Summary
The centrosome, a major organizer of microtubules, has important functions in regulating cell shape, polarity, cilia formation and intracellular transport as well as the position of cellular structures, including the mitotic spindle. By means of these activities, centrosomes have important roles during animal development by regulating polarized cell behaviors, such as cell migration or neurite outgrowth, as well as mitotic spindle orientation. In recent years, the pace of discovery regarding the structure and composition of centrosomes has continuously accelerated. At the same time, functional studies have revealed the importance of centrosomes in controlling both morphogenesis and cell fate decision during tissue and organ development. Here, we review examples of centrosome and centriole positioning with a particular emphasis on vertebrate developmental systems, and discuss the roles of centrosome positioning, the cues that determine positioning and the mechanisms by which centrosomes respond to these cues. The studies reviewed here suggest that centrosome functions extend to the development of tissues and organs in vertebrates.
Key words: Centrosome, Development, Mitotic spindle orientation
Introduction
The centrosome of animal cells (Fig. 1A) consists of a pair of centrioles that is surrounded by a cloud of pericentriolar material (PCM), which contains proteins that are responsible for microtubule nucleation and anchoring (Azimzadeh and Bornens, 2007). Each centriole is composed of nine-triplet microtubules. The older of the two centrioles in a pair is termed the mother centriole, whereas the younger is termed the daughter centriole. Upon exit from the cell cycle, the mother centriole acts as a nucleation site for the growth of primary cilia. During progression through the cell cycle, the centrosome reproduces and the centrioles in each centrosome replicate (Nigg and Stearns, 2011). Then two centrosomes move to opposite ends of the nucleus, and from each centrosome, microtubules grow into a spindle, which is responsible for separating replicated chromosomes into two daughter cells. Centrosomes occupy non-random locations that differ between cell types. This Commentary will focus on the role of centrosome positioning during vertebrate development. We will first discuss how the position of the centrosome affects the structure and function of cells at interphase or post mitotic phase. We then further summarize some recent exciting findings in understanding how centrosome position affects the mitotic spindle orientation in cells at mitotic phase. Finally, we will briefly discuss some molecular mechanisms that control centrosome positioning.
Fig. 1.
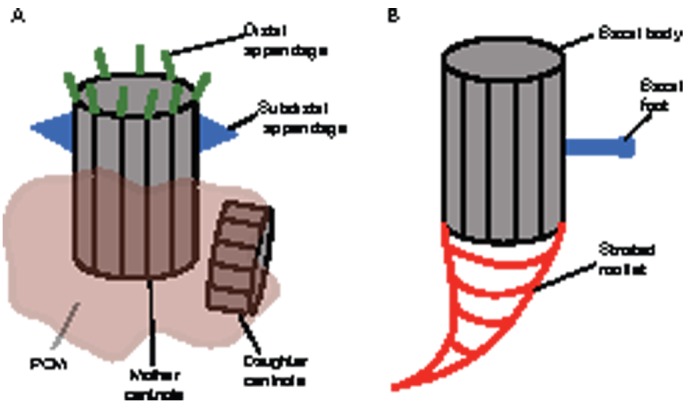
The anatomy of vertebrate centrosomes and centrioles. (A) Schematic diagram of typical centrosome and centriole organization in most vertebrate cells. Centrosomes are composed of two orthogonally oriented centrioles that are surrounded by PCM. The mother centriole is distinguished by two sets of appendages, the subdistal and distal appendages (Paintrand et al., 1992), which are thought to be required for anchoring microtubules at the centriole or forming transitional fibers that contact the cell cortex, respectively (Dawe et al., 2007). (B) Organization of the basal body (centriole) in a cell from a multiciliated epithelium. The proximal side of the basal body is associated with a matrix, which extends into specific and striated structures called rootlets (Klotz et al., 1986). PCM, pericentriolar material.
Centrosome position in cell migration and polarization
In interphase or post-mitotic cells, centrosomes dictate the organization of microtubules, which is important for determining cell shape, polarity and motility (Desai and Mitchison, 1997; Keating and Borisy, 1999). In many nonpolarized cells, such as interphase fibroblasts, the centrosome is located near the cell center and is physically linked to the nucleus, with microtubules radiating out to the cell cortex (Fig. 2A). In some cases, the association between centrosome and nucleus is so close that the centrosome actually resides within an invagination of the nuclear envelope (Hulspas et al., 1994), suggesting that the forces that center the centrosome are strong enough to deform neighboring organelles. In some cell types, centrioles also have defined orientations of their long axis. For instance, in pig kidney embryo cells, the mother centrioles tend to be oriented perpendicular to the substrate (Vorobjev and Chentsov, 1982).
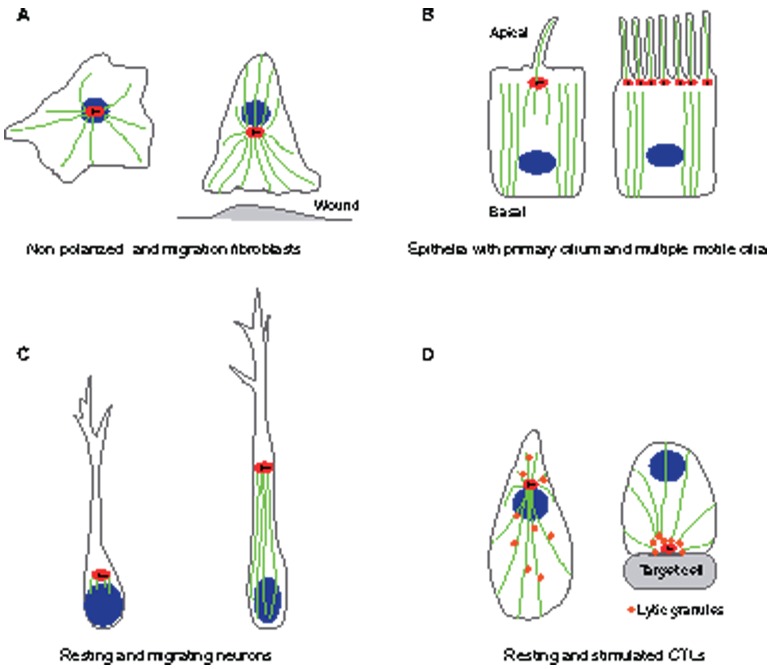
Fig. 2.
Examples of centrosome positioning that depend on cell type and cell state. (A) Fibroblasts: in non-polarized fibroblasts (left), the centrosome is located near the center of the cell and is physically linked to the nucleus, with microtubules radiating from the centrosome to the cell cortex. During wound healing (right), the fibroblast centrosome often becomes oriented between the nucleus and the leading edge. (B) Epithelial cells: in polarized epithelial cells (left), centrosomes are located on the apical surface of the cell. The apical localization of the centrosome is accompanied by a loss of radial microtubule organization and the formation of a predominantly apical–basal array of microtubules. The mother centriole of the centrosome becomes a basal body, which gives rise to a primary cilium. In multiciliated epithelial cells (right), hundreds of centrioles are assembled at once in a single cell, leading to the formation of multiple cilia. (C) Neurons: in resting neurons (left), the centrosome is found in close proximity to the neurite that becomes the axon. In migrating neurons (right), the centrosome is positioned ahead of the nucleus, with microtubules connecting the centrosome and the nucleus. (D) Lymphocytes: in cytotoxic T lymphocytes (CTLs) that are not interacting with a target (left), the centrosome is located near the nucleus, and lytic granules are distributed all along the microtubules. After the CTL is stimulated (right), the centrosome directs the delivery of lytic granules by moving along microtubules to the plasma membrane and then to the point of secretion for releasing lytic granules to the immunological synapse. Red ovals indicate the centrosome, with centrioles indicated as black lines within. Blue ovals indicate nuclei and green lines indicate microtubules.
In most epithelial cells in mammary gland, airway, intestinal duct and liver and epidermal cells, centrioles move to the apical cell surface (Dylewski and Keenan, 1984; Müsch, 2004), which is accompanied by loss of radial microtubule organization, the formation of a predominantly apical-basal array of MTs, and assembly of a primary cilium (Fig. 2B) (Rieder et al., 2001). The microtubule-based reorientation of the secretory and endocytic apparatus along the apical-basolateral polarity axis might ensure the targeting of vesicles to a specific surface domain (Müsch, 2004). The formation of an apical–basal microtubule array is itself a centrosome-dependent process, with the centrosome moving to the cell apical surface and depositing some PCM proteins (Feldman and Priess, 2012). Thus, centrosome positioning has a profound effect on the geometry of the entire cell.
To better convey the variety of centrosome positions in animals, we consider several examples of specialized cell types below.
Cochlea
Hair cells in the organ of Corti (in the mammalian cochlea) sense mechanical stimulation though microvilli called stereocilia, which form a V-shape bundle on the apical surface (Frolenkov et al., 2004). The orientation of the stereociliary bundle is predetermined by the polarized position of the centriole, which assembles a single primary cilium. If the centrioles are relocated to a central, non-polarized position, stereocilia become distributed in a round symmetric pattern (Frolenkov et al., 2004). Therefore, it appears that centriole positioning determines the orientation and morphology of stereociliary bundles. Furthermore, it is likely that the polarized basal bodies also have important functions in directing the uniform alignment of all the cells across the organ of Corti (Jones et al., 2008).
Neurons
The centrosome is often found in close proximity to the neurite that becomes the axon, suggesting a role in determining the site of axon outgrowth (Bellion et al., 2005; de Anda et al., 2005; Zmuda and Rivas, 1998). Doubling the number of centrosomes by inhibiting cytokinesis results in two axons that form adjacent to the two centrosomes, whereas inactivation of the centrosome in cultured Drosophila melanogaster neurons impairs axon formation (de Anda et al., 2005). However, axonogenesis in mouse tegmental hindbrain nuclei neurons clearly occurs distant from the centrosome (Distel et al., 2010).
In migrating neurons (Fig. 2C), the centrosome is sometimes positioned ahead of the nucleus, suggesting that it drives the forward movement of the nucleus along microtubules (Rivas and Hatten, 1995; Solecki et al., 2004; Tsai et al., 2007; Xie et al., 2003). However, live imaging of radial migration of granule cells in cultured developing mouse cerebellum demonstrated that nucleus migration is not always correlated with the movement of the centrosome (Umeshima et al., 2007). Thus, the exact relationship between centrosomes and either nuclear movement or axon outgrowth remains unclear.
Immune cells
Cytotoxic T lymphocytes (CTLs) destroy infected cells by releasing so-called lytic granules within the immunological synapse that is formed between CTLs and their targets (Stinchcombe et al., 2001). The centrosome moves to the immunological synapse by cortical pulling (Kim and Maly, 2009) and directs the delivery of lytic granules (Stinchcombe et al., 2006; Tsun et al., 2011) (Fig. 2D). Components of the intraflagellar transport system, a motile process involved in building cilia onto centrioles, also appear to be involved in trafficking to the immunological synapse (Finetti et al., 2009), suggesting an evolutionary relationship between ciliogenesis and formation of the immune synapse that might help explain a shared function for centrosomes in both processes.
Wound healing and cell migration
In wounded monolayers of fibroblasts, the centrosome often becomes oriented between the nucleus and the leading edge (Gotlieb et al., 1981). This orientation positions both the Golgi complex and the endocytic recycling compartment between the nucleus and the leading edge. However, whether the centrosome is ahead of or behind the nucleus depends on the cell type (Yvon et al., 2002). For example, the centrosome is located ahead of the nucleus during migration in eosinophils (Koonce et al., 1984) and Chinese hamster ovary (CHO) cells (Yvon et al., 2002), but behind the nucleus in PtK cells in wounded epithelial sheets (Yvon et al., 2002). However, when PtK cells migrate individually instead of in the context of a continuous cell sheet, the position of the centrosome relative to the nucleus does not correlate with the direction of cell migration (Danowski et al., 2001). Centriole rotational orientation is also regulated in migrating cells. In lymphocytes and macrophages, centrioles are oriented vertically with respect to the cell surface over which the cells are migrating (Gudima et al., 1988). Furthermore, primary cilia in migrating cells tend to point in the direction of cell movement (Albrecht-Buehler, 1977; Katsumoto et al., 1994; Schneider et al., 2010).
Even within a single cell type, the relative position of the centrosome and nucleus can vary as a function of myosin II activity (Szabó et al., 2011) or the type of substrate (Schütze et al., 1991). Further complicating the connection between centrosome position and cell migration is the fact that ablation or removal of centrosomes appears to affect cell migration in some cell types, but not in others (Koonce et al., 1984; Wakida et al., 2010). In polymorphonuclear leukocytes and keratocytes, centrosomes are not required for cell movement or chemotaxis (Huang et al., 1991; Verkhovsky et al., 1999), whereas in the specific case of wound healing they are more important (Schneider et al., 2010; Wakida et al., 2010).
Convergent extension
During development, vertebrate embryos undergo a dramatic change in shape. The lengthening and narrowing of a field of cells, termed convergent extension, contributes to a variety of morphogenetic processes including gastrulation. During zebrafish gastrulation, the centrosome position is highly polarized along the anteroposterior and mediolateral embryonic axes (Sepich et al., 2011). Centrosomes first polarize along a superficial-deep axis through the ectoderm and mesoderm, and later become polarized within the planes of the ectoderm and mesoderm between mid and late gastrulation. This planar polarization of centrosomes might reflect changes in the movements of polarized cells; specifically, medial or lateral positioned centrosomes can reflect medial or lateral cell rearrangement, respectively.
Multiciliated epithelia
In cells that form primary cilia, the centrosome migrates to the cortex of the cell where the mature centriole nucleates formation of the cilium (Satir and Christensen, 2007). In multiciliated epithelia (Fig. 2B), hundreds of centrioles are assembled at once in a single cell, leading to the formation of multiple cilia with a defined orientation of beating (Marshall and Kintner, 2008), which is in turn dictated by the rotational orientation of the centrioles (Tamm et al., 1975). Mis-orientation of centrioles, and subsequently of the cilia, can lead to a failure of cilia-driven fluid flows even when the individual cilia themselves have normal motility (Marshall and Kintner, 2008). In some cases, such a mis-orientation appears to be sufficient to cause respiratory disease in human patients (Rutland and de Iongh, 1990). However, the molecular pathways that orient the rotation of centrioles remain unclear.
Rotational orientation is determined after centrioles have docked at the cell cortex and begun to form cilia (Boisvieux-Ulrich et al., 1985; Frisch and Farbman, 1968). However, once a specific orientation is set up, it becomes locked and the cilia will not reorient themselves if the tissue is experimentally repositioned (Boisvieux-Ulrich and Sandoz, 1991). The cues that orient centrioles to generate an oriented field of cilia appear to be a combination of planar cell polarity (Borovina et al., 2010; Guirao et al., 2010; Mitchell et al., 2009; Park et al., 2008) and of fluid flow, such that the cilia coordinate the direction of their beating with neighboring cells (Mitchell et al., 2007; Guirao et al., 2010).
Sensory cilia
Cilia act as sensors for flow and mechanical deformation in vertebrate tissues. Cilia in epithelial tubes function as flow sensors (Masyuk et al., 2006; Liu, 2003; Praetorius and Spring, 2001), for example sensing the flow of urine in the kidney. To function properly, the cilia must face the lumen of the tube, because otherwise they would sense the wrong compartment. In tendons, primary cilia in tenocytes are oriented parallel to the collagen fibers along the long axis of the tendon (Donnelly et al., 2010). Cilia are oriented in chondrocytes relative to the cartilage layer (Farnum and Wilsman, 2011) and in vascular smooth muscle cells relative to the direction of stress (Lu et al., 2008). These directed orientations presumably allow mechanosensory cilia to respond to strains in particular directions. Cilia also mediate a response to chemical ligands in the extracellular environment (Singla and Reiter, 2006). A proper response to ligands requires centrioles to be positioned on the correct side of the cell to ensure that they sense the correct environment (for example the lumen of a duct rather than its outer surface).
Breaking of left–right symmetry
One of the most dramatic illustrations of centrosome positioning and cilia function in a develomental context is the breaking of left–right symmetry in mammalian embryos. Leftward fluid flow in the ventral node is responsible for the breaking the left–right symmetry in the mouse embryo (Hirokawa et al., 2009; Hirokawa et al., 2006; Hashimoto et al., 2010; Nonaka et al., 2002; Nonaka et al., 2005). Unidirectional flow is generated by the rotational movement of the node cilia, which are tilted towards the posterior. This posterior tilt, which is essential for generating leftward flow, appears to result from the posterior position of the centriole within each cell of the node (Nonaka et al., 2005; Okada et al., 2005).
Centrosome position in oriented cell division during development
Oriented planar cell division
In dividing cells, the centrosome determines the position at which the spindle poles will form, through interactions between astral microtubules and capture sites that are located on the cortical surface of the cell (Hyman, 1989; Labbé et al., 2004; Lutz et al., 1988; Miller and Rose, 1998). These cortical sites can be established from intracellular breaking events during symmetrical cell divisions or cell–cell interactions in a developing tissue (Goldstein, 1995). Although centrosomes are not strictly required for the formation of a bipolar spindle, for example during female meosis (Heald et al., 1996), they facilitate assembly of astral microtubules (de Saint Phalle and Sullivan, 1998; Hornick et al., 2011; Wilson et al., 1997). In the absence of centrosomes, bipolar spindles form but are not attached to the cell cortex and instead float around within the cell (Khodjakov and Rieder, 2001). Consequently, cells that lack centrosomes, or cells, in which centrosome function is abrogated, form spindles in random orientations (Giansanti et al., 2001; Louvet-Vallée et al., 2005). Because the spindle dictates the position and orientation of the cleavage furrow during cytokinesis (Oliferenko et al., 2009), spindle orientation mediated by centrosome position ultimately determines the orientation of cell division.
Oriented cell division has an important function in morphogenesis of epithelial tissues. When the mitotic spindle is aligned within the plane of the epithelium, it is termed planar spindle orientation. Because oriented planar cell division (OPCD) is ultimately a consequence of centrosome positioning, below we will consider several examples in which OPCD and hence centrosome position has an important role in axial elongation or determining organ shape.
During early neurulation and notochord extension in avian and mouse embryos, mitotic spindles are preferentially oriented parallel to the long axis of the notochord (Schoenwolf and Alvarez, 1989). The majority of notochord extension involves a combination of orientated cell division and cell rearrangement (Sausedo and Schoenwolf, 1994). During gastrulation in zebrafish, the epiblast dramatically elongates along the anterior–posterior axis of the embryo, driven at least in part by OPCD within the epiblast (Gong et al., 2004).
Many organs, such as lung, kidney and blood vessels develop from epithelial tubes that branch into complex networks. During organ development, each branch grows to attain the final size and shape that are appropriate for its position and function in the branching network. The orientation of cell division with respect to the longitudinal axis of the epithelial tube is thought to have a major effect on the shape of the tube (Fig. 3A). In kidney tubules, for example, cells primarily divide along the proximal–distal (longitudinal) axis of the epithelium, leading to lengthening of the tubule, while maintaining a constant diameter. In cystic kidney disease mouse and rat models, the orientation of tubule epithelial cell divisions is randomized, leading to increased tubular diameter and subsequent cysts (Fischer et al., 2006). Similar orientation of spindles along the long axis of developing tubes is seen in blood vessel development (Zeng et al., 2007). Therefore, interestingly, centrioles in blood vessel endothelial cells tend to be located on the same side as the heart, regardless of the direction of blood flow (Kiosses et al., 1997; Rogers et al., 1985). Longitudinally oriented cell divisions occur in the developing lung, and using a mathematical model, we recently demonstrated that a change in airway shape can be explained entirely on the basis of the distribution of spindle angles, without requiring oriented changes in other cellular processes, such as proliferation or cell shape (Tang et al., 2011).
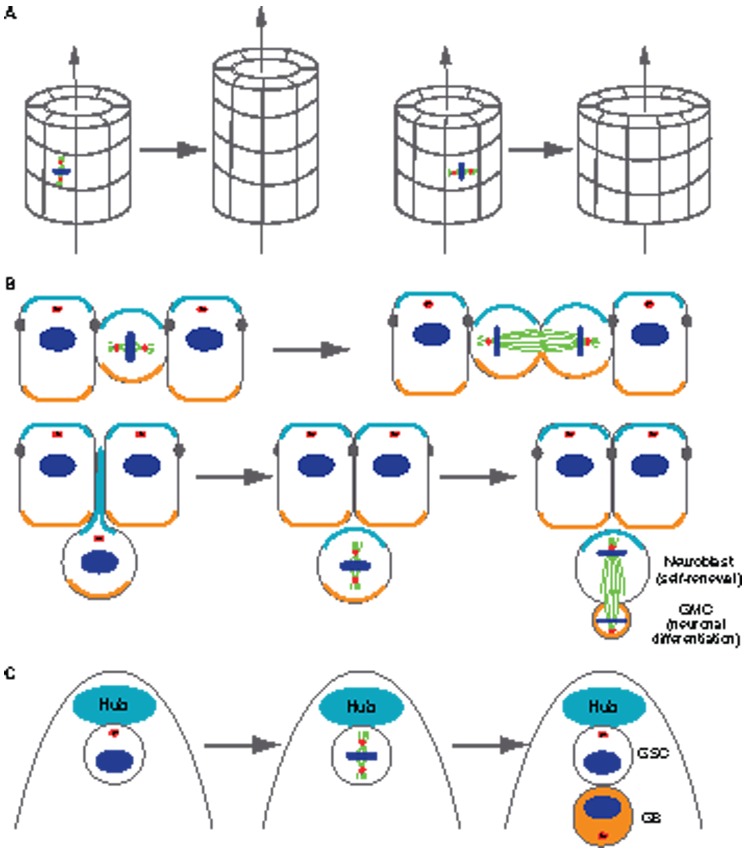
Fig. 3.
Spindle orientation mediated by centrosome–cortex interactions has functional roles and effects on tubular morphogenesis (A) and specification of cell fate (B and C). (A) Mitotic spindle orientation with respect to the longitudinal axis of an epithelial tube has a major effect on its shape. When the spindle orientation is parallel to the longitudinal axis of the tube (shown on the left), this cell division only increases tube length but not the circumference. By contrast, when the spindle orientation is perpendicular to the longitudinal axis of the tube (shown on the right), the cell division only increases tube circumference but not the length. (B) Intrinsic asymmetrically localized cortical cues (represented by the orange and turquoise coloring) determine the different fates of daughter cells in neuroblasts. Symmetrical divisions (top row), in which the mitotic spindles are perpendicular to the apical–basal axis, generate two identical daughter cells that inherit both apical and basal cortical cues. Asymmetric cell divisions (bottom row), in which mitotic spindles are parallel to the apical–basal axis, generate two daughter cells that inherit either apical or basal cortical cues. One daughter cell self-renews to maintain the pool of neuroblasts, whereas the other differentiates to populate the central nervous system. (C) Extrinsic polarity cues determine the different fates of daughter cells in the Drosophila male germline stem cell (GSC). During cell division, the mitotic spindle forms perpendicular to the interface with the stem cell niche (Hub), such that one daughter cell retains contact with the niche and its sustaining signals, whereas the other daughter cell loses contact with the niche and initiates differentiation (gonialblast, shown in orange). GMC, ganglion mother cell; GB, gonialblast.
One pathway that is involved in regulating OPCD is the planar cell polarity (PCP) pathway. Disruption of the PCP pathway affects OPCD and correlates with a reduction in elongation of the epiblast consistent with a role for oriented division in elongation. The PCP pathway appears to be important for tubular morphogenesis in some cases (Saburi et al., 2008), but not in others (Tang et al., 2011).
Asymmetric cell division
Asymmetric cell division generates daughter cells of distinct identities by separating asymmetrically localized proteins, RNA transcripts and other macromolecules, which are called cell fate determinants, to only one of the daughter cells. To do so, the mitotic spindle must be aligned parallel to the axis along which the fate determinants are distributed in the cell. Asymmetric cell division is, thus, a special case of oriented cell division, requiring an accurate positioning of centrosomes to ensure proper orientation of the mitotic spindle.
In the developing Drosophila nervous system, most neurons and glia arise from neural stem cells known as neuroblasts. Symmetric divisions of neuroblasts expand the progenitor pool, whereas asymmetrical divisions of neuroblasts generate postmitotic cells. Neuroblasts that form their mitotic spindles along the apical–basal axis generate two different fates (Fig. 3B). One daughter cell self-renews to maintain the pool of neuroblasts, whereas the other differentiates to populate the central nervous system (Doe, 2008), owing to the asymmetric distribution of proteins that specify either self-renewal or differentiation in separate apical and basal cortical domains during mitosis. The new centrosome is retained in the neuroblast during asymmetric division (Januschke et al., 2011), indicating a precise positioning of the centriole during asymmetric division. For a more detailed discussion of centrosome behavior during neuroblast division, we direct the reader to a recent review (Nigg and Stearns, 2011).
The centrosome also has a function in orienting divisions during brain development in mammals. Lack of the centrosomal abnormal spindle-like microcephaly associated protein (ASPM) in mice leads to defects in planar spindle orientation and a small brain size (Fish et al., 2006). Defects in centrosome-associated proteins are also associated with microcephaly in human patients (Guernsey et al., 2010; Kumar et al., 2009; Rauch et al., 2008; Yu et al., 2010), presumably owing to defects in asymmetric divisions in neuronal precursor cells, although a role in neuronal migration cannot currently be ruled out.
The stratified cell layers of the skin are generated by asymmetric cell division of basal epidermal cells, which generates a committed suprabasal cell and a self-renewed basal cell. Suprabasal cells enter a differentiation program to form the barrier. During this process, the apical localization of cortical polarity determinants is essential to align the spindle (Lechler and Fuchs, 2005), presumably through centrosome positioning.
The stem cell niche itself can also determine whether a stem cell division is asymmetric or symmetric by regulating the orientation of the mitotic spindle (Fuchs et al., 2004; Spradling et al., 2001). For example, in Drosophila male germline stem cells (GSCs) (Fig. 3C), the mitotic spindle is oriented perpendicular to the niche interface. When the cell divides, one daughter cell stays associated with the niche and its sustaining signals, and another daughter cell loses contact with the niche and starts to differentiate (Kiger et al., 2001; Tulina and Matunis, 2001; Yamashita et al., 2007). In GSCs, the mother centrosome is always positioned close to the niche throughout the cell cycle. After centrosome duplication, the daughter centrosome migrates towards the opposite side of the cell, thereby setting up the spindle orientation (Yamashita et al., 2007). Therefore, the mother centrosome contains the oldest centriole that is always inherited by the stem cell. Whether the niche regulates the different behaviors of mother and daughter centrioles in other types of asymmetric cell division remains an important area of investigation.
How important are centrosomes for animal development?
The many roles of centrosome positioning in cell migration, cell polarity, oriented planar cell division and the determination of asymmetric cell fate, imply that centrosomes should have important functions throughout development. However, genetic experiments in Drosophila have shown that centrosomes can be lost from developing flies during the course of development and give rise to adult flies that entirely lack centrosomes (Basto et al., 2006). These experiments had to be performed carefully to make sure that centrosomes were still present during early development, because the loss of centrosomes in early fly development results in developmental failure (Rodrigues-Martins et al., 2008; Stevens et al., 2007; Varmark et al., 2007).
However, there are reports of animal species that have completely lost centrosomes during evolution. For example, the planarian flatworm Schmidtea mediterranea has been shown to contain centrioles but lack centrosomes, as shown by ultrastructural studies that show the absence of centrioles at spindle poles, and by genomic comparisons that indicate that its genome lacks key proteins that are required to assemble a centrosome around a centriole (Azimzadeh et al., 2012). Interestingly, the loss of centrosomes in Schmidtea, as well as in the related parasitic flatworm Schistosoma (Jurberg et al., 2009), appears to correlate with a shift in early development from a highly mosaic form of embryogenesis, in which spindle orientations are precisely controlled, to an entirely regulative embryogenesis, in which spindle orientations are apparently random. These results suggest that the main function for centrosomes in development is in oriented divisions, and, therefore, if there is no strict requirement for oriented division, centrosomes are no longer required. Consistent with this idea, centrosomes are apparently absent during the earliest divisions of the mouse embryo, as cell fates during early mouse embryogenesis are determined in a completely regulative mode and, thus, do not require oriented divisions that are so important for mosaic development (Calarco-Gillam et al., 1983). It has also been reported that Myxozoa, a class of parasites possibly related to Cnidaria, lack centrioles (Canning et al., 2007). Unlike Planaria, which retained a normal bilaterian body plan with complex tissues despite their loss of centrosomes during evolution, Myxozoa have highly degenerate body plans that range from clusters of individual cells to simple sac-like structures (Canning et al., 2007). It is interesting to consider whether the loss of complex tissue morphogenesis in Myxozoa could be a consequence of the loss of centrioles and/or centrosomes. Understanding which types of developmental processes can, and cannot, take place in organisms that lack centrosomes might enable us to gain new insights into the functional relevance of the centrosome in animal development, as well as revealing potential centrosome-independent mechanisms for the regulation of oriented division (Cabernard et al., 2010).
Molecular pathways underlying centrosome positioning
Centrosome positioning requires that there is a source of information that dictates where the centrosome should go, and the existence of a mechanism by which centrosomes respond to this information. Microtubule asters can center themselves within cell fragments (Rodionov and Borisy, 1997), or even within microfabricated chambers (Holy et al., 1997; Laan et al., 2012). However, the means by which a centrosome-mediated aster of microtubules could find the center of a geometrical region remains an area of debate. Both pushing forces that are generated by the microtubules emanating from the centrosome and pulling forces that are exerted on these microtubules by cortical motor proteins have been proposed to underlie the centering mechanism, as suggested in a recent computational study (Zhu et al., 2010). However, in many cell types, centrosomes are not positioned in the center, which raises the important question; what are the mechanisms that result in a deviation from the default central position?
The positional information that specifies centrosome position could be generated within the cell itself, or it could be provided by the external environment of the cell, either by other cells, by the extracellular matrix or by morphogens acting at a tissue level. We refer to these two broad types of positional cues as intrinsic and extrinsic cues in the sections below. In many cases, cells are able to reorient their intrinsic polarity in response to external cues.
Intrinsic and extrinsic polarity cues
The Par complex (see Box 1) is a set of evolutionarily conserved intrinsic polarity proteins, consisting of Par3, Par6 and atypical protein kinase C (aPKC), which are asymmetrically distributed at the cell cortex and have important functions in the determination of cell polarity and the orientation of the mitotic spindle (Pieczynski and Margolis, 2011). The Par complex is a main determinant of polarity. It interacts with the Gαi–Pins–Mud, members of an evolutionarily conserved receptor-independent G-protein pathway, and dynactin complexes (see Box 1) to govern spindle orientation through the attachment of astral microtubule plus-ends to cortically-anchored dynein–dynactin motor complexes, resulting in pulling forces that move the centrosomes towards the cortex. In vertebrate cells, dynein, dynactin and Lis1 (see Box 1) are all required for positioning of the mitotic spindle in response to intrinsic cortical polarity cues. Coupling of cell polarity and dynein function is in part mediated through the nuclear mitotic apparatus (NuMA) protein, which is the vertebrate homolog of Mud. NuMA, whose activity is regulated by both the Par complex and Pins (Galli et al., 2011; Sun and Schatten, 2006) uses its cross-linking properties to mediate spindle anchoring at the cell cortex, and functions together with dynein in positioning of the centrosome and spindle (Siller and Doe, 2009).
Box 1. The Par complex and dynein.
The Par complex, consisting of Par3, Par6 and atypical protein kinase C (aPKC), localizes to the apical cortex of epithelial cells in vertebrates, the anterior cortex in the C. elegans zygote and the apical cortex in Drosophila neuroblasts (Pieczynski and Margolis, 2011). Par3 contains multiple PDZ domains and provides anchorage to assemble the Par complex at the apical–lateral border by binding Par6 and recruiting Par6-associated proteins. Par6 is a scaffolding protein that contains PDZ, PB1 and CRIB domains, which provide a direct interaction with aPKC and Par3. The Par complex is crucial in regulating cell polarity during embryogenesis, epithelial morphogenesis, neuronal differentiation and cell migration. In addition, in healthy cell types the Par complex contributes to maintain cell homeostasis and prevent abnormal growth and cell migration.
Dynein is a large multi-subunit motor protein complex, which contains multiple ATP binding sites (Vallee et al., 2004). Dynein uses ATP hydrolysis to provide the energy for its movement along microtubules towards the minus-end of the microtubule. Dynactin is another multi-subunit protein complex that is required for almost all types of cytoplasmic dynein activity in eukaryotes. Dynactin interacts with dynein directly through the binding of dynein intermediate chains with the p150Glued subunit of dynactin, and allows the motor to traverse the microtubule lattice over long distances. Another dynein-interacting protein is LIS1, which consists of seven spaced WD-40 repeats and is involved in the regulation of microtubule transport (Vallee et al., 2004). The loss of one allele of the gene encoding LIS1 causes lissencephaly (smooth brain), a developmental brain malformation, in which the surface of the brain is smooth because of a defect in the migration of the cortical neuron. The dynein–dynactin complex is required for the regulation of organelle transport and position, centrosome assembly and spindle orientation.
Planar cell polarity (PCP, Box 2) is an intrinsic polarity system that is coordinated between neighboring cells, and can transmit information for an orientation that is perpendicular to the axis of apical–basal polarity across the entire plane of a tissue (Bayly and Axelrod, 2011; Goodrich and Strutt, 2011; Zallen, 2007). The ability of PCP genes to influence centrosome position and spindle orientation has been well demonstrated in Drosophila (Baena-López et al., 2005; Gho and Schweisguth, 1998), and zebrafish (Gong et al., 2004; Ciruna et al., 2006). Furthermore, PCP genes have been linked to oriented cell division in kidneys and the gastrointestinal tract (Matsuyama et al., 2009; Saburi et al., 2008). Recent work (Ségalen et al., 2010) shows that the PCP pathway might influence centrosome positioning through the Mud–NuMA–Dynein cortical polarity pathway.
Box 2. The PCP pathway.
Planar cell polarity (PCP) is a signaling pathway that indicates the orientation within the sheet of an epithelium, so it is perpendicular to the apical–basal axis (Zallen, 2007). PCP influences multiple cellular processes, including orientated cell movement and cell division, and the orientation or formation of cilia. Most of the evolutionarily conserved genes in the PCP pathway were first identified in Drosophila. These genes fall into two groups, which act together to coordinate the establishment of PCP. The first group includes, but is not limited to, dishevelled, frizzled, prickle, Van Gogh (also known as strabismus) and starry night (also known as flamingo) (for more detailed information, please see review by Lawrence et al., 2007). Mutations in genes of this first group disturb cell polarity but have only subtle effects on the overall tissue pattern. The second group includes genes encoding non-classical cadherins, such as fat and dachsous, as well as a gene encoding the transmembrane Golgi-complex protein, Four-jointed. Mutations in genes of the second group not only change the polarity of the cell, but also alter the shapes of wings and legs and can disturb growth. In vertebrates, mutant studies of known PCP genes have provided evidence that the PCP pathway has important functions in a wide variety of development processes and in organogenesis, including gastrulation, neurulation, left-right determination, cardiogenesis, inner ear development, and kidney and lung development (Karner et al., 2006; Yates et al., 2010).
Centrosome position and spindle orientation are also regulated by cell–cell adhesion. E-cadherin provides a polarity cue for GSCs to position their centrosomes (Inaba et al., 2010), and also acts to orient the planar spindle during symmetric cell division (den Elzen et al., 2009). The architecture and extracellular matrix tension can also affect the orientation of cell divisions (Théry et al., 2007; Théry et al., 2005) by controlling the segregation of cortical components that are used for spindle orientation. Even in interphase cells, the shape of the extracellular matrix attachment pattern can dictate the position of the centrosome (Théry et al., 2006). Thus, the extrinsic cues to which centrosome positioning might respond include both molecular and mechanical cues.
Force generation and anchoring
Once a suitable polarity cue is present, the question arises of how the centrosome is physically moved in response to this cue. Diffusion by Brownian motion might be too slow to allow centrosome capture at a defined site within a time frame that would allow cell polarization during a typical cell cycle (Rafelski et al., 2011). Possibly for this reason, centrosome positioning in many systems involves an active force generation by both the microtubule- and actin-based cytoskeletons (Buendia et al., 1990; Burakov et al., 2003; Euteneuer and Schliwa, 1985; Euteneuer and Schliwa, 1992). In some cell types, the daughter centriole is substantially more motile than the mother centriole, and, in these cases, disruption of either the actin or tubulin cytoskeletons fails to halt the motion (Piel et al., 2000). However, if the actin and tubulin cytoskeleton are both disassembled, the daughter centriole stops moving (Piel et al., 2000), suggesting that forces that are generated by both act on centrioles in parallel. Before cytokinesis, centrioles can move along spindle microtubules (Jonsdottir et al., 2010), further implicating microtubules in centriole movement.
During centrosome re-positioning in wound healing, the flow of cortical actin appears to be particularly important (Gomes et al., 2005), but centrosome positioning in these cells also involves dynein, suggesting that microtubules also have a function in this process (Schmoranzer et al., 2009). Similarly, the movement of centrioles to the cell cortex during ciliogenesis also involves actin, potentially at multiple stages, that is, not only in propelling the centrioles out of the cell surface but also in anchoring them into the cortex (Boisvieux-Ulrich et al., 1990; Dawe et al., 2009; Hirota et al., 2010; Pan et al., 2007; Panizzi et al., 2007; Tamm and Tamm, 1988).
In order for the actin and tubulin cytoskeleton to exert force on the centrosome, mechanically stable attachment points are required. Without centrioles, centrosomes fragment when microtubules push on them (Abal et al., 2005), suggesting that the centriole is a structural core that allows forces to pull the centrosome in a coherent way, instead of ripping out small pieces. Centriole–cytoskeleton interactions might, thus, be central for positioning of the centrosome. The centriole extends three main sets of fibrous structures: the basal foot, the distal and subdistal appendages, and the striated rootlet, any of which could potentially serve as attachment points or ‘handles’, onto which the cytoskeleton could exert force to drive orientation (Fig. 1B).
The basal foot (Fig. 1B) is a cone-shaped protrusion from the basal body, which points in the direction of ciliary beat in a multiciliated epithelium (Boisvieux-Ulrich and Sandoz, 1991; Gibbons, 1961). The basal foot also acts as a focal point for microtubules in ciliated tissues, suggesting that it functions as a handle to allow the microtubule cytoskeleton to exert forces on the basal body during orientation of the centrosome (Chailley et al., 1989; Hagiwara et al., 2000). Formation of the basal foot requires the ODF2 protein, and, in mice lacking functional ODF2, basal bodies become randomly oriented, resulting in ineffective cilia-driven flow (Kunimoto et al., 2012).
The other appendage attached to the basal body is the striated rootlet, which points away from the cortex in an opposite direction to the basal foot. Interestingly, proteins that localize to the mitotic spindle midzone during cytokinesis (such as Protein regulator of cytokinesis 1, mitotic kinesin-like protein, Inner centromere protein, and centriolin) relocate to striated rootlets during ciliogenesis (Gromley et al., 2003; Smith et al., 2011). Because these central spindle components can modulate actin and microtubule dynamics (Mishima and Glotzer, 2003), they might mediate interactions between the rootlets and the rest of the cytoskeleton.
Similar to the basal foot acting as a microtubule attachment point for basal bodies in multiciliated epithelia, the subdistal appendages (Fig. 1A) might have a similar function in epithelial cells that have only a single centriole pair (Mogensen et al., 2000). Centrioles in these cells lack basal feet, which appear to be a specialization of multiciliated cells. ODF2, which is required for assembly of the basal foot in multiciliated epithelia (Kunimoto et al., 2012), is required for formation of subdistal appendages in cells with a single primary cilium (Ishikawa et al., 2005), but when centrioles are lacking these distal appendages, ciliogenesis itself is defective, instead of ciliary orientation, suggesting that unlike the basal foot, the subdistal appendages are required for additional functions besides rotational orientation, the most likely one being docking onto the cell cortex or recruitment of ciliogenesis proteins (Dawe et al., 2007).
Another candidate for linking centriole orientation to cell polarity cues is TBCC domain containing I (TBCCD1), a centrosome-associated protein that has been identified in a genetic screen for defects in centriole positioning in Chlamydomonas (Feldman et al., 2007; Feldman and Marshall, 2009), and which was later shown to be crucial for centrosome positioning during wound healing in mammalian cells (Gonçalves et al., 2010). The mechanistic role of TBCCD1 remains unknown. Another important protein linking the cytoskeleton to centrioles is ninein, a microtubule-anchoring component of the subdistal appendages (Delgehyr et al., 2005; Mogensen et al., 2000). Ninein is required for tubular morphogenesis in mammalian endothelial cells, suggesting a role for ninein-mediated microtubule anchoring in oriented cell divisions (Matsumoto et al., 2008). The association of ninein with centrioles requires ODF2 (Ibi et al., 2011). Furthermore, ninein is required for maintaining radial glia progenitors in the developing mammalian neocortex (Wang et al., 2009). It will be of great interest to see the function of such proteins in other developmental processes.
Conclusions and future perspectives
Our understanding of centrosome positioning in animal development is currently hampered by the fact that centrosome behavior varies widely between different types of cells, making it extremely difficult to deduce any general mechanisms. Thus, there is a clear need for systematic, organism-wide surveys of centrosome position and orientation across tissues and across developmental time. A second major challenge is to link the behavior of centrosomes at the organelle level with the large-scale behavior of developing tissues, such as the migration of cell collectives, non-isotropic expansion of epithelial sheets and epithelial–mesenchymal transitions. This issue of how subcellular events influence extracellular processes is a question that clearly lies at the interface of cell and developmental biology. Addressing both of these goals will require a new effort, combining systematic description with multi-scale modeling approaches.
Footnotes
Funding
This work was supported by a grant from the National Institutes of Health (NIH) [grant number R01 GM077004 to W.F.M.]. N.T. was supported by an AHA Scientist Development grant and an NIH training grant [grant number 5T32HL007185 to N.T.]. Deposited in PMC for release after 12 months.
References
- Abal M., Keryer G., Bornens M. (2005). Centrioles resist forces applied on centrosomes during G2/M transition. Biol. Cell 97, 425–434 10.1042/BC20040112 [DOI] [PubMed] [Google Scholar]
- Albrecht–Buehler G. (1977). Phagokinetic tracks of 3T3 cells: parallels between the orientation of track segments and of cellular structures which contain actin or tubulin. Cell 12, 333–339 10.1016/0092-8674(77)90109-X [DOI] [PubMed] [Google Scholar]
- Azimzadeh J., Bornens M. (2007). Structure and duplication of the centrosome. J. Cell Sci. 120, 2139–2142 10.1242/jcs.005231 [DOI] [PubMed] [Google Scholar]
- Azimzadeh J., Wong M. L., Downhour D. M., Sánchez Alvarado A., Marshall W. F. (2012). Centrosome loss in the evolution of planarians. Science 335, 461–463 10.1126/science.1214457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena–López L. A., Baonza A., García–Bellido A. (2005). The orientation of cell divisions determines the shape of Drosophila organs. Curr. Biol. 15, 1640–1644 10.1016/j.cub.2005.07.062 [DOI] [PubMed] [Google Scholar]
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C. G., Khodjakov A., Raff J. W. (2006). Flies without centrioles. Cell 125, 1375–1386 10.1016/j.cell.2006.05.025 [DOI] [PubMed] [Google Scholar]
- Bayly R., Axelrod J. D. (2011). Pointing in the right direction: new developments in the field of planar cell polarity. Nat. Rev. Genet. 12, 385–391 10.1038/nrg2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion A., Baudoin J. P., Alvarez C., Bornens M., Métin C. (2005). Nucleokinesis in tangentially migrating neurons comprises two alternating phases: forward migration of the Golgi/centrosome associated with centrosome splitting and myosin contraction at the rear. J. Neurosci. 25, 5691–5699 10.1523/JNEUROSCI.1030-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvieux–Ulrich E., Sandoz D. (1991). Determination of ciliary polarity precedes differentiation in the epithelial cells of quail oviduct. Biol. Cell 72, 3–14 10.1016/0248-4900(91)90072-U [DOI] [PubMed] [Google Scholar]
- Boisvieux–Ulrich E., Laine M. C., Sandoz D. (1985). The orientation of ciliary basal bodies in quail oviduct is related to the ciliary beating cycle commencement. Biol. Cell 55, 147–150 10.1111/j.1768-322X.1985.tb00417.x [DOI] [PubMed] [Google Scholar]
- Boisvieux–Ulrich E., Lainé M. C., Sandoz D. (1990). Cytochalasin D inhibits basal body migration and ciliary elongation in quail oviduct epithelium. Cell Tissue Res. 259, 443–454 10.1007/BF01740770 [DOI] [PubMed] [Google Scholar]
- Borovina A., Superina S., Voskas D., Ciruna B. (2010). Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat. Cell Biol. 12, 407–412 10.1038/ncb2042 [DOI] [PubMed] [Google Scholar]
- Buendia B., Bré M. H., Griffiths G., Karsenti E. (1990). Cytoskeletal control of centrioles movement during the establishment of polarity in Madin-Darby canine kidney cells. J. Cell Biol. 110, 1123–1135 10.1083/jcb.110.4.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burakov A., Nadezhdina E., Slepchenko B., Rodionov V. (2003). Centrosome positioning in interphase cells. J. Cell Biol. 162, 963–969 10.1083/jcb.200305082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabernard C., Prehoda K. E., Doe C. Q. (2010). A spindle-independent cleavage furrow positioning pathway. Nature 467, 91–94 10.1038/nature09334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarco–Gillam P. D., Siebert M. C., Hubble R., Mitchison T., Kirschner M. (1983). Centrosome development in early mouse embryos as defined by an autoantibody against pericentriolar material. Cell 35, 621–629 10.1016/0092-8674(83)90094-6 [DOI] [PubMed] [Google Scholar]
- Canning E. U., Curry A., Hill S. L., Okamura B. (2007). Ultrastructure of Buddenbrockia allmani n. sp. (Myxozoa, Malacosporea), a parasite of Lophopus crystallinus (Bryozoa, Phylactolaemata). J. Eukaryot. Microbiol. 54, 247–262 10.1111/j.1550-7408.2007.00261.x [DOI] [PubMed] [Google Scholar]
- Chailley B., Nicolas G., Lainé M. C. (1989). Organization of actin microfilaments in the apical border of oviduct ciliated cells. Biol. Cell 67, 81–90 10.1111/j.1768-322X.1989.tb03012.x [DOI] [PubMed] [Google Scholar]
- Ciruna B., Jenny A., Lee D., Mlodzik M., Schier A. F. (2006). Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439, 220–224 10.1038/nature04375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danowski B. A., Khodjakov A., Wadsworth P. (2001). Centrosome behavior in motile HGF-treated PtK2 cells expressing GFP-gamma tubulin. Cell Motil. Cytoskeleton 50, 59–68 10.1002/cm.1041 [DOI] [PubMed] [Google Scholar]
- Dawe H. R., Farr H., Gull K. (2007). Centriole/basal body morphogenesis and migration during ciliogenesis in animal cells. J. Cell Sci. 120, 7–15 10.1242/jcs.03305 [DOI] [PubMed] [Google Scholar]
- Dawe H. R., Adams M., Wheway G., Szymanska K., Logan C. V., Noegel A. A., Gull K., Johnson C. A. (2009). Nesprin-2 interacts with meckelin and mediates ciliogenesis via remodelling of the actin cytoskeleton. J. Cell Sci. 122, 2716–2726 10.1242/jcs.043794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Anda F. C., Pollarolo G., Da Silva J. S., Camoletto P. G., Feiguin F., Dotti C. G. (2005). Centrosome localization determines neuronal polarity. Nature 436, 704–708 10.1038/nature03811 [DOI] [PubMed] [Google Scholar]
- de Saint Phalle B., Sullivan W. (1998). Spindle assembly and mitosis without centrosomes in parthenogenetic Sciara embryos. J. Cell Biol. 141, 1383–1391 10.1083/jcb.141.6.1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgehyr N., Sillibourne J., Bornens M. (2005). Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 118, 1565–1575 10.1242/jcs.02302 [DOI] [PubMed] [Google Scholar]
- den Elzen N., Buttery C. V., Maddugoda M. P., Ren G., Yap A. S. (2009). Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Mol. Biol. Cell 20, 3740–3750 10.1091/mbc.E09-01-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Mitchison T. J. (1997). Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13, 83–117 10.1146/annurev.cellbio.13.1.83 [DOI] [PubMed] [Google Scholar]
- Distel M., Hocking J. C., Volkmann K., Köster R. W. (2010). The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. J. Cell Biol. 191, 875–890 10.1083/jcb.201004154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe C. Q. (2008). Neural stem cells: balancing self-renewal with differentiation. Development 135, 1575–1587 10.1242/dev.014977 [DOI] [PubMed] [Google Scholar]
- Donnelly E., Ascenzi M. G., Farnum C. (2010). Primary cilia are highly oriented with respect to collagen direction and long axis of extensor tendon. J. Orthop. Res. 28, 77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylewski D. P., Keenan T. W. (1984). Centrioles in the mammary epithelium of the rat. J. Cell Sci. 72, 185–193 [DOI] [PubMed] [Google Scholar]
- Euteneuer U., Schliwa M. (1985). Evidence for an involvement of actin in the positioning and motility of centrosomes. J. Cell Biol. 101, 96–103 10.1083/jcb.101.1.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U., Schliwa M. (1992). Mechanism of centrosome positioning during the wound response in BSC-1 cells. J. Cell Biol. 116, 1157–1166 10.1083/jcb.116.5.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnum C. E., Wilsman N. J. (2011). Orientation of primary cilia of articular chondrocytes in three-dimensional space. Anat. Rec. (Hoboken) 294, 533–549 10.1002/ar.21330 [DOI] [PubMed] [Google Scholar]
- Feldman J. L., Marshall W. F. (2009). ASQ2 encodes a TBCC-like protein required for mother-daughter centriole linkage and mitotic spindle orientation. Curr. Biol. 19, 1238–1243 10.1016/j.cub.2009.05.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. L., Priess J. R. (2012). A role for the centrosome and PAR-3 in the hand-off of MTOC function during epithelial polarization. Curr. Biol. 22, 575–582 10.1016/j.cub.2012.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. L., Geimer S., Marshall W. F. (2007). The mother centriole plays an instructive role in defining cell geometry. PLoS Biol. 5, e149 10.1371/journal.pbio.0050149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finetti F., Paccani S. R., Riparbelli M. G., Giacomello E., Perinetti G., Pazour G. J., Rosenbaum J. L., Baldari C. T. (2009). Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat. Cell Biol. 11, 1332–1339 10.1038/ncb1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Legue E., Doyen A., Nato F., Nicolas J. F., Torres V., Yaniv M., Pontoglio M. (2006). Defective planar cell polarity in polycystic kidney disease. Nat. Genet. 38, 21–23 10.1038/ng1701 [DOI] [PubMed] [Google Scholar]
- Fish J. L., Kosodo Y., Enard W., Pääbo S., Huttner W. B. (2006). Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc. Natl. Acad. Sci. USA 103, 10438–10443 10.1073/pnas.0604066103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch D., Farbman A. I. (1968). Development of order during ciliogenesis. Anat. Rec. 162, 221–232 10.1002/ar.1091620209 [DOI] [PubMed] [Google Scholar]
- Frolenkov G. I., Belyantseva I. A., Friedman T. B., Griffith A. J. (2004). Genetic insights into the morphogenesis of inner ear hair cells. Nat. Rev. Genet. 5, 489–498 10.1038/nrg1377 [DOI] [PubMed] [Google Scholar]
- Fuchs E., Tumbar T., Guasch G. (2004). Socializing with the neighbors: stem cells and their niche. Cell 116, 769–778 10.1016/S0092-8674(04)00255-7 [DOI] [PubMed] [Google Scholar]
- Galli M., Muñoz J., Portegijs V., Boxem M., Grill S. W., Heck A. J., van den Heuvel S. (2011). aPKC phosphorylates NuMA-related LIN-5 to position the mitotic spindle during asymmetric division. Nat. Cell Biol. 13, 1132–1138 10.1038/ncb2315 [DOI] [PubMed] [Google Scholar]
- Gho M., Schweisguth F. (1998). Frizzled signalling controls orientation of asymmetric sense organ precursor cell divisions in Drosophila. Nature 393, 178–181 10.1038/30265 [DOI] [PubMed] [Google Scholar]
- Giansanti M. G., Gatti M., Bonaccorsi S. (2001). The role of centrosomes and astral microtubules during asymmetric division of Drosophila neuroblasts. Development 128, 1137–1145 [DOI] [PubMed] [Google Scholar]
- Gibbons I. R. (1961). The relationship between the fine structure and direction of beat in gill cilia of a lamellibranch mollusc. J. Biophys. Biochem. Cytol. 11, 179–205 10.1083/jcb.11.1.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B. (1995). Cell contacts orient some cell division axes in the Caenorhabditis elegans embryo. J. Cell Biol. 129, 1071–1080 10.1083/jcb.129.4.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E. R., Jani S., Gundersen G. G. (2005). Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451–463 10.1016/j.cell.2005.02.022 [DOI] [PubMed] [Google Scholar]
- Gonçalves J., Nolasco S., Nascimento R., Lopez Fanarraga M., Zabala J. C., Soares H. (2010). TBCCD1, a new centrosomal protein, is required for centrosome and Golgi apparatus positioning. EMBO Rep. 11, 194–200 10.1038/embor.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y., Mo C., Fraser S. E. (2004). Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430, 689–693 10.1038/nature02796 [DOI] [PubMed] [Google Scholar]
- Goodrich L. V., Strutt D. (2011). Principles of planar polarity in animal development. Development 138, 1877–1892 10.1242/dev.054080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlieb A. I., May L. M., Subrahmanyan L., Kalnins V. I. (1981). Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J. Cell Biol. 91, 589–594 10.1083/jcb.91.2.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromley A., Jurczyk A., Sillibourne J., Halilovic E., Mogensen M., Groisman I., Blomberg M., Doxsey S. (2003). A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J. Cell Biol. 161, 535–545 10.1083/jcb.200301105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudima G. O., Vorobjev I. A., Chentsov Y. S. (1988). Centriolar location during blood cell spreading and motion in vitro: an ultrastructural analysis. J. Cell Sci. 89, 225–241 [DOI] [PubMed] [Google Scholar]
- Guernsey D. L., Jiang H., Hussin J., Arnold M., Bouyakdan K., Perry S., Babineau–Sturk T., Beis J., Dumas N., Evans S. C.et al. (2010). Mutations in centrosomal protein CEP152 in primary microcephaly families linked to MCPH4. Am. J. Hum. Genet. 87, 40–51 10.1016/j.ajhg.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B., Meunier A., Mortaud S., Aguilar A., Corsi J. M., Strehl L., Hirota Y., Desoeuvre A., Boutin C., Han Y. G.et al. (2010). Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 12, 341–350 10.1038/ncb2040 [DOI] [PubMed] [Google Scholar]
- Hagiwara H., Kano A., Aoki T., Ohwada N., Takata K. (2000). Localization of gamma-tubulin to the basal foot associated with the basal body extending a cilium. Histochem. J. 32, 669–671 10.1023/A%3A1004163315822 [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Blank T., Sandaltzopoulos R., Becker P., Hyman A., Karsenti E. (1996). Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425 10.1038/382420a0 [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Tanaka Y., Okada Y., Takeda S. (2006). Nodal flow and the generation of left-right asymmetry. Cell 125, 33–45 10.1016/j.cell.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Tanaka Y., Okada Y. (2009). Left-right determination: involvement of molecular motor KIF3, cilia, and nodal flow. Cold Spring Harb. Perspect. Biol. 1, a000802 10.1101/cshperspect.a000802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Meunier A., Huang S., Shimozawa T., Yamada O., Kida Y. S., Inoue M., Ito T., Kato H., Sakaguchi M.et al. (2010). Planar polarity of multiciliated ependymal cells involves the anterior migration of basal bodies regulated by non-muscle myosin II. Development 137, 3037–3046 10.1242/dev.050120 [DOI] [PubMed] [Google Scholar]
- Holy T. E., Dogterom M., Yurke B., Leibler S. (1997). Assembly and positioning of microtubule asters in microfabricated chambers. Proc. Natl. Acad. Sci. USA 94, 6228–6231 10.1073/pnas.94.12.6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick J. E., Mader C. C., Tribble E. K., Bagne C. C., Vaughan K. T., Shaw S. L., Hinchcliffe E. H. (2011). Amphiastral mitotic spindle assembly in vertebrate cells lacking centrosomes. Curr. Biol. 21, 598–605 10.1016/j.cub.2011.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. J., Silverstein S. C., Malawista S. E. (1991). Cryopreserved cytoplasts from human neutrophils migrate across monolayers of human endothelial cells in response to a chemoattractant gradient. J. Leukoc. Biol. 50, 624–627 [DOI] [PubMed] [Google Scholar]
- Hulspas R., Houtsmuller A. B., Bauman J. G., Nanninga N. (1994). The centrosome moves out of a nuclear indentation in human lymphocytes upon activation. Exp. Cell Res. 215, 28–32 10.1006/excr.1994.1310 [DOI] [PubMed] [Google Scholar]
- Hyman A. A. (1989). Centrosome movement in the early divisions of Caenorhabditis elegans: a cortical site determining centrosome position. J. Cell Biol. 109, 1185–1193 10.1083/jcb.109.3.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibi M., Zou P., Inoko A., Shiromizu T., Matsuyama M., Hayashi Y., Enomoto M., Mori D., Hirotsune S., Kiyono T.et al. (2011). Trichoplein controls microtubule anchoring at the centrosome by binding to Odf2 and ninein. J. Cell Sci. 124, 857–864 10.1242/jcs.075705 [DOI] [PubMed] [Google Scholar]
- Inaba M., Yuan H., Salzmann V., Fuller M. T., Yamashita Y. M. (2010). E-cadherin is required for centrosome and spindle orientation in Drosophila male germline stem cells. PLoS ONE 5, e12473 10.1371/journal.pone.0012473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Kubo A., Tsukita S., Tsukita S. (2005). Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat. Cell Biol. 7, 517–524 10.1038/ncb1251 [DOI] [PubMed] [Google Scholar]
- Januschke J., Llamazares S., Reina J., Gonzalez C. (2011). Drosophila neuroblasts retain the daughter centrosome. Nat. Commun. 2, 243 10.1038/ncomms1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C., Roper V. C., Foucher I., Qian D., Banizs B., Petit C., Yoder B. K., Chen P. (2008). Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat. Genet. 40, 69–77 10.1038/ng.2007.54 [DOI] [PubMed] [Google Scholar]
- Jonsdottir A. B., Dirks R. W., Vrolijk J., Ogmundsdottir H. M., Tanke H. J., Eyfjörd J. E., Szuhai K. (2010). Centriole movements in mammalian epithelial cells during cytokinesis. BMC Cell Biol. 11, 34 10.1186/1471-2121-11-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurberg A. D., Gonçalves T., Costa T. A., de Mattos A. C., Pascarelli B. M., de Manso P. P., Ribeiro–Alves M., Pelajo–Machado M., Peralta J. M., Coelho P. M.et al. (2009). The embryonic development of Schistosoma mansoni eggs: proposal for a new staging system. Dev. Genes Evol. 219, 219–234 10.1007/s00427-009-0285-9 [DOI] [PubMed] [Google Scholar]
- Karner C., Wharton K. A., Jr and Carroll T. J. (2006). Planar cell polarity and vertebrate organogenesis. Semin. Cell Dev. Biol. 17, 194–203 10.1016/j.semcdb.2006.05.003 [DOI] [PubMed] [Google Scholar]
- Katsumoto T., Higaki K., Ohno K., Onodera K. (1994). The orientation of primary cilia during the wound response in 3Y1 cells. Biol. Cell 81, 17–21 10.1016/0248-4900(94)90050-7 [DOI] [PubMed] [Google Scholar]
- Keating T. J., Borisy G. G. (1999). Centrosomal and non-centrosomal microtubules. Biol. Cell 91, 321–329 [PubMed] [Google Scholar]
- Khodjakov A., Rieder C. L. (2001). Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J. Cell Biol. 153, 237–242 10.1083/jcb.153.1.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiger A. A., Jones D. L., Schulz C., Rogers M. B., Fuller M. T. (2001). Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294, 2542–2545 10.1126/science.1066707 [DOI] [PubMed] [Google Scholar]
- Kim M. J., Maly I. V. (2009). Deterministic mechanical model of T-killer cell polarization reproduces the wandering of aim between simultaneously engaged targets. PLoS Comput. Biol. 5, e1000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiosses W. B., McKee N. H., Kalnins V. I. (1997). The distribution of centrosomes in endothelial cells of the rat aorta and inferior vena cava. Artery 22, 251–265 [PubMed] [Google Scholar]
- Klotz C., Bordes N., Laine M. C., Sandoz D., Bornens M. (1986). A protein of 175,000 daltons associated with striated rootlets in ciliated epithelia, as revealed by a monoclonal antibody. Cell Motil. Cytoskeleton 6, 56–67 10.1002/cm.970060108 [DOI] [PubMed] [Google Scholar]
- Koonce M. P., Cloney R. A., Berns M. W. (1984). Laser irradiation of centrosomes in newt eosinophils: evidence of centriole role in motility. J. Cell Biol. 98, 1999–2010 10.1083/jcb.98.6.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Girimaji S. C., Duvvari M. R., Blanton S. H. (2009). Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am. J. Hum. Genet. 84, 286–290 10.1016/j.ajhg.2009.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto K., Yamazaki Y., Nishida T., Shinohara K., Ishikawa H., Hasegawa T., Okanoue T., Hamada H., Noda T., Tamura A.et al. (2012). Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell 148, 189–200 10.1016/j.cell.2011.10.052 [DOI] [PubMed] [Google Scholar]
- Laan L., Pavin N., Husson J., Romet–Lemonne G., van Duijn M., López M. P., Vale R. D., Jülicher F., Reck–Peterson S. L., Dogterom M. (2012). Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell 148, 502–514 10.1016/j.cell.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J. C., McCarthy E. K., Goldstein B. (2004). The forces that position a mitotic spindle asymmetrically are tethered until after the time of spindle assembly. J. Cell Biol. 167, 245–256 10.1083/jcb.200406008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. A., Struhl G., Casal J. (2007). Planar cell polarity: one or two pathways? Nat. Rev. Genet. 8, 555–563 10.1038/nrg2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T., Fuchs E. (2005). Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 10.1038/nature03922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Xu S., Woda C., Kim P., Weinbaum S., Satlin L. M. (2003). Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am. J. Physiol. Renal Physiol. 285, F998–F1012 [DOI] [PubMed] [Google Scholar]
- Louvet–Vallée S., Vinot S., Maro B. (2005). Mitotic spindles and cleavage planes are oriented randomly in the two-cell mouse embryo. Curr. Biol. 15, 464–469 10.1016/j.cub.2004.12.078 [DOI] [PubMed] [Google Scholar]
- Lu C. J., Du H., Wu J., Jansen D. A., Jordan K. L., Xu N., Sieck G. C., Qian Q. (2008). Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press. Res. 31, 171–184 10.1159/000132462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz D. A., Hamaguchi Y., Inoué S. (1988). Micromanipulation studies of the asymmetric positioning of the maturation spindle in Chaetopterus sp. oocytes: I. Anchorage of the spindle to the cortex and migration of a displaced spindle. Cell Motil. Cytoskeleton 11, 83–96 10.1002/cm.970110202 [DOI] [PubMed] [Google Scholar]
- Marshall W. F., Kintner C. (2008). Cilia orientation and the fluid mechanics of development. Curr. Opin. Cell Biol. 20, 48–52 10.1016/j.ceb.2007.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk A. I., Masyuk T. V., Splinter P. L., Huang B. Q., Stroope A. J., LaRusso N. F. (2006). Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131, 911–920 10.1053/j.gastro.2006.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Schiller P., Dieterich L. C., Bahram F., Iribe Y., Hellman U., Wikner C., Chan G., Claesson–Welsh L., Dimberg A. (2008). Ninein is expressed in the cytoplasm of angiogenic tip-cells and regulates tubular morphogenesis of endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28, 2123–2130 10.1161/ATVBAHA.108.169128 [DOI] [PubMed] [Google Scholar]
- Matsuyama M., Aizawa S., Shimono A. (2009). Sfrp controls apicobasal polarity and oriented cell division in developing gut epithelium. PLoS Genet. 5, e1000427 10.1371/journal.pgen.1000427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. K., Rose M. D. (1998). Kar9p is a novel cortical protein required for cytoplasmic microtubule orientation in yeast. J. Cell Biol. 140, 377–390 10.1083/jcb.140.2.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima M., Glotzer M. (2003). Cytokinesis: a logical GAP. Curr. Biol. 13, R589–R591 10.1016/S0960-9822(03)00521-9 [DOI] [PubMed] [Google Scholar]
- Mitchell B., Jacobs R., Li J., Chien S., Kintner C. (2007). A positive feedback mechanism govens the polarity and motion of motile cilia. Nature 447, 97–101 [DOI] [PubMed] [Google Scholar]
- Mitchell B., Stubbs J. L., Huisman F., Taborek P., Yu C., Kintner C. (2009). The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr. Biol. 19, 924–929 10.1016/j.cub.2009.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen M. M., Malik A., Piel M., Bouckson–Castaing V., Bornens M. (2000). Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J. Cell Sci. 113, 3013–3023 [DOI] [PubMed] [Google Scholar]
- Müsch A. (2004). Microtubule organization and function in epithelial cells. Traffic 5, 1–9 10.1111/j.1600-0854.2003.00149.x [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Stearns T. (2011). The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat. Cell Biol. 13, 1154–1160 10.1038/ncb2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S., Shiratori H., Saijoh Y., Hamada H. (2002). Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418, 96–99 10.1038/nature00849 [DOI] [PubMed] [Google Scholar]
- Nonaka S., Yoshiba S., Watanabe D., Ikeuchi S., Goto T., Marshall W. F., Hamada H. (2005). De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol. 3, e268 10.1371/journal.pbio.0030268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Takeda S., Tanaka Y., Izpisua Belmonte J. C., Hirokawa N. (2005). Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell 121, 633–644 [DOI] [PubMed] [Google Scholar]
- Oliferenko S., Chew T. G., Balasubramanian M. K. (2009). Positioning cytokinesis. Genes Dev. 23, 660–674 10.1101/gad.1772009 [DOI] [PubMed] [Google Scholar]
- Paintrand M., Moudjou M., Delacroix H., Bornens M. (1992). Centrosome organization and centriole architecture: their sensitivity to divalent cations. J. Struct. Biol. 108, 107–128 10.1016/1047-8477(92)90011-X [DOI] [PubMed] [Google Scholar]
- Pan J., You Y., Huang T., Brody S. L. (2007). RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J. Cell Sci. 120, 1868–1876 10.1242/jcs.005306 [DOI] [PubMed] [Google Scholar]
- Panizzi J. R., Jessen J. R., Drummond I. A., Solnica–Krezel L. (2007). New functions for a vertebrate Rho guanine nucleotide exchange factor in ciliated epithelia. Development 134, 921–931 10.1242/dev.02776 [DOI] [PubMed] [Google Scholar]
- Park T. J., Mitchell B. J., Abitua P. B., Kintner C., Wallingford J. B. (2008). Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 40, 871–879 10.1038/ng.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieczynski J., Margolis B. (2011). Protein complexes that control renal epithelial polarity. Am. J. Physiol. Renal Physiol. 300, F589–F601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel M., Meyer P., Khodjakov A., Rieder C. L., Bornens M. (2000). The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149, 317–330 10.1083/jcb.149.2.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius H. A., Spring K. R. (2001). Bending the MDCK cell primary cilium increases intracellular calcium. J. Membr. Biol. 184, 71–79 10.1007/s00232-001-0075-4 [DOI] [PubMed] [Google Scholar]
- Rafelski S. M., Keller L. C., Alberts J. B., Marshall W. F. (2011). Apparent diffusive motion of centrin foci in living cells: implications for diffusion-based motion in centriole duplication. Phys. Biol. 8, 026010 10.1088/1478-3975/8/2/026010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch A., Thiel C. T., Schindler D., Wick U., Crow Y. J., Ekici A. B., van Essen A. J., Goecke T. O., Al–Gazali L., Chrzanowska K. H.et al. (2008). Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science 319, 816–819 10.1126/science.1151174 [DOI] [PubMed] [Google Scholar]
- Rieder C. L., Faruki S., Khodjakov A. (2001). The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol. 11, 413–419 10.1016/S0962-8924(01)02085-2 [DOI] [PubMed] [Google Scholar]
- Rivas R. J., Hatten M. E. (1995). Motility and cytoskeletal organization of migrating cerebellar granule neurons. J. Neurosci. 15, 981–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov V. I., Borisy G. G. (1997). Self-centring activity of cytoplasm. Nature 386, 170–173 10.1038/386170a0 [DOI] [PubMed] [Google Scholar]
- Rodrigues–Martins A., Riparbelli M., Callaini G., Glover D. M., Bettencourt–Dias M. (2008). From centriole biogenesis to cellular function: centrioles are essential for cell division at critical developmental stages. Cell Cycle 7, 11–16 10.4161/cc.7.1.5226 [DOI] [PubMed] [Google Scholar]
- Rogers K. A., McKee N. H., Kalnins V. I. (1985). Preferential orientation of centrioles toward the heart in endothelial cells of major blood vessels is reestablished after reversal of a segment. Proc. Natl. Acad. Sci. USA 82, 3272–3276 10.1073/pnas.82.10.3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutland J., de Iongh R. U. (1990). Random ciliary orientation. A cause of respiratory tract disease. N. Engl. J. Med. 323, 1681–1684 10.1056/NEJM199012133232406 [DOI] [PubMed] [Google Scholar]
- Saburi S., Hester I., Fischer E., Pontoglio M., Eremina V., Gessler M., Quaggin S. E., Harrison R., Mount R., McNeill H. (2008). Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat. Genet. 40, 1010–1015 10.1038/ng.179 [DOI] [PubMed] [Google Scholar]
- Satir P., Christensen S. T. (2007). Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 69, 377–400 10.1146/annurev.physiol.69.040705.141236 [DOI] [PubMed] [Google Scholar]
- Sausedo R. A., Schoenwolf G. C. (1994). Quantitative analyses of cell behaviors underlying notochord formation and extension in mouse embryos. Anat. Rec. 239, 103–112 10.1002/ar.1092390112 [DOI] [PubMed] [Google Scholar]
- Schmoranzer J., Fawcett J. P., Segura M., Tan S., Vallee R. B., Pawson T., Gundersen G. G. (2009). Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr. Biol. 19, 1065–1074 10.1016/j.cub.2009.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L., Cammer M., Lehman J., Nielsen S. K., Guerra C. F., Veland I. R., Stock C., Hoffmann E. K., Yoder B. K., Schwab A.et al. (2010). Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell. Physiol. Biochem. 25, 279–292 10.1159/000276562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwolf G. C., Alvarez I. S. (1989). Roles of neuroepithelial cell rearrangement and division in shaping of the avian neural plate. Development 106, 427–439 [DOI] [PubMed] [Google Scholar]
- Schütze K., Maniotis A., Schliwa M. (1991). The position of the microtubule-organizing center in directionally migrating fibroblasts depends on the nature of the substratum. Proc. Natl. Acad. Sci. USA 88, 8367–8371 10.1073/pnas.88.19.8367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségalen M., Johnston C. A., Martin C. A., Dumortier J. G., Prehoda K. E., David N. B., Doe C. Q., Bellaïche Y. (2010). The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Dev. Cell 19, 740–752 10.1016/j.devcel.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepich D. S., Usmani M., Pawlicki S., Solnica–Krezel L. (2011). Wnt/PCP signaling controls intracellular position of MTOCs during gastrulation convergence and extension movements. Development 138, 543–552 10.1242/dev.053959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller K. H., Doe C. Q. (2009). Spindle orientation during asymmetric cell division. Nat. Cell Biol. 11, 365–374 10.1038/ncb0409-365 [DOI] [PubMed] [Google Scholar]
- Singla V., Reiter J. F. (2006). The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313, 629–633 10.1126/science.1124534 [DOI] [PubMed] [Google Scholar]
- Smith K. R., Kieserman E. K., Wang P. I., Basten S. G., Giles R. H., Marcotte E. M., Wallingford J. B. (2011). A role for central spindle proteins in cilia structure and function. Cytoskeleton (Hoboken) 68, 112–124 10.1002/cm.20498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki D. J., Model L., Gaetz J., Kapoor T. M., Hatten M. E. (2004). Par6alpha signaling controls glial-guided neuronal migration. Nat. Neurosci. 7, 1195–1203 10.1038/nn1332 [DOI] [PubMed] [Google Scholar]
- Spradling A., Drummond–Barbosa D., Kai T. (2001). Stem cells find their niche. Nature 414, 98–104 10.1038/35102160 [DOI] [PubMed] [Google Scholar]
- Stevens N. R., Raposo A. A., Basto R., St Johnston D., Raff J. W. (2007). From stem cell to embryo without centrioles. Curr. Biol. 17, 1498–1503 10.1016/j.cub.2007.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe J. C., Bossi G., Booth S., Griffiths G. M. (2001). The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 10.1016/S1074-7613(01)00234-5 [DOI] [PubMed] [Google Scholar]
- Stinchcombe J. C., Majorovits E., Bossi G., Fuller S., Griffiths G. M. (2006). Centrosome polarization delivers secretory granules to the immunological synapse. Nature 443, 462–465 10.1038/nature05071 [DOI] [PubMed] [Google Scholar]
- Sun Q. Y., Schatten H. (2006). Role of NuMA in vertebrate cells: review of an intriguing multifunctional protein. Front. Biosci. 11, 1137–1146 10.2741/1868 [DOI] [PubMed] [Google Scholar]
- Szabó B., Ünnep R., Markó K., Környei Z., Méhes E., Czirók A. (2011). Inhibition of myosin II triggers morphological transition and increased nuclear motility. Cytoskeleton (Hoboken) 68, 325–339 10.1002/cm.20515 [DOI] [PubMed] [Google Scholar]
- Tamm S., Tamm S. L. (1988). Development of macrociliary cells in Beroë. I. Actin bundles and centriole migration. J. Cell Sci. 89, 67–80 [DOI] [PubMed] [Google Scholar]
- Tamm S. L., Sonneborn T. M., Dippell R. V. (1975). The role of cortical orientation in the control of the direction of ciliary beat in Paramecium. J. Cell Biol. 64, 98–112 10.1083/jcb.64.1.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Marshall W. F., McMahon M., Metzger R. J., Martin G. R. (2011). Control of mitotic spindle angle by the RAS-regulated ERK1/2 pathway determines lung tube shape. Science 333, 342–345 10.1126/science.1204831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry M., Racine V., Pépin A., Piel M., Chen Y., Sibarita J. B., Bornens M. (2005). The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7, 947–953 10.1038/ncb1307 [DOI] [PubMed] [Google Scholar]
- Théry M., Racine V., Piel M., Pépin A., Dimitrov A., Chen Y., Sibarita J. B., Bornens M. (2006). Anisotropy of cell adhesive microenvironment governs cell internal organization and orientation of polarity. Proc. Natl. Acad. Sci. USA 103, 19771–19776 10.1073/pnas.0609267103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry M., Jiménez–Dalmaroni A., Racine V., Bornens M., Jülicher F. (2007). Experimental and theoretical study of mitotic spindle orientation. Nature 447, 493–496 10.1038/nature05786 [DOI] [PubMed] [Google Scholar]
- Tsai J. W., Bremner K. H., Vallee R. B. (2007). Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nat. Neurosci. 10, 970–979 10.1038/nn1934 [DOI] [PubMed] [Google Scholar]
- Tsun A., Qureshi I., Stinchcombe J. C., Jenkins M. R., de la Roche M., Kleczkowska J., Zamoyska R., Griffiths G. M. (2011). Centrosome docking at the immunological synapse is controlled by Lck signaling. J. Cell Biol. 192, 663–674 10.1083/jcb.201008140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulina N., Matunis E. (2001). Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 294, 2546–2549 10.1126/science.1066700 [DOI] [PubMed] [Google Scholar]
- Umeshima H., Hirano T., Kengaku M. (2007). Microtubule-based nuclear movement occurs independently of centrosome positioning in migrating neurons. Proc. Natl. Acad. Sci. USA 104, 16182–16187 10.1073/pnas.0708047104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B., Williams J. C., Varma D., Barnhart L. E. (2004). Dynein: An ancient motor protein involved in multiple modes of transport. J. Neurobiol. 58, 189–200 10.1002/neu.10314 [DOI] [PubMed] [Google Scholar]
- Varmark H., Llamazares S., Rebollo E., Lange B., Reina J., Schwarz H., Gonzalez C. (2007). Asterless is a centriolar protein required for centrosome function and embryo development in Drosophila. Curr. Biol. 17, 1735–1745 10.1016/j.cub.2007.09.031 [DOI] [PubMed] [Google Scholar]
- Verkhovsky A. B., Svitkina T. M., Borisy G. G. (1999). Self-polarization and directional motility of cytoplasm. Curr. Biol. 9, 11–20 10.1016/S0960-9822(99)80042-6 [DOI] [PubMed] [Google Scholar]
- Vorobjev I. A., Chentsov Y. S. (1982). Centrioles in the cell cycle. I. Epithelial cells. J. Cell Biol. 93, 938–949 10.1083/jcb.93.3.938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakida N. M., Botvinick E. L., Lin J., Berns M. W. (2010). An intact centrosome is required for the maintenance of polarization during directional cell migration. PLoS ONE 5, e15462 10.1371/journal.pone.0015462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tsai J. W., Imai J. H., Lian W. N., Vallee R. B., Shi S. H. (2009). Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461, 947–955 10.1038/nature08435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P. G., Fuller M. T., Borisy G. G. (1997). Monastral bipolar spindles: implications for dynamic centrosome organization. J. Cell Sci. 110, 451–464 [DOI] [PubMed] [Google Scholar]
- Xie Z., Sanada K., Samuels B. A., Shih H., Tsai L. H. (2003). Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell 114, 469–482 10.1016/S0092-8674(03)00605-6 [DOI] [PubMed] [Google Scholar]
- Yamashita Y. M., Mahowald A. P., Perlin J. R., Fuller M. T. (2007). Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science 315, 518–521 10.1126/science.1134910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates L. L., Schnatwinkel C., Murdoch J. N., Bogani D., Formstone C. J., Townsend S., Greenfield A., Niswander L. A., Dean C. H. (2010). The PCP genes Celsr1 and Vangl2 are required for normal lung branching morphogenesis. Hum. Mol. Genet. 19, 2251–2267 10.1093/hmg/ddq104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T. W., Mochida G. H., Tischfield D. J., Sgaier S. K., Flores–Sarnat L., Sergi C. M., Topçu M., McDonald M. T., Barry B. J., Felie J. M.et al. (2010). Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat. Genet. 42, 1015–1020 10.1038/ng.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon A. M., Walker J. W., Danowski B., Fagerstrom C., Khodjakov A., Wadsworth P. (2002). Centrosome reorientation in wound-edge cells is cell type specific. Mol. Biol. Cell 13, 1871–1880 10.1091/mbc.01-11-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen J. A. (2007). Planar polarity and tissue morphogenesis. Cell 129, 1051–1063 10.1016/j.cell.2007.05.050 [DOI] [PubMed] [Google Scholar]
- Zeng G., Taylor S. M., McColm J. R., Kappas N. C., Kearney J. B., Williams L. H., Hartnett M. E., Bautch V. L. (2007). Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood 109, 1345–1352 10.1182/blood-2006-07-037952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Burakov A., Rodionov V., Mogilner A. (2010). Finding the cell center by a balance of dynein and myosin pulling and microtubule pushing: a computational study. Mol. Biol. Cell 21, 4418–4427 10.1091/mbc.E10-07-0627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmuda J. F., Rivas R. J. (1998). The Golgi apparatus and the centrosome are localized to the sites of newly emerging axons in cerebellar granule neurons in vitro. Cell Motil. Cytoskeleton 41, 18–38 10.1002/(SICI)1097-0169 [DOI] [PubMed] [Google Scholar]