Abstract
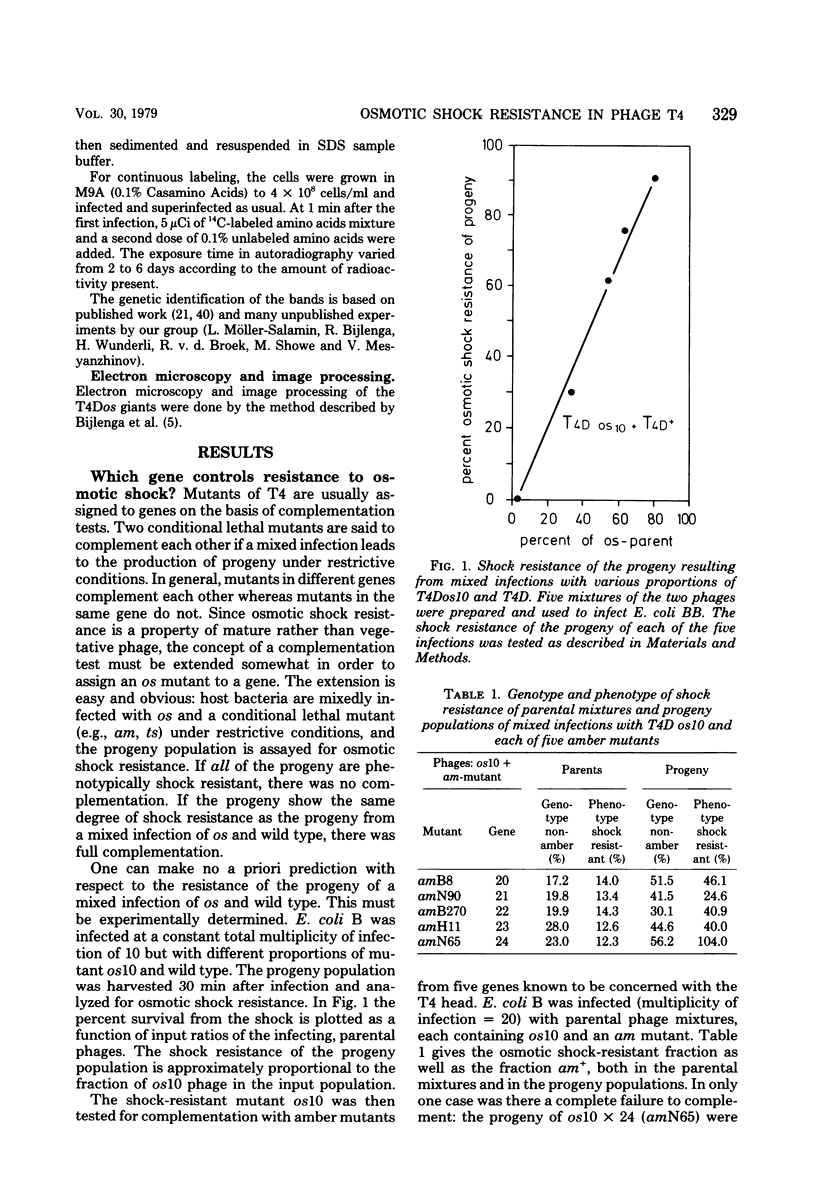
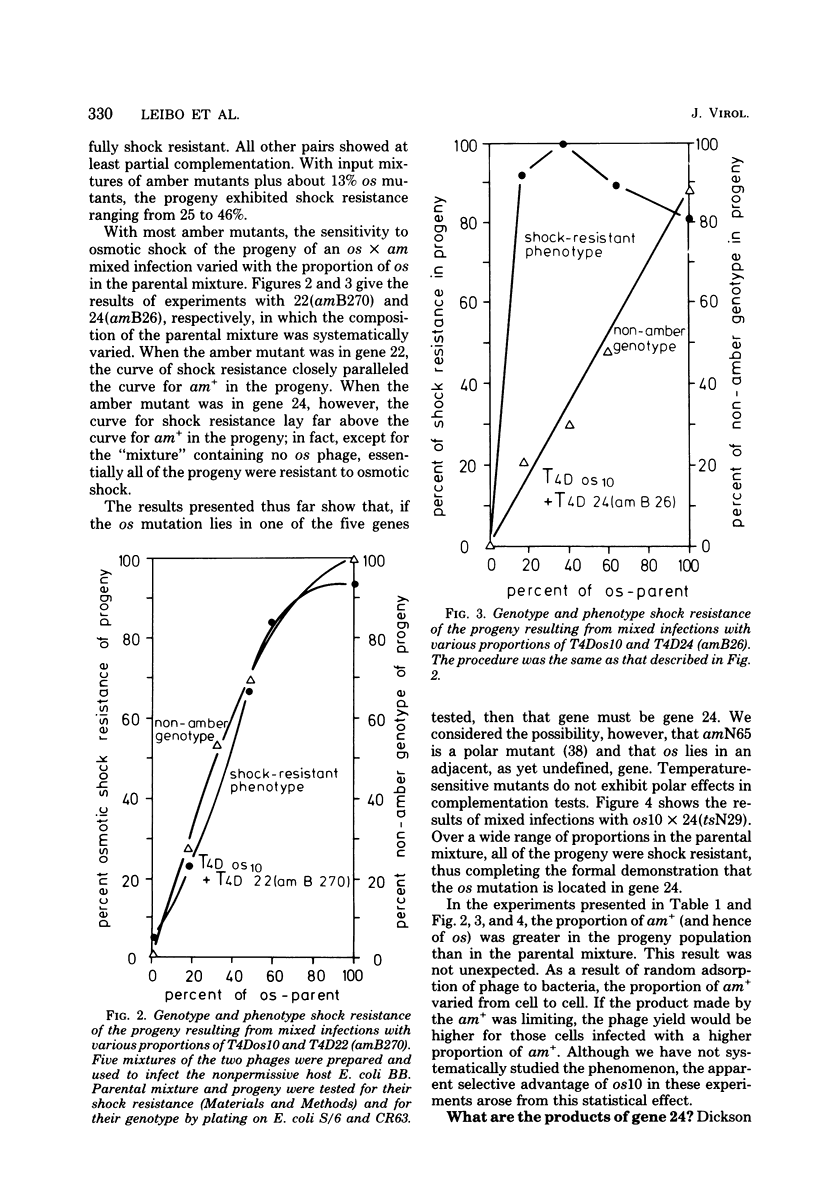
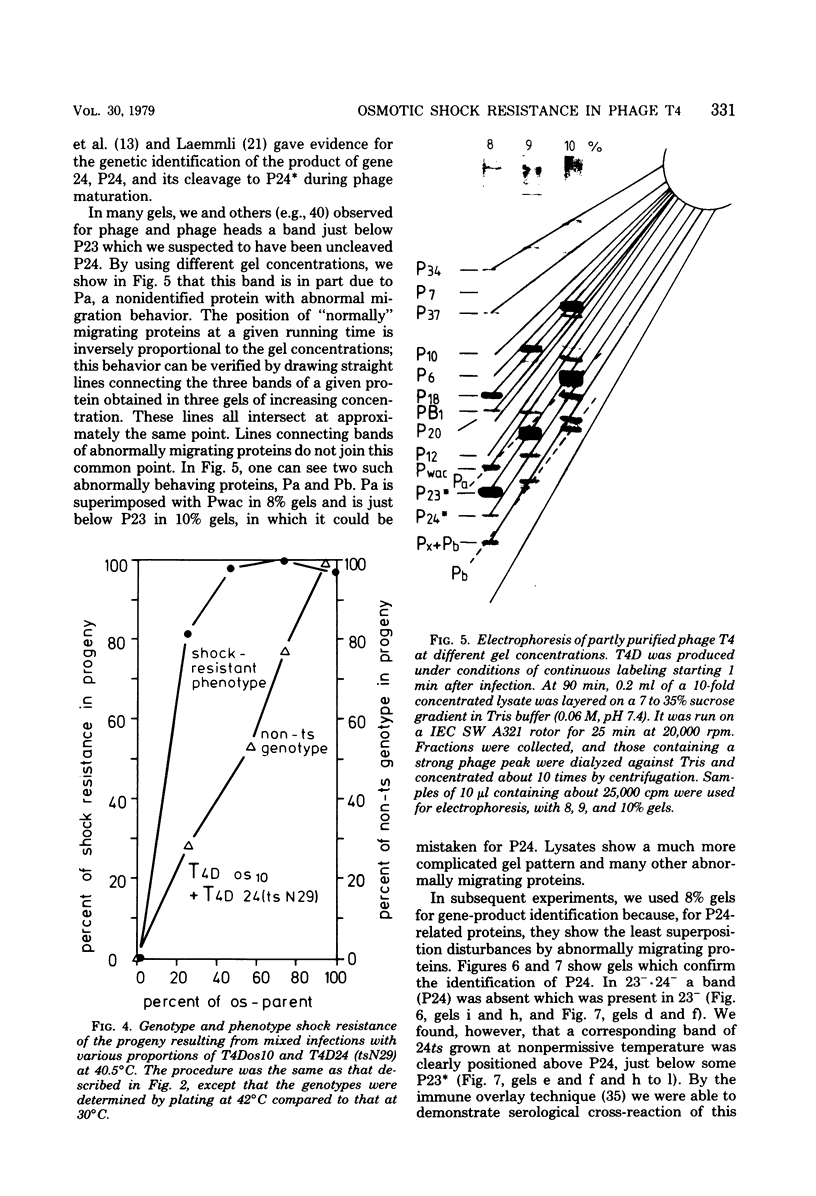
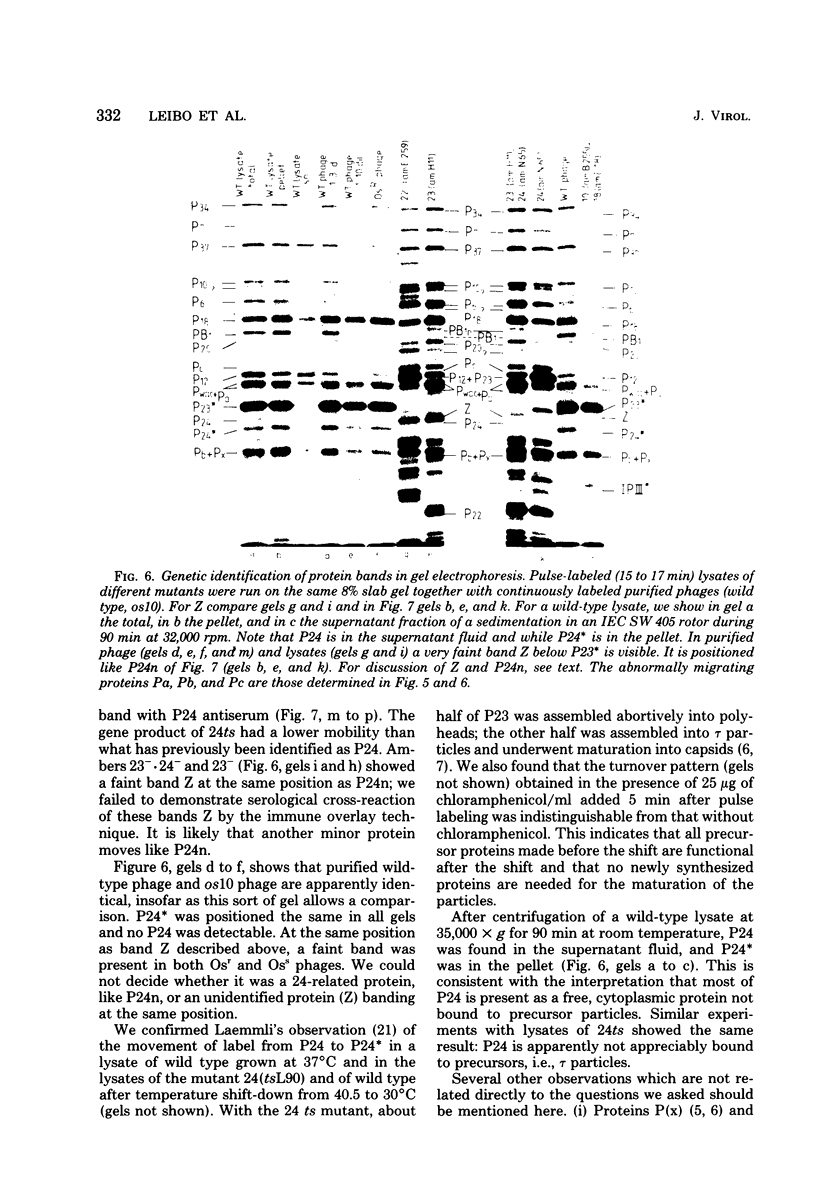
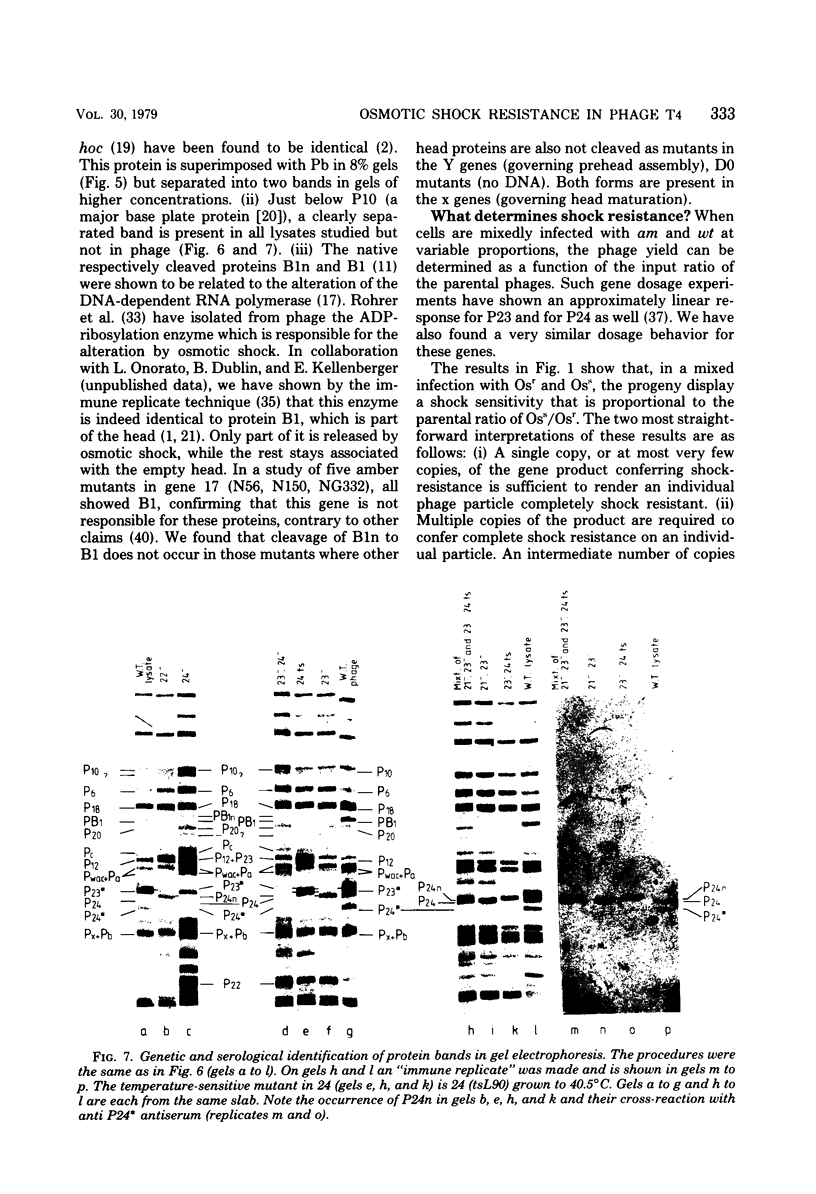
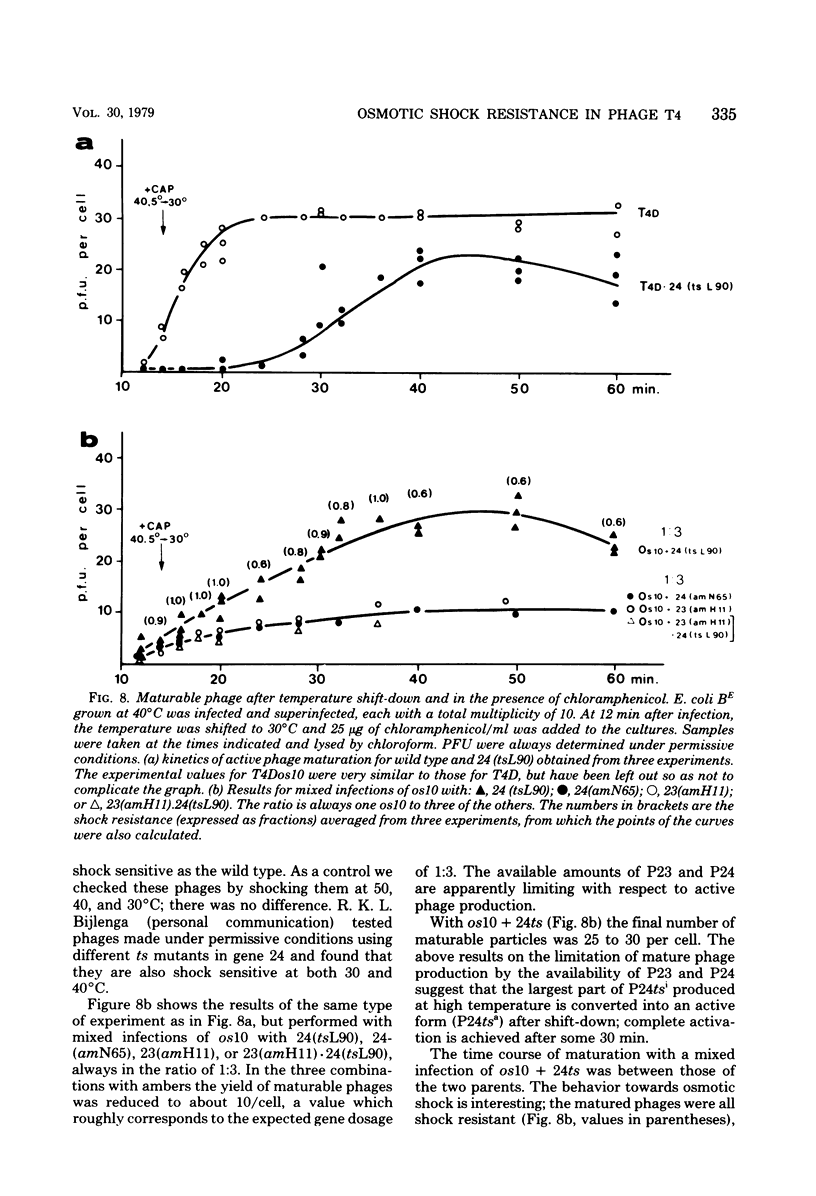
By use of mixed infections with conditional lethal mutations in the head genes and an osmotic shock-resistant mutant we have demonstrated that osmotic shock resistance is controlled by gene 24. Using acrylamide gel electrophoresis combined with the "immune replicate" technique, we confirmed the positions of gene products 24 and 24* (P24 and P24*). In this paper we have still used the notation "P24," etc., for designating the product of gene 23, etc., although we prefer and use in general the designation "gp23" as introduced by Casjens and King (Annu. Rev. Biochem. 44:585, 1975). The reason for using the old notation is because the illustrations were prepared several years ago.) P24 ts showed a significantly slower mobility. Both osmotic shock-resistant and -sensitive mature phages contain 24*. Giants constructed with the Osr phage showed the same surface lattice as normal phage. Through temperature-shift experiments with 24(tsL90) alone and in combinations, we studied the phages which are matured after the shift to permissive temperature in the absence of new protein synthesis. Our results strongly suggest that only a fraction of the total phage complement of gene 24-controlled proteins is involved in determining the phenotype of shock resistance, and the remainder is necessary to mature the head.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- ANDERSON T. F., RAPPAPORT C., MUSCATINE N. A. On the structure and osmotic properties of phage particles. Ann Inst Pasteur (Paris) 1953 Jan;84(1):5–14. [PubMed] [Google Scholar]
- Aebi U., Bijlenga R., v d Broek J., v d Broek H., Eiserling F., Kellenberger C., Kellenberger E., Mesyanzhinov V., Müller L., Showe M. The transformation of tau particles into T4 heads. II. Transformations of the surface lattice and related observations on form determination. J Supramol Struct. 1974;2(2-4):253–275. doi: 10.1002/jss.400020218. [DOI] [PubMed] [Google Scholar]
- Aebi U., van Driel R., Bijlenga R. K., ten Heggeler B., van den Broek R., Steven A. C., Smith P. R. Capsid fine structure of T-even bacteriophages. Binding and localization of two dispensable capsid proteins into the P23* surface lattice. J Mol Biol. 1977 Mar 15;110(4):687–698. doi: 10.1016/s0022-2836(77)80084-3. [DOI] [PubMed] [Google Scholar]
- BRENNER S., BARNETT L. Genetic and chemical studies on the head protein of bacteriophages T2 and T4. Brookhaven Symp Biol. 1959 Nov;12:86–94. [PubMed] [Google Scholar]
- Bijlenga R. K., Aebi U., Kellenberger E. Properties and structure of a gene 24-controlled T4 giant phage. J Mol Biol. 1976 May 25;103(3):469–498. doi: 10.1016/0022-2836(76)90213-8. [DOI] [PubMed] [Google Scholar]
- Bijlenga R. K., Broek R vd, Kellenberger E. The transformation of rho-particles into T4 heads. I. Evidence for the conservative mode of this transformation. J Supramol Struct. 1974;2(1):45–59. doi: 10.1002/jss.400020106. [DOI] [PubMed] [Google Scholar]
- Bijlenga R. K., Scraba D., Kellenberger E. Studies on the morphopoiesis of the head of T-even phage. IX. Gamma-particles: their morphology, kinetics of appearance and possible precursor function. Virology. 1973 Nov;56(1):250–267. doi: 10.1016/0042-6822(73)90304-8. [DOI] [PubMed] [Google Scholar]
- CUMMINGS D. J., KOZLOFF L. M. Various properties of the head protein of T2 bacteriophage. J Mol Biol. 1962 Jul;5:50–62. doi: 10.1016/s0022-2836(62)80060-6. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Smith J. D., Brenner S. Correlation between genetic and translational maps of gene 23 in bacteriophage T4. Nat New Biol. 1973 Jan 31;241(109):130–132. doi: 10.1038/newbio241130a0. [DOI] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Abortive bacteriophage T4 head assembly in mutants of Escherichia coli. J Mol Biol. 1973 May 5;76(1):61–87. doi: 10.1016/0022-2836(73)90081-8. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Forrest G. L., Cummings D. J. Head proteins from T-even bacteriophages. II. Physical and chemical characterization. J Virol. 1971 Jul;8(1):41–55. doi: 10.1128/jvi.8.1.41-55.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R. Bacteriophage T4 mutants deficient in alteration and modification of the Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):739–750. doi: 10.1016/0022-2836(74)90537-3. [DOI] [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Yanagida M. The two dispensable structural proteins (soc and hoc) of the T4 phage capsid; their purification and properties, isolation and characterization of the defective mutants, and their binding with the defective heads in vitro. J Mol Biol. 1977 Feb 5;109(4):487–514. doi: 10.1016/s0022-2836(77)80088-0. [DOI] [PubMed] [Google Scholar]
- King J., Mykolajewycz N. Bacteriophage T4 tail assembly: proteins of the sheath, core and baseplate. J Mol Biol. 1973 Apr 5;75(2):339–358. doi: 10.1016/0022-2836(73)90025-9. [DOI] [PubMed] [Google Scholar]
- LOVELOCK J. E. The haemolysis of human red blood-cells by freezing and thawing. Biochim Biophys Acta. 1953 Mar;10(3):414–426. doi: 10.1016/0006-3002(53)90273-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Mölbert E., Showe M., Kellenberger E. Form-determining function of the genes required for the assembly of the head of bacteriophage T4. J Mol Biol. 1970 Apr 14;49(1):99–113. doi: 10.1016/0022-2836(70)90379-7. [DOI] [PubMed] [Google Scholar]
- Leibo S. P., Mazur P. Effect of osmotic shock and low salt concentration on survival and density of bacteriophages T4B and T4Bo1. Biophys J. 1966 Nov;6(6):747–772. doi: 10.1016/S0006-3495(66)86693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibo S. P., Mazur P. Freezing of bacteriophage T4: temperature and rate effects as a function of salt concentration. Virology. 1969 Aug;38(4):558–566. doi: 10.1016/0042-6822(69)90176-7. [DOI] [PubMed] [Google Scholar]
- McNicol L. A., Simon L. E. A mutation which bypasses the requirement for p24 in bacteriophage T4 capsid morphogenesis. J Mol Biol. 1977 Oct 25;116(2):261–283. doi: 10.1016/0022-2836(77)90216-9. [DOI] [PubMed] [Google Scholar]
- Mosig G., Carnighan J. R., Bibring J. B., Cole R., Bock H. G., Bock S. Coordinate variation in lengths of deoxyribonucleic acid molecules and head lengths in morphological variants of bacteriophage T4. J Virol. 1972 May;9(5):857–871. doi: 10.1128/jvi.9.5.857-871.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Salamin L., Onorato L., Showe M. K. Localization of minor protein components of the head of bacteriophage T4. J Virol. 1977 Oct;24(1):121–134. doi: 10.1128/jvi.24.1.121-134.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Rohrer H., Zillig W., Mailhammer R. ADP-ribosylation of DNA-dependent RNA polymerase of Escherichia coli by an NAD+: protein ADP-ribosyltransferase from bacteriophage T4. Eur J Biochem. 1975 Dec 1;60(1):227–238. doi: 10.1111/j.1432-1033.1975.tb20995.x. [DOI] [PubMed] [Google Scholar]
- SARABHAI A. S., STRETTON A. O., BRENNER S., BOLLE A. CO-LINEARITY OF THE GENE WITH THE POLYPEPTIDE CHAIN. Nature. 1964 Jan 4;201:13–17. doi: 10.1038/201013a0. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Isobe E., Onorato L. Bacteriophage T4 prehead proteinase. II. Its cleavage from the product of gene 21 and regulation in phage-infected cells. J Mol Biol. 1976 Oct 15;107(1):55–69. doi: 10.1016/s0022-2836(76)80017-4. [DOI] [PubMed] [Google Scholar]
- Simon L. D. Infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope: T4 head morphogenesis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):907–911. doi: 10.1073/pnas.69.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snustad D. P. Dominance interactions in Escherichia coli cells mixedly infected with bacteriophage T4D wild-type and amber mutants and their possible implications as to type of gene-product function: catalytic vs. stoichiometric. Virology. 1968 Aug;35(4):550–563. doi: 10.1016/0042-6822(68)90285-7. [DOI] [PubMed] [Google Scholar]
- Stahl F. W., Crasemann J. M., Yegian C., Stahl M. M., Nakata A. Co-transcribed cistrons in bacteriophage T4. Genetics. 1970 Feb;64(2):157–170. doi: 10.1093/genetics/64.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Vanderslice R. W., Yegian C. D. The identification of late bacteriophage T4 proteins on sodium dodecyl sulfate polyacrylamide gels. Virology. 1974 Jul;60(1):265–275. doi: 10.1016/0042-6822(74)90384-5. [DOI] [PubMed] [Google Scholar]
- Wunderli H., Couture E., Vince D. A., Kellenberger E. Studies related to the head-maturation pathway of bacteriophages T4 And T2:II. nuclear disruption, protein synthesis and particle formation with the mutant 43-.30-.46-. J Supramol Struct. 1977;7(2):163–190. doi: 10.1002/jss.400070203. [DOI] [PubMed] [Google Scholar]
- Wunderli H., vd Broek J., Kellenberger E. Studies related to the head-maturation pathway of bacteriophages T4 and T2:I. morphology and kinetics of intracellular particles produced by mutants in the maturation genes. J Supramol Struct. 1977;7(2):135–161. doi: 10.1002/jss.400070202. [DOI] [PubMed] [Google Scholar]