Abstract
Purpose
Previously we reported that a program of resistance + impact training stopped bone loss at the spine in older, postmenopausal BCS, but had no effect on bone mineral density (BMD) at the hip. Aging may blunt the responsiveness of the hip to mechanical loading so we conducted a secondary data analysis to evaluate age moderated the effect of exercise on hip BMD.
Methods
We analyzed data from our randomized, controlled trial in older (≥ 50 years of age at diagnosis), postmenopausal, post-adjuvant treatment BCS (n=106) comparing women assigned to impact + resistance exercise (POWIR) or to a control program of low-intensity stretching (FLEX). We examined effect modification by age on BMD at three hip sites (greater trochanter, femoral neck, and total hip) using hierarchical linear modeling adjusting for time since diagnosis and use of adjuvant hormone therapy.
Results
Age moderated the effect of exercise on total hip BMD such that younger women in POWIR were more likely to see a positive net benefit than FLEX compared to older women where there was little difference between groups (p=0.02).
Conclusions
The skeletal response to loading at the hip within postmenopausal BCS diminishes with age. Whether more vigorous exercise programs and/or longer training periods are required to favorably change hip health in older BCS will require future study and careful thought about the risks and benefits of tougher training programs.
Keywords: osteoporosis, resistance exercise, neoplasms, aging, physical activity
INTRODUCTION
Cancer treatment-induced bone loss contributes to increased fracture risk in breast cancer survivors (BCS). Chemotherapy, premature ovarian failure and aromatase inhibitor (AI) therapy each cause bone loss at the hip and spine and the combined and cumulative effects increase skeletal fragility and fracture risk [1,2]. In the Women’s Health Initiative trial, hip fracture rates were significantly increased after a breast cancer diagnosis in postmenopausal women (HR = 1.55, CI = 1.13–2.11)[2]. Using a population-based cohort, Neuner et al tracked fractures among older BCS (n=2748; mean age: 72.8 years) for 3 years after breast cancer surgery [3]. Hip fractures rates were higher than those for the general older adult population and compared to tamoxifen users were significantly greater among BCS on AIs (HR: 3.24; 95% CI: 1.05, 9.98) and BCS receiving no adjuvant endocrine therapy (HR: 3.32; 95% CI: 1.14, 9.65). While bisphosphonates are increasingly prescribed to prevent bone loss in BCS and to reduce the rate of skeletal metastases, their efficacy at reducing hip fracture risk in BCS remains unclear [4] and patient concerns about adverse drug effects may threaten compliance. Complementary approaches, such as exercise, that could reduce hip bone loss and fall risk should be considered for BCS.
Exercise has been investigated as a means to reduce cancer-treatment-induced bone loss at the spine and hip in BCS [5,6]; however, only one group has reported a positive effect of exercise on hip BMD. In that study, an aerobic + impact exercise program stopped bone loss at the femoral neck in premenopausal, but not postmenopausal BCS [6]. The relative effectiveness of exercise to stop bone loss that is unrelated to cancer treatment depends mostly upon the type and dose of loading, but can also be influenced by estrogen availability, nutritional status (i.e., calcium and vitamin D sufficiency) and age [7]. There appears to be a blunting of the exercise response to mechanical loading with age, where skeletal adaptations are greatest during growth and progressively diminish toward advanced age [8]. We recently reported that a one-year program of impact + resistance exercise (e.g., POWIR) stopped spine, but not hip bone loss in older (50+ yrs), postmenopausal BCS [9]. Since the age range of BCS in our recent trial was broad, we felt it prudent to conduct a secondary data analysis to determine whether age moderates the effectiveness of POWIR on hip BMD in postmenopausal BCS. We hypothesized that younger women may demonstrate a more favorable response to POWIR at the hip compared to older women within our postmenopausal cohort.
METHODS
The design, sample and measures for this study have been previously described in the original report [9]. We will briefly summarize important elements. We conducted a 12-month single-blind randomized controlled trial comparing two groups: 1) moderate-intensity impact + resistance training (POWIR: Prevent Osteoporosis With Impact + Resistance) and 2) exercise placebo (FLEX) measured over three timepoints (pre, mid-, and post-intervention). Testing and exercise training took place at Oregon Health & Science University (OHSU). The OHSU Institutional Review Board approved the study and procedures.
Participants
Eligibility criteria for the study were as follows: diagnosis of stage 0–3a breast cancer at age 50+ years old, postmenopausal at breast cancer diagnosis based on absence of menstrual cycles for >12 months or oophorectomy, ≥ 1 year post chemotherapy or radiation therapy, non-osteoporotic, no medication to stop bone loss, physician clearance to exercise, no regular participation in resistance and/or impact exercise and, ability to complete study testing. Participant flow across the trial has been described previously [9]. In short, 106 women enrolled in the trial and were randomized to POWIR (n=52) or FLEX (n=54).
Study Programs
Participants in both groups were prescribed an exercise program consisting of two, 1-hr supervised classes and one 45-minute home-based session per week for 12 months. POWIR is an impact (jump) + free-weight resistance training program designed to specifically load the hip and spine because selected resistance exercises utilize musculature with attachments directly on these skeletal sites and because ground reaction forces generated by jump landings create a direct osteogenic stimulus to the hip. Participants in the FLEX performed a series of whole body stretching and relaxation exercises in a seated or lying position to minimize weight-bearing forces on the skeleton.
Procedures
At baseline, written informed consent was obtained followed by completion of questionnaires and DXA testing. Randomization followed baseline testing to protect against bias.
Bone mineral density (g/cm2) of the proximal femur (total hip, greater trochanter, femoral neck) and anterior-posterior lumbar spine (L1–L4) were assessed by dual energy x-ray absorptiometry (DXA; Hologic QDR Discovery Wi; software version 12.0). DXA scans were performed by licensed technicians blinded to participant group and were analyzed by the same technician. Coefficients of variation for DXA measures in our laboratory are 1%–1.5%.
Demographic data, health history, and health behaviors were obtained by self-report using both investigator developed and standardized questionnaires [9]. Medication use was updated at 6 and 12-month visits. Chronic medical conditions were assessed by the Charlson Comorbidity Index. To account for changes in physical activity and diet outside of the intervention that could affect BMD outcomes, we measured habitual physical activity with the Community Health Activity Model Program for Seniors (CHAMPS) physical activity questionnaire validated for older adults (kcal/day in all activities) [10] and habitual calcium (dietary + supplemental) and total energy intake with the 2005 Block Food Frequency Questionnaire at each visit.
Statistical Analysis
The intent-to-treat (ITT) approach was used for data analysis and performed using Hierarchical Linear Modeling (HLM; HLM 6.08 software) analyzing each participant according to her originally assigned group and regardless of missing follow-up data. To examine the influence of age on the skeletal response to POWIR, we added an interaction term representing the product of treatment and age. Significant interactions were graphed and interpreted to determine the nature of the effect modification. Analyses were adjusted for time since diagnosis and use of adjuvant hormone therapy. To aid interpretation of moderator effects we created age groups by tertiles of age and compared demographic and clinical characteristics of the subgroups using one-way ANOVA with Bonferroni post-hoc testing for continuous data and chi-squares tests for categorical data.
RESULTS
Tertiles of age separated our group of older women into 3 age ranges of youngest-old (53–58 years old), middle-old (59–64 years old), and oldest-old (65–83 years old (Table 1). The oldest-old tertile was significantly further from breast cancer diagnosis than the youngest-old tertile (p < 0.01), but other clinical characteristics (e.g., stage, treatment types) were not significantly different. Baseline BMD at the femoral neck was significantly different across age tertiles with post-hoc tests revealing significantly lower BMD among the oldest-old versus youngest-old tertile (p<0.03). BMD at the other two hip sites did not significantly differ by age tertile. Physical activity levels, energy intake and calcium consumption were similar across age tertiles. Sixty-seven women completed final testing. Adherence to supervised classes (% of classes attended) for participants who completed the study did not differ across age tertiles.
Table 1.
Baseline demographic, clinical and behavioral characteristics and bone mineral density of participants by tertile of age. Data presented as unadjusted mean (SD) for continuous data and % of sample for categorical data.
| Youngest-old (n=37) | Middle-old (n=35) | Oldest-old (n=34) | ||
|---|---|---|---|---|
| Characteristic | Mean (SD) or % sample |
Mean (SD) or % sample |
Mean (SD) or % sample |
p- value* |
| Age | 55.8 (1.7) | 61.2 (1.9) | 70.4 (4.8) | <0.01 |
| Comorbidity Index | 1.9 (1.9) | 1.8 (1.9) | 1.6 (1.2) | 0.79 |
| BMI (kg/m2) | 29.0 (6.0) | 31.2 (5.9) | 28.3 (4.8) | 0.08 |
| Time since diagnosis (months) | 47.4 (21.4)† | 58.2 (33.1) | 77.4 (49.0) | <0.01 |
| Stage 0 (%) | 3% | 10% | 5% | 0.64 |
| Stage I (%) | 45% | 39% | 45% | |
| Stage II (%) | 45% | 45% | 45% | |
| Stage IIIa (%) | 7% | 6% | 5% | |
| Received chemotherapy (%) | 71% | 62% | 51% | 0.23 |
| Received radiation therapy (%) | 77% | 88% | 95% | 0.08 |
| Currently taking AI (%) | 26% | 50% | 48% | 0.07 |
| Currently taking SERM (%) | 29% | 6% | 12% | |
| Energy expenditure (kcal/d)a | 254 (197) | 240 (244) | 194 (256) | 0.53 |
| Energy intake (kcal/d) | 1468 (456) | 1401 (565) | 1389 (504) | 0.78 |
| Calcium intake (mg/day)b | 741 (286) | 664 (282) | 764 (361) | 0.38 |
| Exercise adherencec | 70% | 74% | 76% | 0.72 |
| Total hip BMD (g/cm2) | 0.891 (0.101) | 0.868 (0.090) | 0.843 (0.092) | 0.11 |
| Greater trochanter BMD (g/cm2) | 0.672 (0.085) | 0.673 (0.080) | 0.634 (0.087) | 0.10 |
| Femoral neck BMD (g/cm2) | 0.768 (0.101)† | 0.737 (0.092) | 0.710 (0.077) | 0.03 |
Energy expenditure calculated from CHAMPS physical activity survey and includes energy expended in moderate-vigorous intensity activities
Calcium intake includes calcium obtained both from dietary sources and from dietary supplements.
Adherence calculated as % of supervised classes attended across one-year study on those participants completing baseline and final time points (n=67)
p-value comparing age groups using one-way ANOVA for continuous variables or chi-squares test for categorical variables. For outcomes of cancer stage and adjuvant hormone therapy use comparisons tested for differences in the distribution of each variable among age groups.
youngest-old tertile significantly different from oldest-old tertile, p<0.05
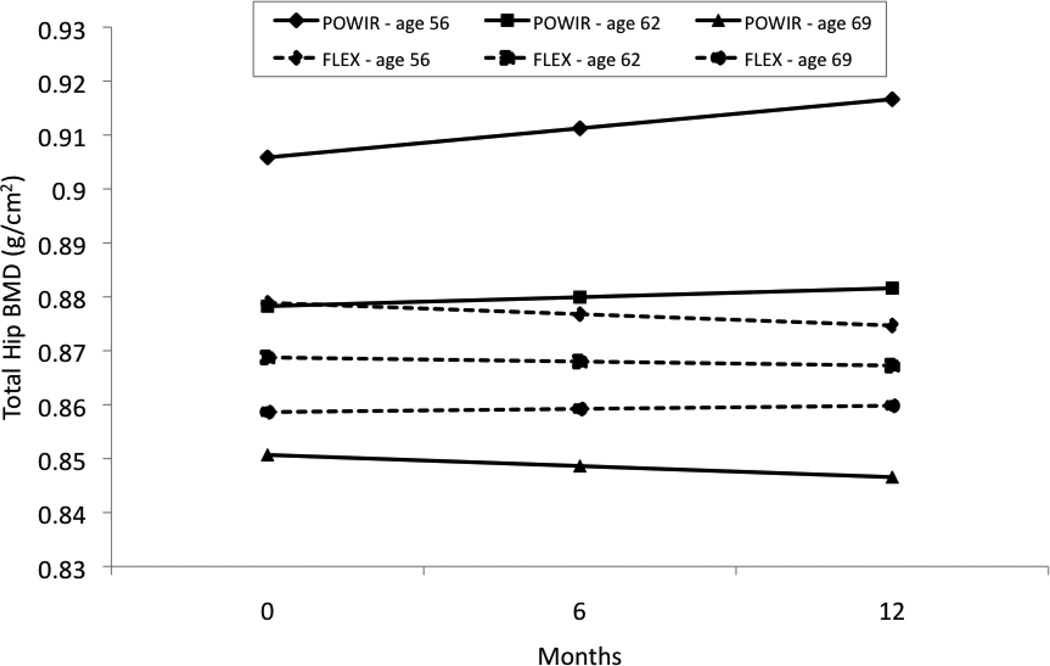
There was a significant moderating effect of age on group × time differences for total hip BMD (Coefficient on slope of time = −0.0008, SE=0.0003, t(98)=−2.36, p=0.02). Age did not significantly affect group × time differences at other sites within the proximal femur. However, there was a trend toward significance at the femoral neck, with a pattern similar to that observed for the total hip site (Coefficient on slope of time = −0.0007, SE=0.0004, t(98)= −1.815, p=0.07). Figure 1 shows that total hip BMD increases at a younger age within POWIR, but that POWIR becomes less effective at stopping bone loss as age increases. Relatively little change in total hip BMD occurs across time within FLEX, regardless of age.
Figure 1.
Predicted values for total hip bone mineral density (BMD; g/cm2) at each study timepoint for three ages of women representing the mean age of the study sample (62 years old) and 1 standard deviation below (56 years old) and 1 standard deviation above (69 years old) the mean. Predicted BMD values were calculated using the regression equation derived from hierarchical linear modeling analysis using observed data.
DISCUSSION
The original analysis of our data failed to show that POWIR, which was effective at preventing bone loss at the spine, could also improve hip outcomes [9]. However, this secondary analysis revealed that age moderates the effectiveness of POWIR on hip BMD. POWIR participants in the younger end of the age range of our sample demonstrated an increase in hip BMD compared to losses in controls; however, as age increased the effectiveness of POWIR diminished. Our finding of age-dependent differences in the degree of skeletal adaptations to exercise at the hip is consistent with observations in women with and without cancer. Saarto reported that an impact + aerobic exercise program stopped hip bone loss in premenopausal BCS (ages 36–57 years old) but had little effect in postmenopausal patients (ages 48–68) [6]. Similarly, Bassey reported that hip BMD increased in premenopausal, but not postmenopausal women without cancer who participated in the same one-year jumping program [11]. In our prior work in women without cancer who trained in programs similar to POWIR, hip BMD increased in premenopausal women [12], but not in elderly postmenopausal women [13] after 9–12 months of training. However, a follow-up study in the elderly women who continued to exercise after the original intervention concluded showed that bone loss at the hip was eventually stopped after many years of exercise training [14]. Animal studies that subject young and old animals to the same carefully applied degree of mechanical loading can isolate the effects of age on skeletal responsiveness. Two such studies reported that young animals could mount an osteogenic response to a given dose of mechanical loading, but that older animals failed to improve measures of bone mineralization or strength from the same loads [15,16].
Age-related changes in systemic factors that influence bone (i.e., hormones, oxidative stress, growth factors) could modify the skeletal response to exercise. The estrogen environment can modulate the bone response to loading [8] and may partially explain why postmenopausal women did not adapt to the same exercise loads that stopped or improved femoral BMD in premenopausal BCS [6] or premenopausal women without cancer [11], respectively. However, over half of BCS in Saarto’s premenopausal cohort became postmenopausal during the intervention and still showed a better bone response at the hip compared to BCS who were postmenopausal at study start. All of the BCS in our study were postmenopausal at study enrollment, eliminating estrogen as a reason why age groups differed on hip outcomes. Increases in oxidative stress and declines in growth factors that contribute to age-related bone loss might also influence skeletal adaptability and are worth further consideration [8]. It has been advanced that cancer treatment accelerates the aging process [17], which could thereby accelerate the same age-related changes that blunt skeletal responsiveness.
While physiologic aging could underlie the diminished skeletal response to loading, it is also reasonable to consider that older women are not training at the same level of effort as younger women. Saarto reported less improvement in performance outcomes to their impact + aerobic intervention among postmenopausal versus premenopausal BCS which may reflect a lower dose to the skeleton among the older women [6]. However, Bassey’s pre- and postmenopausal groups exercised at a similar effort [11]. In our sample, adherence was similar across age groups (Table 1) and a separate analysis found that muscular strength adaptations to the intervention were independent of age [18]. Assessing the intensity of jumps, however, is difficult and depends on both the vest weight and jump height. While vest weight was similar across women because it was prescribed as % body weight, jump height was not as easily dictated nor monitored. Women were encouraged to jump to a height of 1” off the floor, but in practice women could have jumped more or less than that and it may be that older women did not jump as high as younger women. Our data suggest that skeletal adaptations to a given degree of loading and across a given timeframe lessen with age, but this does not necessarily indicate a reduction in the fundamental capacity of the aging skeleton to respond to mechanical loading. In our past work in older cancer-free women, the effect of exercise on hip BMD was not detected in the first year of training, but did emerge at 5-year follow-up [14]. In Turner’s animal study, older animals demonstrated the same degree of improvement in bone outcomes, once applied loads were increased to 70% above the load magnitude that was effective for younger animals [15]. These data suggest that the older skeleton may have a blunted response to exercise training, but still retains the capacity to adapt. Whether a longer time period and/or a greater load are necessary to improve hip outcomes for older BCS requires further, systematic study. Extending the timeframe over which to detect skeletal adaptations may be a safer approach than increasing exercise intensity, but would require additional strategies to ensure long-term adherence.
This secondary data analysis has strengths and limitations. By evaluating age as an effect modifier we were able to test for the influence of age on the skeletal response to the same prescribed dose of exercise. We also examined other indicators of actual dose received such as adherence and the degree of strength improvements. Similarly, we considered factors that also change with age and might have moderated exercise responses, such as different clinical characteristics (e.g., time since diagnosis and adjuvant hormone therapy) or health behaviors (e.g., physical activity and calcium intake). However, we did not design the study to specifically investigate age-related differences in bone adaptations across a very broad age range of BCS nor to compare to similar age groups of women without cancer. Since the resistance of a bone to fracture is a function of both its mass and architecture, exercise-induced improvements in structural indices of bone strength may also confer some protection against fracture. We did not evaluate changes in bone geometry from POWIR, either through hip structural analysis of DXA data or through other types of imaging (e.g, computed tomography). Others have reported improvements in hip bone geometry from resistance or impact training in older women without cancer [19], thus it is possible that POWIR could have this additional benefit. Future trials in cancer populations would gain a better picture about favorable skeletal adaptations to exercise training if both BMD and geometry were evaluated.
POWIR appears to improve hip bone health in postmenopausal BCS at the younger end of the age spectrum. Given the limitations of this secondary data analysis, adequately powered controlled trials are needed in order to confirm our findings. The most appropriate and targeted exercise programs to reduce risk factors for hip fracture among BCS in the older age ranges is still unclear. Since falls are an equally important risk factor for fracture as skeletal fragility, fracture risk reduction exercise programs should both strengthen bone and lower fall risk. Resistance training reduces risk factors for falls by increasing muscle strength and improving stability [20]. POWIR improved lower extremity muscle strength in older BCS regardless of age [18], lowering a top risk factor for falls. Whether POWIR can also prevent bone loss at the hip across a broad age range of older BCS may require a longer period of evaluation and/or program modification.
Acknowledgements
Supported by Susan G. Komen Race for the Cure and the National Cancer Institute (1R01 CA120123, to Dr. Winters-Stone) and with partial support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. We thank the Oregon State Cancer Registry for their assistance with recruitment efforts for the study. Thera-band provided elastic bands for home exercise programs. We thank Ms. Jessica Dobek for data management and manuscript preparation, Ms. Ann Reiner for helping to manage the study and Mr. Nathan Brooks, Ms. Camella Potter, and Mr. Anton Stupnitskiy for their assistance with data collection. We also thank Ms. Janice Hoffman, Ms. Laurie Iverson, and Ms. Lisa Domenico for their assistance with exercise training.
REFERENCES
- 1.Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, Pritchard KI. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008;26(33):5465–5476. doi: 10.1200/JCO.2008.18.4184. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z MM, Aragaki AK, Mouton C, Arendell L, Lopez AM, Bassford T, Chlebowski RT. Fracture risk increases after diagnosis of breast or other cancers in postmenopausal women: results from the Women's Health Initiative. Osteoporos Int. 2009;20(4):527–536. doi: 10.1007/s00198-008-0721-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuner J, Yen T, Sparapani R, Laud P, Nattinger A. Fracture risk and adjuvant hormonal therapy among a population-based cohort of older female breast cancer patients. Osteoporosis International. 2011;22(11):2847–2855. doi: 10.1007/s00198-010-1493-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valachis A, Polyzos NP, Georgomicronulias V, Mavroudis D, Mauri D. Lack of evidence for fracture prevention in early breast cancer bisphosphonate trials: A metaanalysis. Gynecol Oncol. 2010;117(1):139–145. doi: 10.1016/j.ygyno.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Winters-Stone KM, Schwartz A, Nail LM. A review of exercise interventions to improve bone health in adult cancer survivors. J Cancer Surviv. 2010;4(3):187–201. doi: 10.1007/s11764-010-0122-1. [DOI] [PubMed] [Google Scholar]
- 6.Saarto T, Sievanen H, Kellokumpu-Lehtinen P, Nikander R, Vehmanen L, Huovinen R, Kautiainen H, Jarvenpaa S, Penttinen HM, Utriainen M, Jaaskelainen AS, Elme A, Ruohola J, Palva T, Vertio H, Rautalahti M, Fogelholm M, Luoto R, Blomqvist C. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int. 2011 doi: 10.1007/s00198-011-1761-4. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 7.Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;(7):CD000333. doi: 10.1002/14651858.CD000333.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohrt WM. Aging and the osteogenic response to mechanical loading. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S137–S142. doi: 10.1123/ijsnem.11.s1.s137. [DOI] [PubMed] [Google Scholar]
- 9.Winters-Stone K, Dobek J, Nail L, Bennett JA, Naik A, Schwartz A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2011;27(2):447–456. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Bassey E, Rothwell M, Littlewood J, Pye D. Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J Bone Miner Res. 1998;13(12):1805–1813. doi: 10.1359/jbmr.1998.13.12.1805. [DOI] [PubMed] [Google Scholar]
- 12.Winters KM, Snow CM. Detraining reverses positive effects of exercise on the musculoskeletal system in premenopausal women. J Bone Miner Res. 2000;15:2495–2503. doi: 10.1359/jbmr.2000.15.12.2495. [DOI] [PubMed] [Google Scholar]
- 13.Shaw JM, Snow CM. Weighted vest exercise improves indices of fall risk in older women. J Gerontol. 1998;53:M53–M58. doi: 10.1093/gerona/53a.1.m53. [DOI] [PubMed] [Google Scholar]
- 14.Snow CM, Shaw JM, Winters KM, Witzke KA. Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2000;55(9):M489–M491. doi: 10.1093/gerona/55.9.m489. [DOI] [PubMed] [Google Scholar]
- 15.Turner CH, Takano Y, Owan I. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Min Res. 1995;10(10):1544–1549. doi: 10.1002/jbmr.5650101016. [DOI] [PubMed] [Google Scholar]
- 16.Rubin CT, Bain SD, McLeod KJ. Suppression of the osteogenic response in the aging skeleton. Calcified tissue international. 1992;50(4):306–313. doi: 10.1007/BF00301627. [DOI] [PubMed] [Google Scholar]
- 17.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypoth. 2006;67(2):212–215. doi: 10.1016/j.mehy.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. J Cancer Surviv. 2011 doi: 10.1007/s11764-011-0210-x. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton C, Swan V, Jamal S. The effects of exercise and physical activity participation on bone mass and geometry in postmenopausal women: a systematic review of pQCT studies. Osteoporos Int. 2010;21(1):11–23. doi: 10.1007/s00198-009-0967-1. [DOI] [PubMed] [Google Scholar]
- 20.Korpelainen R, Keinanen-Kiukaanniemi S, Nieminen P, Heikkinen J, Vaananen K, Korpelainen J. Long-term Outcomes of Exercise: Follow-up of a Randomized Trial in Older Women With Osteopenia. Arch Intern Med. 2010;170(17):1548–1556. doi: 10.1001/archinternmed.2010.311. [DOI] [PubMed] [Google Scholar]