Abstract
Background
Statin-induced lung injury (SILI) is an uncommon but serious complication of statins. The clinical features and outcome of patients with SILI vary widely. Clinical data relevant to diagnosis and outcome of patients with SILI were investigated in this study.
Method
Four cases of SILI diagnosed at our institute and 12 cases reported in the English literature from 1995 to 2010 were studied. The patients were further divided into favourable and unfavourable outcome groups and compared.
Results
Compared with the 12 previously reported cases, fever (p=0.008) and consolidation (p=0.027) were more common and duration of statin treatment was significantly shorter (p=0.030) in our patients. Foamy alveolar macrophages in bronchoalveolar lavage fluid (BALF) were found in our four patients. Patients with cough (p=0.024), fever (p=0.026) and alveolar infiltrates (p=0.036), especially ground-glass opacity (GGO) (p=0.001) shown on thoracic high-resolution CT (HRCT), had a favourable outcome. Conversely, those with fibrosis shown on HRCT (p=0.008) had an unfavourable outcome. Stepwise logistic regression analysis demonstrated that cough (p=0.011), fever (p=0.005), and alveolar infiltrates (p=0.017), GGO (p<0.001) and fibrosis (p=0.002) shown on thoracic HRCT were independent factors affecting the outcome of SILI.
Conclusions
For patients with SILI, pulmonary phospholipidosis, as shown by foamy alveolar macrophages in BALF, may be valuable in diagnosis, and clinical symptoms and thoracic HRCT findings are of value in predicting the outcome.
Introduction
Pulmonary drug toxicity is an increasing cause of acute and chronic lung diseases. Many drugs can cause adverse pulmonary reactions, and establishing a diagnosis of drug-induced lung injury is difficult because the clinical, radiological and physiological findings are often non-specific.
Statins, competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, are initially used to decrease plasma low-density lipoprotein cholesterol and reduce the risk of adverse cardiovascular events and death.1 Although statins seem safe and well tolerated clinically, major adverse effects such as hepatotoxicity and myotoxicity have been reported.2 3
In 1995, lung injury, an uncommon but potentially fatal complication of statin use, was first reported by Hill and co-investigators.4 Several cases of lung injury caused by different statins were subsequently reported.5–11 Using the database from the US Food and Drug Administration adverse event reporting system, as of June 2007, Fernandez and colleagues reported that interstitial lung disease (ILD), comprising a group of disorders with similar pathological findings including cellular infiltration, scarring and/or architectural disruption of the pulmonary parenchyma involving alveolar lining cells, small and large airways, endothelial basement membranes and occasionally the pleura, was found in 0.01–0.4% of patients who had statin-induced adverse events.12 Recently, Xu et al13 reported a positive correlation between statin use and ILD in the cohort of smokers in the COPDGene Study, with 38% of subjects with ILD taking statins compared with 27% of subjects without ILD.
However, clinical features and outcomes of reported patients with statin-induced lung injury (SILI) vary widely. In this study, we reviewed and analysed the clinical data of 16 patients with SILI: 12 patients reported in the English literature and four patients whose condition was diagnosed at our institute. The clinical data relevant to the outcome were explored. In addition, the role of bronchoalveolar lavage (BAL) in diagnosing SILI was investigated.
Methods
Patients with SILI diagnosed at our institute
The four patients with SILI diagnosed at our institute met the following criteria for presumed drug-induced lung injury caused by statins: (1) treatment with statins (rosuvastatin, n=3; atorvastatin, n=1); (2) newly developed pulmonary lesions presenting as ILD on the imaging studies after statin treatment; (3) other causes of lung diseases extensively excluded clinically; (4) obvious clinical and radiological improvement after discontinuation of statins.12 14 Because of severe lung injury, rechallenge with statins was not performed. However, one patient (case 1) experienced increased dyspnoea 1 week after inadvertent re-administration of rosuvastatin prescribed by a physician with no knowledge of the patient's history of SILI.
Patients with SILI reported in the English literature
Articles related to SILI published in English from 1 January 1995 to 31 December 2010 were searched for using PubMed and the keywords ‘statins’ or ‘HMG-CoA reductase inhibitors’ in combination with the terms ‘pneumonitis’, ‘lung injury/toxicity’, ‘pulmonary toxicity/injury’ or ‘interstitial lung disease’. Relevant articles were selected and reviewed in detail, including the references of each article. Because of limited information, case reports and case series were included. Finally, 12 cases selected from six English articles were included in this study.4–9
Data collection
The clinical data of 16 patients with SILI were reviewed and analysed. The role of BAL in diagnosing SILI and the factors affecting the outcome of the patients were explored. The outcome was favourable when resolution of clinical symptoms or regression of lung lesions was found after discontinuation of statins and/or treatment with steroids. The outcome was unfavourable when worsening of clinical symptoms or death occurred irrespective of discontinuation of statins and/or treatment with steroids.
Statistical analysis
The data were expressed as mean and SD or case number. Two group comparisons of continuous data were made using the Mann–Whitney U test. Comparisons of categorical data between two groups were performed using the χ2 test and/or Fisher exact test when appropriate. Independent factors for the outcome were assessed by stepwise logistic regression analysis. Statistical significance was defined as p<0.05 (two-tailed). Statistical analysis was performed using SPSS V.15.0.
Results
Clinical data of the four patients with SILI diagnosed at our institute
Four cases of SILI were diagnosed at our institute during the period 1 January 2006 to 31 December 2010. The demographic and clinical data are summarised in table 1. All four patients were male, aged 41–85 years (mean±SD, 67.5±20.7 years). Rosuvastatin was administered in three patients, and atorvastatin in one. The duration of statin treatment ranged from 0.33 to 12 months (mean±SD, 4.0±5.4 months). All patients presented with dyspnoea and fever, and two of them also complained of a dry cough. Thoracic high-resolution CT (HRCT) showed diffusely bilateral ground-glass opacity (GGO) in four patients, consolidations in three, minimal bilateral pleural effusions in two, and interstitial infiltrates predominantly in the upper lungs in one. Restrictive ventilatory impairment was noted in two of the three patients who underwent pulmonary function testing, and diffusing capacity for carbon monoxide (DLCO) had decreased significantly in all three patients. Lung biopsy—transbronchial or open—was not performed in our patients because of impaired renal function, compromised cardiopulmonary function and reluctance of the patients. Instead, diagnostic BAL was performed in all four patients. Microbiological culture of BAL fluid (BALF) yielded no growth for all four patients. The cellular profile of BALF varied: a neutrophilic pattern in case 2, a mixed pattern with increased lymphocytes and eosinophils in case 3, and predominance of alveolar macrophages in cases 1 and 4. Of note, a foamy appearance of the majority of alveolar macrophages (figure 1) which stained positive for Sudan black (figure 2) was found in all four cases. The four patients were managed with immediate withdrawal of statins and administration of prednisolone (0.5 mg/kg body weight per day or methylprednisolone in bioequivalent dose), and all of them improved clinically. Marked resolution of lung lesions, as evidenced by chest radiogram and/or thoracic HRCT, was found in two patients at 1 month, in one patient by chest radiogram at 2.5 months, and in a further patient by chest radiogram at 9 months.
Table 1.
Demographic and clinical data of four patients with statin-induced lung injury diagnosed at our institute
| Variable | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Age, years | 41 | 61 | 85 | 83 |
| Statin/daily dose, mg | Rosuvastatin/10 | Rosuvastatin/10 | Atorvastatin/5 | Rosuvastatin/5 |
| Duration of treatment, months | 0.33 | 12 | 1.75 | 2 |
| Clinical symptoms | Dyspnoea, fever, cough | Dyspnoea, fever | Dyspnoea, fever, cough | Dyspnoea, fever |
| HRCT findings | G | G, C, P | G, C, I, P | G, C |
| Bronchoalveolar lavage fluid | ||||
| Celluar profile, % (M/L/N/E) | 94.5/2.5/3/0 | 74/4/22/0 | 58.5/33/2.5/6 | 94.5/4.5/1/0 |
| Foamy macrophages | + | + | + | + |
| Sudan black smear | + | + | + | + |
| Pulmonary function testing | ||||
| Spirometry | Not done | Restrictive | Normal | Restrictive |
| DLCO, % of predicted | Not done | 44 | 44 | 39 |
| Laboratory findings* | ||||
| ALT/AST | 33/63 | Not done | 24/36 | 33/23 |
| CK | 240 | Not done | 79 | 10 |
| CRP | 69.2 | 4.39 | 4.99 | 25.5 |
| Treatment | ||||
| Discontinuation of statin | + | + | + | + |
| Steroid/daily dose, mg | Prednisolone/45 | Prednisolone/30 | Methylprednisolone/24 | Prednisolone/30 |
| Outcome | Resolution | Resolution | Resolution | Resolution |
*ALT (reference, 0–40 U/l), AST (reference, 5–45 U/l), CK (reference, 27–168 U/l), CRP (reference, 0–0.5 mg/l).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; C, consolidation; CK, creatinine kinase; CRP, C-reactive protein; DLCO, carbon monoxide diffusing capacity; E, eosinophil; G, ground-glass opacities; HRCT, high-resolution CT; I, interstitial opacities; L, lymphocyte; M, macrophage; N, neutrophil; P, pleural changes.
Figure 1.
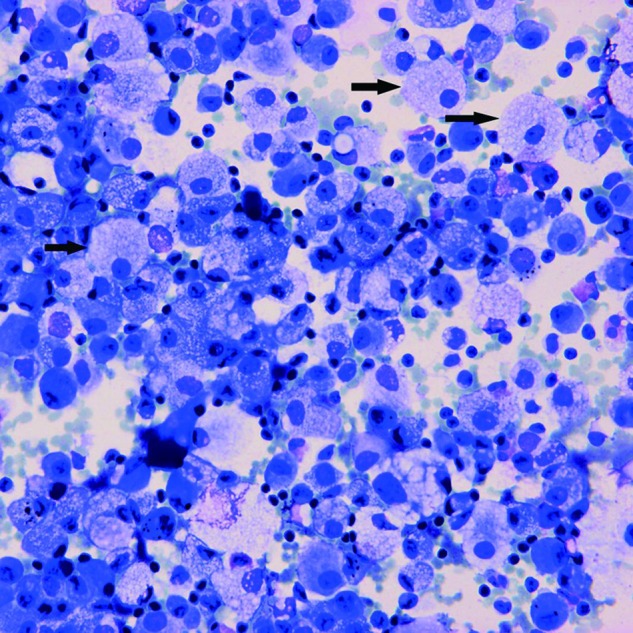
Riu's stain smear of bronchoalveolar lavage fluid shows a foamy appearance of the alveolar macrophages (arrows) (original magnification ×400).
Figure 2.
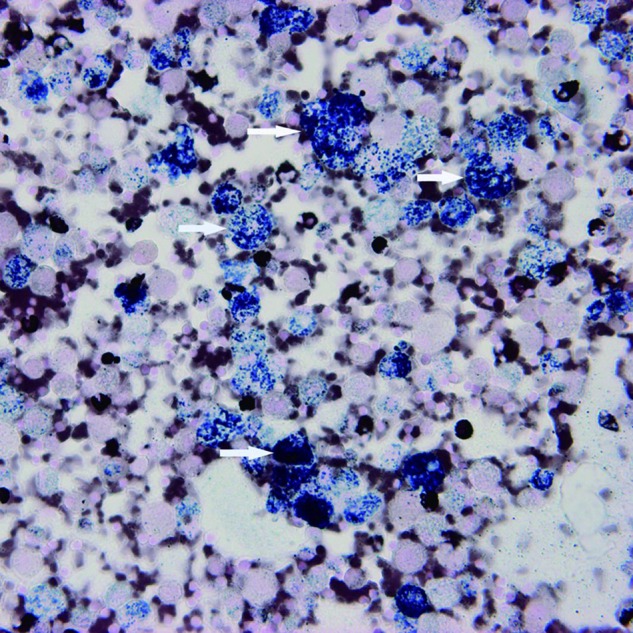
Sudan black-stained smear of bronchoalveolar lavage fluid showing positive staining of alveolar macrophages (arrows) (original magnification ×400).
Clinical data of the 16 studied subjects
Sixteen cases of SILI including four cases diagnosed at our institute and 12 reported cases selected from English articles were investigated. Simvastatin was the most common statin reported to cause lung injury, found in six cases. The time from the start of statin treatment to the onset of lung injury varied widely, ranging from 0.25 to 120 months (mean±SD, 34.3±34.9 months). Cardiovascular disease was the most common underlying comorbidity, found in 14 of 16 patients. Dyspnoea, cough and fever were the most common presenting symptoms.
Radiological features of the 16 studied subjects
Alveolar infiltrates shown on thoracic HRCT were found in 11 patients (68.8%). GGO was the main finding in nine of these 11 patients. Six patients presented with varying degrees of fibrosis, and pleural changes including effusion and pleural thickening were seen in three patients.
Results of pulmonary function testing of the 16 studied subjects
The results of pulmonary function testing showed a restrictive ventilatory defect in six of 10 patients studied. Hypoxaemia was found in all six patients undergoing arterial blood gas analysis. Marked reduction of DLCO was found in all 13 patients studied, and the values of DLCO measured in 12 patients ranged from 14% to 46% (mean±SD, 35.8±10.5%) of normal predicted value.
Histopathology of the 16 studied subjects
Lung biopsy was performed in nine patients. The reported main histopathological findings in eight of the nine patients were as follows: fibrosis in three, non-specific interstitial pneumonitis (NSIP) in two, diffuse alveolar damage in two, and hypersensitivity pneumonitis in one. The histopathological findings were non-diagnostic in the remaining patient. Of note, a foamy appearance of the alveolar macrophages, type II pneumocytes and interstitial histiocytes was found and documented as heterogeneous intralysosomal lamellar inclusions in the cytoplasm of these cells under an electron microscope, highly suggestive of pulmonary phospholipidosis, in one patient.8
Management and outcome of the 16 studied subjects
Statins were discontinued and steroids were administered in 15 of the 16 patients. Clinical or radiological improvement was noted in 10 patients, who were classified in the favourable outcome group. Of the remaining six patients with unfavourable outcome, two had slowly progressive disease complicated by persistent dyspnoea. Four patients died from respiratory or cardiac failure, including the patient receiving continuous simvastatin treatment.9
Comparison between our four patients and the 12 previously reported patients
Comparisons of clinical features between our four patients and the 12 patients reported in the English literature are shown in table 2. The duration of statin treatment was significantly shorter (4.0±5.4 vs 41.0±36.0 months, p=0.030) and fever more common in our patients (4/4 vs 2/12, p=0.008). Of note, most alveolar macrophages in the BALF of our four patients were found to be foamy in appearance and stained positive with Sudan black. However, no morphological change in alveolar macrophages was mentioned in six of the 12 reported patients undergoing BAL. In addition, consolidation was more common in our patients (3/4 vs 1/12, p=0.027).
Table 2.
Comparisons between patients with statin-induced lung injury diagnosed at our institute and reported in the English literature
| Variable | Our patients (N=4) | Reported patients (N=12) | p Value |
|---|---|---|---|
| Age, years | 67.5±20.7 | 69.7±8.8 | 0.953 |
| Gender (male/female) | 4/0 | 7/5 | 0.181 |
| Duration of statin treatment, months | 4.0±5.4 | 41.0±36.0 | 0.030 |
| Symptoms | |||
| Dyspnoea | 4 (100.0) | 11 (91.7) | 0.750 |
| Cough | 2 (50.0) | 7 (58.3) | 0.608 |
| Fever | 4 (100.0) | 2 (16.7) | 0.008 |
| Laboratory findings | |||
| Leucocytosis | 1/4 (25.0) | 1/2 (50.0) | 0.600 |
| Elevated CK level | 1/3 (33.3) | 2/3 (66.7) | 0.500 |
| Impaired LFT | 1/3 (33.3) | 2/2 (100.0) | 0.300 |
| HRCT findings | |||
| Alveolar infiltrates | 4 (100.0) | 7 (58.3) | 0.181 |
| Consolidation | 3 (75.0) | 1 (8.3) | 0.027 |
| GGO | 4 (100.0) | 5 (41.7) | 0.069 |
| Unspecified | 0 (0) | 2 (16.7) | 0.550 |
| Interstitial infiltrates | 1 (25.0) | 1 (8.3) | 0.450 |
| Fibrosis | 0 (0) | 6 (50.0) | 0.115 |
| Pleural changes | 2 (50.0) | 1 (8.3) | 0.136 |
| Spirometry | |||
| Restrictive | 2/3 (66.7) | 4/7 (57.1) | 0.667 |
| Obstructive | 0/3 (0) | 3/7 (42.9) | 0.292 |
| DLCO, % of predicted | 42.3±2.9 | 33.7±11.3 | 0.373 |
| BALF cytology | |||
| Foamy macrophages | 4/4 (100.0) | 0/6 (0) | 0.005 |
| Neutrophils, > 3% | 1/4 (25.0) | 4/6 (66.7) | 0.262 |
| Lymphocytes, > 20% | 1/4 (25.0) | 1/6 (16.7) | 0.667 |
| Eosinophils, > 0.5% | 1/4 (25.0) | 4/6 (66.7) | 0.044 |
Data are expressed as mean±SD or n (%).
BALF, bronchoalveolar lavage fluid; CK, creatinine kinase; DLCO, carbon monoxide diffusion capacity; GGO, ground glass opacity; HRCT, high-resolution CT; LFT, liver function test.
Comparisons between favourable and unfavourable outcome groups
Comparisons of demographic and clinical data between 10 patients with favourable outcome and six patients with unfavourable outcome are summarised in table 3. Cough (p=0.024) and fever (p=0.026) were more common in the patients with favourable outcome. The findings of thoracic HRCT appeared to be of value in predicting the outcome of patients. Alveolar infiltrates (p=0.036), in particular GGO (p=0.001), were more common in the patients with favourable outcome. In contrast, fibrosis (p=0.008) was more common in the patients with unfavourable outcome. After stepwise logistic regression analysis, cough (OR=0.050, 95% CI 0.050 to 0.706, p=0.011), fever (OR=0.040, 95% CI 0.187 to 0.855, p=0.005), and alveolar infiltrates (OR=0.056, 95% CI 0.004 to 0.805, p=0.017), GGO (OR=0.100, 95% CI 0.016 to 0.642, p<0.001) and fibrosis (OR=45.0, 95% CI 2.287 to 885.601, p=0.002) shown on HRCT were independent factors affecting the outcome of patients with SILI.
Table 3.
Comparisons between patients with statin-induced lung injury with favourable and unfavourable outcome
| Variable | Favourable outcome (N=10) | Unfavourable outcome (N=6) | p Value (univariable) | p Value (multivariable) |
|---|---|---|---|---|
| Age, years | 65.6±13.4 | 75.0±6.3 | 0.147 | |
| Gender, M/F | 8/2 | 3/3 | 0.242 | |
| Duration of statin treatment, months | 32.2±31.0 | 31.0±44.3 | 0.958 | |
| Symptoms | ||||
| Dyspnoea | 9 (90.0) | 6 (100.0) | 0.625 | |
| Cough | 8 (80.0) | 1 (16.7) | 0.024 | 0.011 |
| Fever | 6 (60.0) | 0 (0.0) | 0.026 | 0.005 |
| Laboratory findings | ||||
| Leucocytosis | 2 (20.0) | 3 (50.0) | 0.714 | |
| Elevated CK | 2/4 (50.0) | 1/2 (50.0) | 0.600 | |
| Impaired LFT | 2/4 (50.0) | 1/1 (100.0) | 0.600 | |
| HRCT findings | ||||
| Alveolar infiltrates | 9 (90.0) | 2 (16.7) | 0.036 | 0.017 |
| Consolidation | 4 (40.0) | 0 (0.0) | 0.115 | < 0.001 |
| GGO | 9 (90.0) | 0 (0.0) | 0.001 | 0.002 |
| Unspecified | 0 (0.0) | 2 (16.7) | 0.125 | |
| Interstitial infiltrates | 2 (20.0) | 0 (0.0) | 0.375 | |
| Fibrosis | 1 (10.0) | 5 (83.3) | 0.008 | |
| Pleural changes | 3 (30.0) | 0 (0.0) | 0.214 | |
| Spirometry | ||||
| Restrictive | 4/7 (57.1) | 2/3 (67.7) | 0.667 | |
| Obstructive | 2/7 (28.6) | 1/3 (33.3) | 0.708 | |
| Normal | 1/7 (14.3) | 0/3 (0.0) | 0.383 | |
| DLCO, % of predicted | 35.3±10.3 | 37.3±13.3 | 0.600 | |
| Lung biopsy | ||||
| Fibrosis | 2/5 (40.0) | 2/3 (66.7) | 0.500 | |
| HP | 1/5 (20.0) | 0/3 (0.0) | 0.625 | |
| NSIP | 2/5 (40.0) | 0/3 (0.0) | 0.357 | |
| DAD | 0/5 (0.0) | 2/3 (66.7) | 0.107 | |
| BALF cytology | ||||
| Foamy macrophages | 4/8 (50.0) | 0/2 | 0.333 | |
| Neutrophils, >3% | 3/8 (37.5.) | 2/2 | 0.467 | |
| Lymphocytes, >20% | 2/8 | 0/2 | 0.622 | |
| Eosinophils, >0.5% | 3/8 | 2/2 | 0.071 | |
Data are expressed as mean±SD or n (%).
BALF, bronchoalveolar lavage fluid; CK, creatinine kinase; DAD, diffuse alveolar damage; DLCO, carbon monoxide diffusion capacity; GGO, ground glass opacity; HP, hypersensitivity pneumonitis; HRCT, high-resolution CT; LFT, liver function test; NSIP, non-specific interstitial pneumonitis.
Discussion
Our results indicate that pulmonary phospholipidosis, as evidenced by a foamy appearance of alveolar macrophages in BALF, may be of value in diagnosing SILI, as shown in our four cases. Furthermore, the clinical symptoms and findings on HRCT may be of value in predicting the outcome of patients with SILI.
The duration between drug initiation and onset of respiratory symptoms varied widely, ranging from 0.25 to 120 months (mean±SD, 34.3±34.9 months) in the 16 patients studied. Compared with the 12 patients reported in the English literature, the duration of statin treatment was significantly shorter in our four patients, as shown in table 2. This can be explained in part by the clinical experience and high index of suspicion of SILI of our team. Of more importance, foamy appearance of most alveolar macrophages in BALF which showed positive Sudan black staining in the cytological smear used to detect pulmonary phospholipidosis was found in our four patients. This finding may be of value in diagnosing SILI because pulmonary phospholipidosis may be the representative pathological pattern of lung injury caused by statin because foamy appearance of macrophages, type II pneumocytes and interstitial histiocytes demonstrated on lung biopsy was reported in a patient with statin-induced fibrotic non-specific interstitial pneumonitis (NSIP).8
Pulmonary phospholipidosis is neither diagnostic nor pathognomonic for SILI, and can be induced by a variety of drugs and chemicals15 16 including a well-studied anti-arrhythmic agent, amiodarone.17 18 However, a foamy appearance of most alveolar macrophages in BALF may help narrow the list of differential diagnoses for a patient with ILD and aid the diagnosis of SILI in the appropriate clinical setting. Furthermore, the findings may be of clinical importance because SILI is a serious adverse pulmonary reaction with high morbidity (6/16, 37.5%) and mortality (4/16, 25%) as shown in this study (table 2).
Our results indicate that the patients with clinical symptoms of cough and/or fever had a favourable outcome. It may be that the presence of such acute symptoms prompts the patient to seek medical help and avoid delay in diagnosis and treatment. However, these clinical symptoms, in addition to thoracic radiological findings, may mimic pulmonary infection and confuse physicians who are unaware of SILI.
The main findings of thoracic HRCT were alveolar infiltrates, in particular GGO, and varying degrees of fibrosis in SILI, which were similar to the radiological features described in previous reports.12 13 These image findings have limited value in the diagnosis of drug-induced lung injury,19–21 but may affect the patient's outcome. Patients with alveolar infiltrates, in particular GGO, had a favourable outcome. In contrast, those with fibrosis had an unfavourable outcome. These results are consistent with previous studies on the prognostic role of thoracic HRCT in interstitial pneumonitis, including NSIP and idiopathic pulmonary fibrosis.22 23 These studies suggested that inflammatory (GGO/consolidation) predominant findings were indicative of a better outcome, whereas fibrotic (reticular/honeycombing) predominant findings were suggestive of a worse prognosis. Further studies with larger numbers of subjects are needed to address the role of thoracic HRCT in predicting the outcome of patients with SILI.
This study had some limitations. First, the number of patients studied was inadequate because of the stringent selection criteria and limited cases reported in the English literature. Limited cases may confer potential error and bias. Second, pathological findings characteristic of pulmonary phospholipidosis were not obtained in our four patients because lung biopsy was not performed because of compromised cardiopulmonary function and reluctance of the patients. Third, SILI might be underdiagnosed because of the unusually prolonged duration of statin treatment and low index of suspicion of the physicians. Fourth, the patients enrolled in this study may have varying degrees of severity, and therefore conclusions must be drawn with caution. Fifth, the morphological changes in alveolar macrophages in BALF of the patients with SILI were not addressed in previous reports. Sixth, the risk factors for statin-associated ILD, including cigarette smoking, occupational dust exposure and hydrophilicity of statins, could not be evaluated because of incomplete clinical information drawn from previous reports and limited case number. However, the results of the present study may be of clinical relevance. Pulmonary phospholipidosis, as shown by morphological changes in alveolar macrophages in BALF, may be of value in diagnosing SILI and thereby avoiding delay in treatment. In addition, our results indicate that some clinical data may be relevant to the outcome of the patients. To our knowledge, this is the first study to investigate these issues in patients with SILI.
In summary, the duration of statin treatment varies widely in patients with SILI, and the clinical features of the disease are protean. A high index of suspicion and the presence of pulmonary phospholipidosis, shown by a foamy appearance of the majority of alveolar macrophages which stain positive for Sudan black, may be of considerable value in diagnosing SILI. Clinical symptoms of cough and fever and thoracic HRCT findings of alveolar infiltrates, GGO and fibrosis are of value in predicting the outcome of SILI. Further studies with larger populations are needed to clarify these issues.
Main messages.
Statin-induced lung injury (SILI) is an uncommon but serious pulmonary complication. The clinical features and outcome of patients with SILI vary widely.
Foamy appearance of the majority of alveolar macrophages showing positive Sudan black staining in bronchoalveolar lavage fluid may be of value in the diagnosis of SILI, especially after exclusion of other possible causal agents and entities.
The high-resolution CT (HRCT) findings in SILI are non-specific, but may be of prognostic value. Alveolar infiltrates, especially ground-glass opacity, indicate favourable outcome, while fibrosis shown on HRCT suggests unfavourable outcome.
Current research questions.
What is the underlying mechanism of statin-induced lung injury (SILI)?
Are there any risk factors for the occurrence of SILI?
How can physicians become more aware of SILI in their clinical practice?
Key references.
Lantuejoul S, Brambilla E, Brambilla C, et al. Statin-induced fibrotic nonspecific interstitial pneumonia. Eur Respir J 2002;19:577–80.
Fernandez AB, Karas RH, Alsheikh-Ali AA, et al. Statins and interstitial lung disease: a systematic review of the literature and of food and drug administration adverse event reports. Chest 2008;134:824–30.
Xu JF, Washko GR, Nakahira K, et al. Statins and pulmonary fibrosis: the potential role of NLRP3 inflammasome activation. Am J Respir Crit Care Med 2012;185:547–56.
Schmitz G, Grandl M. Endolysosomal phospholipidosis and cytosolic lipid droplet storage and release in macrophages. Biochim Biophys Acta 2009;1791:524–39.
Footnotes
Contributors: LKH and SCC were jointly responsible for the conception, design of the study and preparation of the manuscript. LKH, MJT, HSC, FCL and SCC were responsible for performing bronchoscopy, retrieving the specimen, and analysing the cytological results. LKH was responsible for collection of the data. HCT was responsible for statistical analysis of the data. SCC was responsible for conducting the processing of the study and preparation of the final manuscript.
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Open Access:This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/
References
- 1.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486–97 [DOI] [PubMed] [Google Scholar]
- 2.Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interact. Circulation 2004;109(Suppl 1):III50–7 [DOI] [PubMed] [Google Scholar]
- 3.Farmer JA, Torre-Amione G. Comparative tolerability of the HMG-CoA reductase inhibitors. Drug Saf 2000;23:197–213 [DOI] [PubMed] [Google Scholar]
- 4.Hill C, Zeitz C, Kirkham B. Dermatomyositis with lung involvement in a patient treated with simvastatin. Aust N Z J Med 1995;25:745–6 [DOI] [PubMed] [Google Scholar]
- 5.De Groot RE, Willems LN, Dijkman JH. Interstitial lung disease with pleural effusion caused by simvastatin. J Intern Med 1996;239:361–3 [DOI] [PubMed] [Google Scholar]
- 6.Sridhar MK, Abdulla A. Fatal lupus-like syndrome and ARDS induced by fluvastatin (letter). Lancet 1998;352:114. [DOI] [PubMed] [Google Scholar]
- 7.Liebhaber MI, Wright RS, Gelberg HJ, et al. Polymyalgia, hypersensitivity pneumonitis and other reactions in patients receiving HMG-CoA reductase inhibitors: a report of ten cases. Chest 1999;115:886–9 [DOI] [PubMed] [Google Scholar]
- 8.Lantuejoul S, Brambilla E, Brambilla C, et al. Statin-induced fibrotic nonspecific interstitial pneumonia. Eur Respir J 2002;19:577–80 [DOI] [PubMed] [Google Scholar]
- 9.Walker T, McCaffery J, Steinfort C. Potential link between HMG-CoA reductase inhibitor (statin) use and interstitial lung disease. MJA 2007;186:91–4 [DOI] [PubMed] [Google Scholar]
- 10.Veyac G, Cellerin L, Jolliet P. A case of interstitial lung disease with atorvastatin (Tahor) and a review of the literature about these effects observed under statins. Therapie 2006;61:57–67 (In French English abstract) [DOI] [PubMed] [Google Scholar]
- 11.Liscoët-Loheac N, André N, Couturaud F, et al. Hypersensitivity pneumonitis in a patient taking pravastatin (in French). Rev Mal Respir 2001;18(4 Pt 1):426–8 [PubMed] [Google Scholar]
- 12.Fernandez AB, Karas RH, Alsheikh-Ali AA, et al. Statins and interstitial lung disease: a systematic review of the literature and of food and drug administration adverse event reports. Chest 2008;134:824–30 [DOI] [PubMed] [Google Scholar]
- 13.Xu JF, Washko GR, Nakahira K, et al. Statins and pulmonary fibrosis: the potential role of NLRP3 inflammasome activation. Am J Respir Crit Care Med 2012;185:547–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flieder DB, Travis WD. Pathologic characteristics of drug-induced lung disease. Clin Chest Med 2004;25:37–45 [DOI] [PubMed] [Google Scholar]
- 15.Costabel U, Uzaslan E, Guzman J. Bronchoalveolar lavage in drug-induced lung disease. Clin Chest Med 2004;25:25–35 [DOI] [PubMed] [Google Scholar]
- 16.Schmitz G, Grandl M. Endolysosomal phospholipidosis and cytosolic lipid droplet storage and release in macrophages. Biochim Biophys Acta 2009;1791:524–39 [DOI] [PubMed] [Google Scholar]
- 17.Israël-Biet D, Venet A, Caubarrère I, et al. Bronchoalveolar lavage in amiodarone pneumonitis: cellular abnormalities and their relevance to pathogenesis. Chest 1987;91:214–21 [DOI] [PubMed] [Google Scholar]
- 18.Chang CL, Chern MS, Chang SC. Amiodarone toxicity in a patient with simultaneous involvement of cornea, thyroid gland, and lung. Am J Med Sci 2000;320:64–8 [DOI] [PubMed] [Google Scholar]
- 19.Rossi SE, Erasmus JJ, McAdams HP, et al. Pulmonary drug toxicity: Radiologic and pathologic manifestations. RadioGraphics 2000;20:1245–59 [DOI] [PubMed] [Google Scholar]
- 20.Cleverley JR, Screaton NJ, Hiorns MP, et al. Drug-induced lung disease: high-resolution CT and histological findings. Clin Radiol 2002;57:292–9 [DOI] [PubMed] [Google Scholar]
- 21.Ryu JH, Daniels CE, Hartman TE, et al. Diagnosis of interstitial lung diseases. Mayo Clin Proc 2007;82:976–86 [DOI] [PubMed] [Google Scholar]
- 22.Screaton NJ, Hiorns MP, Lee KS, et al. Serial high resolution CT in non-specific interstitial pneumonia: prognostic value of the initial pattern. Clin Radiol 2005;60:96–104 [DOI] [PubMed] [Google Scholar]
- 23.Flaherty KR, Thwaite EL, Kazerooni EA, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax 2003;58:143–8 [DOI] [PMC free article] [PubMed] [Google Scholar]


