Abstract
Sand flies Lutzomyia (Psathyromyia) shannoni (Dyar) and Lu. (Helcocyrtomyia) vexator (Coquillet) were collected for the first time in southwest Missouri and southeast Kansas, expanding the known range of these species in North America. Altogether, 680 sand flies (356 males and 324 females) were collected during trapping from May through October 2011 and identified using morphological characters. Of the total sand flies collected 315 were identified as Lu. shannoni, with 181 individuals (or 26.6% of all sand flies) trapped in Missouri and 134 individuals (or 19.7%) trapped in Kansas. Whereas 358 Lu. vexator were identified from SW MO, only a single specimen was trapped in SE KS. One male Lu. vexator with asymmetric gonostyli was trapped in Missouri. We also developed a PCR protocol to consistently and accurately distinguish Lu. shannoni from Lu. vexator based on presence or absence of a 416bp fragment from the cytochrome oxidase I gene.
Keywords: Sand flies, Lutzomyia shannoni, Lutzomyia vexator
Phlebotomine sand flies are blood-feeding dipterans known for their role as vectors for Leishmania, certain phleboviruses and bacteria. Leishmaniasis is prevalent in over 88 countries with 1.5 – 2 million cases of cutaneous leishmaniasis (CL) and 500 thousand cases of visceral leishmaniasis (VL) per year (WHO, 2010). Sand flies of the genus Lutzomyia are present in the New World, with 14 species considered native to North America (Young and Perkins 1984). Among these, Lu. (Psathyromyia) shannoni (Dyar) and Lu. (Helcocyrtomyia) vexator (Coquillet) are frequently reported in sand fly surveys conducted in the eastern United States (Price et al. 2011).
In North America Lu. shannoni is commonly associated with hardwood forest habitats, and recent reports have pointed to an expansion of its historical range. Currently, reports on the presence of Lu. shannoni include 14 U. S. states (AL, AR, DE, FL, GA, LA, MD, MS, NC, SC, NJ, KY, OH, TN, and TX) (Young and Perkins 1984, Comer et al. 1990, Haddow et al. 2008, Claborn et al. 2009, Minter et al. 2009, Price et al. 2011).
Lutzomyia vexator is commonly found in sympatry with Lu. shannoni and has been reported in 21 states within the U. S., including eastern and western portions of the country (Young and Perkins 1984, Ostfeld et al. 2004, Haddow et al. 2008, Minter et al. 2009). Lutzomyia shannoni is of particular interest due to its role as a vector of vesicular stomatitis virus (Comer et al. 1990) and as a potential vector of leishmaniasis, including VL (Ferro et al. 1998, Travi et al. 2002). Cases of canine VL were previously identified in foxhounds in Missouri and Kansas (Duprey et al. 2006, Petersen and Barr 2009), without any previous reports of sand flies.
Here, we report for the first time the presence of sand flies (Lu. shannoni and Lu. vexator) in Kansas and Missouri based on trapping in one location in KS and two in MO from May to October 2011. We also describe a PCR protocol to molecularly distinguish Lu. shannoni from Lu. vexator, and report on a Lu. vexator specimen displaying asymmetric gonostyli.
Materials and Methods
Sand fly trapping and sites
Sand flies were trapped from dusk to dawn using CO2-baited CDC miniature light traps (model #512, John W. Hock). Sand flies were microscopically identified using the morphological characteristics of external genitalia for males and of the spermatheca for females (Young and Perkins 1984, Young and Duncan 1994).
Trapping locations were in southeastern KS and southwestern MO. Wilderness Park (WP; 37°27′14″ N 94°42′50″ W), on the northern boundary of Pittsburg, KS (population density of 623.5/km2 within 32.4 km2, 2010 Census), is a roughly 0.4 km2 public access area on an un-reclaimed coal strip mine that has become an oak-hickory forest containing numerous water bodies formed in strip pits and a creek running from the north to the south. It is surrounded by forested land, residential development and farmland. In Missouri, two locations were sampled: Springfield with a population density of 800/km2 within an area of 191.1 km2 (Census 2010), and the Bull Shoals Field Station (BSFS; 36°34′ N, 93°4′ W), a preserve located in the Drury-Mincy Conservation Area southeast of Branson, MO. Springfield was further subdivided into three focal sites located on the outskirts of town, with Focal Site 1 located in an area of hardwood and hickory adjacent to a pasture for a horse farm (37°6′33″ N, 93°19′12″ W); Focal Site 2 was a wood and pasture habitat in a residential area (37°6′24″ N, 93°17′52″ W); and Focal Site 3 was borderline between a secondary forest patch and a surrounding pasture (37°5′54″ N, 93°19′51″ W). Distances between focal sites ranged from 1.5 to 3.0 Km.
The number of light traps set at each location varied due to logistics and environmental factors. At WP, as few as three and as many as 11 light traps were placed when sampling the location. For the focal sites in Springfield the number of light traps used varied from eight to nine traps per night at the Focal Site 1, to three traps per night for Focal Sites 2 and 3. A single trapping event took place in site three, on June 12. At the BSFS location, between seven and 11 light traps were used per night. Generally, the distance between traps placed at any given location or focal site (in the case of Springfield) ranged from 10 m to 100 m. The distances between the three locations used for this study were: approximately 138 Km between WP (Kansas) and any of the three focal sites in Springfield (Missouri); 192 Km between WP and the BSFS (Missouri); and 65 Km between Springfield and the BSFS (http://www.gpsvisualizer.com/calculators#distance_address).
Effect of Weather on Flight Activity
Because of variations in trapping frequency and trap density among the three locations, data were pooled and the mean number of sand flies trapped within a two-week period (referred to as trapping periods below) was analyzed (the total number of sand flies trapped at any given location divided by the total number of traps used during a 2-week period). Trapping results from the three focal sites in Springfield were pooled and treated as a single location.
The effects of weather on sand fly activity at WP were assessed by performing linear regressions of the proportion of sand flies per trap captured during each two-week interval against 1) the average daily temperature for those two-week intervals 2) the average daily humidity for those two-week intervals and 3) total precipitation for those two-week intervals. Weather data were obtained from the weather station KKSFRONT2 located in Frontenac, KS, and 1.21 Km (or 0.75 miles) from Wilderness Park (http://www.wunderground.com/weatherstation/WXDailyHistory.asp?ID=KKSFRONT2&day=4&year=2011&month=6&graphspan=year). Linear regressions were performed using R programming for Statistical Language v2.14 (http://www.r-project.org).
Sex Ratios
We assessed the sex ratios using the binomial tests (R programming for Statistical Language v2.14) for sand flies trapped during each 2-week period for sample sizes greater than five individuals.
DNA extraction and polymerase chain reaction (PCR)
Genomic DNA from 47 Lu. shannoni and 23 Lu. vexator females was extracted using 10% Chelex 100 resin beads (Bio Rad). Sand flies were homogenized individually in 20 μl of molecular grade water, heated in 120 μl of Chelex 10% solution at 95 °C for 30 min, centrifuged briefly (6 sec) at 14,000 × g, and the supernatant transferred to a new tube. DNA extraction was confirmed by amplification of a 285bp fragment of the internal transcribed spacer region 2 (ITS-2) using GoTaq Colorless Master Mix (Promega, Madison, WI), 2μM each forward ACTGCATGGACCACGTATGG and reverse CACATATGAGTTGAGATCGC primers, in 10 μl reaction. ITS2 PCR conditions were: 94 °C for 2 min, two cycles of 94 °C for 30 sec and 72 °C for 45 sec, two cycles of 94 °C for 30 sec and 68 °C for 45 sec, 30 cycles of 94 °C for 30 sec, 58 °C for 30 sec, and 72 °C for 45 sec; with final extension at 72 °C for 10 min.
A Lu. shannoni-specific PCR was developed to amplify a 416bp fragment of the mitochondrial cytochrome oxydase C subunit 1 (CO1) in GeneBank. Forward and reverse primers were ATTTGGAAATTGATTGGTCC and TAAAAGTATGGTAATTGCAC, and PCR conditions done as indicated above were set at 94°C 2 min, 30 cycles of 94°C 1 min, 54 °C for 1 min, 72°C for 1 min, with a final extension of 72°C for 10 min. PCR products were visualized following electrophoresis on ethidium bromide-stained 1.2% agarose gel. To verify that the 416bp PCR product was indeed that of the CO1 gene, amplified fragments from several individuals were sequenced with 100% sequence identity to Lu. shannoni CO1 (accession # GU597891.1). Also, the blood source from the single engorged Lu. shannoni trapped at WP in late July was determined by amplification of an 850bp fragment of the cytB gene followed by DNA sequencing. All DNA sequencing was carried out using an ABI3730 DNA Analyzer at the Sequencing and Genotyping Facility at Kansas State University.
Results
Sand fly trappings
Phlebotomine (Lu. shannoni and Lu. vexator) sand flies were trapped for the first time in KS and MO. A total of 680 sand flies comprising 315 Lu. shannoni and 359 Lu. vexator were identified. Among the Lu. shannoni, 42.5% were trapped in SE KS, with 39.4% and 18.1% trapped in Springfield and BSFS, respectively. Of the Lu. vexator, 59.9% were trapped in Springfield and 39.8% in BSFS. A single specimen (0.3%) was trapped in SE KS. Specimens are deposited as voucher number 222 in the KSU Museum of Entomological and Prairie Arthropod Research.
At Wilderness Park (WP), 135 sand flies were trapped between mid-June and mid-October using 118 trap nights. The sand fly activity increased from 0.08 flies per trap night in June to 1.8 flies per trap night in July and 2.0 flies per trap night in August. By the first half of September it fell to one fly per trap (Fig. 1A). There was no significant correlation between the abundance of sand flies trapped at WP and the environmental variables tested (temperature: F1,7= 0.05, p= 0.82; precipitation: F1,7= 0.89, p= 0.37; relative humidity: F1,7= 0.13, p= 0.73). Also, a Lu. shannoni engorged with blood from white-tailed deer (Odocoileus virginianus) was collected in late August.
Figure 1. Sand flies trapped.
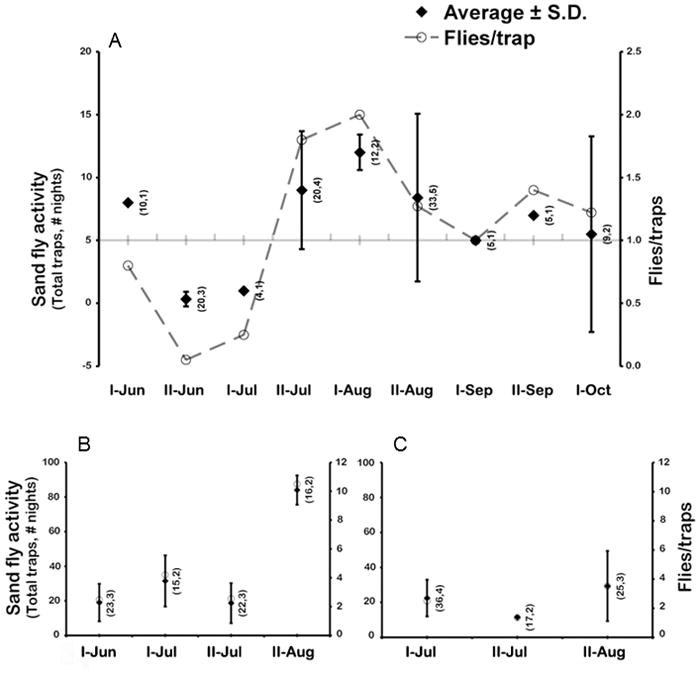
Sand fly activity according to sites, trapping period, trapnights and number of traps used. (A) Wilderness Park, Pittsburg, KS; (B) Springfield, MO; and (C) Bull Shoals Field Station, MO. The average number of sand flies trapped per night (diamond; +/− SD) was calculated for each 2-week period. Total number of traps used and number of nights are listed in parenthesis. Number of sand flies per trapnight (open circles) is shown on the right Y axis. A trend line was added in (A) for apparent activity of Lu. shannoni at WP.
In Springfield, MO, 344 sand flies were collected during 10 nights (76 trap nights in total) for all three focal sites, with 124 identified as Lu. shannoni, 215 identified as Lu. vexator, and five unidentified individuals (either no DNA was obtained or identification via morphological features was not possible). During trappings in June and July, 2.5 to 4.2 flies per trap night were observed. In late August, rates averaged 10.5 flies per trap night with a total of 168 sand flies collected in two nights (Fig. 1B). At the BSFS, 201 sand flies (57 Lu. shannoni, 143 Lu. vexator, and one unidentified female) were trapped during nine nights (Fig. 1C). Trapping at BSFS occurred three times, twice in July for a total of 113 flies with an average of 2.13 flies/trap/night (six nights), and 88 flies trapped in August for an average of 3.52 flies/trap/night over three nights. Overall, more Lu. shannoni males than females were trapped at WP (p = 0.046; Table 1 and Suppl. Fig), but this difference was not significant when individually considering each of the six 2-week trapping periods analyzed (Table 1).
Table 1.
Lu. shannoni sex ratio and number of individuals trapped in the Wilderness Park.
|
Lu. shannoni
|
|||
|---|---|---|---|
| ♀ | ♂ | p | |
| I-Jun | 3 | 5 | 0.727 |
| II-Jun | 0 | 1 | N/A |
| I-Jul | 1 | 0 | N/A |
| II-Jul | 13 | 23 | 0.133 |
| I-Aug | 7 | 17 | 0.064 |
| II-Aug | 21 | 20 | 1 |
| I-Sep | 5 | 0 | N/A |
| II-Sep | 2 | 5 | 0.453 |
| I-Oct | 3 | 8 | 0.227 |
| Total | 55 | 79 | 0.046 |
Two-week trapping periods shown on left column. Statistical significance difference was found only in the total of all trapped individuals (p-value in bold and italic). Probability (p) according to Binomial test (alpha = 0.05) is shown for n>5; N/A, analysis was not performed, n≤5.
Lu. shannoni sex ratio for Springfield alternated according to trapping periods, with significantly more females trapped in June (p = 0.001) and significantly more males trapped in July and August (p = 0.036 and p = 0.001, respectively) (Table 2 and Suppl. Fig). At the BSFS, the Lu. shannoni sex ratio based on three trapping periods was evenly distributed (Table 2 and Suppl. Fig). For Lu. vexator, no significant differences in the sex ratio were observed for any of the trapping periods at any of the locations (Table 2). In addition, twenty-eight gravid females (6 Lu. shannoni and 22 Lu. vexator) with fully developed eggs were collected during the months of June through September (Table 3).
Table 2.
Sex ratio and total sand flies trapped in Springfield and at the Bull Shoals Field Station (BSFS)
|
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Springfield | BSFS | |||||||||||
|
| ||||||||||||
| Lu. shannoni | Lu. vexator | Lu. shannoni | Lu. vexator | |||||||||
|
| ||||||||||||
| ♀ | ♂ | p | ♀ | ♂ | p | ♀ | ♂ | p | ♀ | ♂ | p | |
| II-June | 35 | 12 | 0.001 | 6 | 2 | 0.289 | - | - | - | - | - | - |
| I-July | 2 | 2 | N/A | 31 | 25 | 0.504 | 6 | 5 | 1.000 | 40 | 39 | 1.000 |
| II-July | 8 | 20 | 0.036 | 18 | 10 | 0.185 | 7 | 9 | 0.804 | 4 | 1 | N/A |
| II- Aug | 11 | 34 | 0.001 | 52 | 71 | 0.104 | 14 | 15 | 1.000 | 28 | 31 | 0.795 |
| Total | 56 | 68 | 0.323 | 107 | 108 | 0.576 | 27 | 29 | 0.894 | 72 | 71 | 1.000 |
Sex ratio and numbers of sand flies collected during 2-week trapping periods showing in the left column. Significant statistical difference was found between number of females and males trapped in Springfield (p-value in bold and italic). Probability (p) according to Binomial test (alpha = 0.05) is shown for n>5; N/A, analysis not performed, n≤5.
Table 3.
Distribution of gravid females trapped.
|
|
||||||
|---|---|---|---|---|---|---|
| Lu. shannoni | Lu. vexator | |||||
|
| ||||||
| WP N (%) |
Springfield N (%) |
BSFS N (%) |
WP N (%) |
Springfield N (%) |
BSFS N (%) |
|
| July | 0 (0) | 0 (0) | 1 (7) | 0 | 4 (8) | 6 (14) |
| August | 1 (3) | 2 (18) | 1 (7) | 0 | 11 (21) | 1 (4) |
| September | 1 (14) | N/T | N/T | 0 | N/T | N/T |
N indicates number of gravid flies trapped; % of gravid per total females trapped each month is shown in parenthesis. N/T, trapping for sand flies did not occur at these locations. WP, Wilderness Park; BSFS, Bull Shoals Field Station.
Three peaks of Lu. shannoni activity were observed in the Wilderness Park, beginning with the first trapping period in June (Figure 1A). The last successful trapping at that location was on October 8th, with a total of 11 flies collected in five traps. In Springfield, both Lu. shannoni and Lu. vexator were trapped in all four trapping periods in this location. In Springfield however, 97% of all sand flies were trapped in late August (Fig. 1B). Moreover, twice as many sand flies per trap were collected in Focal site 1 than the other two focal sites combined.
Morphological variations in Lu. shannoni male genitalia were reported by Florin (Florin et al. 2010, Florin et al. 2011) that can lead to misidentification of this species as Lu. vexator. One individual Lu. shannoni from Springfield matching the variation reported by Florin (the presence of five spines in one of the gonostylus) (Florin et al. 2010) was observed and one Lu. vexator male displaying asymmetric gonostyles (Fig. 2) also was identified.
Figure 2.

Lu. vexator male with asymmetric gonostylus. (A) shows a superimposed image of (B) and (C), with white arrows pointing to the four spines in one gonostylus, and the black arrows pointing the five spines characteristics of the species. Note one missing spine in the end of gonostylus in (A) and (B).
Lu. shannoni specific CO1 PCR amplification
The PCR protocol designed to specifically amplify the 416bp fragment from the CO1 gene was effective in distinguishing Lu. shannoni from Lu. vexator. Specimens from all three sites were identified using morphological characters and DNA isolated. In addition, DNA samples from 20 male sand flies of each species and from each of the three locations (with the exception of WP, where a single Lu. vexator was captured) were PCR amplified with the specific primers. Only the DNA obtained from Lu. shannoni was amplified in any of these samples. In addition, no amplification of the CO1 gene was observed with DNA isolated from colonized Lu. longipalpis and Phlebotomus papatasi (from our laboratory colonies), using the PCR conditions described. Following amplification, the 416bp fragment was matched to 100% nucleotide sequence identity to Lu. shannoni CO1.
Twenty three sand fly specimens preserved in 70% ethanol were identified based solely on the PCR described above. These included eight Lu. shannoni females (six from Springfield and two from BSFS), 11 Lu. vexator females (nine from Springfield and two from the BSFS site), two Lu. vexator males (one each from Springfield and BSFS), and two male Lu. shannoni from Springfield (Fig. 3).
Figure 3.
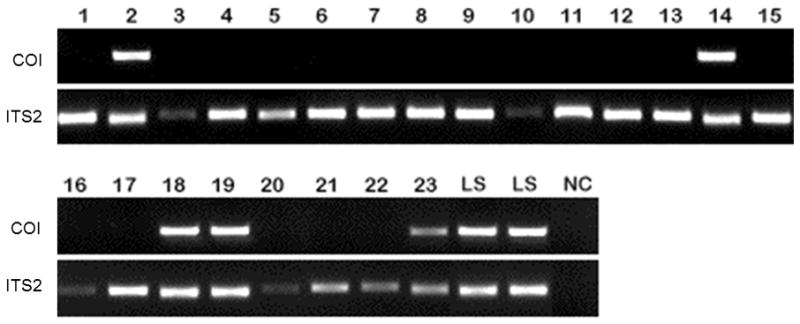
Lu. shannoni PCRTwenty-three sand flies not identified by morphological characters were identified by presence (Lu. shannoni) or absence (Lu. vexator) of the 416bp fragment of the CO1 gene. ITS2 amplification was used to confirm DNA isolation. Lanes 1, 3–13, 15–17, 20–22 were identified as Lu. vexator; lanes 2, 14, 18,19, and 23 were identified as Lu. shannoni. LS, DNA from Lu. shannoni females identified via morphological characters. NC, negative control (no DNA).
Discussion
Here, we report for the first time the presence of Lu. shannoni and Lu. vexator in Kansas and Missouri, adding to the known distribution of sand flies in North America. We did not observe a significant statistical correlation between weather and sand fly abundance for the analyses performed for the sand flies trapped at Wilderness Park. However, this might be due in part to the fact that our results were based on a single sand fly trapping season. Our data suggest at least two generations of Lu. shannoni occurring per year in Pittsburg and Springfield, matching previous reports (Minter 2010). In contrast to what has been reported for Lu. shannoni in Florida (Mann and Kaufman 2010), no sand flies were trapped after October 8th in KS, suggesting that they undergo diapause to survive the winter months. We expect this to also be the case in Missouri. Likely, Lu. shannoni undergoes facultative diapause during the winter months as reported for Lu. diabolica (Lawyer and Young 1991).
We also established a PCR protocol that specifically amplifies a 416bp fragment of the CO1 gene in Lu. shannoni and not Lu. vexator. This protocol can be applied as a molecular tool in situations where confounding morphological differences may be present in Lu. shannoni, such as the ones described by Florin et al. (2010).
Due to the introduction of Leishmania infantum through importation of dogs from endemic areas, the potential exists for the parasite to become endemic in North America. The role of sand flies in the current canine outbreak is unknown; however, the presence of a vector species that feeds on mammals may be significant. Fourteen sand fly species are native to North America, including two, Lu. authophora (Addis) and Lu. diabolica (Hall), that are proven vectors of Le. mexicana (Endris et al. 1987, Lawyer and Young 1987, Lawyer et al. 1987). Lu. shannoni, also native to North America, is a suspected vector of Le. mexicana in Mexico (Pech-May et al. 2010, González et al. 2011), was shown to be susceptible to infection with Old World parasites, such as Le. major (Claborn et al. 2009), and has also been incriminated in the transmission of pathogens, including Le. infantum (Travi et al. 2002). If this sand fly is indeed able to transmit Le. infantum to and from domestic dogs, it may also transmit to wild canids (e.g., coyotes and foxes) as well as other animals, creating a scenario for the establishment of the parasite in North America. Further studies on the bionomics and current distribution of Lu. shannoni, as well as its potential as vector for canine visceral leishmaniasis in North America, will be important for assessing disease risk due to sand fly-borne disease in North America.
Supplementary Material
Acknowledgments
Thanks to Sarah Mull and Nathan Elliot for assistance with trappings. This work was funded by NIAID-NIH grant R21 AI088051. Samantha Young was partially supported by a Student Scholar Award from the Kansas IDeA Network of Biomedical Research Excellence (K-INBRE). NIH grant number P20 RR016475 from the National Center for Research Resources. This manuscript is Kansas State Research and Extension contribution number 12-406-J.
References Cited
- Claborn DM, Rowton ED, Lawyer PG, Brown GC, Keep LW. Species diversity and relative abundance of phlebotomine sand flies (Diptera: Psychodidae) on three Army installations in the southern United States and susceptibility of a domestic sand fly to infection with Old World Leishmania major. Mil Med. 2009;174:1203–1208. doi: 10.7205/milmed-d-00-4309. [DOI] [PubMed] [Google Scholar]
- Comer JA, Tesh RB, Modi GB, Corn JL, Nettles VF. Vesicular stomatitis virus, New Jersey serotype: replication in and transmission by Lutzomyia shannoni (Diptera: Psychodidae) Am J Trop Med Hyg. 1990;42:483–490. doi: 10.4269/ajtmh.1990.42.483. [DOI] [PubMed] [Google Scholar]
- Duprey ZH, Steuer FJ, Rooney JA, Kirchhoff LV, Jackson JE, Rowton ED, Schantz PM. Canine visceral leishmaniasis, United States and Canada, 2000–2003. Emerg Infect Dis. 2006;12:440–446. doi: 10.3201/eid1203.050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endris RG, Young DG, Perkins PV. Experimental transmission of Leishmania mexicana by a North American sand fly, Lutzomyia anthophora (Diptera: Psychodidae) J Med Entomol. 1987;24:243–247. doi: 10.1093/jmedent/24.2.243. [DOI] [PubMed] [Google Scholar]
- Ferro C, Cárdenas E, Corredor D, Morales A, Munstermann LE. Life cycle and fecundity analysis of Lutzomyia shannoni (Dyar) (Diptera: Psychodidae) Mem Inst Oswaldo Cruz. 1998;93:195–199. doi: 10.1590/s0074-02761998000200011. [DOI] [PubMed] [Google Scholar]
- Florin DA, Lawyer P, Rowton E, Schultz G, Wilkerson R, Davies SJ, Lipnick R, Keep L. Morphological anomalies in two Lutzomyia (Psathyromyia) shannoni (Diptera: Psychodidae: Phlebotominae) specimens collected from Fort Rucker, Alabama, and Fort Campbell, Kentucky. J Med Entomol. 2010;47:952–956. doi: 10.1603/me10088. [DOI] [PubMed] [Google Scholar]
- Florin DA, Davies SJ, Olsen C, Lawyer P, Lipnick R, Schultz G, Rowton E, Wilkerson R, Keep L. Morphometric and molecular analyses of the sand fly species Lutzomyia shannoni (Diptera: Psychodidae: Phlebotominae) collected from seven different geographical areas in the southeastern United States. J Med Entomol. 2011;48:154–166. doi: 10.1603/me10199. [DOI] [PubMed] [Google Scholar]
- González C, Rebollar-Téllez EA, Ibáñez-Bernal S, Becker-Fauser I, Martínez-Meyer E, Peterson ATS-CV. Current knowledge of Leishmania vectors in Mexico: how geographic distributions of species relate to transmission areas. Am J Trop Med Hyg. 2011;85:839–846. doi: 10.4269/ajtmh.2011.10-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow A, Curler G, Moulton J. New records of Lutzomyia shannoni and Lutzomyia vexator (Diptera: Psychodidae) in eastern Tennessee. J Vector Ecol. 2008;33:393–396. doi: 10.3376/1081-1710-33.2.393. [DOI] [PubMed] [Google Scholar]
- Lawyer P, Young D. Diapause and quiescence in Lutzomyia diabolica (Diptera:Psychodidae) Parassitologia. 1991;33(Suppl):353–360. [PubMed] [Google Scholar]
- Lawyer PG, Young DG. Experimental transmission of Leishmania mexicana to hamsters by bites of phlebotomine sand flies (Diptera: Psychodidae) from the United States. J Med Entomol. 1987;24:458–462. doi: 10.1093/jmedent/24.4.458. [DOI] [PubMed] [Google Scholar]
- Lawyer PG, Young DG, Butler JF, Akin DE. Development of Leishmania mexicana in Lutzomyia diabolica and Lutzomyia shannoni (Diptera: Psychodidae) J Med Entomol. 1987;24:347–355. doi: 10.1093/jmedent/24.3.347. [DOI] [PubMed] [Google Scholar]
- Mann RS, Kaufman PE. The seasonal abundance of phlebotomine sand flies, Lutzomyia species in Florida. J Am Mosq Control Assoc. 2010;26:10–17. doi: 10.2987/09-5901.1. [DOI] [PubMed] [Google Scholar]
- Minter L, Kovacic B, Claborn DM, Lawyer P, Florin D, Brown GC. New State Records for Lutzomyia shannoni (Dyar) and Lutzomyia vexator (Coquillett) J Med Entomol. 2009;46:965–968. doi: 10.1603/033.046.0432. [DOI] [PubMed] [Google Scholar]
- Minter LM. (Diptera: Psychodidae) in deciduous habitats of the eastern United States. MS, University of Kentucky; Lexington KY: 2010. Mesoscale spatial and temporal distribution of Lutzomyia spp. [Google Scholar]
- Ostfeld RS, Roy P, Haumaier W, Canter L, Keesing F, Rowton ED. Sand fly (Lutzomyia vexator) (Diptera: Psychodidae) populations in upstate New York: abundance, microhabitat, and phenology. J Med Entomol. 2004;41:774–778. doi: 10.1603/0022-2585-41.4.774. [DOI] [PubMed] [Google Scholar]
- Pech-May A, Escobedo-Ortegón FJ, Berzunza-Cruz M, Rebollar-Téllez EA. Incrimination of four sandfly species previously unrecognized as vectors of Leishmania parasites in Mexico. Med Vet Entomol. 2010;24:150–161. doi: 10.1111/j.1365-2915.2010.00870.x. [DOI] [PubMed] [Google Scholar]
- Petersen CA, Barr SC. Canine leishmaniasis in North America: emerging or newly recognized? Vet Clin North Am Small Anim Pract. 2009;39:1065–1074. doi: 10.1016/j.cvsm.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DC, Gunther DE, Gaugler R. First collection records of phlebotomine sand flies (Diptera: Psychodidae) from New Jersey. J Med Entomol. 2011;48:476–478. doi: 10.1603/me10170. [DOI] [PubMed] [Google Scholar]
- Travi BL, Ferro C, Cadena H, Montoya-Lerma J, Adler GH. Canine visceral leishmaniasis: dog infectivity to sand flies from non-endemic areas. Res Vet Sci. 2002;72:83–86. doi: 10.1053/rvsc.2001.0527. [DOI] [PubMed] [Google Scholar]
- World Health Organization Technical Report Series no. 949: Control of the leishmaniases. Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases; Geneva. 22–26 March 2010. [Google Scholar]
- Young D, Duncan M. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae) Mem Amer Entomol Inst. 1994;54:1–881. [Google Scholar]
- Young DG, Perkins PV. Phlebotomine sand flies of North America (Diptera:Psychodidae) Mosq News. 1984;44:263–304. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


