Abstract
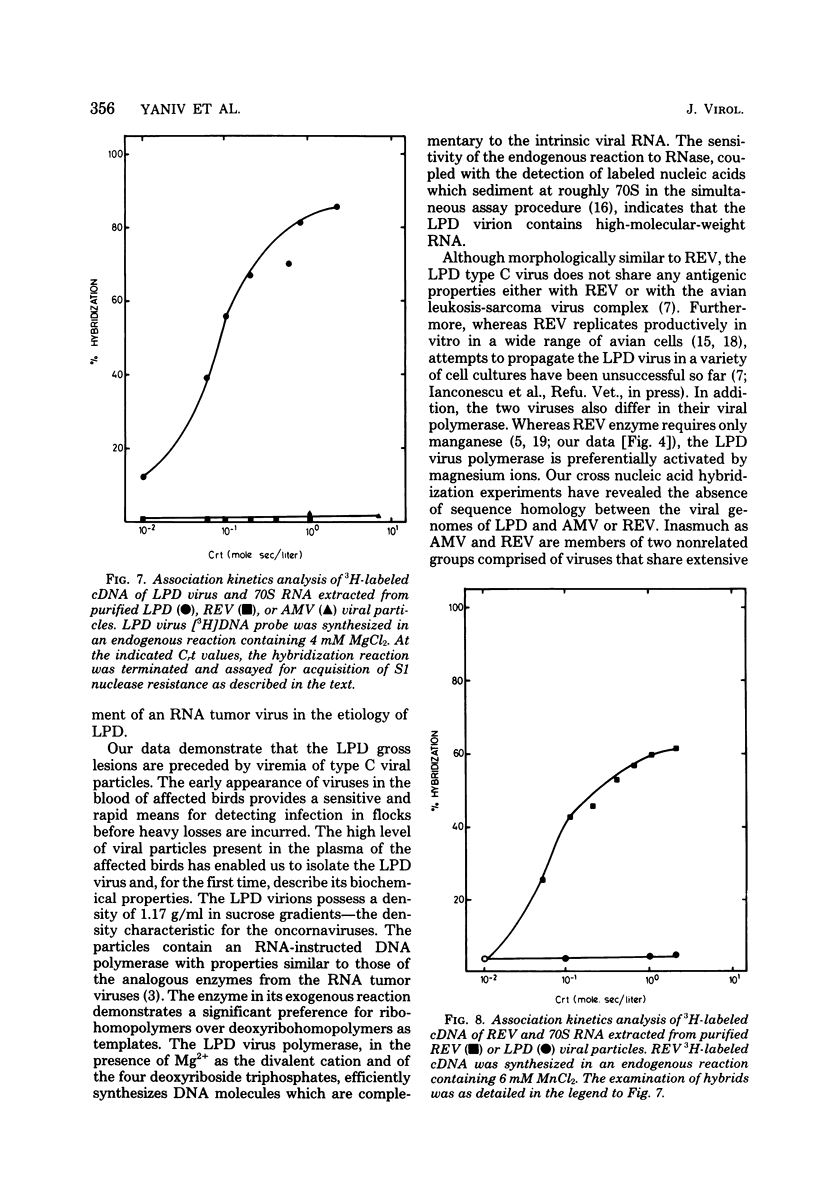
Turkeys inoculated with spleen extracts from lymphoproliferative disease (LPD)-affected birds developed viremia, followed by typical LPD lesions. Electron microscopy and biochemical characterization established that the virus present in the blood of infected turkeys is a type C retrovirus. The viral particles possess a buoyant density of 1.17 g/ml in sucrose gradients; they contain high-molecular-weight RNA and an RNA-instructed DNA polymerase with efficient exogenous and endogenous activity. The LPD virus polymerase is preferentially activated by magnesium ions. Cross nucleic acid hybridization assays revealed no sequence homology between the viral genome of LPD and avian myeloblastosis virus or reticuloendotheliosis virus, thus indicating that the LPD virus belongs to a distinct group unrelated to the avian leukosis-sarcoma virus complex or to the reticuloendotheliosis virus group.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Green M., Gerard G. F. RNA-directed DNA polymerase--properties and functions in oncogenic RNA viruses and cells. Prog Nucleic Acid Res Mol Biol. 1974;14(0):187–334. [PubMed] [Google Scholar]
- Halpern M. S., Wade E., Rucker E., Baxter-Gabbard K. L., Levine A. S., Friis R. R. A study of the relationship of reticuloendotheliosis virus to the avian leukosis-sarcoma complex of viruses. Virology. 1973 Jun;53(2):287–299. doi: 10.1016/0042-6822(73)90206-7. [DOI] [PubMed] [Google Scholar]
- Kang C. Y. Characterization of endogenous RNA-directed DNA polymerase activity of reticuloendotheliosis viruses. J Virol. 1975 Oct;16(4):880–886. doi: 10.1128/jvi.16.4.880-886.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R. L., Bose H. R. Relationship of reticuloendotheliosis virus to the avian tumor viruses: nucleic acid and polypeptide composition. J Virol. 1973 May;11(5):741–747. doi: 10.1128/jvi.11.5.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Specific serological relationships among partially purified DNA polymerases of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and avian cells. J Virol. 1974 May;13(5):1020–1029. doi: 10.1128/jvi.13.5.1020-1029.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Gelderblom H., Pauli G., Friis R. A comparative study of the avian reticuloendotheliosis virus: relationship to murine leukemia virus and viruses of the avian sarcoma-leukosis complex. Virology. 1975 Jun;65(2):546–557. doi: 10.1016/0042-6822(75)90059-8. [DOI] [PubMed] [Google Scholar]
- Paul P. S., Pomeroy K. A., Sarma P. S., Johnson K. H., Barnes D. M., Kumar M. C., Pomeroy B. S. Naturally occurring reticuloendotheliosis in turkeys: transmission. J Natl Cancer Inst. 1976 Feb;56(2):419–422. doi: 10.1093/jnci/56.2.419. [DOI] [PubMed] [Google Scholar]
- Peterson D. A., Baxter-Gabbard K. L., Levine A. S. Avian reticuloendotheliosis virus (strain T): V. DNA polymerase. Virology. 1972 Jan;47(1):251–254. doi: 10.1016/0042-6822(72)90259-0. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Ludford C., Nazerian K., Cox H. W. A new group of oncogenic viruses: reticuloendotheliosis, chick syncytial, duck infectious anemia, and spleen necrosis viruses. J Natl Cancer Inst. 1973 Aug;51(2):489–499. [PubMed] [Google Scholar]
- Purchase H. G., Witter R. L. The reticuloendotheliosis viruses. Curr Top Microbiol Immunol. 1975;71:103–124. doi: 10.1007/978-3-642-66193-8_3. [DOI] [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Guntaka R. V., Varmus H. E., Bishop J. M. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976 Mar 5;101(3):349–365. doi: 10.1016/0022-2836(76)90152-2. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Kassner V. K. Replication of reticuloendotheliosis viruses in cell culture: acute infection. J Virol. 1974 Feb;13(2):291–297. doi: 10.1128/jvi.13.2.291-297.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite M. R., Allen P. T. RNA-directed DNA polymerase activity of reticuloendotheliosis virus: characterization of the endogenous and exogenous reactions. J Virol. 1975 Oct;16(4):872–879. doi: 10.1128/jvi.16.4.872-879.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv A., Kleinman R., Eylan E. RNA-instructed DNA polymerase associated with C-type particles produced in vivo by murine myeloma cells. J Gen Virol. 1976 Aug;32(2):301–309. doi: 10.1099/0022-1317-32-2-301. [DOI] [PubMed] [Google Scholar]