Abstract
Background
Limited data exist on the prevalence, associations and prognosis of individuals with asymptomatic left ventricular systolic dysfunction (ALVSD), especially in populations without prior clinical cardiovascular disease (CVD).
Methods and Results
Kaplan-Meier and Cox proportional hazard analyses were used to assess the association between ALVSD, defined as left ventricular ejection fraction less than 50%, and adjudicated incident congestive heart failure (CHF), all-cause mortality, and CVD events.
Out of 5004 participants, 112 participants had CHF, 321 had a CVD event, and 278 died after 9 years of follow-up. The overall prevalence of ALVSD was 1.7%, with a higher prevalence in African Americans (2.6%). ALVSD had worse cardiovascular risk profile and was also associated with increased risk in unadjusted and adjusted models for incident CHF [HR (95%): 12.0(7.04 – 20.3), p<0.0001 and 8.69(4.89 – 15.45), p<0.001 respectively], CVD [HR (95%):3.32(1.98 -5.58), p<0.001 and 2.21(1.30 – 3.73), p=0.003 respectively] and all-cause mortality [HR(95%):3.47(2.03 – 5.94), p<0.0001 and 2.00(1.13-3.54), p=0.017 respectively]. A 10% decrement in LVEF at baseline was associated with increase in risk in unadjusted and adjusted models for clinical CHF [HR (95%CI): 2.17(1.82 -2.63), p<0.0001 and 2.13(1.73 - 2.51), p<0.001 respectively] and all-cause mortality [HR (95%CI): 1.22(1.05 – 1.41), p=0.009 and 1.17(1.00 – 1.36), p=0.047 respectively]. Among the subset of participants with ALVSD, LVMI was particularly informative about risk for incident CHF (c- index = 0.74).
Conclusions
ALVSD is uncommon in individuals without prior clinical CVD, but is associated with high risk for CHF, CVD, and all-cause mortality. LVMI had good discrimination for incident CHF in MESA participants with ALVSD.
Keywords: heart failure, death, cardiovascular diseases, magnetic resonance imaging, population
Introduction
Despite recent advances in heart failure management, individuals with congestive heart failure still endure high morbidity and mortality1. Except for congestive heart failure (CHF) precipitated by an extensive myocardial infarction, most patients with heart failure appear to progress from an asymptomatic phase (American Heart Association stage B) to symptomatic phases (American Heart Association stage C and D)2-4. The poor prognosis in subjects with symptomatic heart failure is already well established5. Current evidence suggests that treatments targeting the asymptomatic phase may slow the progression to the symptomatic phase, and reduce subsequent morbidity and mortality6,7. However, current data on the prevalence and prognosis of asymptomatic left ventricular systolic dysfunction (ALVSD) are limited. Most data were obtained from the non-interventional arm of clinical trials and generally include subjects with prior cardiovascular disease (CVD)6-15. Similar to the clinical trial data6-8, in the few published epidemiologic studies to date, most subjects had a history of myocardial infarction (MI) and even though prior CVD/MI is accounted for in their models, it limits the generalizability of their findings14. Moreover most ALVSD subjects with prior MI have had contact with healthcare professionals and are likely to have been prescribed the recommended therapy, questioning the impact of public health screening for them in our communities. Finally, almost all previously published data on this subject are based on echocardiographic measures of left ventricular structure and function rather than MRI-based measures, which have superior accuracy and reproducibility, especially for measures of LV mass16.
To address some of these limitations and better characterize the prevalence, associations and prognosis of individuals with asymptomatic left ventricular systolic dysfunction (ALVSD) without prior CVD, we assessed the nine-year incidence of CHF, CVD and total mortality in participants with ALVSD assessed using cardiac MRI from the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
Study Population and Data Collection
A detailed study design for MESA has been published elsewhere17. In brief, MESA is a prospective cohort study begun in July 2000 to investigate the prevalence, correlates, and progression of subclinical CVD in individuals without known CVD at baseline. The cohort includes 6814 women and men aged 45–84 years old recruited from 6 US communities (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan, NY; and St. Paul, MN). MESA participants were 38% white (n = 2624), 28% black (n = 1895), 22% Hispanic (n = 1492) and 12% Chinese (n = 803). Individuals with a history of physician-diagnosed myocardial infarction, angina, heart failure, stroke or transient ischemic attack, or who had undergone an invasive procedure for CVD (coronary artery bypass graft, angioplasty, valve replacement, pacemaker placement or other vascular surgeries) were excluded. This study was approved by the Institutional Review Boards of each study site and written informed consent was obtained from all participants.
Demographics, medical history, anthropometric and laboratory data for this study were obtained at the first MESA examination (July 2000 to August 2002). Current smoking was defined as having smoked a cigarette in the last 30 days. Diabetes mellitus was defined as fasting glucose ≥126 mg 100 ml−1 or the use of hypoglycemic medications. Use of antihypertensive and other medications was based on the review of prescribed medication containers. Resting blood pressure was measured three times in seated position, and the average of the second and third readings was recorded. Hypertension was defined as a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg or use of medication prescribed for hypertension. Body mass index was calculated as weight (kg)/height2 (m2). Total and high-density lipoprotein cholesterol were measured from blood samples obtained after a 12-h fast. Low-density lipoprotein cholesterol was estimated by the Friedewald equation18.
Cardiac Magnetic Resonance Imaging
Consenting participants underwent a cardiac MRI scan a median of 16 days after the baseline evaluation; 95% were completed by 11 weeks after the baseline examination. Participation in the MRI exam was voluntary. All imaging was done with a four-element phased-array surface coil positioned anteriorly and posteriorly, electrocardiographic gating, and brachial artery blood pressure monitoring19. Imaging consisted of fast gradient echo cine images of the left ventricle with time resolution < 50 ms. Functional parameters and mass were determined by volumetric imaging. Imaging data were read using MASS software (version 4.2, Medis, Leiden, the Netherlands) at a single reading center by trained readers blinded to risk factor information. Papillary muscles were included in the LV volumes and excluded from LV mass. LV end-diastolic volume and LV end-systolic volume were calculated using Simpson’s rule (the summation of areas on each separate slice multiplied by the sum of slice thickness and image gap). LV mass was determined by the sum of the myocardial area (the difference between endocardial and epicardial contour) times slice thickness plus image gap in the end-diastolic phase multiplied by the specific gravity of myocardium (1.05 g/mL). LVEF was calculated as LV stroke volume/ LV end-diastolic volume X 100. The interobserver variability in estimating LV parameters was: LVM (6.0 gm, 95% CI, 4.6, 7.4); LVEF (5.1%, 95% CI 3.6, 6.7) and intraobserver variability in estimating LV parameters was: LVM (6.3 gm, 95% CI, 5.17, 7.38); LVEF (3.9%, 95% CI, 3.06, 4.72)
Ascertainment of Outcomes
Outcomes in MESA are adjudicated by a committee which includes a cardiologist, a cardiovascular physician-epidemiologist and a neurologist. Reviewers/ adjudicators classified incident CHF as definite, probable, or absent. Definite or probable CHF required heart failure symptoms, such as shortness of breath or edema; probable CHF required CHF diagnosed by a physician and patient receiving medical treatment for CHF. Definite CHF required one or more other criteria, such as pulmonary edema/congestion by chest X-ray; dilated ventricle or poor LV function by echocardiocardiography or ventriculography; or echocardiography evidence of left ventricular diastolic dysfunction. Participants who had only a physician diagnosis of CHF without any other evidence, were classified as “no CHF”. Individuals with adjudicated definite or probable CHF were used in our analysis.
An incident cardiovascular event is a composite of adjudicated myocardial infarction, stroke, resuscitated cardiac arrest, angina if followed by percutaneous coronary intervention or coronary bypass grafting, and cardiovascular disease death. In order to make our results comparable to prior published data9,14, asymptomatic left ventricular systolic dysfunction (ALVSD) was defined as participants with LVEF <50%. Left ventricular mass index (LVMI) was calculated using the formula LVMI = LV mass/ body surface area (g/m2).
Statistical Analysis
Demographic characteristics of participants with ALVSD are reported as mean ±SD for continuous variables and as frequency or percentages for categorical variables compared with those without ALVSD during the MESA baseline exam. Kaplan-Meier analysis and log rank test were used to explore the association between ALVSD and incident CHF, CVD and all-cause mortality. Cox proportional hazard analysis was also used to assess the association between ALVSD and outcomes (incident CHF, CVD, and all-cause mortality) in unadjusted and adjusted models adjusting for covariates including age, gender, race/ethnicity, diabetes mellitus, body mass index (BMI), systolic blood pressure, total cholesterol, HDL, triglycerides, cigarette smoking status, blood pressure medication use (ACE inhibitors or beta blockers) and statin use. Interim myocardial infarction was adjusted for in the adjusted Cox models for incident CHF and all-cause mortality. These covariates were chosen based on their associations in the present study and also in prior publications. The extended Cox model, which used the time-dependent variable approach, was used to test for the proportionality assumption. The discriminative ability of LV mass index for incident CHF in the subset with ALVSD was evaluated in a Cox model using the approach proposed by Pencina et al20 and a time dependent receiver operating characteristic curve was constructed (at 4 years of follow up) using the approach by Heagerty et al21 (TDROC macro in sas authored by Mithat Gonen)
Subsequently, the association between LVEF as a continuous variable and outcomes (incident CHF, CVD, and all-cause mortality) was explored using Cox proportional hazard analysis in unadjusted and adjusted analyses adjusting for the covariates listed above. We estimated the discriminative ability of a Cox model with LVEF and LVEF +LVMI for incident CHF using the approach by Pencina et al20. Time dependent Reciever operating characteristic (ROC) curves were constructed (at 4years of follow up) with LVEF /LVEF+ LVMI in the model and the area under the ROC curves compared. A 2-tailed value of P<0.05 was considered significant. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
From 6,814 overall participants, 5,004 participants with no clinical cardiovascular disease, including valvular heart disease and CHF, underwent cardiac MRI for assessment of left ventricular ejection fraction. 1.7% of the cohort had asymptomatic left ventricular systolic dysfunction (ALVSD). This was highest in African Americans (AA) (2.6%) followed by Caucasians (1.7%), Hispanic Americans (1.6%), and Chinese Americans (0.15%). After an average of 7.5 years of follow-up (maximum of 9 years), 112 participants had adjudicated incident CHF, 321 had an incident cardiovascular event, and 278 died from various causes.
MESA participants with ALVSD were more likely to be male, African American, have high LV mass index and in general, poor cardiovascular risk profiles compared with participants without ALVSD (Table 1). Use of statins, ACE inhibitors, beta blockers, diuretics, and blood pressure medications did not differ between participants with and without ALVSD. Participants with ALVSD were not significantly obese (using BMI) compared with those without ALVSD (Table 1). In addition, participants with ALVSD who developed congestive heart failure during the follow up period were not significantly obese compared with those who did not develop congestive heart failure.
Table 1.
Demographic characteristics of study participants.
| Variable | No ALVSD (N=4918) ( Mean ±SD) |
ALVSD (N= 86) (Mean ±SD) |
P value |
|---|---|---|---|
| Age (years) | 61.5 ±10.1 | 62.4 ±10.7 | 0.432 |
| Female (%) | 2607(53.1) | 15(17.4) | <0.0001 |
|
Race/ Ethnicity (%)
Caucasian Chinese African American Hispanics |
1923(39.1) 652(13.3) 1252(25.4) 1091(22.2) |
34(39.5) 1(1.2) 33(38.4) 18(20.9) |
<0.001 |
| BMI( Kg/m2) | 27.7± 4.9 | 28.2 ±4.9 | 0.353 |
| Systolic BP(mmHg) | 125.4 ±21.3 | 129.7 ±22.3 | 0.06 |
| Diastolic BP(mmHg) | 71.7 ±10.3 | 76.9 ±11.6 | <0.001 |
| Diabetes Mellitus (%) | 503(10.2) | 15(17.4) | 0.07 |
|
Cholesterol(mg/dl)
Total LDL HDL Triglycerides |
194.4 ±35.3 117.2± 31.3 51.2 ±15.0 131.1± 85.1 |
191.2± 39.6 115.8 ±32.5 46.8 ±13.1 140.6 ±88.9 |
0.414 0.685 0.006 0.306 |
|
Cigarette Smoking (%)
Never Former Current |
2546(51.9) 1749(35.6) 612(12.5) |
23(27.7) 37(44.6) 23(27.7) |
<0.0001 |
| L V mass index (gm/m2) | 77.5 ± 15.6 | 103.1 ± 29.3 | <0.0001 |
| In(CAC +1) | 2.1 ± 2.5 | 3.1 ± 2.6 | 0.003 |
| Framingham Risk Score | 8.4 ± 8.0 | 13.3 ± 8.8 | <0.001 |
| Any Diuretic (%) | 602 (12.3) | 8(9.3) | 0.408 |
| Beta Blocker Use (%) | 421(8.6) | 5(5.8) | 0.364 |
| STATIN use (%) | 716(14.6) | 11(12.1) | 0.640 |
| ACE Inhibitor Use (%) | 531(10.8) | 14(16.3) | 0.110 |
| BP medication Use (%) | 1541(31.3) | 24(27.9) | 0.496 |
| LVEF (%) | 69.4 ±6.7 | 44.4 ± 6.0 | <0.0001 |
Caucasians with ALVSD were more likely to be males but were not likely to either have diabetes mellitus, cigarette smoker or have hypertension compared with those without ALVSD. African Americans with ALVSD were more likely to be males, smoke cigarette, have hypertension but were not likely to have diabetes mellitus compared with those without ALVSD. Hispanics with ALVSD were more likely to be males, smoke cigarette and have hypertension but were not likely to have diabetes mellitus compared with those without ALVSD. Characteristic of Chinese with ALVSD is not provided due to very small sample size.
Asymptomatic Left Ventricular Systolic Dysfunction and Outcomes
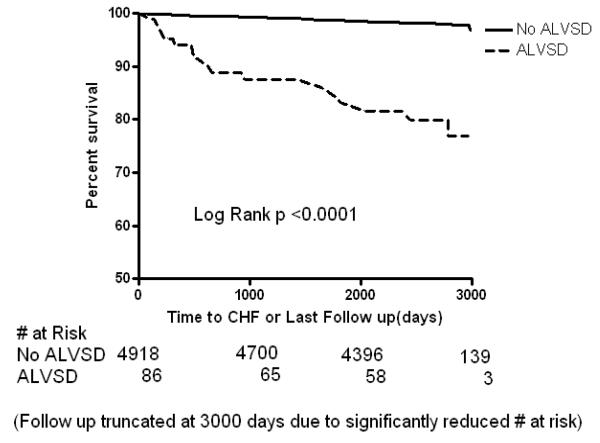
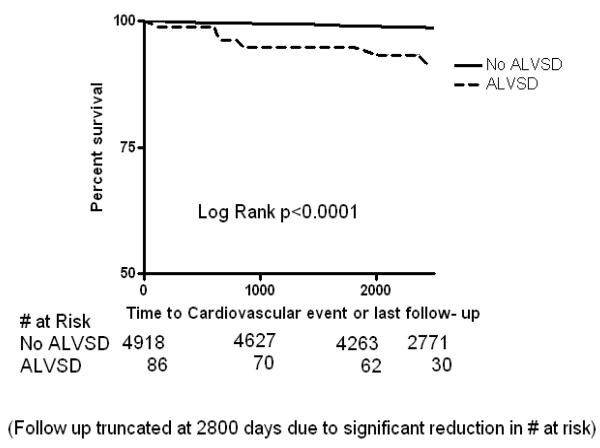
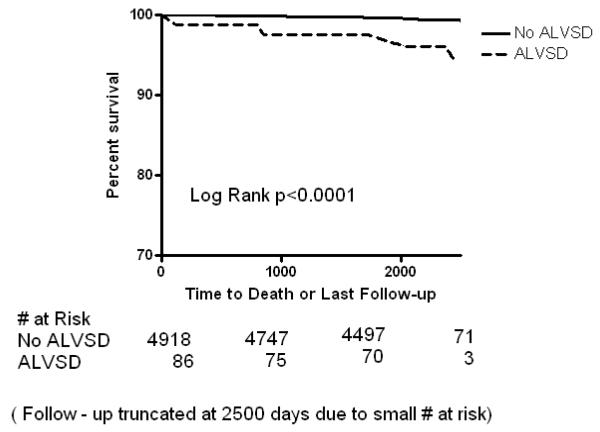
In Kaplan-Meier analysis, participants with ALVSD had higher incident CHF, CVD and all-cause mortality compared with participants without ALVSD (Figure 1A, 1B and 1C). Similarly, ALVSD was associated with higher incident CHF, CVD, and all-cause mortality in our unadjusted and adjusted models (Table 2). Similar estimates were obtained when a measure of socio economic status (level of education or income level) was forced into the model (data not shown).
Figure 1a.
Kaplan Meier curves showing the event free survival of participants with asymptomatic left ventricular systolic dysfunction (ALVSD) and those without ALVSD for incident congestive heart failure after a maximum of 9 years in MESA
Figure 1b.
Kaplan Meier curves showing the event free survival of participants with asymptomatic left ventricular systolic dysfunction (ALVSD) and those without ALVSD for incident cardiovascular events after a maximum of 9 years in MESA
Figure 1c.
Kaplan Meier curves showing the event free survival of participants with asymptomatic left ventricular systolic dysfunction (ALVSD) and those without ALVSD for all cause mortality after a maximum of 9 years in MESA
Table 2.
Association of Asymptomatic left ventricular systolic dysfunction and incident cardiovascular events, congestive heart failure and all-cause mortality. MESA
| Outcome | Unadjusted | Adjusted* | |||
|---|---|---|---|---|---|
| # of Events |
Hazard Ratio(95%CI) | P value | Hazard Ratio(95%CI) | P value | |
|
Congestive Heart
Failure |
112 | 11.97(7.04 -20.3) | <0.0001 | 8.69(4.89 -15.45) | <0.0001 |
| Cardiovascular Event | 321 | 3.32(1.98 – 5.58) | <0.0001 | 2.21(1.30 -3.73) | 0.003 |
| All Cause mortality | 278 | 3.47(2.03 -5.94) | <0.0001 | 2.14(1.21 – 3.77) | 0.009 |
Adjusted model adjusted for age, gender, race/ethnicity, diabetes mellitus, BMI, systolic blood pressure, total cholesterol, HDL, triglycerides, cigarette smoking status, blood pressure medication use (beta blocker use, ACE inhibitor use) and statin use.
For congestive heart failure and all cause mortality, interim myocardial infarction was adjusted for in the adjusted model.
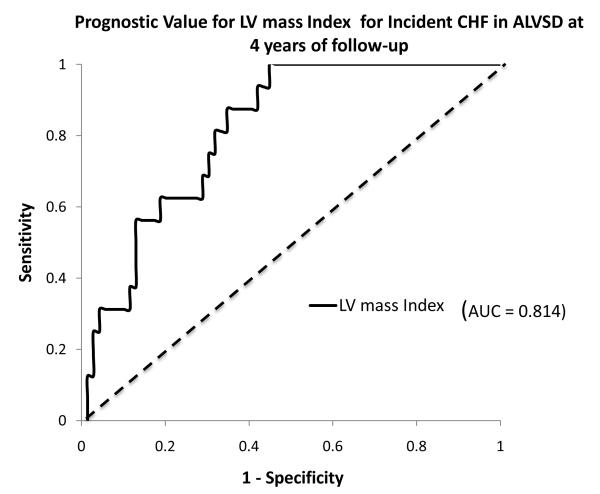
18.6 percent of participants with ALVSD developed incident CHF during the follow-up period. Left ventricular mass index had a good discriminative ability for incident CHF in participants with baseline ALVSD[c-index (95%CI); 0.74(0.56 - 0.85). Area under time dependent ROC curve at 4years of follow up is as shown in Figure 2.
Figure 2.
Receiver operator curve showing the discrimination afforded by left ventricular mass index (LVMI) for incident congestive heart failure in the subset with asymptomatic left ventricular systolic dysfunction at 4 years of follow-up in MESA.
Left Ventricular Ejection Fraction and Outcomes
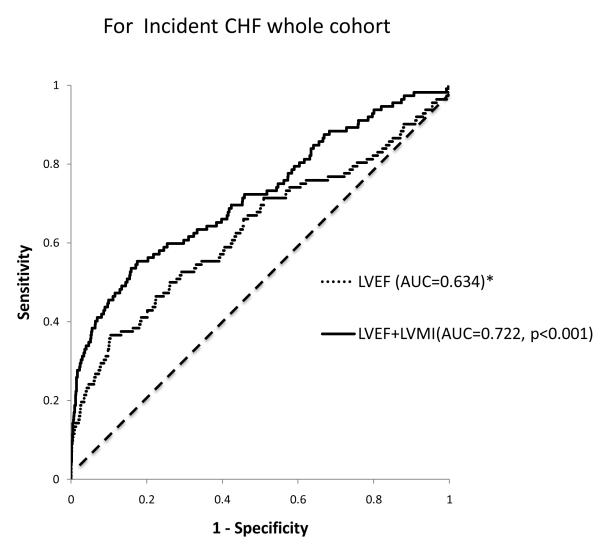
In the current cohort, a 10% decrement in left ventricular ejection fraction at baseline was associated with significant increase in incident CHF, CVD and all-cause mortality in unadjusted analysis. In the adjusted analysis, a 10 percent decrement in LVEF was associated with an increase in incident CHF and all –cause mortality but not CVD events (Table 3). Left ventricular ejection fraction was predictive of incident CHF. The c index (95% CI) of LVEF alone was 0.60(0.41-0.77). Adding LVMI to LVEF improved the discriminative ability for incident CHF [c-index (95%): 0.67(0.51-0.84)]. Comparison of the area under the time dependent ROC curve at 4 years of follow up for LVEF and LVEF + LVMI is as shown in Figure 3.
Table 3.
Association of a 10 percent decrement in left ventricular ejection fraction at baseline and incident cardiovascular events, congestive heart failure and all-cause mortality. MESA
| Outcome | Unadjusted | Adjusted* | |||
|---|---|---|---|---|---|
| # of Events |
Hazard Ratio(95%CI) | P value | Hazard Ratio(95%CI) | P value | |
|
Congestive Heart
Failure |
112 | 2.17(1.82-2.63) | <0.0001 | 2.13(1.73-2.51) | <0.0001 |
| Cardiovascular Event | 321 | 1.22(1.05 -1.41) | 0.007 | 1.12(0.97-1.30) | 0.104 |
| All Cause mortality | 278 | 1.22(1.05 -1.41) | 0.009 | 1.18(1.01 -1.37) | 0.038 |
Adjusted model adjusted for age, gender, race/ethnicity, diabetes mellitus, BMI, systolic blood pressure, total cholesterol, HDL, triglycerides, cigarette smoking status, blood pressure medication use (beta blocker use, ACE inhibitor use) and statin use.
For congestive heart failure and all cause mortality, interim myocardial infarction was adjusted for in the adjusted model.
Figure 3.
Receiver operator curves showing the improvement in discrimination afforded by the addition of left ventricular mass index ( LVMI) to Left ventricular ejection fraction (LVEF) for incident congestive heart failure at 4years follow up in MESA.
Discussion
The current study used the MESA cohort, composed of adults free of clinical cardiovascular disease (including myocardial infarction), to show that ALVSD is uncommon (prevalence of 1.7%) but nonetheless associated with significant morbidity (CHF and cardiovascular events) and mortality. LVMI improved the accuracy of LVEF for identifying individuals at risk for future incident CHF in the whole cohort and also had a good discrimination among individuals with ALVSD for incident CHF during the follow-up period.
The prevalence of ALVSD has been estimated to occur in 0.9 – 12.9% of the population14. The wide range is due to differences in study design, setting, characteristics of the study sample, and LVEF threshold used to define ALVSD. The LVEF threshold used in previous studies ranged from 30-54 %14. McDonagh et al defined ALVSD as LVEF < 30% in a cross-sectional survey of a selected urban cohort; in that study, the prevalence of ALVSD was 1.5% 13. Older males were more likely to have ALVSD, and 83% had evidence of ischemic heart disease as the underlying etiology. In another large practice-based multi-ethnic cohort, using an LVEF threshold of 40%, prevalence of ALVSD was 0.89%12. In a subset of the Strong Heart Study (some of whom had prevalent CHD and clinical CHF), Devereux et al reported that the prevalence of left ventricular dysfunction in Indian Americans, defined as LVEF <54%, was 14% (10). Gottdiener et al, in an elderly cohort (mostly Caucasian) reported a prevalence of ALVSD of about 0.8%22. In the Framingham Heart Study, Wang et al showed that the prevalence of ALVSD (defined as LVEF <50%) in participants with and without prior MI was 3%14. These studies are limited by differences in the LVEF threshold, small sample sizes, lack of racial diversity, and the use of echocardiography in estimating LVEF. In addition, almost all included participants with and without prior MI. The present study used a large multi-ethnic cohort and a more accurate measure of LVEF, cardiac MRI. We found that within population based adults free of clinical cardiovascular disease, the prevalence of ALVSD is 1.7% and is mostly in men and African Americans.
There is no consensus on how to best identify individuals with ALVSD in the community, and the best most cost-effective way to do so14. The use of natruiretic peptides to screen for ALVSD in communities has yielded mixed results23-25. Despite the relatively rare prevalence of ALVSD, its presence identified a group of participants at high risk for incident CHF, CVD, and all-cause mortality. Our data also suggest that individuals with ALVSD may not be more likely to have incident myocardial infarction, compared to those without ALVSD. Thus, these individuals may evade detection until late in their disease course, increasing the cost of therapy and worsening their prognosis.
African Americans with left ventricular dysfunction appear to be at higher risk for progression of heart failure and death from any cause than similarly treated whites26. In the present cohort, African American males had the highest mean blood pressure and LVMI, and were more likely to have ALVSD and develop clinical CHF compared with other MESA participants. This suggests that community-wide screening for ALVSD and preemptive interventions in hypertensive African American men could be explored as an appropriate and cost-effective public health strategy to reduce heart failure burden in our communities. Current debate should focus on how best to screen for ALVSD in individuals without clinical CVD, since our data suggest these individuals are at higher risk for CHF, CVD, and all-cause mortality. More research is needed to determine if preemptive interventions might reduce the risk for CHF, CVD, and death in this high risk subgroup.
Unlike echocardiography, cardiac MRI more accurately measures left ventricular volumes, from which LVEF is derived16. In addition, other variables, such as LVMI, can be measured during the cardiac MRI scan. The present study shows that adding LVMI as part of a cardiac risk assessment would significantly improve the prognostic accuracy of LVEF in predicting incident CHF. Furthermore, in individuals with ALVSD, LVMI would discriminate accurately among those most likely to progress to clinical CHF. Studies in other cohorts are needed to replicate and extend our findings.
The strengths of this study include the large sample size, long duration of follow-up, adjudicated outcomes, use of cardiac MRI, the multi-ethnic nature of the cohort and the fact that unlike other studies, all participants were free of clinical cardiovascular disease at baseline. However, given the relatively small prevalence of ALVSD, we did not explore stratified analysis due to limited statistical power. In addition, MESA is an observational study; although we adjusted for most covariates in our adjusted models, our results may still have been influenced by residual confounding. The cardiac MRI results including left ventricular ejection fraction was made available to participants and their clinicians (if participants consented). MESA does not include other ethnic groups such as American Indians and other Asian groups except Chinese. In addition the proportion of each ethnic group in MESA does not accurately reflect that of the US population. This limits the generalizability of our findings. Presently other cardiac MRI data including left atrial size and structure etc, likely to influence the development of congestive heart failure, cardiovascular events and mortality are not available in MESA. Inclusion of such data and a more comprehensive analysis of the MESA cardiac MRI data may further inform risk prediction of these outcomes in this population. Lastly, because the present study involved individuals without clinical cardiovascular disease at baseline, our results may not be applicable to other populations.
Conclusions
In a population-based multi-ethnic adult population free of clinical cardiovascular disease, the prevalence of ALVSD was relatively low but carries significant risk for incident CHF, CVD, and all-cause mortality. LVMI may be a good screening tool for identifying individuals with ALVSD who would develop CHF. LVMI may also improve the discriminative ability of LVEF for identifying adults free of clinical CVD who are at risk for CHF.
Commentary.
Individuals with congestive heart failure still endure high morbidity and mortality despite recent advances in management. Studies have shown that early identification and treatment of individuals with the asymptomatic phase of heart failure could slow the progression to the symptomatic phase and reduce subsequent morbidity and mortality. However data on the prevalence and prognosis of individuals with asymptomatic left ventricular systolic dysfunction (ALVSD) are limited. The few available data included ALVSD individuals with known myocardial infarction, a subgroup who most likely have had contact with healthcare professionals and have been prescribed the needed therapy. The public health impact of screening for this subgroup of ALVSD is likely to be of limited value. The present study used a large multi ethnic cohort, free of clinical cardiovascular disease including myocardial infarction to show that ALVSD is uncommon (prevalence of 1.7%). This low prevalence however translates into several hundred thousand, if not millions of community dwelling Americans. The highest prevalence was in African Americans (2.6%) and the least in Chinese Americans (0.15%). African American males with hypertension had the highest prevalence of ALVSD. The risk of developing congestive heart failure was about nine times higher in individuals with ALVSD compared with those without ALVSD after nine years of follow up. Individuals with ALVSD were about twice as likely to die or develop a cardiovascular event compared with those without ALVSD during the follow up period. Current debate and more research should focus on how best to screen for ALVSD in individuals without clinical cardiovascular disease, since our data suggest these individuals are at higher risk for congestive heart failure, cardiovascular disease events, and all-cause mortality
Acknowledgements
The authors would like to thank the investigators, the staff, and the participants of the MESA study for their valuable contributions. We also want to thank Karen P. Klein MS for editing this paper.
Funding Sources: This research was supported by contracts N01-HC-95159 through N01-HC-95167 and a Diversity Supplement to R01HL098445 (PI: J. Jeffrey Carr). A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Conflict of Interest Disclosures: None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL. Mechanism and models in heart failure: a combinatorial approach. Circulation. 1999;100:999–1008. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81:1161–172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JV, McDonagh TA, Davie AP, Cleland JG, Francis CM, Morrison C. Should we screen for asymptomatic left ventricular dysfunction to prevent heart failure. Eur Heart J. 1998;19:842–846. doi: 10.1093/eurheartj/19.6.842. [DOI] [PubMed] [Google Scholar]
- 5.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russell RO, Smith SC, Jr, American College of Cardiology/American Heart Association ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology /American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2001;38:2011–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 6.The SOLVD investigators Effects of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the survival and ventricular enlargement trial. The SAVE investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 8.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomized trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 10.Devereux RB, Roman MJ, Paranicas M, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Rodeheffer RJ, Cowan LD, Howard BV. A population-based assessment of left ventricular systolic dysfunction in middle-aged and older adults: the Strong Heart Study. Am Heart J. 2001;141:439–46. doi: 10.1067/mhj.2001.113223. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Bella JN, Palmieri V, Oberman A, Kitzman DW, Hopkins PN, Rao DC, Morgan D, Paranicas M, Fishman D, Arnett DK, Hypertension Genetic Epidemiology Network Study Group Left ventricular systolic dysfunction in a biracial sample of hypertensive adults: The Hypertension Genetic Epidemiology Network (HyperGEN) Study. Hypertension. 2001;38:417–23. doi: 10.1161/01.hyp.38.3.417. [DOI] [PubMed] [Google Scholar]
- 12.Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, Wosornu D, Lancashire RJ. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet. 2001;358:439–44. doi: 10.1016/s0140-6736(01)05620-3. [DOI] [PubMed] [Google Scholar]
- 13.McDonagh TA, Morrison CE, Lawrence A, Ford I, Tunstall-Pedoe H, McMurray JJ, Dargie HJ. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet. 1997;350:829–33. doi: 10.1016/S0140-6736(97)03033-X. [DOI] [PubMed] [Google Scholar]
- 14.Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: Implications for screening. Ann Intern Med. 2003;138:907–916. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]
- 15.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 16.Keenan NG, Pennell DJ. CMR of ventricular function. Echocardiography. 2007;24:185–93. doi: 10.1111/j.1540-8175.2007.00375.x. [DOI] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. Am J Roentgenol. 2006;186:S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 21.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. 200. [DOI] [PubMed] [Google Scholar]
- 22.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcomes of congestive heart failure in elderly persons: influences of left ventricular systolic function: The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 23.Ng LL, Loke IW, Davies JE, Geeranavar S, Khunti K, Stone MA, Chin DT, Squire IB. Community screening for left ventricular systolic dysfunction using plasma and urinary natruiretic peptides. J Am Coll Cardiol. 2005;45:1043–50. doi: 10.1016/j.jacc.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 24.Vasan RS, Benjamin EJ, Larson MG, Leip EP, Wang TJ, Wilson PW, Levy D. Plasma natruiretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: The Framingham Heart Study. JAMA. 2002;288:1252–9. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 25.De Lemos JA, McGuire DK, Khera A, Das SR, Murphy SA, Omland T, Drazner MA. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natruiretic peptides. Results from the Dallas Heart Study. Am Heart J. 2009;157:746–53. doi: 10.1016/j.ahj.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med. 1999;340:609–616. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]