Abstract
Background: Calcinosis cutis—the deposition of insoluble calcium salts in the skin and the soft tissue—occurs in the following five settings: calciphylaxis, dystrophic, iatrogenic, idiopathic, and metastatic. Idiopathic calcinosis cutis of the penis is rare. Purpose: This paper describes a man with idiopathic calcinosis cutis of the penis, summarizes the clinical features of previously reported men with this condition, and also reviews dystrophic, iatrogenic, and metastatic penile calcinosis. Methods: A 27-year-old Pakistan man presented with concurrent, asymptomatic, individual nodules on the right mid-ventral penile shaft and left side of scrotum and two additional papules on the right side of the scrotum. Evaluation and treatment included the excision of all lesions. Reports of patients with penile calcinosis were identified using a medical search engine (PubMed Central) and referenced citations from the published papers on this subject. Results: Microscopic examination of the patient's nodules showed idiopathic and dystrophic calcinosis cutis of the penis and scrotum, respectively; the scrotal papules were fibroepithelial polyps. Including this individual, idiopathic calcinosis cutis of the penis has only been reported in 11 men. It presents as either an asymptomatic nodule (5 patients) or multiple lesions (6 patients) of less than one-year duration, on either the penile shaft (distal in 4 patients, mid in 2 patients, both in 1 patient, and site unspecified in 1 patient) or the prepuce (3 patients) of uncircumcised men less than 30 years of age. Concurrent scrotal calcification was noted in two patients. Dermal deposits of calcium are found in the dermis—often with surrounding histiocytes and multinucleated giant cells; concurrent features of dystrophic penile shaft calcification, such as calcium within syringomas or transepidermal elimination of calcium through eccrine sweat ducts, was only noted in two men. The nodules do not recur following excision. Conclusion: Idiopathic calcinosis cutis of the penis is extraordinary and has only been reported in 11 men. It presents as an asymptomatic nodule or nodules on mid- to distal penile shaft or foreskin. Concurrent scrotal calcinosis cutis was noted in two men. Microscopic examination shows calcium deposits in the dermis, usually with associated histiocytes and multinucleated giant cells; concurrent changes of dystrophic calcification were also present in two men. Excision of the penile nodules not only provides the diagnosis, but also successfully resolves the condition without recurrence.
Calcinosis cutis is a condition in which insoluble calcium salts are deposited in the skin and subcutaneous tissue.1,2 Penile shaft calcinosis is rare. The authors present the case of a 27-year-old man with concurrent calcinosis cutis of the penis and the scrotum whose condition adds further insight into the pathogenesis of calcinosis cutis occurring at these locations.
Case Report
A 27-year-old healthy Pakistan man presented with a six-month history of an asymptomatic nodular lesion on the penile skin. Beginning one year ago, he also developed other asymptomatic lesions on his left and right scrotum. He had no systemic symptoms and there was no history of either trauma or an inflammatory process affecting the penis or the scrotum.
Clinical examination showed a firm, smooth, 8x6mm, reddish-brown to flesh-colored nodule covered by normal skin on the right mid-ventral shaft of the penis lateral to the median raphe (Figure 1). There was a hard, smooth, 5x5mm, reddish-brown to flesh-colored nodule on the left side of the scrotum (Figure 2) and two smaller, soft, flesh-colored (3x3mm and 2x2mm) papules on the right side of the scrotum (Figure 3). The remaining physical examination revealed no other physical abnormalities.
Figures 1A and 1B.

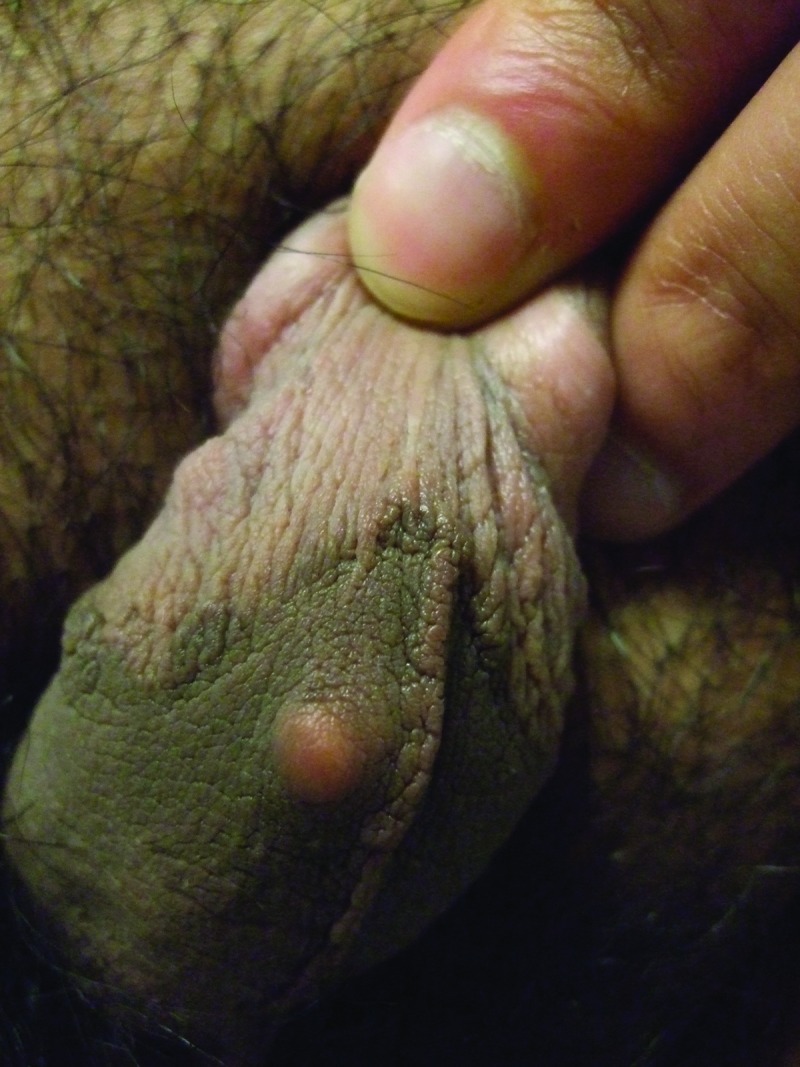
Distant and closer views of idiopathic calcinosis cutis of the penis presenting as an asymptomatic smooth firm reddish-brown to flesh-colored nodule on the right side of the mid ventral penile shaft in a 27-year-old man.
Figures 2A and 2B.

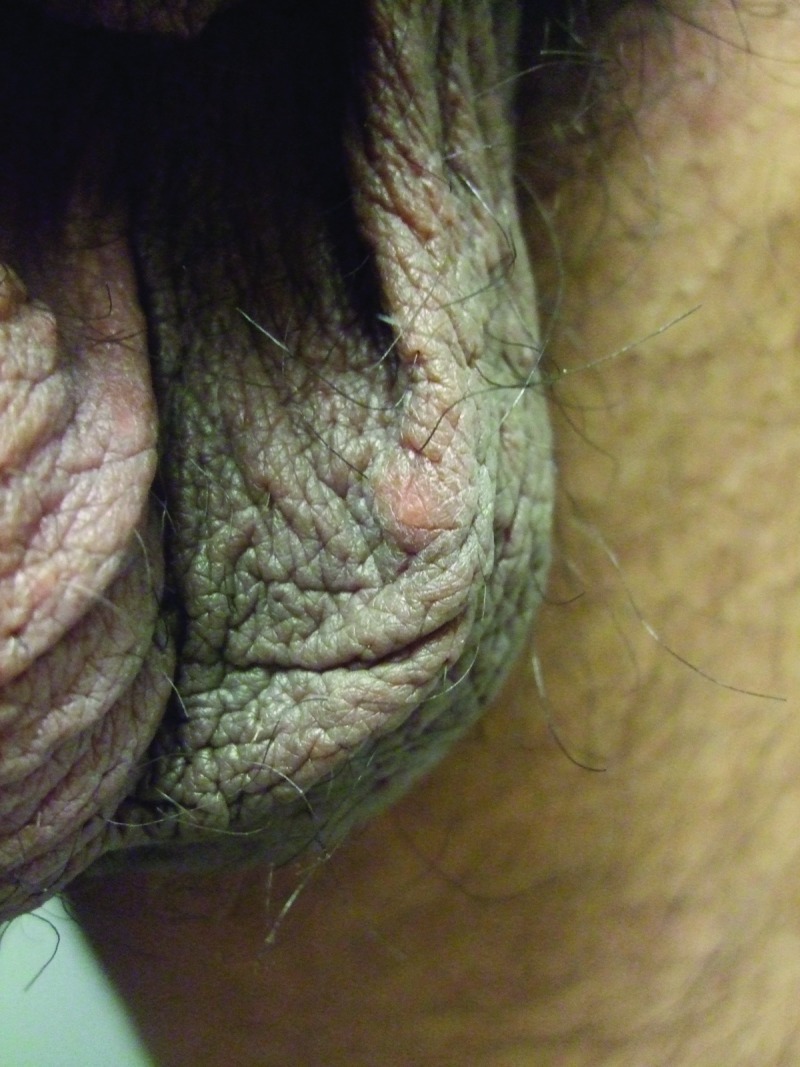
Distant and closer views of concurrently present scrotal calcinosis in the same 27-year-old man appearing as an asymptomatic smooth hard reddish-brown to flesh-colored nodule on the left side of the scrotum.
Figures 3A and 3B.
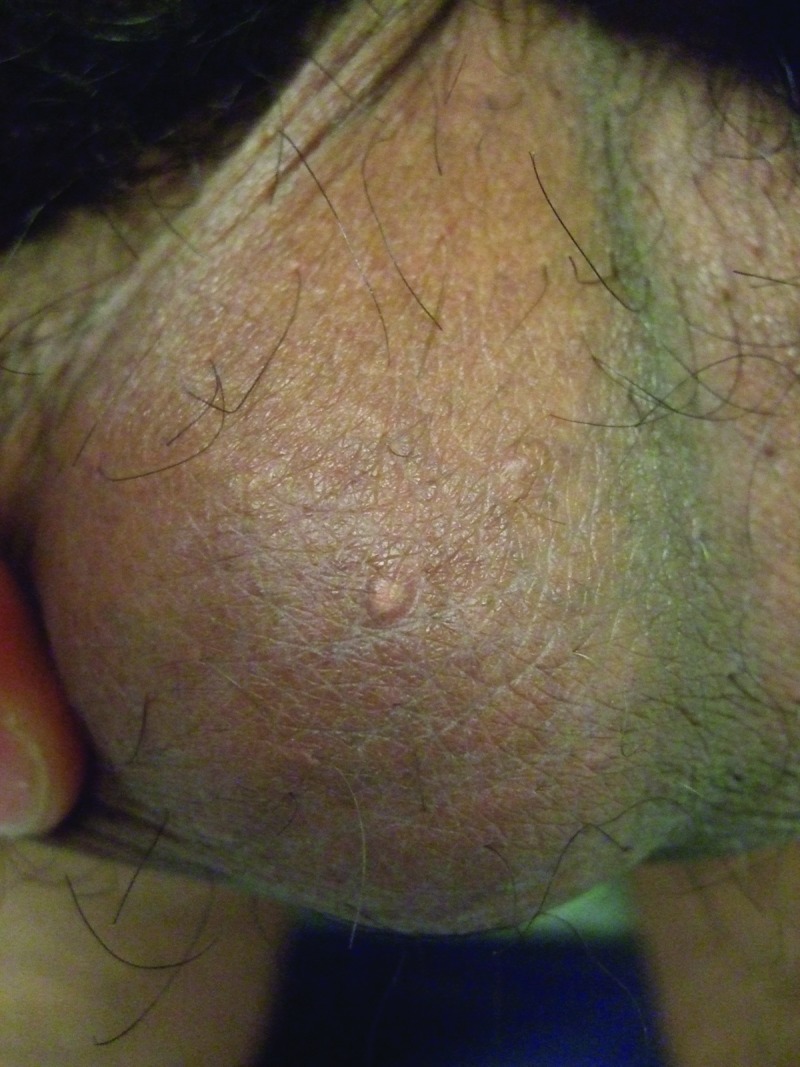
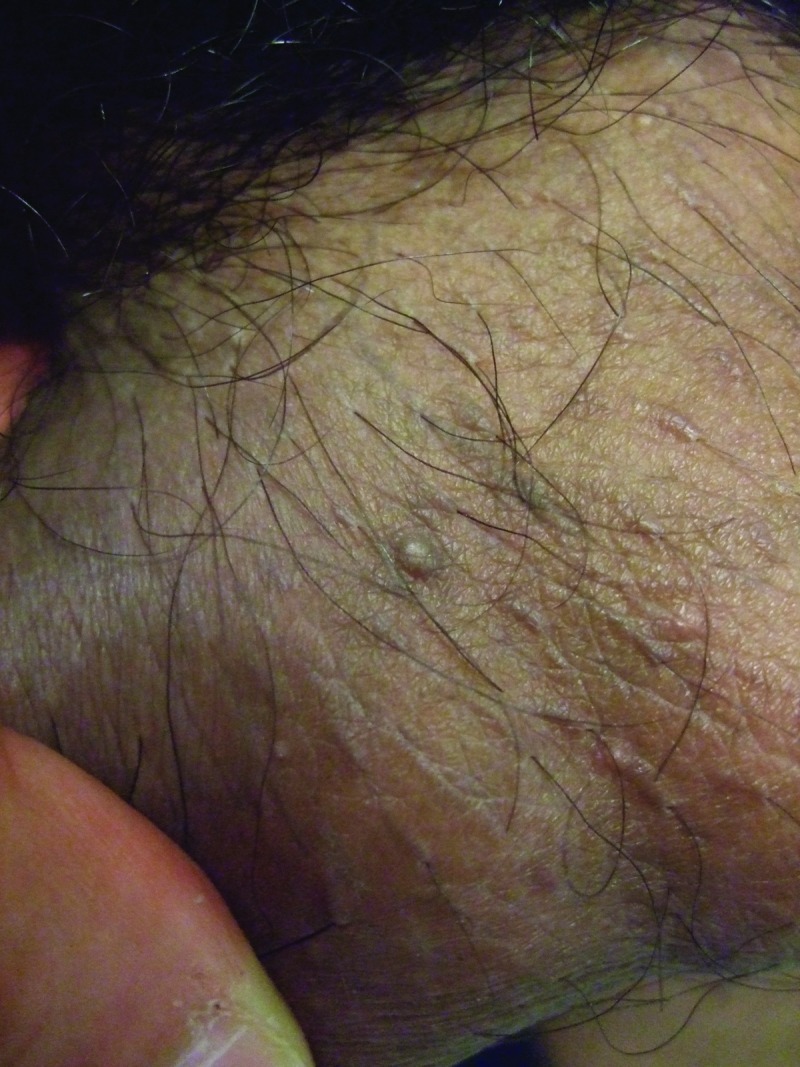
Larger and smaller, biopsy-confirmed, fibroepithelial polys that were also concurrently present on the right side of the 27-year-old man's scrotum.
The lesions were excised under local anesthesia. Microscopic examination of the penile nodule showed a normal epidermis and deposits of basophilic staining calcium crystals in the dermis; there was neither fibrotic tissue nor inflammation surrounding the calcium. Neither cysts nor adnexal structures were observed in the multiple deeper sections that were examined (Figure 4).
Figures 4A and 4B.
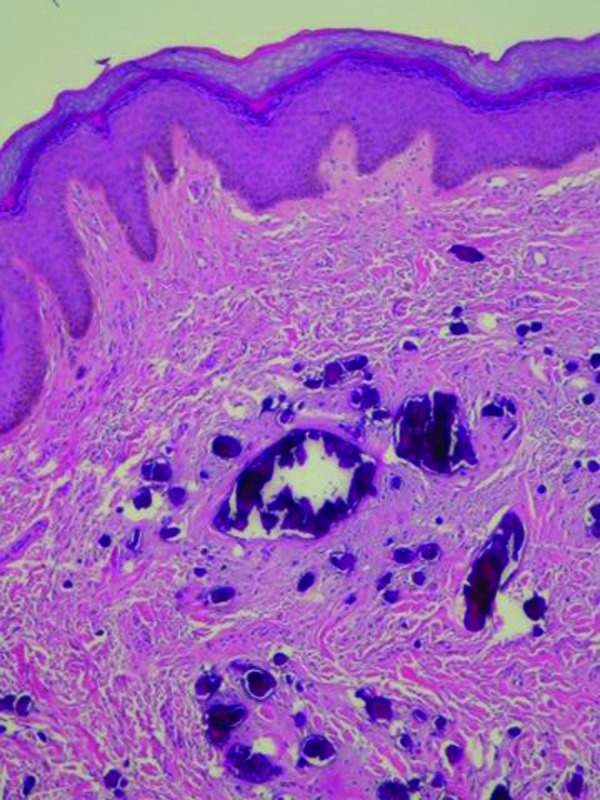
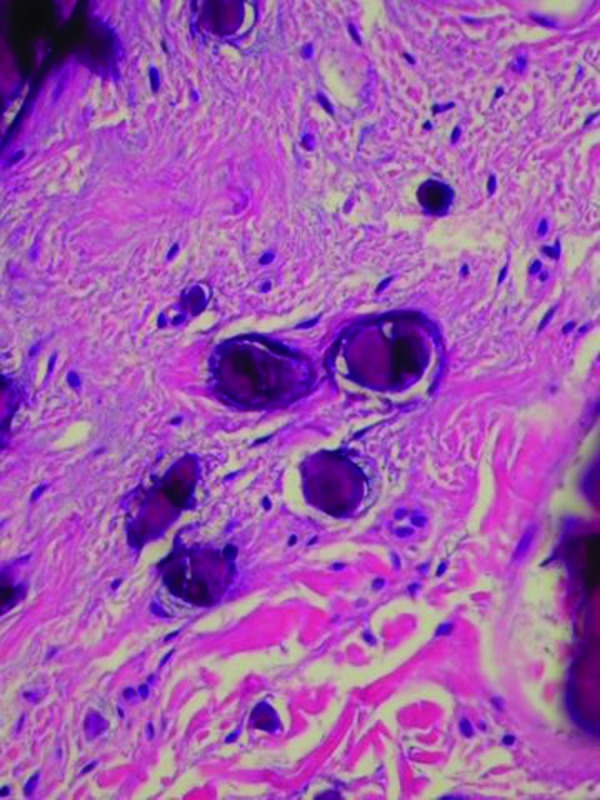
Microscopic examination—lower (left) and higher (right) magnification—of the penile nodule shows basophilic-staining deposits of calcium crystal in the dermis without any surrounding epithelial lining or inflammation (hematoxylin and eosin: X4; X20).
The left scrotum nodule showed a single aggregate of basophilic staining calcium crystal in the dermis surrounded by an epithelial lined cystic structure. Eccrine structures, composed of similar-appearing epithelium, were located adjacent to the calcium-containing cystic lesion in the dermis. There was no inflammation (Figure 5). Both of the papules on the right scrotum showed edema between the collagen bundles consistent with fibroepithelial polyps. Complete healing of the excision sites occurred. At follow up three months later, no new penile or scrotal lesions were detected.
Figures 5A and 5B.
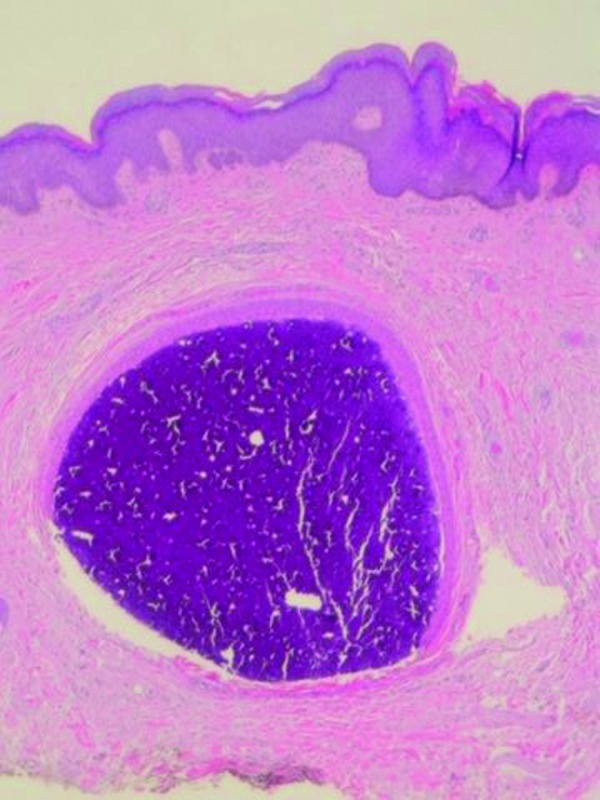
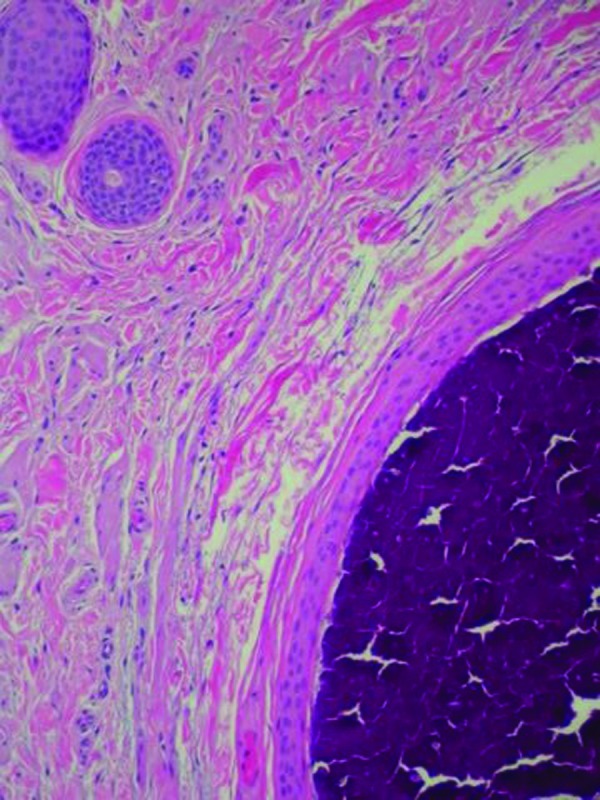
Microscopic examination—lower (left) and higher (right) magnification—of scrotal nodule shows basophilic-staining calcified mass surrounded by an epithelial lining in the dermis; eccrine structures, composed of similar appearing cells as the epithelial lining surrounding the amorphous mass of calcium, are present in the adjacent dermis (hematoxylin and eosin: X4; X10).
Discussion
Calcinosis cutis has previously been divided into the following four subtypes: dystrophic (following local tissue damage in people with normal serum calcium and phosphate levels), iatrogenic (resulting from a therapy-associated side effect), idiopathic (occurring in individuals without a metabolic disorder or lesion-related tissue trauma), and metastatic (presenting in patients with abnormal calcium and/or phosphate metabolism, which promotes calcium precipitation).1 Calciphylaxis—with calcification of the small vessels in the dermis and subcutaneous fat—has recently been added as a fifth category of calcinosis cutis.2 Calcinosis cutis may present with penile shaft lesions, scrotal nodules, or both.
Calcinosis of the scrotum is not uncommon; however, the etiology for this condition remains to be definitively established. Pathological findings vary and support either a dystrophic etiology (when an epithelial-lined cystic space surrounds the calcium)3–8 or an idiopathic etiology (when there is free calcium crystals in the dermis with or without adjacent inflammation).9,10 More recently, several investigators have postulated that in some men with 'idiopathic' calcinosis cutis of the scrotum, the mechanism of pathogenesis may indeed be dystrophic calcification of the dartos muscle.11–13 Albiet less commonly observed in women, calcinosis of the vulva—which occasionally presents with pruritus of the labia—is an analogous condition to scrotal calcinosis.14–16
Calcinosis of the penis is rare. In patients with end stage renal disease on dialysis, calcinosis of the penile shaft vessels has been observed and is favored to be metastatic in etiology (Table 1).17–21 Less commonly, dystrophic22–29 or iatrogenic30,31 calcinosis cutis of the soft tissue of the penis has also been noted (Tables 2 and 3).22–31
TABLE 1.
Characteristics of end stage renal disease patients with penile calcinosis cutisa
| INCIDENCE |
| 19% (6 of 32 patients) |
| ONSET AGE |
| 32–58 years; median=56 years |
| DIALYSIS TYPE |
| Hemodialysis (6 patients): onset after 17 to 82 months; median=52 months |
| Peritoneal dialysis (1 patient: 27 months of prior hemodialysis): onset after 30 months |
| PRIMARY DIAGNOSIS |
| Nephrosclerosis (3 patients) |
| Nephropathy: analgesic (1 patient) and diabetic (1 patient) |
| Chronic glomerulonephritis (1 patient) |
| MECHANISM OF PATHOGENESIS |
| Metastatic calcinosis cutis associated with linear calcification of the penile artery |
| ASSOCIATED COMPLICATIONS |
| Erectile dysfunction: 100% (6 patients)b |
| Diffuse bone demineralization: 100% (6 patients) |
| Secondary hyperparathyroidism (roentgenogram evidence): 100% (6 patients) |
| Serum alkaline phosphatase level elevation: 100% (6 patients)c |
| Serum parathyroid hormone elevation: 33% (2 patients) |
| Gangrene of penis: 17% (1 patient)d |
Data summarized from reference 17.
There was no improvement in sexual function despite intensive hemodialysis, use of phosphorus-binders, and/or subtotal parathyroidectomy (2 patients).
The patients' serum alkaline phosphatase averaged 230 IU (normal=40–130 IU).
TABLE 2.
Conditions associated with dystrophic calcification of the penile shaft
Peyronie's disease is a localized connective tissue disorder characterized by single or multiple areas of fibrosis involving the tunica albugine and the corpus cavernosum of the penis. Clinical presentation varies from asymptomatic palpable nodules to painful erection with marked penile deformity and—in several patients—impotentia coeundi secondary to impeded or precluded intercourse. One study of 66 consecutive patients with Peyronie's disease noted dystrophic calcification in 33% (22) of the patients.22 Another investigator—evaluating a larger group of patients—noted calcification in 25% of patients with Peyronie's disease.23 These studies confirm earlier observations of penile calcification in Peyronie's disease in which either ultrasound imaging24 or computed tomography25 were utilized. The recent studies also support the proposed mechanism of dystrophic calcification in Peyronie's disease that were based on scanning and transmission electron micrograph findings performed on excised penile plaques from men with the condition.26
A 22-year-old Japanese man with a one-year history of asymptomatic penile shaft calcinosis that presented as a gradual increasing nodule accompanied by many smaller papules demonstrated histological features of both idiopathic calcinosis cutis (with nodular collections of basophilic, von Kossa-positive calcific material in the dermal connective tissue without any epithelial cell lining them) and dystrophic calcinosis cutis (with syringoma or sweat duct milia with epithelial-lined cystic structures partially or completely filled with calcific deposits). Similar changes have been noted in patients with scrotal calcinosis cutis.3
Trauma to the penis with subsequent calcification or ossification of the penis is uncommon. Hecker, in 1844, reported a man who developed a penile ossification four months after severe trauma to his penis.28 More recently, Joffe described a 53-year-old African man with trauma-induced radiographically confirmed calcification of the penis: 2 years earlier, he was the recipient of a kick on the penis; 18 months later he became aware of an asymptomatic “hardness of the penis” and approximately six months thereafter “he had began to experience slight pain when the organ became turgid.”29
TABLE 3.
Conditions associated with iatrogenic calcification of the penile shaft
Reversible penile calcifications associated with bleomycin-induced pulmonary toxicity was described in a 55-year-old White man whose diffuse histiocytic lymphoma was treated with combination chemotherapy consisting of cyclophosphamide, adriamycin, vincristine, bleomycin, and prednisone. Prior to treatment, he had hypercalcemia, which rapidly regressed after receiving the initial treatment in January 1974. Two months later, in March 1974, he developed rales and progressively worsening dyspnea; a chest roentgenogram revealed pulmonary infiltrates and bleomycin pulmonary toxicity was clinically suspected and confirmed with an open lung biopsy. He was treated with prednisone and oxygen and was able to resume chemotherapy (without bleomycin) in May 1974; his lymphoma entered complete remission but the basilar rales and diffuse infiltrative process on chest roentgenogram persisted. In May 1974, the patient noticed painless rigidity of the proximal third of his penis; a roentgenogram of the organ showed calcification to be located within the sinusoids of the corpora cavernosa and about the periphery of the corpora cavernosa. By July 1974 his pulmonary symptoms had improved and steroids were withdrawn; in August 1974, a 67 gallinium scan of his lungs (which had shown increased uptake in March 1974) showed normal lung uptake. In November 1974, his proximal penile rigidity was gone and the penile calcification was almost totally resolved; only minimal calcium in the mid-portion of he penis was noted on roentgenogram. Also in November 1974, only a few basilar rales were present and his chest roentgenogram only showed a few chronic changes at the bases.
Intracavernosal self-injection of vasoactive drugs has been used in the treatment of erectile dysfunction. Pery et al, in 1990, reported penile induration in 6 of 120 patients with erectile dysfunction who practiced intracavernosal self-injection of papaverine-phentolamine; cavernosal calcifications were demonstrated by means of roentgenogram or ultrasound or both in 4 of these 6 individuals. The patients ranged in age from 31 to 63 and the duration of treatment prior to presenting with a palpable penile mass was 6 to 25 months with a frequency of 1 to 3 injections per week. The dose, injected each time, was individually adjusted: 4–40mg of papaverine and 0.1–2.0mg of phentolamine. The indurations in the 6 patients were painless, found at the site of injection, measured 10–15mm in diameter, and did not cause deviation of the penis. In 4 of these patients, amorphous calcifications (up to 1.5cm in diameter) developed that were localized in the intracavernosal septum, the tunica albuginea, or the corpus cavernosum. The calcifications were of various shapes and sizes, single or multiple, and linear or geographic. In those patients with multiple calcifications, the site of calcification was not always related to the site of injection and induration. None of the patients with penile calcification and/or induration complained of responsiveness to the self-injection of papaverine and no increase in dosage was required.
Idiopathic calcinosis cutis of the penis, to the best of the authors' knowledge, has only been reported in 11 men—including the patient described herein (Table 4).27,32–40 The age of diagnosis ranged from 10 to 29 years (mean=19 years; median=19 years). The lesion was initially noted 6 to 12 months (mean=9 months; median=8 months) prior to the patient seeking medical evaluation.
TABLE 4.
| CASE | A | D | NL | SOL | MORPHOLOGY OF LESIONS | LOP | C | REF |
|---|---|---|---|---|---|---|---|---|
| 1c | 10 | 12 | >20 | Ps, Ph–Pp | Firm, white Ps papules (Prep, S, and thighs); Ph-Pp lesions (IF) | Prep, Sc, thighs | ? | 32 |
| 2 | 13 | 9 | 2 | 10x10 | Nodules | DS,MS | — | 33 |
| 3d | 16 | S | ≥2 | 15x15 | Hard nodules | S-NOS | ? | 34 |
| 4e | 16 | 8 | >5 | 4x4 | Smooth and hard (largest central pap) | DS | — | 35 |
| 5f | 16 | >6 | S | 2x2 | White tan firm papules | S-NOS | ? | 36 |
| 6 | 19 | 7 | 1 | 10x10 | Hard nodule | DS | — | 37 |
| 7 | 21 | 10 | 1 | 15x10 | Hard, bilobular nodule | Prep | — | 38 |
| 8g | 22 | 12 | >20 | 17x13, punct | Soft, skin-colored, with irregular surface nodule (1); punct yellowish papules (many) | MS | — | 27 |
| 9 | 25 | 6 | 1 | 20x15 | Nodule | Prep | — | 39 |
| 10h | 27 | 6 | 1 | 8x6 | Firm smooth reddish-brown to flesh-colored nodule | MS, Sc, VS, | — | CR |
| 11 | 29 | 11 | 1 | 10x10 | Firm flat hard nodular mass | DS | ? | 40 |
Abbreviations: A=age at diagnosis (years); C=circumcised; CR=current report; D=duration of lesion (months); DS=distal shaft; IF=inguinal folds (lesions located superior and inferior); LOP=location on penis; MS=mid shaft; NL=number of lesions on penis; Pap=papule; Ph=pinhead; PP=pinpoint; Prep=prepuce (foreskin); Ps=pea-sized; punct=punctiformis; Ref=reference; S=several; Sc=scrotum; SM=several months; SOL=size of lesions (millimeters); S-NOS=shaft-not otherwise specified; VS=ventral shaft; — = no; ?=not reported
For all of the patients, there was either no (or no reported): (1) symptoms for the lesions, (2) history of trauma or inflammation to the penis, (3) family history of similar lesions, and (4) abnormality of calcium or phosphorus serum level. Excision of the lesion (in 8 patients) not only provided tissue for pathology evaluation but also resulted in successfully treated the lesion without subsequent recurrence27,33,34,37–40,CR; biopsy only, for diagnosis confirmation, was performed in three patients.32,35,36 Microscopic examination of the lesion, in all patients, showed deposits of calcium (without surrounding epithelial-lined structures) in the dermis.
There were about 20 of the Ps paps and innumerable Ph-pp size lesions. In addition to the penis, lesions were not only present on the thighs but also the scrotum. In addition to the calcium deposits filling the papillary dermis, they were also “penetrating through the thickened epidermis and sweat ducts.”32
The nodules increased to the size of “hazels” they were whitish and brittle at cross-section.
“Examination revealed a 4mm diameter smooth hard papule on the shaft of the penis surrounded by a cluster of smaller papuless.”
The Caucasian patient had papules that were “ranging in size from 1–2mm in diameter…[and]…appeared clinically to be closed comedones or molluscum lesion.”
The Japanese patient had one nodule that was gradually increasing in size accompanied by small papules; four histological patterns were observed: (1) cystic structure, compatible with syringoma or sweat duct milia, filled with homogenous eosinophilic, von Kossa-negative contents; (2) similar homogeneous eosinophilic material-filled cystic 'syringoma' structure which also contains some basophilic, von Kossa-positive calcific deposits; (3) similar cystic 'syringoma' structure that is completely filled with amorphous, basophilic, von Kossa-positive calcific mass (and no eosinophilic material); and (4) numerous nodular collections of basophilic calcific material surrounded by thick fibrosis in the dermal connective tissue.
The Pakastan patient had a calcified nodule not only on his penile shaft but also on his scrotum; in contrast to the penile lesion which only demonstrated calcium crystals in the dermis, the scrotal lesion showed an epithelial-lined cystic structure that was completely filled with the basophilic calcified mass in the dermis and adjacent eccrine structures composed of similar-appearing epithelium. In addition, he had two fibroepithelial polyps that presented as small papules on the right scrotum.
Idiopathic calcinosis cutis of the penis lesions typically presented as firm, flesh-colored, asymptomatic nodules of the distal penile shaft or foreskin. The nodules were often progressively increasing in size. Most of the men were uncircumcised.
Examination often reveals a single large nodule ranging in greatest diameter from 1 to 2cm. However, 55 percent of the patients had multiple lesions—either two (1 man),33 greater than or equal to two, which had “developed to the size of hazels” (1 man),34 or more than five (4 men).27,32,35,36 In the latter group of men, the lesions presented either as a larger central “…4mm diameter smooth hard papule on the shaft of the penis surrounded by a cluster of smaller papules”35 or as “several…papules on the shaft of his penis…[which]…appeared clinically to be closed comedones or molluscum”36 or as “20 pea-sized firm white papules on both the upper thighs, scrotum and foreskin…presenting as milia”32 or as a soft, skin-colored “…asymptomatic nodule…accompanied by many punctiformis yellowish papules”.27
The diagnosis of calcinosis cutis was biopsy confirmed in all patients. There were small or large aggregates of calcium deposits in the dermis. Inflammation surrounding the dermal calcification—consisting of histiocytes and foreign body giant cells or lymphocytes or both—was present in 82 percent of the lesions. The calcium aggregates were also surrounded by fibrosis in five individuals.27,35,37,38,40 There was neither an epithelial lining surrounding the calcium nor adjacent adnexal structures with the exception of one man27; however, one patient's lesion also demonstrated transepidermal elimination of the calcium either directly through the epidermis or within eccrine sweat ducts.32
The deposits of calcium were basophilic on hematoxylin and eosin stained tissue sections. Several of the investigators confirmed the identity of the dermal deposits as calcium by demonstrating black staining of the material using the von Kossa stain.32,37–40 In addition, some of the researchers excluded epithelial-lined or adnexal structures by demonstrating negative results using either special stains (periodic acid-Schiff and alcian blue)39 or an immunoperoxidase stain (AE1/AE3) for cytokeratin.37,38,40
The suspected idiopathic etiology for the dermal calcification was further supported by demonstrating normal levels of calcium, phosphorus, and parathyroid hormone in several of the patients.27,32,33,35–38,40 None of the patients had a prior history of end stage renal disease, hyperparathyroidism, Peyronie's disease, or trauma to their penis.
The calcinosis cutis was restricted to the penile shaft in 82 percent (9/11) of the patients. However, two patients had additional sites of calcinosis. One child had “…about 20 pea-sized firm white papules on both upper thighs, scrotum, and foreskin for a year. Innumerable pinpoint size lesions were [also] located superior and inferior to the inguinal folds”.32 The photomicrograph of a biopsied lesion demonstrated not only “dermal papillary calcium deposits beneath an acanthotic epidermis [but also] calcium deposits eliminate transepidermally through a sweat duct”32; however, the site of biopsy—either thighs, scrotum, or penis—was not specified.
The reported patient also had calcinosis cutis of his penis and left scrotum. Microscopic examination of his scrotal nodule showed an enlarged epithelial-lined structure in the dermis containing a larger aggregate of calcium. There was not any surrounding inflammatory response; however, several sweat gland ductal structures were also noted adjacent to the calcified lesion. Concurrent dystrophic calcification of syringoma or sweat duct milia and idiopathic calcinosis within the soft tissue of the penile shaft has also been observed in one patient.27
Interestingly, the reported patient also had two fibroepithelial polyps on his right scrotum. These presented at the same time as his other scrotal and penile lesions. Indeed, the patient was concerned that his skin lesion represented a possible sexually transmitted disease; serologic evaluation was negative for syphilis (rapid plasma reagin) and HIV infection. Hence, his scrotal achrochordons were favored to represent a coincidental occurrence and not to be associated with his calcinosis cutis.
The clinical differential of idiopathic calcinosis cutis of the penis predominantly includes either an epidermal inclusion cyst33,35 or milia.32 Other papular penile lesions include either a benign adnexal tumor, verruca vulgaris, or molluscum contagiosum.36 Also, some of the patients inquired about the possibility of a sexually transmitted disease—such as a condyloma acuminata. An excisional biopsy not only establishes the diagnosis, but also effectively results in removal of the lesion without recurrence.
The clinical findings in all of the men with idiopathic calcinosis cutis of the penis exclude either a metastatic or an iatrogenic etiology for the condition. The individuals are otherwise healthy with normal levels of serum calcium, phosphorus, and parathyroid hormone, thereby eliminating a metabolic mechanism of calcinosis cutis. Similarly, an iatrogenic etiology can be eliminated since none of the patients received either intravenous bleomycin or intrapenile papaverine.
The absence of a prior history of trauma to the affected sites and the pathological findings of calcium without evidence of an epithelial cyst or adnexal lining surrounding the dermal calcification also supports the idiopathic pathogenesis of cutaneous calcification in all of the patients with this form of penile calcinosis cutis. However, the concurrent presence of dystrophic calcinosis cutis of the scrotum (2 patients) or the penile shaft (1 patient) is unusual. Indeed, calcinosis cutis in these unique individuals raises the possibility that more than one mechanism of pathogenesis of calcinosis cutis—idiopathic (with or without dystrophic) for the penis and dystrophic for the scrotum—can coincidentally occur in the same individual.
Conclusion
Idiopathic calcinosis cutis of the penis is a benign, albeit rare, condition appearing as asymptomatic nodules on the mid to distal penile shaft or foreskin of predominantly uncircumcised men. It presented as a single, enlarging mass in nearly half of the individuals; however, six patients had multiple nodules and one-third of the men had more than five lesions. Microscopic examination usually reveals dermal deposits of calcium typically associated with inflammation consisting of histiocytes and multinucleated giant cells. In most of the patients, adnexal structures were not present and an epithelial lining did not surround the calcification. Yet, two patients concurrently demonstrated either calcification within syringomas or trans-epidermal elimination of calcium through eccrine sweat ducts. Excision of the lesion not only provides successful resolution without recurrence but also establishes the diagnosis. Correlation of the clinical history and presentation with the pathological findings excludes either a metabolic disorder (with elevated calcium, phosphorus and/or parathyroid serum levels) or an iatrogenic etiology (from systemic or local drug administration) in patients with idiopathic calcinosis cutis of the penis; however, in some individuals, concurrent dystrophic calcinosis cutis of either the penis or the scrotum may be present.
Footnotes
DISCLOSURE:The authors report no relevant conflicts of interest.
References
- 1.Walsh JS, Fairley JA. Calcifying disorders of the skin. J Am Acad Dermatol. 1995;33:693–706. doi: 10.1016/0190-9622(95)91803-5. [DOI] [PubMed] [Google Scholar]
- 2.Reiter N, El-Shabrawi L, Leinweber B, et al. Calcinosis cutis: Part I. Diagnostic pathway. J Am Acad Dermatol. 2011;65:1–12. doi: 10.1016/j.jaad.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 3.Dare AJ, Axelsen RA. Scrotal calcinosis: origin from dystrophic calcification of eccrine duct milia. J Cutan Pathol. 1988;15:142–149. doi: 10.1111/j.1600-0560.1988.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 4.Ronchese F. Calcification and ossification of steatomas of the scrotum: report of a case. Arch Dermatol. 1944;49:12–15. [Google Scholar]
- 5.Swinehart JM, Golitz LE. Scrotal calcinosis: dystrophic calcification of epidermoid cysts. Arch Dermatol. 1982;118:985–988. doi: 10.1001/archderm.118.12.985. [DOI] [PubMed] [Google Scholar]
- 6.Akosa AB, Gillliland EA, Ali MH, Khoo CTK. Idiopathic scrotal calcinosis: a possible aetiology reaffirmed. Br J Plast Surg. 1989;42:324–327. doi: 10.1016/0007-1226(89)90155-0. [DOI] [PubMed] [Google Scholar]
- 7.Ruiz-Genao DP, Rios-Buceta L, Herrero L, et al. Massive scrotal calcinosis. Dermatol Surg. 2002;28:745–747. doi: 10.1046/j.1524-4725.2002.02022.x. [DOI] [PubMed] [Google Scholar]
- 8.Saad AG, Zaatari GS. Scrotal calcinosis: is it idiopathic? Urology. 2001;57:365xi–365xiii. doi: 10.1016/s0090-4295(00)01007-4. [DOI] [PubMed] [Google Scholar]
- 9.Wright S, Navsaria H, Leigh IM. Idiopathic scrotal calcinosis is idiopathic. J Am Acad Dermatol. 1991;24:727–730. doi: 10.1016/0190-9622(91)70110-n. [DOI] [PubMed] [Google Scholar]
- 10.Fisher BK, Dvoretzky I. Idiopathic calcinosis of the scrotum. Arch Dermatol. 1978;114:957. [PubMed] [Google Scholar]
- 11.King DT, Brosman S, Hirose FM, Gillespie LM. Idiopathic calcinosis of scrotum. Urology. 1979;14:92–94. doi: 10.1016/0090-4295(79)90225-5. [DOI] [PubMed] [Google Scholar]
- 12.Kelten EC, Akbulut M, Colakoglu N, et al. Scrotal calcinosis: is it idiopathic or dystrophic? Aegean Pathology J. 2005;2:4–7. [Google Scholar]
- 13.Pabuccuoglu U, Canda MS, Guray M, et al. The possible role of dartoic muscle degeneration in the pathogenesis of idiopathic scrotal calcinosis [letter] Br J Dermatol. 2003;148:827–829. doi: 10.1046/j.1365-2133.2003.05251.x. [DOI] [PubMed] [Google Scholar]
- 14.Jamaleddine FN, Salman SM, Shbaklo Z, et al. Idiopathic vulvar calcinosis: the counterpart of idiopathic scrotal calcinosis. Cutis. 1988;41:273–275. [PubMed] [Google Scholar]
- 15.Bernardo BD, Huettner PC, Merritt DF, Ratts VS. Idiopathic calcinosis cutis presenting as labial lesions in children: report of two cases with literature review. J Pediatr Adolesc Gynecol. 1999;12:157–160. doi: 10.1016/s1038-3188(99)00010-8. [DOI] [PubMed] [Google Scholar]
- 16.Balfour PJT, Vincenti AC. Idiopathic vulvar calcinosis. Histopathology. 1991;18:183–184. doi: 10.1111/j.1365-2559.1991.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 17.Dalal S, Gandhi VC, Yu AW, et al. Penile calcification in maintenance hemodialysis patients. Urology. 1992;40:422–424. doi: 10.1016/0090-4295(92)90455-6. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi VC, Pakkala BD, Bhate DV, et al. Penile vascular calcification in patients treated with maintenance dialysis [abstract]. The American Society of Nephrology, Washington, DC, November 21-22, 1977. Kidney Int. 1977;12:481. [Google Scholar]
- 19.Ashouri OS, Perez RA. Vascular calcification presenting as necrosis of penis in patient with chronic renal failure. Urology. 1986;28:420–423. doi: 10.1016/0090-4295(86)90077-4. [DOI] [PubMed] [Google Scholar]
- 20.Massry SG, Coburn JW. Divalent ion metabolism and renal osteodystrophy (Chapter 11) In: Massry SG, Sellers AL, editors. Clinical Aspects of Uremia and Dialysis. Springfield, IL: CC Thomas Co.; 1976. pp. 304–387. [Google Scholar]
- 21.Marcen R, Moreno J, Garcia F, et al. Os penis in a hemodialysis patient. Eur Urol. 1987;13:132–133. doi: 10.1159/000472753. [DOI] [PubMed] [Google Scholar]
- 22.Gelbard MK. Dystrophic penile calcification in Peyronie's disease. J Urol. 1988;139:738–740. doi: 10.1016/s0022-5347(17)42617-6. [DOI] [PubMed] [Google Scholar]
- 23.Devine CJ., Jr Dystrophic penile calcification in Peyronie's disease [Editorial Comment] J Urol. 1988;139 doi: 10.1016/s0022-5347(17)42617-6. [DOI] [PubMed] [Google Scholar]
- 24.Gelbard MK. Dystrophic penile calcification in Peyronie's disease. J Urol. 1988;139:738–740. doi: 10.1016/s0022-5347(17)42617-6. [DOI] [PubMed] [Google Scholar]
- 25.Rollandi GA, Tentarelli T, Vespier M. Computed tomographic findings in Peyronie's disease. Urol Radiol. 1985;7:153–156. doi: 10.1007/BF02926875. [DOI] [PubMed] [Google Scholar]
- 26.Vande Berg JS, Devine CJ, Jr, Horton CE, et al. Mechanisms of calcification in Peyronie's disease. J Urol. 1982;127:52–54. doi: 10.1016/s0022-5347(17)53599-5. [DOI] [PubMed] [Google Scholar]
- 27.Katoh N, Okabayashi K, Wakabayashi S, et al. Dystrophic calcinosis of the penis. J Dermatol. 1993;20:114–117. doi: 10.1111/j.1346-8138.1993.tb03842.x. [DOI] [PubMed] [Google Scholar]
- 28.Hecker A. A case of trauma to the penis followed by ossification. Archiv fur physiologische Heilkunde. 1844;3:269–272. [Google Scholar]
- 29.Joffe N. Calcification in the penis. Br J Radiology. 1961;34:198–199. doi: 10.1259/0007-1285-34-399-198. [DOI] [PubMed] [Google Scholar]
- 30.Ihde DC, Gormley PE, Francis RS, DeVita VT. Reversible penile calcifications associated with bleomycin (NSC-125066)-induced pulmonary toxicity. Cancer Chemotherapy Reports. 1975;59:1039–1041. [PubMed] [Google Scholar]
- 31.Pery M, Rosenberger A, Kaftori JK, Vardi Y. Intracorporeal calcifications after self-injection of papaverine. Radiology. 1990;176:81–83. doi: 10.1148/radiology.176.1.2191374. [DOI] [PubMed] [Google Scholar]
- 32.Eng AM, Mandrea E. Perforating calcinosis cutis presenting as milia. J Cutan Pathol. 1981;8:247–250. doi: 10.1111/j.1600-0560.1981.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 33.Hutchinson IF, Abel BJ, Susskind W. Idiopathic calcinosis cutis of the penis. Br J Dermatol. 1980;102:341–343. doi: 10.1111/j.1365-2133.1980.tb08151.x. [DOI] [PubMed] [Google Scholar]
- 34.Wozniak F, Maslankiewicz B. Idiopathic calcinosis cutins of the scrotum and penis. Ann Univ Mariae Curie Sklodowska Med. 1990;45:107–109. [PubMed] [Google Scholar]
- 35.Lucke T, Fallowfield M, McHenry P. Idiopathic calcinosis cutis of the penis [letter] Br J Dermatol. 1997;137:1025–1026. doi: 10.1111/j.1365-2133.1997.tb01581.x. [DOI] [PubMed] [Google Scholar]
- 36.Cangelosi J, Matherne R, Sanchez R. Idiopathic calcinosis cutis of the penis. Poster presented at American Society of Dermatopathology 47th Annual Meeting; October 7-10, 2010; Atlanta, Georgia. J Cutan Pathol. 2011;38:174–175. [Google Scholar]
- 37.Sanchez-Merino JM, Bouso-Montero M, Fernandez-Flores A, Garcia-Alonso J. Idiopathic calcinosis cutis of the penis. J Am Acad Dermatol. 2004;51:S118–S119. doi: 10.1016/j.jaad.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 38.Cecchi R, Giomi A. Idiopathic calcinosis cutis of the penis. Dermatology. 1999;198:174–175. doi: 10.1159/000018100. [DOI] [PubMed] [Google Scholar]
- 39.Corsi A, Salvi PF, Diomesi Camassei F, Bosman C. Non-metastatic calcinosis localized in the prepuce. Clinicopathological study of a case and review of the literature. Pathologica. 1997;89:536–539. [PubMed] [Google Scholar]
- 40.Tainwala R, Sharma YK, Gaur N. Idiopathic calcinosis cutis of the penis. Indian Dermatol Online J. 2010;1:36–38. doi: 10.4103/2229-5178.73258. [DOI] [PMC free article] [PubMed] [Google Scholar]


