Abstract
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy is a rare autoimmune disorder. The clinical spectrum of symptoms is diverse; the diagnosis relying on the presence of at least two out of the three main conditions defining the syndrome: chronic mucocutaneous candidiasis, hypoparathyroidism, and Addison's disease.
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), also called autoimmune polyendocrine syndrome type I (APS-1), is a rare organ-specific autosomal recessive disease (OMIM 240300).1–3 Although APECED may occur worldwide, it remains most common in Iranian Jews (1:9,000),4 Sardinians (1:14,500),5–12 Finns (1:25,000),13 Slovenians (1:43,000),14 Norwegians (1:80,000),15 Polish (1:1,29,000),16 and Japanese (1:10,000,000).17 Multiple organ failures in APECED are caused by a progressive loss of tolerance against self-antigens resulting in an immunological attack and secondary destruction of the adrenals, parathyroid glands, β cells of islet of Langerhans,18 stomach, and small intestine.13 Many of the auto-antigens have been identified and characterized.9,12,19
Diagnosis
The diagnosis of APECED requires the presence of at least two of the following three classical conditions (diagnostic dyad): chronic mucocutaneous candidiasis (CMC), hypoparathyroidism, or Addison's disease (adrenocortical failure).20 Other endocrine or nonendocrine manifestations of APECED may include thyroid autoimmune disease (autoimmune thyroiditis, Grave's disease), gastrointestinal manifestations (chronic active hepatitis, malabsorption, asplenia, juvenile onset pernicious anemia), dermatological conditions (alopecia areata, vitiligo), primary hypogonadism, kidney disease, and oral or esophageal cancer. The intestinal dysfunction has been attributed to bacterial overgrowth, pancreatic insufficiency, lymphectesia, candidal infection, and cholecystokinin deficiency. Humoral immunodeficiency may also be seen in rare cases.21 All of the previously mentioned pathological characteristics are secondary to an autoimmune phenomenon.21
Besides these aforementioned conditions, features of ectodermal dystrophy, such as enamel hypoplasia, punctuate nail dystrophy, atrophy, and calcification of tympanic membrane, and disorders of sweat glands, may also be observed.22 This condition exhibits a wide variation in its clinical picture,4,23 and less common disease manifestations may also dominate the picture for many years. The early diagnosis of APECED can help prevent potentially life-threatening conditions, such as Addison's disease.
Immunological Studies
The innate, humoral, and adaptive arms of the immune system help provide protection against Candida albicans.24 Protection is provided against systemic infections by neutrophils as well as the T helper cell 2 (Th2) immune response. In cases involving the mucous membranes, protection largely depends on cell-mediated immunity by the generation and activation of a dominant, antigen-specific Th1-cell response.25,26 Patients with CMC are noted to display an impaired or low proliferation response against Candida antigens.27 Despite this idea, Arulanantham et al28 reported that one patient in their study demonstrated an increased T-cell response, whereas two other patients developed an adequate response. To understand this paradox, Brannstrom et al29 examined the role that the autoimmune regulator (AIRE) gene plays in the defense against C. albicans. They investigated the proliferative response in AIRE-deficient mice and APS-1 patients challenged with a Candida cell wall extract; or more specifically, T-cell activation and the ability of antigen presenting cells to internalize and become activated against Candida antigens. T-cells from AIRE-deficient mice and from APS-1 patients both demonstrated an increased proliferation against Candida antigens, yet did not offer the expected protection from mucocutaneous candidiasis. However, it was noted that the exaggerated T-cell mediated response caused destruction of multiple endocrine organs in APECED/APS-1. It was eventually observed that there was an altered and delayed receptor mediated internalization of zymosan (yeast cell wall derivate) in AIRE-deficient antigen presentation. Therefore, it was concluded that T-cells from AIRE-deficient individuals have a competent proliferative response against C. albicans. However, monocytes from APS-1 patients exhibit defective internalization and intracellular signaling after exposure to Candida antigens. This defect likely alters the effectiveness in clearing muco-cutaneous candidiasis. Recently, autoantibodies against Th17-related cytokines interleukin-22 (IL-22), IL-17A, and IL-17F were discovered in the sera of APS-1 patients, suggesting a probable role of the Th17 immune response in the pathogenesis of this disease.30,31
Dermatological Features
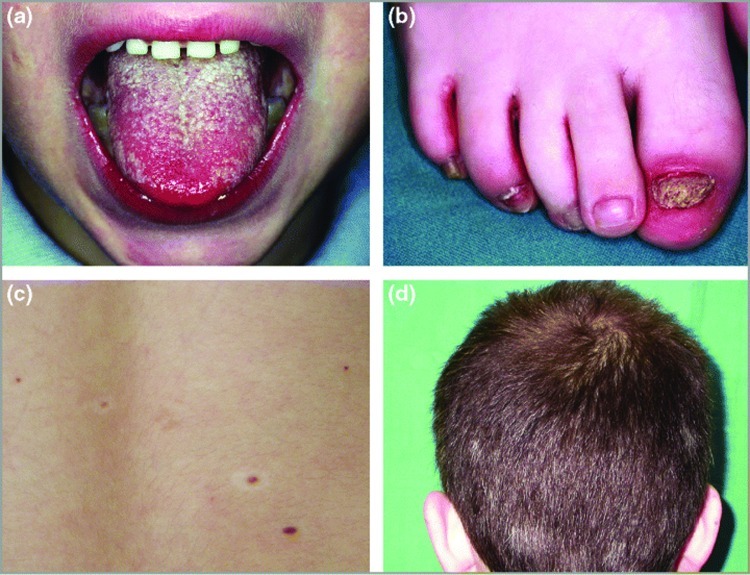
The main skin manifestations of APECED include CMC, nail dystrophy, alopecia areata (AA), vitiligo, and Addison-related hyperpigmentation (Figure 1). Less common manifestations include recurrent urticarial vasculitis, scleroderma, Sjogren's syndrome, lichen planus, and oral squamous cell carcinoma. Candidiasis is usually the first manifestation of the disease and may occur before the age of five years.32
Figure 1.
Dermatological manifestations of autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy syndrome. (a) Oral candidiasis (Patient 5); (b) candidal onychomycosis and paronychia (Patient 5); (c) halo nevi (Patient 14); (d) poliosis (Patient 14). Reproduced with permission from John Wiley and Sons.33
Collins et al33 examined the dermatological manifestations of APECED in an Irish case series with focus on the timing of their appearance, association with disease severity, and genotype-phenotype correlation. In their series of 18 patients, all showed evidence of CMC, but its course and severity widely varied, including presentations of angular cheilitis, oral (leukoplakia) and esophageal candidiasis, esophageal web, and stricture. A large percentage (72%) showed candidal paronychia and/or onychomycosis. The age of onset ranged from infancy to 18 years with fingernails being more commonly affected than toes and the thumb being the most commonly affected finger. The majority of patients failed treatment with systemic antifungals. Patchy AA was observed in 33 percent of the patients either as a single episode or a recurrence. All the patients with AA also suffered from concomitant hypoparathyroidism. The investigators otherwise failed to find a correlation between the genotype and dermatological phenotype.
Endocrinological Features
Hypoparathyroidism may appear before 10 years of age, while adrenal insufficiency can present before 15 years of age.34 Hypoparathyroidism and Addison's disease (adrenal insufficiency) are two of the autoimmune conditions that patients with APECED are predisposed to developing. At least one study demonstrated that 54 of 68, or 79 percent, of patients had hypoparathyroidism.13 Clinical symptoms of hypoparathyroidism are caused by hypocalcemia due to inadequate parathyroid hormone secretion. The presenting symptoms include tetany, seizures, altered mental status, congestive heart failure, stridor, twitching spasms, muscle cramping, parasthesias, and numbness. The acute phase of hypoparathyroidism can be life threatening. The reason for the development of the autoimmunity has been attributed to a loss of tolerance resulting from mutation in the AIRE gene. AIRE normally expresses a diverse number peripheral self-antigens in the thymic medullary epithelial cells, thereby facilitating negative selection of T cells that recognize these peripheral antigens strongly.35,36 Animal models of this autoimmune disorder have suggested that genes within the major histocompatibility complex, particularly the immune-response genes (similar to the HLA-DQ and HLA-DR alleles in humans) are responsible for the alteration in autoimmunity.
Adrenal insufficiency can be difficult to diagnose due to its gradual and vague symptoms unless the physician's suspicion is high. The classic symptoms include chronic fatigue, muscle weakness, loss of appetite, weight loss, nausea, vomiting, diarrhea, hyperpigmentation of the skin, and low blood pressure. Children may develop hypoglycemia while females may suffer from primary or secondary amenorrhea. Careful observation and knowledge of APECED can help detect early symptoms of an adrenal crisis—a situation that should be avoided and is preventable if the diagnosis is made early. Adrenal crisis typically presents with vomiting, diarrhea, dehydration, low blood pressure, and loss of consciousness.
Genetic Features
The underlying genetic defect most often associated with APECED is a mutation in the AIRE gene, which is located on chromosome 21q22.3. AIRE is mainly expressed in a subset of the medullary thymic epithelial cells (mTEC) and in dendritic cells in the spleen and lymph nodes.37,38 Various gene defects, such as point mutations, insertions, and deletions, spread over the entire coding region of the AIRE gene have been identified and demonstrated to be responsible for the clinical presentations of APECED.39–44 More than 50 different mutations have been identified to date and are known to affect different aspects of intracellular targeting and transcription regulation functions.21,40,45
The most prevalent mutation, R257X, found in the Finnish population, was present in 89 percent of the Finnish chromosomes examined.40 The most common mutation in British,46 Irish,47 North American,42,48 and Norwegian patients is 1094–1106del13 (or 967–979del13 bp).40 The Y85C mutation is typical among Iranian Jews. In Sardinia, the AIRE gene mutation known as R139X causes a nonsense mutation on exon 3, resulting from a C to T transition. Associations between certain alleles of the human leukocyte antigen (HLA) class I (HLA A, B, Cw) and Class II (DR, DQ, DP) and autoimmune manifestations of APECED have also been suspected. Halonen et al49 studied the AIRE and HLA class II genotypes in a series of 104 patients with APECED.49 They demonstrated that direct associations between the APECED phenotype and the AIRE mutation may exist for the R257X mutation and the high frequency of mucocutaneous candidiasis. Addison's disease was associated with HLA-DRB1*03, alopecia with HLA-DRB1*04-DQB1*0302, whereas type I diabetes showed a negative correlation with HLADRB1*15-DQB1*0602. They concluded that mutation of AIRE per se has little influence on the APECED phenotype, whereas HLA class II is a significant determinant.
Management
Currently, the management of APECED/APS-1 involves the treatment of its individual conditions. Knowledge and a high index of suspicion of this rare syndrome can lead to its early detection and prevention of most of the complications. It must be remembered that in case of simultaneous hypothyroidism and Addison's disease, glucocorticoid replacement should be performed prior to thyroid hormone replacement to avoid an adrenal crisis. The development of Type 1A diabetes should be expected and closely monitored. The challenge is to detect the syndrome in a timely fashion and to anticipate its other manifestations. For example, in a patient with known Type 2 autoimmune polyendocrine syndrome, but no features of Addison's disease, regular screening for antibodies against 21-hydroxylase (a feature of Addison's) may prompt early intervention and hydrocortisone replacement to prevent the characteristic crises. Hypocalcemia associated with hypoparathyrodism should be approached depending on its severity.50 In the acute situation, intravenous calcium gluconate should be used along with 5% dextrose or 0.9% sodium chloride given over five minutes.50 In chronic hypoparathyroidism, oral calcium supplements 1,000 to 1,500mg per day along with calcitrol 2µg per day should be used to bring the calcium level within the low normal range.50 Supplementing too much calcium should be avoided to decrease the risk of inducing nephrolithiasis.50
Footnotes
DISCLOSURE:The authors report no relevant conflicts of interest.
References
- 1.Esselborn VM, Landing BH, Whitaker J, Williams RR. The syndrome of familial juvenile hypoadrenocorticism, hypoparathyroidism and superficial moniliasis. J Clin Endocrinol Metab. 1956;16:1374–1387. doi: 10.1210/jcem-16-10-1374. [DOI] [PubMed] [Google Scholar]
- 2.Neufeld M, Maclaren NK, Blizzard RM. Two types of autoimmune Addison’s disease associated with different polyglandular autoimmune (PGA) syndromes. Medicine (Baltimore) 1981;60:355–362. doi: 10.1097/00005792-198109000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) In: Eriksson AW, Forsius HR, Workman PL, Norio RK, editors. Population Structure and Genetic Disorders. London: Academic Press: 1980. pp. 583–588. [Google Scholar]
- 4.Zlotogora J, Shapiro MS. Polyglandular autoimmune syndrome type I among Iranian Jews. J Med Genet. 1992;29:824–826. doi: 10.1136/jmg.29.11.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosatelli MC, Meloni A, Devoto M, et al. A common mutation in Sardinian autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients. Hum Genet. 1998;103:428–434. doi: 10.1007/s004390050846. [DOI] [PubMed] [Google Scholar]
- 6.Consortium TF-GA. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 7.Norio R. The Finnish Disease Heritage III: the individual diseases. Hum Genet. 2003;112:470–526. doi: 10.1007/s00439-002-0877-1. [DOI] [PubMed] [Google Scholar]
- 8.Myllarniemi S, Perheentupa J. Oral findings in the autoimmune polyendocrinopathy-candidosis syndrome (APECS) and other forms of hypoparathyroidism. Oral Surg Oral Med Oral Pathol. 1978;45:721–729. doi: 10.1016/0030-4220(78)90147-0. [DOI] [PubMed] [Google Scholar]
- 9.Tarkkanen A, Merenmies L. Corneal pathology and outcome of keratoplasty in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) Acta Ophthalmol Scand. 2001;79:204–207. doi: 10.1034/j.1600-0420.2001.079002204.x. [DOI] [PubMed] [Google Scholar]
- 10.Perheentupa J. APS-I/APECED: the clinical disease and therapy. Endocrinol Metab Clin North Am. 2002;31(vi):295–320. doi: 10.1016/s0889-8529(01)00013-5. [DOI] [PubMed] [Google Scholar]
- 11.Gylling M, Kaariainen E, Vaisanen R, et al. The hypoparathyroidism of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protective effect of male sex. J Clin Endocrinol Metab. 2003;88:4602–4608. doi: 10.1210/jc.2003-030700. [DOI] [PubMed] [Google Scholar]
- 12.Soderbergh A, Myhre AG, Ekwall O, et al. Prevalence and clinical associations of 10 defined autoantibodies in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 2004;89:557–562. doi: 10.1210/jc.2003-030279. [DOI] [PubMed] [Google Scholar]
- 13.Ahonen P, Myllarniemi S, Sipila I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–1836. doi: 10.1056/NEJM199006283222601. [DOI] [PubMed] [Google Scholar]
- 14.Podkrajsek KT, Bratanic N, Krzisnik C, Battelino T. Autoimmune regulator-1 messenger ribonucleic acid analysis in a novel intronic mutation and two additional novel AIRE gene mutations in a cohort of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients. J Clin Endocrinol Metab. 2005;90:4930–4935. doi: 10.1210/jc.2005-0418. [DOI] [PubMed] [Google Scholar]
- 15.Myhre AG, Halonen M, Eskelin P, et al. Autoimmune polyendocrine syndrome type 1 (APS I) in Norway. Clin Endocrinol (Oxf) 2001;54:211–217. doi: 10.1046/j.1365-2265.2001.01201.x. [DOI] [PubMed] [Google Scholar]
- 16.Stolarski B, Pronicka E, Korniszewski L, et al. Molecular background of polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome in a Polish population: novel AIRE mutations and an estimate of disease prevalence. Clin Genet. 2006;70:348–354. doi: 10.1111/j.1399-0004.2006.00690.x. [DOI] [PubMed] [Google Scholar]
- 17.Sato K, Nakajima K, Imamura H, et al. A novel missense mutation of AIRE gene in a patient with autoimmune polyendocrinopathy, candidiasis and ectodermal dystrophy (APECED), accompanied with progressive muscular atrophy: case report and review of the literature in Japan. Endocr J. 2002;49:625–633. doi: 10.1507/endocrj.49.625. [DOI] [PubMed] [Google Scholar]
- 18.Eisenbarth GS. Autoimmune polyendocrine syndromes. Adv Exp Med Biol. 2004;552:204–218. [PubMed] [Google Scholar]
- 19.Winqvist O, Gustafsson J, Rorsman F, et al. Two different cytochrome P450 enzymes are the adrenal antigens in autoimmune polyendocrine syndrome type I and Addison’s disease. J Clin Invest. 1993;92:2377–2385. doi: 10.1172/JCI116843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faiyaz-Ul-Haque M, Bin-Abbas B, Al-Abdullatif A, et al. Novel and recurrent mutations in the AIRE gene of autoimmune polyendocrinopathy syndrome type 1 (APS1) patients. Clin Genet. 2009;76:431–440. doi: 10.1111/j.1399-0004.2009.01278.x. [DOI] [PubMed] [Google Scholar]
- 21.Perheentupa J. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2006;91:2843–2850. doi: 10.1210/jc.2005-2611. [DOI] [PubMed] [Google Scholar]
- 22.Meriluoto T, Halonen M, Pelto-Huikko M, et al. The autoimmune regulator: a key toward understanding the molecular pathogenesis of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Keio J Med. 2001;50:225–239. doi: 10.2302/kjm.50.225. [DOI] [PubMed] [Google Scholar]
- 23.Perheentupa J, Miettinen A. Type 1 autoimmune polyglandular disease. Ann Med Interne (Paris) 1999;150:313–325. [PubMed] [Google Scholar]
- 24.Bodey G. Candidiasis: Pathogenesis, Diagnosis, and Treatment. New York: Raven: 1993. ed. [Google Scholar]
- 25.Lilic D, Gravenor I, Robson N, et al. Deregulated production of protective cytokines in response to Candida albicans infection in patients with chronic mucocutaneous candidiasis. Infect Immun. 2003;71:5690–5699. doi: 10.1128/IAI.71.10.5690-5699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4:1–23. doi: 10.1038/nri1255. [DOI] [PubMed] [Google Scholar]
- 27.Ashman RB, Papadimitriou JM. Production and function of cytokines in natural and acquired immunity to Candida albicans infection. Microbiol Rev. 1995;59:646–672. doi: 10.1128/mr.59.4.646-672.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arulanantham K, Dwyer JM, Genel M. Evidence for defective immunoregulation in the syndrome of familial candidiasis endocrinopathy. N Engl J Med. 1979;300:164–168. doi: 10.1056/NEJM197901253000403. [DOI] [PubMed] [Google Scholar]
- 29.Brannstrom J, Hassler S, Peltonen L, et al. Defect internalization and tyrosine kinase activation in Aire deficient antigen presenting cells exposed to Candida albicans antigens. Clin Immunol. 2006;121:265–273. doi: 10.1016/j.clim.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Kisand K, Boe Wolff AS, Podkrajsek KT, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puel A, Doffinger R, Natividad A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betterle C, Greggio NA, Volpato M. Clinical review 93: autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab. 1998;83:1049–1055. doi: 10.1210/jcem.83.4.4682. [DOI] [PubMed] [Google Scholar]
- 33.Collins SM, Dominguez M, Ilmarinen T, et al. Dermatological manifestations of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome. Br J Dermatol. 2006;154:1088–1093. doi: 10.1111/j.1365-2133.2006.07166.x. [DOI] [PubMed] [Google Scholar]
- 34.Betterle C, Volpato M. Adrenal and ovarian autoimmunity. Eur J Endocrinol. 1998;138:16–25. doi: 10.1530/eje.0.1380016. [DOI] [PubMed] [Google Scholar]
- 35.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 36.Anderson MS, Venanzi ES, Chen Z, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Zuklys S, Balciunaite G, Agarwal A, et al. Normal thymic architecture and negative selection are associated with Aire expression, the gene defective in the autoimmune-polyendocrinopathy syndrome type I and Addison’s disease. J Clin Invest. 1976;92:2377. doi: 10.4049/jimmunol.165.4.1976. [DOI] [PubMed] [Google Scholar]
- 38.Halonen M, Pelto-Huikko M, Eskelin P, et al. Subcellular location and expression pattern of autoimmune regulator (Aire), the mouse orthologue for human gene defective in autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) J Histochem Cytochem. 2001;49:197–208. doi: 10.1177/002215540104900207. [DOI] [PubMed] [Google Scholar]
- 39.Nagamine K, Peterson P, Scott HS, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 40.Bjorses P, Halonen M, Palvimo JJ, et al. Mutations in the AIRE gene: effects on subcellular location and transactivation function of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protein. Am J Hum Genet. 2000;66:378–392. doi: 10.1086/302765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott HS, Heino M, Peterson P, et al. Common mutations in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients of different origins. Mol Endocrinol. 1998;12:1112–1119. doi: 10.1210/mend.12.8.0143. [DOI] [PubMed] [Google Scholar]
- 42.Wang CY, Davoodi-Semiromi A, Huang W, et al. Characterization of mutations in patients with autoimmune polyglandular syndrome type 1 (APS1) Hum Genet. 1998;103:681–685. doi: 10.1007/s004390050891. [DOI] [PubMed] [Google Scholar]
- 43.Saugier-Veber P, Drouot N, Wolf LM, et al. Identification of a novel mutation in the autoimmune regulator (AIRE-1) gene in a French family with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Eur J Endocrinol. 2001;144:347–351. doi: 10.1530/eje.0.1440347. [DOI] [PubMed] [Google Scholar]
- 44.An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 45.Pitkanen J, Doucas V, Sternsdorf T, et al. The autoimmune regulator protein has transcriptional transactivating properties and interacts with the common coactivator CREB-binding protein. J Biol Chem. 2000;275:16802–16809. doi: 10.1074/jbc.M908944199. [DOI] [PubMed] [Google Scholar]
- 46.Pearce SH, Cheetham T, Imrie H, et al. A common and recurrent 13-bp deletion in the autoimmune regulator gene in British kindreds with autoimmune polyendocrinopathy type 1. Am J Hum Genet. 1998;63:1675–1684. doi: 10.1086/302145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominguez M, Crushell E, Ilmarinen T, et al. Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in the Irish population. J Pediatr Endocrinol Metab. 2006;19:1343–1352. doi: 10.1515/jpem.2006.19.11.1343. [DOI] [PubMed] [Google Scholar]
- 48.Heino M, Scott HS, Chen Q, et al. Mutation analyses of North American APS-1 patients. Hum Mutat. 1999;13:69–74. doi: 10.1002/(SICI)1098-1004(1999)13:1<69::AID-HUMU8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 49.Halonen M, Eskelin P, Myhre AG, et al. AIRE mutations and human leukocyte antigen genotypes as determinants of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy phenotype. J Clin Endocrinol Metab. 2002;87:2568–2574. doi: 10.1210/jcem.87.6.8564. [DOI] [PubMed] [Google Scholar]
- 50.Khosla S. Hypercalcemia and hypocalcemia. In: Fauci AS, Kasper DL, Hauser SL, et al., editors. Harrison’s Principles of Internal Medicine. 17 ed. New York: McGraw Hill: 2008. pp. 286–287. [Google Scholar]