
HCV: Hepatitis C
NHANES: National Health and Nutrition Examination Survey
Also adjusting for sex, race, and age
Subjects with HIV/HCV had vascular age differences that were 4.1 years greater (Table 4) than the vascular age differences in the general population in adjusted analyses (p=0.01). HCV participants had vascular ages that were 4.4 years greater than the vascular age difference in the general population (p<.001). Among subjects with HIV, the vascular age difference was 2.4 years greater than the vascular age difference in NHANES participants, but was not significantly different (p=0.10) in adjusted analyses.
Table 4.
Beta estimates from multivariable linear regression analyses of infection group and vascular age differences after adjusting for covariates
| HIVa (n=956) |
HIV and HCVa (n=728) |
HCVa (n=668) |
||||
|---|---|---|---|---|---|---|
| β Coefficients for Vascular age differences (95% CI) |
p | β Coefficients for Vascular age differences (95% CI) |
p | β Coefficients for Vascular age differences (95% CI) |
p | |
| Infection versus NHANES | 2.36 (−0.48, 5.21) | 0.10 | 4.12 (0.78, 7.46) | 0.01 | 4.43 (2.33, 6.53) | <.001 |
| Body Mass Index | ||||||
| Underweight versus Normal | 0.10 (−6.63, 6.84) | 0.98 | −0.53 (−5.98, 4.92) | 0.85 | 2.50 (−6.06, 11.05) | 0.56 |
| Overweight versus Normal | 2.49 (0.92, 4.06) | 0.002 | 0.93 (−1.34, 3.20) | 0.42 | 0.76 (−1.64, 3.17) | 0.53 |
| Obese versus Normal | 4.89 (3.32, 6.45) | <.001 | 4.93 (2.57, 7.29) | <.001 | 6.05 (3.73, 8.36) | <.001 |
| Marital status | ||||||
| Single versus married | 2.06 (0.51, 3.61) | 0.001 | 1.80 (−0.44, 4.04) | 0.11 | 3.22 (0.96, 5.48) | 0.005 |
| Formerly married versus married | 1.51 (−0.23, 3.26) | 0.09 | 1.39 (−0.87, 3.65) | 0.23 | 3.94 (1.73, 6.14) | <.001 |
| Lipid lowering medications | 1.90 (−0.04, 3.83) | 0.05 | 2.19 (−0.48, 4.86) | 0.11 | 2.61 (0.20, 5.02) | 0.04 |
| On HIV medications | −0.67 (−3.65, 2.32) | 0.66 | −0.79 (−4.50, 2.93) | 0.68 | ||
CI: Confidence interval
HCV: Hepatitis C
NHANES: National Health and Nutrition Examination Survey
Also adjusting for sex, race, and age
DISCUSSION
Patients with HIV/HCV or HCV had the highest general FRS (approximately 2–3%) and vascular age differences of all groups; significantly higher than the risks in their sex-, race-, and age-matched NHANES counterparts. Although the higher general FRS (2%) is a small difference on an individual level, this difference on a population level is potentially clinically significant. In addition, the larger vascular age differences in our infected groups may translate to even larger long term disparities between virally infected individuals compared to the general, uninfected population. These elevated risks were noted despite more favorable total and non-HDL cholesterol levels.
In contrast to the existing literature [18, 19], the results from this study suggest the risk of CVD based on the general FRS among persons mono-infected with HIV was elevated, but was not significantly greater than the general population. Previous studies assessed the risk of coronary heart disease in HIV infected European populations, whereas we assessed the risk of general cardiovascular disease in ethnically diverse HIV, HCV and HIV/HCV US populations, thus our results may not be directly comparable. In light of the increased CVD incidence in the HIV population as noted in the literature, it is possible that Rochester HIV clinicians may have been aggressively screening for CVD and proactively treating risk factors in our HIV mono-infected population. As we excluded subjects with documented CVD, perhaps aggressive screening and medical treatment in our HIV mono-infected population resulted in only the healthiest HIV subjects being eligible for our study. This is further suggested by the fact that our HIV mono-infected subjects had the highest rate of being on lipid lowering medications, despite being approximately 10 years younger than our HCV and HIV/HCV infected groups. The FRS has previously been reported to underestimate CVD risk among younger individuals with HIV infection, thus our HIV estimates of CVD risk may be an underestimation [20]. The FRS has not been validated in HCV populations. In addition, recently published articles noting elevated risk among subjects on drug holidays suggest that inflammation as a result of immune activation, and not the previously believed lipid changes during HAART is the major mediator of increased risk of CVD [21]. Thus it is possible elevated risk among persons mono-infected with HIV is not due solely based on lipid changes, and thus would not be apparent in the general FRS. Our findings suggest that inflammatory markers ought to be further investigated as part of a CVD prediction tool for use in the HIV population.
The higher general FRS and vascular age differences in our virally infected groups are likely due to their higher prevalence of hypertension and smoking use. These CVD risk factors are similar to those of the HIV, HIV/HCV and HCV infected populations in the US [22] and reflect the reality of the differences of the virally infected individuals compared to their same age, race, and sex uninfected peers. The elevated CVD risk in individuals with HCV or HIV/HCV is in agreement with the literature which reported an increased prevalence of CVD markers and events in persons with HIV/HCV [23, 24] in comparison to uninfected controls, and is consistent with a theory that multiple factors may increase CVD risk among persons with viral infections. Other studies report HCV mono-infected patients have increased aortic stiffness, higher proinflammatory cytokine levels, and increased presence of carotid plaques in comparison to HIV mono-infected patients, or uninfected controls, providing additional evidence of an association between hepatitis infection and cardiovascular disease [25–27].
The results from this study should be interpreted in the context of the following limitations. Our study was cross-sectional with a limited sample size thus we were unable to examine longitudinal effects, or the roles of specific antiretroviral medications on cardiovascular risk. Although the fasting status of our Rochester subjects was not always known, TC and HDL-C are not significantly affected by non-fasting states in comparison to TG or LDL-C [28]. Smoking status and drug use, history of co-morbidities, and adherence to HIV medication were all self-reported. In addition, a distinction cannot be made between participants that were poorly managed by their medical providers for their co-morbidities, and the participants that were not adherent. A number of covariates such as cocaine, alcohol consumption and renal function were not able to be assessed consistently or adequately controlled in this analysis. Data on family history of CVD or diabetes were not available for our Rochester subjects, although research suggests the inclusion of family history minimally affects the FRS [29]. Lastly, because a substantial proportion of our virally infected patients never had a liver biopsy conducted, we are unable to fully control for hepatic disease. However, biopsies are not routinely performed in clinics for a number of reasons and our study exemplifies a reality of the HCV clinical setting [30].
Due to the expected low number of cardiovascular events, this study was not able to examine specific cardiovascular outcomes or events. However, the general FRS has been reported to have an area under the receiver operating characteristics curve of 0.78 to predict carotid intima-media thickness among persons with HIV infection [14], and performed better than the more commonly used coronary heart disease FRS, or metabolic syndrome definitions [31, 32]. In addition, despite the fact that the general FRS has not been validated in HCV and HIV/HCV populations, the ordering of a person’s risk relative to others has been shown to be consistent in the general and HIV population regardless of which equation is used to quantify CVD risk [33]. Thus future studies should prospectively assess if infection groups continue to experience an estimated higher risk of CVD compared to the general population.
To the best of our knowledge, ours is the first study to simultaneously compare the general FRS and vascular ages in the general population to individuals with HIV, HCV, or HIV/HCV. Although the coronary FRS is the more commonly used predictor of CVD, the general FRS has significant public health importance and may be particularly informative for translating heart disease risk to patients. As these populations age, the risk of CVD becomes an increasingly important area of research. Results from this study suggest the risk of CVD is increased in persons co-infected with HIV/HCV, and mono-infected with HCV compared to the general population. In particular, the previously reported ‘protective’ effect [34] of lower lipid profiles in HCV mono-infected and HIV/HCV co-infected patients did not reduce their general FRSs or vascular age in our study. Lower lipid profiles in persons mono-infected with HCV or co-infected with HIV/HCV may incorrectly influence clinicians and patients into believing there is little risk of CVD. This incorrect assumption may translate into paying less attention to other CVD risk factors such as hypertension, diabetes or smoking. Further research should be conducted in elucidating the risk of CVD in these populations, and in validating risk measures in order to lower morbidity and mortality for infected individuals. Notably, the elevated vascular age differences in all groups are a cautionary reminder that CVD risk surveillance is needed for all populations.
What’s known
With effective antiretrovirals, HIV patients are living longer and are at an increased risk for cardiovascular disease. The Framingham Risk Score is often used to predict cardiovascular disease in the general population but data on Framingham Risk Scores in HIV and Hepatitis C patients is limited. A comparison between the risk scores between HIV, Hepatitis C, and HIV/Hepatitis C patients with the general population has yet to be carried out.
What’s new
Our HIV, Hepatitis C, and HIV/Hepatitis C populations (n=588) were age, sex, and race matched 3:1 to uninfected participants from the National Health and Nutrition Examination Survey (NHANES). Although the Hepatitis C and HIV/Hepatitis C patients had more favorable lipid profiles, their Framingham Risk Scores were elevated in comparison to the general uninfected population. This study highlights the need to further investigate potential cardiovascular disease in these virally infected populations.
ACKNOWLEDGEMENTS
This study was supported by a grant from the National Heart Lung and Blood Institute (NHLBI) Preventive Cardiology Training Program #5T32HL007937-10. This publication was also made possible by Grant Number KL2 RR 024136 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NHLBI, NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/.
Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
We gratefully acknowledge the support and assistance from the faculty and staff at the Infectious Diseases Division and Gastroenterology and Hepatology Division at the University of Rochester Medical Center, Unity Health and AIDS Community Health Center.
Footnotes
Disclosures: L.K., R.C.B., M.J.A., S.E.C., B.M., and S.G.F. have no conflicts of interest to declare.
AUTHORSHIP CONTRIBUTION:
All authors were involved with the concept/design, interpretation of data, critical revision and final approval of the article. LK additionally collected the data, conducted the data analysis and drafted the article.
Presented in part at the 18th AIDS International Conference, July 18–23, 2010, Vienna Austria.
References
- 1.Krentz HB, Kliewer G, Gill MJ. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med. 2005;6:99–106. doi: 10.1111/j.1468-1293.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 3.van Vonderen MG, Smulders YM, Stehouwer CD, et al. Carotid intima-media thickness and arterial stiffness in HIV-infected patients: the role of HIV antiretroviral therapy, and lipodystrophy. J Acquir Immune Defic Syndr. 2009;50:153–161. doi: 10.1097/QAI.0b013e31819367cd. [DOI] [PubMed] [Google Scholar]
- 4.Oliviero U, Bonadies G, Apuzzi V, et al. Human immunodeficiency virus per se exerts atherogenic effects. Atherosclerosis. 2009;204:586–589. doi: 10.1016/j.atherosclerosis.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Varriale P, Saravi G, Hernandez E, Carbon F. Acute myocardial infarction in patients infected with human immunodeficiency virus. Am Heart J. 2004;147:55–59. doi: 10.1016/j.ahj.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 7.Ishizaka Y, Ishizaka N, Takahashi E, et al. Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J. 2003;67:26–30. doi: 10.1253/circj.67.26. [DOI] [PubMed] [Google Scholar]
- 8.Bedimo R, Westfall AO, Mugavero M, et al. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010 doi: 10.1111/j.1468-1293.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 9.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44:S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 11.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 12.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 13.Falcone EL, Mangili A, Skinner S, et al. Framingham risk score and early markers of atherosclerosis in a cohort of adults infected with HIV. Antivir Ther. 2011;16:1–8. doi: 10.3851/IMP1682. [DOI] [PubMed] [Google Scholar]
- 14.Schambelan M, Wilson PW, Yarasheski KE, et al. Development of appropriate coronary heart disease risk prediction models in HIV-infected patients. Circulation. 2008;118:e48–e53. doi: 10.1161/CIRCULATIONAHA.107.189627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corey KE, Kane E, Munroe C, et al. Hepatitis C virus infection and its clearance alter circulating lipids: implications for long-term follow-up. Hepatology. 2009;50:1030–1037. doi: 10.1002/hep.23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka H, Miyano M, Ueda H, et al. Changes in serum and red blood cell membrane lipids in patients treated with interferon ribavirin for chronic hepatitis C. Clin Exp Med. 2005;5:190–195. doi: 10.1007/s10238-005-0085-0. [DOI] [PubMed] [Google Scholar]
- 17.New York State Department of Health. New York State Department of Health chronic Hepatitis C surveillance summary 2004–2009. [cited 2012 March 9]; Available from: http://www.health.ny.gov/diseases/communicable/hepatitis/hepatitis_c/2009_chronic_hepatitis_c_short_summary.htm.
- 18.De Socio GV, Martinelli L, Morosi S, et al. Is estimated cardiovascular risk higher in HIV-infected patients than in the general population? Scand J Infect Dis. 2007;39:805–812. doi: 10.1080/00365540701230884. [DOI] [PubMed] [Google Scholar]
- 19.Aboud M, Elgalib A, Pomeroy L, et al. Cardiovascular risk evaluation and antiretroviral therapy effects in an HIV cohort: implications for clinical management: the CREATE 1 study. Int J Clin Pract. 2010;64:1252–1259. doi: 10.1111/j.1742-1241.2010.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parra S, Coll B, Aragones G, et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010;11:225–231. doi: 10.1111/j.1468-1293.2009.00766.x. [DOI] [PubMed] [Google Scholar]
- 21.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vital Signs: HIV Testing and Diagnosis Among Adults --- United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2010;59:1550–1555. [PubMed] [Google Scholar]
- 23.Butt AA, Xiaoqiang W, Budoff M, et al. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225–232. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petta S, Torres D, Fazio G, et al. Carotid atherosclerosis and chronic hepatitis C: A prospective study of risk associations. Hepatology. 2011 doi: 10.1002/hep.25508. [DOI] [PubMed] [Google Scholar]
- 25.Oyake N, Shimada T, Murakami Y, et al. Hepatitis C virus infection as a risk factor for increased aortic stiffness and cardiovascular events in dialysis patients. J Nephrol. 2008;21:345–353. [PubMed] [Google Scholar]
- 26.Oliveira CP, Kappel CR, Siqueira ER, et al. Effects of Hepatitis C virus on cardiovascular risk in infected patients: A comparative study. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Masia M, Padilla S, Robledano C, et al. Evaluation of endothelial function and subclinical atherosclerosis in association with hepatitis C virus in HIV-infected patients: a cross-sectional study. BMC Infect Dis. 2011;11:265. doi: 10.1186/1471-2334-11-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 30.Rocca LG, Yawn BP, Wollan P, Kim WR. Management of patients with hepatitis C in a community population: diagnosis, discussions, and decisions to treat. Ann Fam Med. 2004;2:116–124. doi: 10.1370/afm.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 32.De Socio GV, Martinelli C, Ricci E, et al. Relations between cardiovascular risk estimates and subclinical atherosclerosis in naive HIV patients: results from the HERMES study. Int J STD AIDS. 2010;21:267–272. doi: 10.1258/ijsa.2009.009165. [DOI] [PubMed] [Google Scholar]
- 33.Thomsen TF, Davidsen M, Ibsen H, et al. A new method for CHD prediction and prevention based on regional risk scores and randomized clinical trials; PRECARD and the Copenhagen Risk Score. J Cardiovasc Risk. 2001;8:291–297. doi: 10.1177/174182670100800508. [DOI] [PubMed] [Google Scholar]
- 34.Bedimo R, Ghurani R, Nsuami M, et al. Lipid abnormalities in HIV/hepatitis C virus-coinfected patients. HIV Med. 2006;7:530–536. doi: 10.1111/j.1468-1293.2006.00416.x. [DOI] [PubMed] [Google Scholar]


