Update to: European Journal of Human Genetics (2010) 18, 1071; doi:10.1038/ejhg.2010.77; published online 26 May 2010
1. Disease characteristics
1.1 Name of the disease (synonyms)
MUTYH-associated polyposis (MAP), Autosomal recessive colorectal adenomatous polyposis, Multiple colorectal adenomas, Multiple adenomatous polyps (MAP).
1.2 OMIM# of the disease
608456.
1.3 Name of the analysed genes or DNA/chromosome segments
MUTYH (MYH, outdated name of the gene).
1.4 OMIM# of the gene(s)
604933.
1.5 Mutational spectrum
See Leiden Open Variation Database (LOVD) of the MUTYH gene (www.lovd.org/mutyh).1
Broad, all types of point mutations (missense, splice site, and truncating mutations).2, 3, 4, 5
Mutations were described in almost all exons (except exons 1 and 2).
Gross genomic deletions or duplications seem to be very rare events, only one recurrent large deletion has been described so far.6
In part ethnicity-specific mutational spectrum: two founder mutations – c.536A>G;p.Tyr179Cys (previously annotated as c.494A>G;p.Tyr165Cys or Y165C) in exon 7 and c.1187G>A;p.Gly396Asp (previously annotated as c.1145G>A;p.Gly382Asp or G382D) in exon 13 – dominate in individuals of North-Western European origin (together they account for around 80% of all reported mutant alleles) and are (almost) absent in Asia.
Further ethnic-specific mutations include, for example, c.1437_1439delGGA;p.Glu480del (formerly c.1395_1397delGGA;p.Glu466del) (Southern Europe), c.312C>A;p.Tyr104X (formerly c.270C>A; p.Tyr90X) (Pakistan), and c.1438G>T;p.Glu480X (formerly c.1396G>T;p.Glu466X) (India).
1.6 Analytical methods
Direct sequencing of all 16 exons and flanking intronic sequences on genomic DNA (standard approach in routine diagnostics).
Indirect screening of exons and flanking intronic sequences: dHPLC, CSGE, and melting curve analysis.
Screening for founder mutations (useful only in patients of North-Western European origin): restriction digestion, ARMS, and pyrosequencing.
Some procedures start with a screening for both founder mutations. If only one of them is detected, then the whole gene is searched for a second mutation. However, using this method a considerable proportion (up to 20%) of biallelic mutation carriers is missed.2
Screening for large deletions and duplications with MLPA or other methods for gene dosage analysis can be considered, but is currently not generally recommended as routine diagnostic approach due to the very low frequency of this mutation type.
1.7 Analytical validation
As with other molecular genetic diagnostic tests, analytical results can be validated using standard procedures of internal and external quality assessment (EQA). These may include:
Internal validation through analysis of known mutations (positive controls).
Direct sequencing of both DNA strands (bidirectional sequencing).
Confirmation of mutation in an independent biological sample of the index case or an affected relative.
In some cases (eg, single-exon deletions detected by MLPA), the results of semiquantitative methods should be confirmed by an independent technique (long-range PCR, RNA analysis, or different MLPA kit).
External validation through exchange of DNA control samples with other diagnostic institutions and participation in EQA schemes (eg, www.emqn.org).
1.8 Estimated frequency of the disease
(incidence at birth (‘birth prevalence') or population prevalence)
The frequency of MAP is around 15–20% in unselected APC mutation-negative patients with colorectal adenomatous polyposis of European origin.
The prevalence is <1% in population-based CRC patients of European origin and up to 2% in familial or early onset CRC and Lynch syndrome/HNPCC cohorts.7, 8 Assuming a CRC frequency of 5% in the general European population, the overall prevalence is assumed to be around 1:5000. Of note, up to one-third of biallelic MUTYH mutation carriers identified in population-based CRC studies developed CRC without a colorectal polyposis.7
From the frequency of MUTYH heterozygotes in the general European population (1–1.5%)3, 9, 10 the prevalence of biallelic carriers of MUTYH mutations (clinical plus subclinical biallelic carriers) can be derived as 1:40 000–1:20 000; however, the penetrance of biallelic MUTYH mutations is as yet unknown.4 In Far Eastern Asian populations, no prevalent MUTYH mutations have been identified, and thus the heterozygote frequency is presumed to be lower compared with European populations.
1.9 If applicable, prevalence in the ethnic group of investigated person
The observed mutation patterns are not sufficient to derive ethnicity-specific prevalence rates. However, due to the presence of founder mutations among ethnic groups, differences in the frequency of MAP are likely to exist. The two hotspot mutations c.536A>G;p.Tyr179Cys and c.1187G>A;p.Gly396Asp observed in patients of European origin are almost absent in Far Eastern Asian populations.
1.10 Diagnostic setting

Comment: Prenatal/preimplantation diagnosis is almost never requested. Since MAP is a relatively late-manifesting and treatable disease, it should be performed only exceptionally, in reasonable cases with clear indication and after extensive genetic counselling
2. TEST CHARACTERISTICS
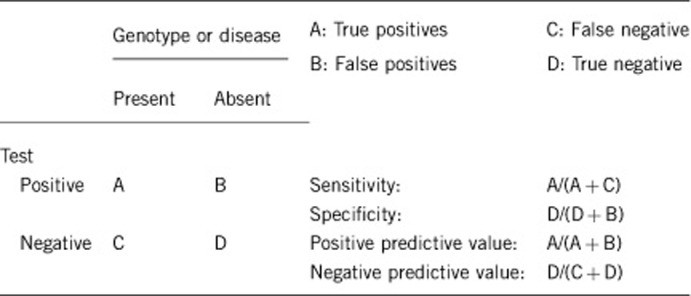
2.1 Analytical Sensitivity
(proportion of positive tests if the genotype is present)
Almost 100% by sequencing of all 16 exons and deletion/duplication screening by methods such as MLPA.
Mutations deep within introns or in regulatory elements are missed with current standard methods.
2.2 Analytical Specificity
(proportion of negative tests if the genotype is not present)
Almost 100%.
2.3 Clinical Sensitivity
(proportion of positive tests if the disease is present)
The clinical sensitivity can be dependent on variable factors such as age or family history. In such cases, a general statement should be given, even if a quantification can only be made case by case.
About 15–20% (depending on severity of disease and family history: up to 60% when the pedigree strongly indicates recessive inheritance).
2.4 Clinical Specificity
(proportion of negative tests if the disease is not present)
The clinical specificity can be dependent on variable factors such as age or family history. In such cases, a general statement should be given, even if a quantification can only be made case by case.
Almost 100% (except for variants of uncertain clinical significance).
2.5 Positive clinical predictive value
(life-time risk to develop the disease if the test is positive)
Penetrance of CRC in proven biallelic mutation carriers, according to present knowledge, is up to 80%. Due to clinical variability, mildly affected persons may not be diagnosed or will be deceased for other reasons during presymptomatic (subclinical) stage of the disease.
Relatives of MAP patients who are monoallelic mutation carriers (simple heterozygotes) may have a slightly increased CRC risk (odds ratio 1.5–2.1) in advanced age (>50 years).11
2.6 Negative clinical predictive value
(Probability not to develop the disease if the test is negative; although the population risk for CRC and adenomas remains)
Assume an increased risk based on family history for a non-affected person. Allelic and locus heterogeneity may need to be considered.
Index case in that family had been tested:
Almost 100% for sibs of index patient.
The obligate heterozygous children of an index patient have a low risk (∼0.5%) to carry a second mutation inherited from the other parent. Since the mutation detection rate in MUTYH is probably below 100%, the negative predictive value in this situation is slightly less than 100%, even if the whole gene is screened. Detection of a variant of uncertain clinical significance in the child of an affected person may not allow a secure decision on whether the test is ‘negative' or not. De novo mutations have not been described so far.
Index case in that family had not been tested:
Since the mutation detection rates in APC and MUTYH strongly depend on the colorectal phenotype (mild versus more florid forms of polyposis) and the family history (dominant versus recessive pedigree pattern), and – particularly in attenuated courses of adenomatous polyposis – more and still unknown genes may be involved (locus heterogeneity), no exact figures can be given.
To test persons at risk without having identified the underlying germline mutations in a clearly affected index patient of the family is not a meaningful approach and should therefore be avoided, since persons at risk who are tested negative may still have a substantial risk and cannot be released from surveillance.
3. Clinical Utility
3.1 (Differential) diagnosis: The tested person is clinically affected
(To be answered if in 1.10 ‘A' was marked)
MUTYH mutation analysis should be considered in patients with multiple (>10) synchronous colorectal adenomas and rather young onset (<60 years of age) in the absence of a clear autosomal dominant inheritance pattern. Since hyperplastic polyps and sessile serrated adenomas are a common finding in MAP and the occurrence of CRC in the absence of colorectal polyps has been described, the clinical presentation might be misdiagnosed as hyperplastic polyposis or sporadic CRC in some patients.7, 12 Phenotypic overlap with Lynch syndrome/ HNPCC has also been reported.13
The frequency of biallelic MUTYH mutations is low (0.3–1.7%) in population-based and early-onset CRC cohorts and in patients with a low number of adenomas (<10) at any age, without an overt family history (and no carcinoma). For these cases, currently no general recommendations regarding MUTYH mutation analysis exist.
3.1.1 Can a diagnosis be made other than through a genetic test?
3.1.2 Describe the burden of alternative diagnostic methods to the patient
Family history: no strain.
3.1.3 How is the cost effectiveness of alternative diagnostic methods to be judged?
Family history: very cost-effective.
3.1.4 Will disease management be influenced by the result of a genetic test?

3.2 Predictive setting: The tested person is clinically unaffected but carries an increased risk based on family history
(To be answered if in 1.10 ‘B' was marked)
3.2.1 Will the result of a genetic test influence lifestyle and prevention?
Yes. If the test result is positive (please describe):
Biallelic mutation carriers: Heightened motivation of siblings to participate in specific preventive checkups, including colonoscopy and upper gastrointestinal endoscopy, eventually colectomy when several adenomas are detected.14
In some cases family planning, choice of profession.
Monoallelic carriers: surveillance regarding CRC as recommended for first degree relatives of a patient with sporadic CRC is discussed.11
If the test result is negative (please describe):
Yes. Release from preventive programme, psychological relief.
3.2.2 Which options in view of lifestyle and prevention does a person at-risk have if no genetic test has been done (please describe)?
Same for siblings of index patients as for proven biallelic carriers: close-meshed early diagnosis and surveillance programmes (in particular, frequent pancolonoscopies).
Children of affected persons, because of their low disease risk (∼0.5%), should not undergo an intensive early diagnosis program. Given their comparatively low a priori disease risk, the offer of predictive diagnostics is, for the present, a matter of discretion.
So far, one study suggested that it may be appropriate to initiate surveillance later for c.1187G>A;p.Gly396Asp homozygotes and c.1187G>A;p.Gly396Asp/c.536A>G;p.Tyr179Cys compound heterozygotes compared with c.536A>G;p.Tyr179Cys homozygotes.15
3.3 Genetic risk assessment in family members of a diseased person
(To be answered if in 1.10 ‘C' was marked)
3.3.1 Does the result of a genetic test resolve the genetic situation in that family?
Yes.
3.3.2 Can a genetic test in the index patient save genetic or other tests in family members?
Yes: by securing the primary cause of the disease, extended diagnostic investigations in other symptomatic relatives can be avoided.
The low recurrence risk in children and exclusion of carriership by predictive diagnostics in siblings of affected persons allow to avoid superfluous preventive checkups and offer psychological relief.
3.3.3 Does a positive genetic test result in the index patient enable a predictive test in a family member?
Yes.
3.4 Prenatal diagnosis
(To be answered if in 1.10 ‘D' was marked)
3.4.1 Does a positive genetic test result in the index patient enable a prenatal diagnostic?
Yes (but see the comment in 2.10).
4. IF APPLICABLE, FURTHER CONSEQUENCES OF TESTING
Please assume that the result of a genetic test has no immediate medical consequences. Is there any evidence that a genetic test is nevertheless useful for the patient or his/her relatives? (Please describe)
For many patients prove of diagnosis is a value in itself – irrespective of a medical benefit – because the disease and its cause can often clearly be named.
When a genetic cause is verified, an assumption of ‘own fault' as cause of disease (exogenous poisons, ‘wrong conduct') often can be lapsed with relief.
The main benefits of genetic diagnostics in MAP are the differentiation from FAP, a precise recurrence risk for close relatives, relief of non-carriers during predictive diagnostics, and a tailored surveillance programme including prophylactic surgery options.
Acknowledgments
This work was supported by EuroGentest, an EU-FP6 supported NoE, contract number 512148 (EuroGentest Unit 3: ‘Clinical genetics, community genetics and public health', Workpackage 3.2), and by the German Cancer Aid (Deutsche Krebshilfe e.V. Bonn, Grant no. 108421).
The authors declare no conflict of interest.
References
- Out AA, Tops CM, Nielsen M, et al. Leiden Open Variation Database of the MUTYH gene. Hum Mutat. 2010;31:1205–1215. doi: 10.1002/humu.21343. [DOI] [PubMed] [Google Scholar]
- Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis (MAP): 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer. 2006;119:807–814. doi: 10.1002/ijc.21905. [DOI] [PubMed] [Google Scholar]
- Poulsen ML, Bisgaard ML. MUTYH associated polyposis (MAP) Curr Genomics. 2008;9:420–435. doi: 10.2174/138920208785699562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JR, Jones N. MUTYH-associated polyposis. Best Pract Res Clin Gastroenterol. 2009;23:209–218. doi: 10.1016/j.bpg.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Molatore S, Russo MT, D'Agostino VG, et al. MUTYH mutations associated with familial adenomatous polyposis: functional characterization by a mammalian cell-based assay. Hum Mutat. 2010;31:159–166. doi: 10.1002/humu.21158. [DOI] [PubMed] [Google Scholar]
- Torrezan GT, da Silva FC, Krepischi AC, et al. Breakpoint characterization of a novel large intragenic deletion of MUTYH detected in a MAP patient: Case report. BMC Med Genet. 2011;12:128. doi: 10.1186/1471-2350-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary SP, Cotterchio M, Jenkins MA, et al. Germline MutY human homologue mutations and colorectal cancer: A multisite case-control study. Gastroenterology. 2009;136:1251–1260. doi: 10.1053/j.gastro.2008.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbe SJ, Di Bernardo MC, Chandler IP, Houlston RS. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J Clin Oncol. 2009;27:3975–3980. doi: 10.1200/JCO.2008.21.6853. [DOI] [PubMed] [Google Scholar]
- Morreau H, Riddell R, Aretz. S.MUTYH-associated polyposis WHO Classification of Tumours of the Digestive System 2010. 4th edn.IARC: Lyon; 156–159. [Google Scholar]
- Farrington SM, Tenesa A, Barnetson R, et al. Germline susceptibility to colorectal cancer due to base-excision repair gene defects. Am J Hum Genet. 2005;77:112–119. doi: 10.1086/431213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N, Vogt S, Nielsen M, et al. Increased colorectal cancer incidence in obligate carriers of heterozygous mutations in MUTYH. Gastroenterology. 2009;137:489–494. doi: 10.1053/j.gastro.2009.04.047. [DOI] [PubMed] [Google Scholar]
- Boparai KS, Dekker E, Van Eeden S, et al. Hyperplastic polyps and sessile serrated adenomas as a phenotypic expression of MYH-associated polyposis. Gastroenterology. 2008;135:2014–2018. doi: 10.1053/j.gastro.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Vogt S, Jones N, Christian D, et al. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology. 2009;137:1976–1985. doi: 10.1053/j.gastro.2009.08.052. [DOI] [PubMed] [Google Scholar]
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP) Gut. 2008;57:704–713. doi: 10.1136/gut.2007.136127. [DOI] [PubMed] [Google Scholar]
- Nielsen M, Joerink-van de Beld MC, Jones N, et al. Analysis of MUTYH genotypes and colorectal phenotypes in patients with MUTYH-associated polyposis. Gastroenterology. 2009;136:471–476. doi: 10.1053/j.gastro.2008.10.056. [DOI] [PubMed] [Google Scholar]


