Introduction
Health hazards from increased lead (Pb) exposure as a result of industrial and environmental pollution are recognized (Juberg et al., 1997). Lead poisoning is considered to be one of the most difficult environmental health problems, since it does not show any unique manifestation during its early stage (Patrick, 2006). Lead has been found to produce wide range of biochemical and physiological dysfunctions in humans and laboratory animals (Courtois et al., 2003). Several mechanisms have been proposed to explain the Pb-induced toxicity. Many investigators proposed that one possible mechanism of Pb toxicity is the disturbance of prooxidant and antioxidant balance by generation of reactive oxygen species (Wang et al. 2001). Adonaylo and Oteiz, (1999) reported that Pb-associated tissue injury in the vital organs is resulted from the oxidative stress. Some studies suggested potential role of antioxidants to ameliorate Pb toxicity (Hsu et al., 1998). The increasing interest in the health properties of tea extract and its main catechin polyphenols have led to a significant rise in scientific investigation for prevention and therapeutics in several diseases (Mandel et al., 2006). Green tea extract (GTE) due to its content of catechins reveals strong antioxidative activity, which is manifested by its ability to inhibit free radical generation, scavenge free radicals and chelate transition metal ions that catalyze free radical reactions (Ostrowska and Skrzydlewska, 2006).
The present study was conducted to investigate the biochemical and histo-pathological effects of Pb toxicity on liver, kidney and brain of rats. Moreover, the antioxidative activity of GTE against oxidative stress induced by Pb toxicity was evaluated. The chelating property of GTE to reduce Pb burden in rat tissues was detected.
Methods
Four groups of male rats (each 15 rats) were utilized as following: Controls, GTE – treated rats (1.5 % w/v), Pb –treated rats (0.4 % lead acetate in dist. H2O), Pb + GTE -treated rats. The rats received GTE and/or lead orally in drinking H2O for 6 weeks. The biochemical measurements were performed in plasma, erythrocytes and tissue of liver, kidney and brain. The histo-pathological examinations of the studied organs of different groups using light microscope were done. The levels of lipid peroxides (LPO), nitric oxides (NO), total antioxidant, glutathione (GSH), glutathione S- transferase (GST), superoxide dismutase (SOD) were detected using colorimetric methods. The concentrations of Pb in whole blood and tissues of liver, kidney and brains were determined using atomic absorption spectrometer.
Results
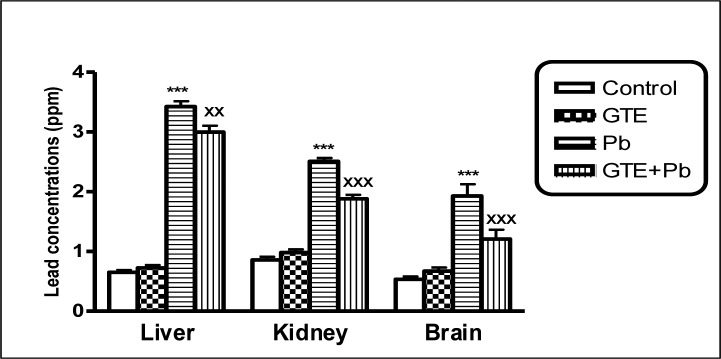
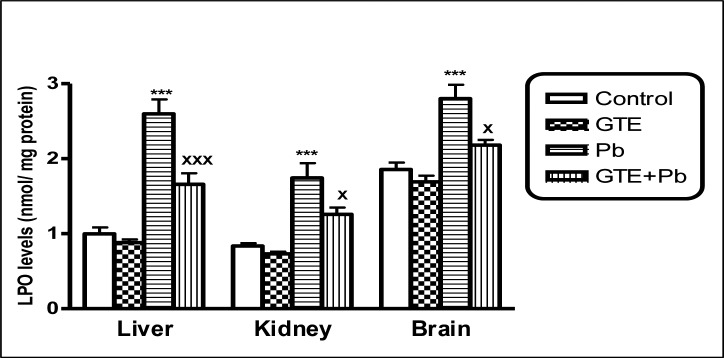
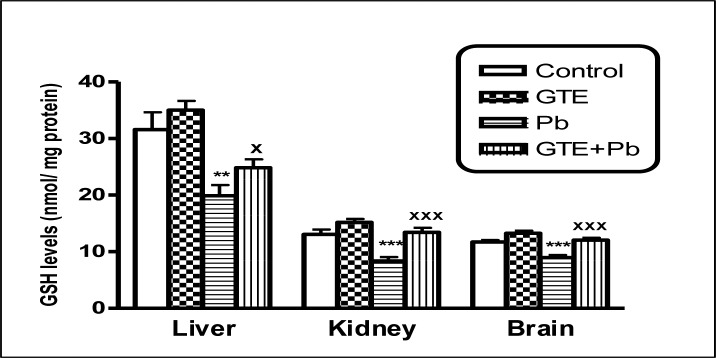
Pb concentrations in Pb-treated rats were significantly higher in whole blood and studied tissues than controls. Levels of LPO in Pb-treated rats were significantly higher in plasma, erythrocytes, liver, kidney and brain than controls. Plasma level of total antioxidant and levels of GSH and SOD activities in all studied tissues and activities of GST in only tissues organs of Pb-exposed rats were significantly reduced in comparison with controls. GTE co-administrated with Pb appeared more effective in reduction of Pb concentrations in tissues of the studied organs and whole blood as well as reductions of oxidative stress indices as detected by a significant decline of LPO in all studied tissues. The antioxidant status was improved in almost the studied tissues in Pb+GTE treated rats comparing to Pb-treated rats. The biochemical results were confirmed by histopathology.
Lead concentrations in tissues of liver, kidney and brain of different treated groups. (mean ± SEM. ***P<0.001 for Pb vs controls; xxP<0.01 and xxxP<0.001 for GTE+Pb vs Pb)
Lipid peroxide (LPO) levels in tissues of liver, kidney and brain of different treated groups (mean ± SEM. ***P<0.001 for Pb vs controls; xP<0.05 and xxxP<0.001 for GTE+Pb vs Pb)
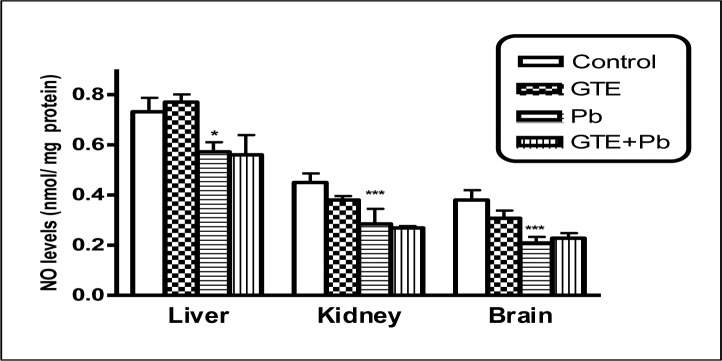
Nitric oxide (NO) levels in tissues of liver, kidney and brain of different treated groups. (mean ± SEM. *P<0.05, ***P<0.001 for Pb vs controls)
Glutathione (GSH) levels in tissues of liver, kidney and brain of different treated groups (mean ± SEM. **P<0.01, ***P<0.001 for Pb vs controls; xP<0.05 and xxxP<0.001 for GTE+Pb vs Pb)
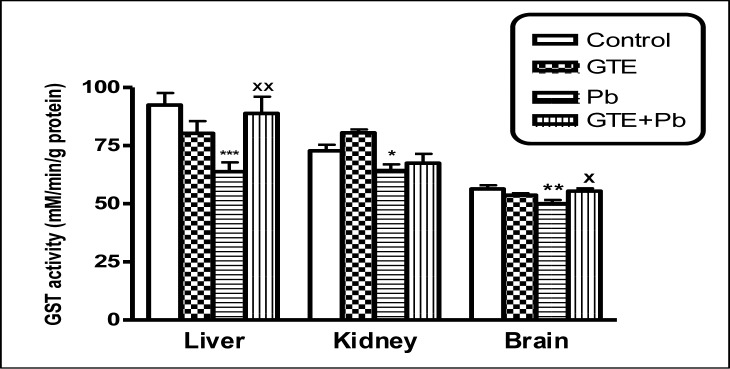
Glutathione S -transferase (GST) activities in tissues of liver, kidney and brain of different treated groups. (mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 for Pb vs controls; xP<0.05 and xxP<0.001 for GTE+Pb vs Pb)
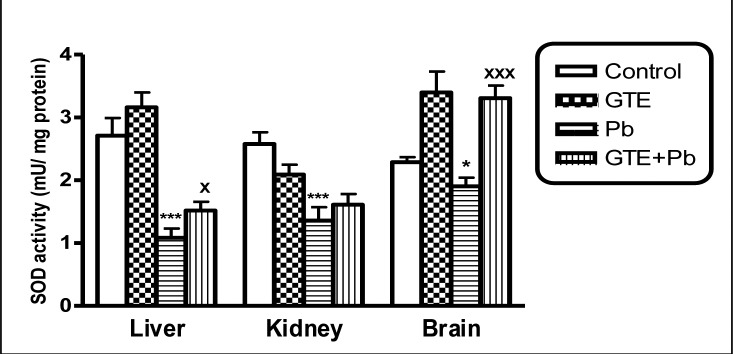
Superoxide dismutase (SOD) activities in tissues of liver, kidney and brain of different treated groups. (mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 for Pb vs controls; xP<0.05 and xxP<0.001 for GTE+Pb vs Pb)
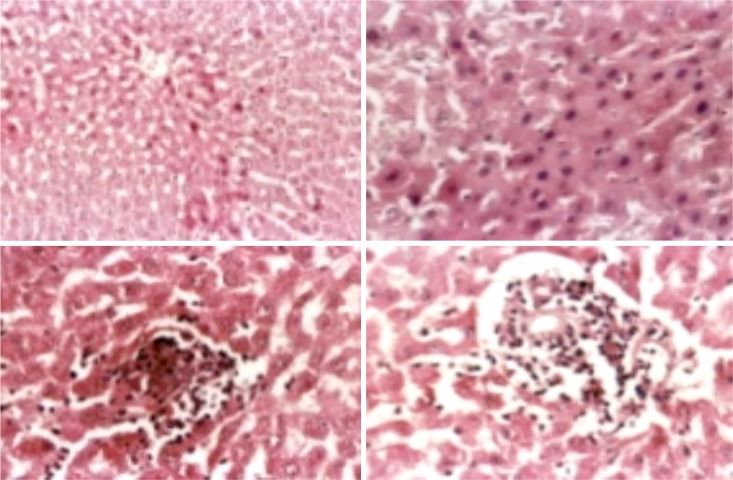
Liver of male rat intoxicated with lead acetate (clockwise): fatty change and hydropic degeneration of hepatocytes and multifocal hepatocytic coagulative necrosis (H&E .X 200); diffuse coagulative necrosis (H&E. X. 400); necrotic cells replaced by mononuclear leucocytes (H&E. X. 400); and interface Hepatitis (H&E.X.400).
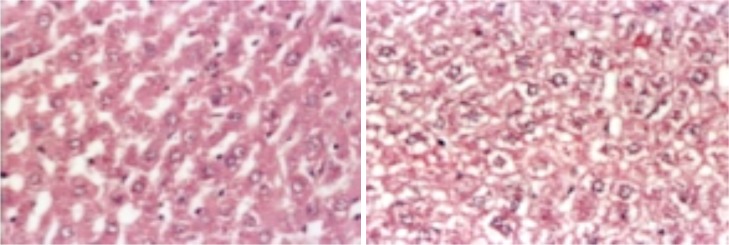
Liver of male rat treated with GTE+Pb showing Hydropic degeneration (L) and vacuolated nuclei (R)
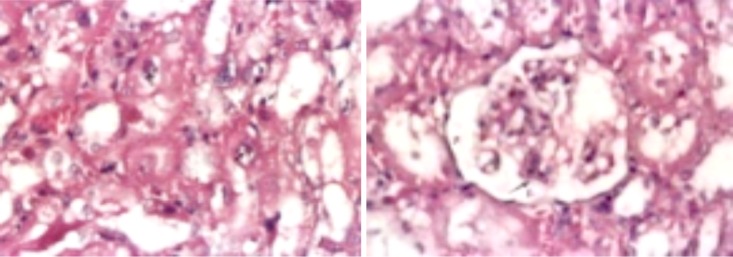
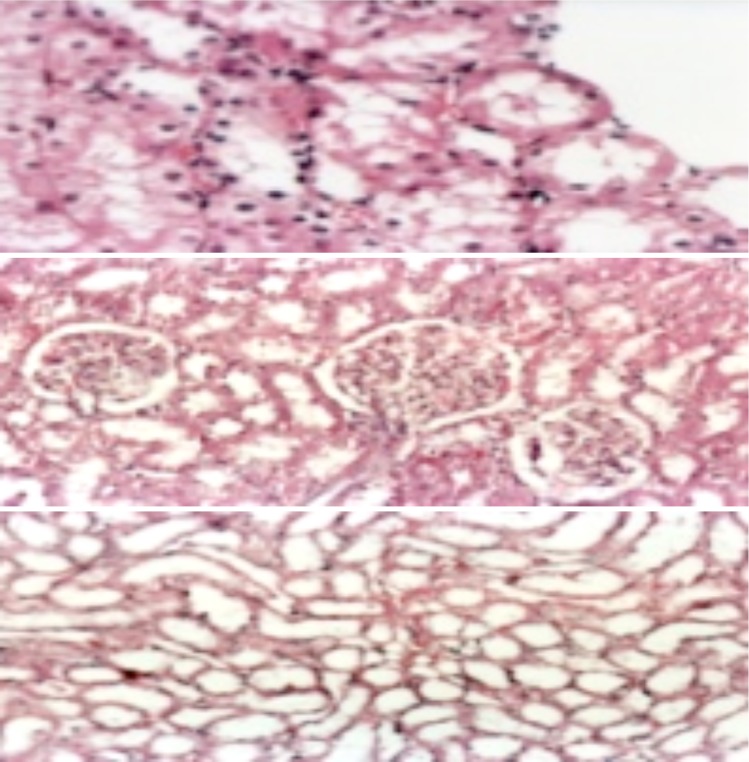
Kidney of male rat treated with Pb showing diffuse type of coagulative necrosis, intranuclear eosinophilic inclusion bodies (arrows ), cast and cystic dilated tubules (H&E.X. 400).
Kidney of male rat treated with GTE+ Pb showing (from above): (I) Nearly normal kidney (just cloudy swelling, hydropic and fatty change besides apoptotic necrotic cells) (H&E.X. 400) and (II and III) Normal renal tubules and glomeruli with mild degree of necrosis (marked improvement in the histological structure) (H&E.X. 200).
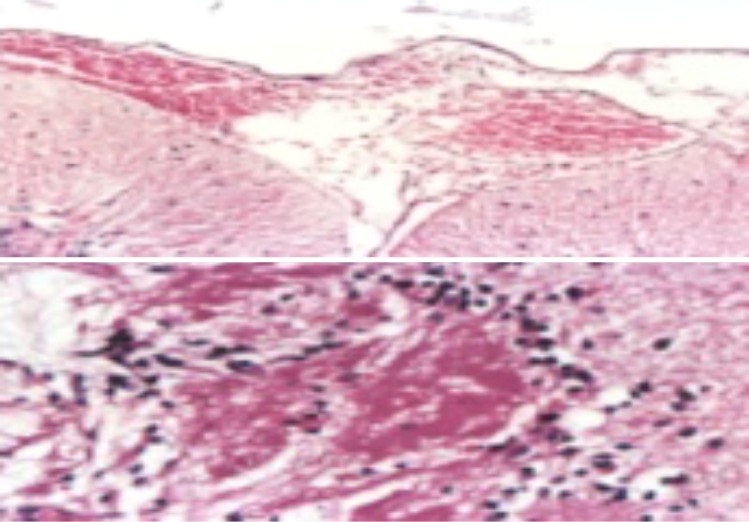
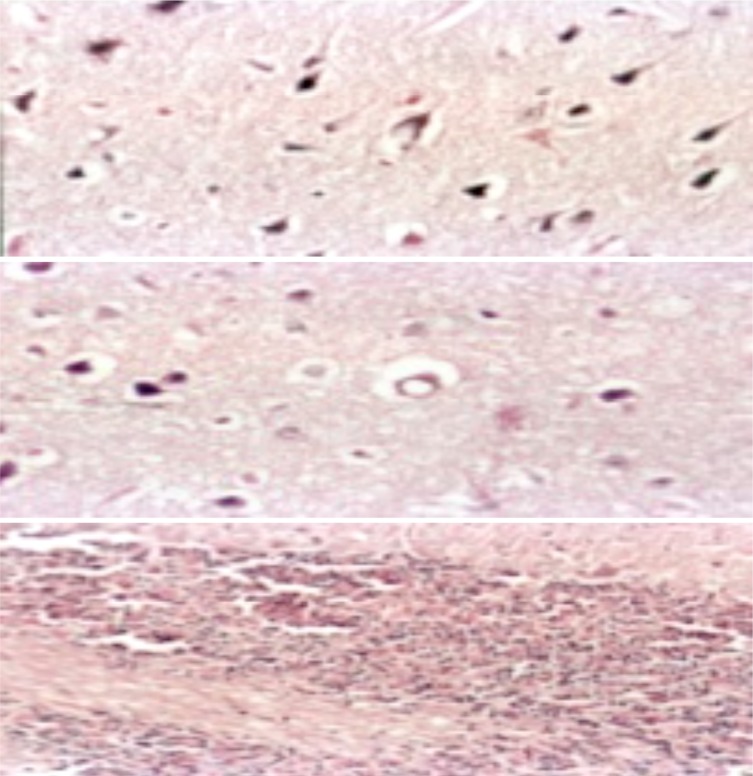
Brain of male rat treated with Pb showing meningeal hemorrhage, edema and congestion (H&E. X. 200) (above), and cerebral infarction with mononuclear cells infiltration (H&E.X. 400) (below).
Brain of male rat treated with GTE+ Pb showing (from above): (I and II) Marked improvement “ just vacuolated neurons (H&E.X. 400) and (III) Normal arrangement of the cerebellum layers and no spaces in between its granular and purkinje layers (H&E.X. 200).
Conclusions
The accumulation of Pb in the studied tissues was associated with oxidative stress and tissue injury as detected by histopathology. The treatment of rats with GTE combined with Pb could enhance antioxidant/ detoxification system which consequently reduced the oxidative stress. The beneficial effect of GTE in the improving of antioxidant status was associated with reduction of Pb burden in the tissue organs, thus potentially reducing Pb toxicity and tissue damage.