Abstract
Obstructive sleep apnea (OSA) is associated with cardiovascular complications including hypertension. Previous findings from our laboratory indicate that exposure to intermittent hypoxia (IH), to mimic sleep apnea, increases blood pressure in rats. IH also increases endothelin-1 (ET-1) constrictor sensitivity in a protein kinase C (PKC) δ–dependent manner in mesenteric arteries. Because phosphoinositide-dependent kinase-1 (PDK-1) regulates PKCδ activity, we hypothesized that PDK-1 contributes to the augmented ET-1 constrictor sensitivity and elevated blood pressure following IH. Male Sprague-Dawley rats were exposed to either sham or IH (cycles between 21% O2/0% CO2 and 5% O2/5% CO2) conditions for 7 h/day for 14 or 21 days. The contribution of PKCδ and PDK-1 to ET-1–mediated vasoconstriction was assessed in mesenteric arteries using pharmacological inhibitors. Constrictor sensitivity to ET-1 was enhanced in arteries from IH-exposed rats. Inhibition of PKCδ or PDK-1 blunted ET-1 constriction in arteries from IH but not sham group rats. Western analysis revealed similar levels of total and phosphorylated PDK-1 in arteries from sham and IH group rats but decreased protein-protein interaction between PKCδ and PDK-1 in arteries from IH- compared with sham-exposed rats. Blood pressure was increased in rats exposed to IH, and treatment with the PDK-1 inhibitor OSU-03012 [2-amino-N-{4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phenyl}-acetamide] (33 mg/day) lowered blood pressure in IH but not sham group rats. Our results suggest that exposure to IH unmasks a role for PDK-1 in regulating ET-1 constrictor sensitivity and blood pressure that is not present under normal conditions. These novel findings suggest that PDK-1 may be a uniquely effective antihypertensive therapy for OSA patients.
Introduction
Obstructive sleep apnea (OSA) occurs due to a cyclical loss of pharyngeal muscle tone during sleep, resulting in airway collapse and subsequent arousal with disruption of sleep and restoration of airflow. Upon airflow restoration, sleep resumes and the cycle repeats. Conversely, central sleep apnea is caused by loss of the central drive to breathe. In both cases, these repeated cycles of apneic events lead to intermittent hypoxia (IH) and are associated with increased risk for hypertension, stroke, coronary artery disease, arrhythmias, and heart failure. It is estimated that 24% of men and 9% of women experience at least five apneic episodes per hour, with an estimated 80% undiagnosed for OSA (Bradley and Floras, 2009). Studies in rodent models demonstrate that chronic exposure to IH to mimic the apneic episodes of sleep apnea causes vascular dysfunction that might underlie the increased cardiovascular complications observed in OSA patients (Dematteis et al., 2008).
Previous studies from our laboratory have shown that 14 days of exposure to IH increases mean arterial blood pressure in rats (Kanagy et al., 2001). This augmented blood pressure is dependent on activation of the endothelin-1 (ET-1) A receptor (ETAR) and generation of reactive oxygen species (ROS); moreover, ROS mediate increased levels of plasma ET-1 in IH-exposed rats (Troncoso Brindeiro et al., 2007). Along with increased levels of circulating ET-1, IH-exposed rats show augmented constrictor responses in mesenteric arteries to ET-1 but not to phenylephrine or depolarizing concentrations of KCl (Allahdadi et al., 2005). This increased constrictor response is dependent on greater calcium sensitivity as opposed to greater increases in intracellular calcium levels. Arteries from IH-exposed rats also have increased expression of the ETAR, and the increased vasoconstrictor sensitivity to ET-1 in IH rats can be attenuated by pharmacologically blocking ETAR (Allahdadi et al., 2005, 2008a). We subsequently reported that IH-augmented vasoconstriction is mediated by the δ-isoform of protein kinase C (PKCδ) in a calcium-independent manner and that ET-1 increases PKCδ autophosphorylation levels (Allahdadi et al., 2008b). However, it is not clear what increases ET-1 activation of PKCδ-dependent contraction in vascular smooth muscle from IH-exposed rats.
Similar to all members of the protein kinase A, B, and C families, PKCδ has multiple phosphorylation sites that differentially regulate function. PKC isoforms in particular are phosphorylated as part of the “maturation” process. Specifically, phosphoinositide-dependent kinase-1 (PDK-1), which activates at least 23 members of the AGC family of protein kinases, including PKC isoforms (Le Good et al., 1998) and Akt (Bayascas et al., 2008), phosphorylates the activation loop domain in PKC, “priming” PKC for diacyl glycerol binding, autophosphorylation, and activation (reviewed in Newton, 2009). For PKCδ, Thr505 is the PDK-1 activation site, whereas Ser643 and Ser662 are the turn motif and hydrophobic domain autophosphorylation sites, respectively (Kikkawa et al., 2002).
Regulation of PDK-1 activity is not as well defined as that of its downstream targets. Of interest, ROS and insulin are two known activators of PDK-1 phosphorylation and activity. Specifically, H2O2 increases serine and tyrosine phosphorylation of PDK-1 in cultured cells (Prasad et al., 2000), whereas Zou et al. (2003) observed increased phosphorylation of Ser241 in bovine aortic endothelial cells following exposure to peroxynitrite. In light of the previously reported increase in ROS levels following IH exposure, we hypothesized that increased PDK-1 activity mediates the increased ET-1 constrictor sensitivity and elevation in blood pressure following IH exposure. To test this hypothesis, we examined the effect of PDK-1 inhibition on ET-1-dependent vasoconstriction in mesenteric arteries from control and IH-exposed rats and used the orally active PDK-1 inhibitor OSU-03012 to investigate a role for PDK-1 in the IH-induced increase in blood pressure.
Materials and Methods
Rodent Model of Sleep Apnea.
Male Sprague-Dawley rats (250–275 g) were exposed to either sham or IH conditions as described previously (Allahdadi et al., 2005; Troncoso Brindeiro et al., 2007). Briefly, all rats were housed in enclosed Plexiglas boxes with free access to food and water. IH exposure consisted of cycling between room air (21% O2/0% CO2) and hypoxia (5% O2/5% CO2), whereas sham conditions consisted of exposure to room air only in identical boxes that subjected control rats to sounds and airflow similar to that produced by the hypoxia cycler. Rats were exposed to 20 hypoxic episodes per hour for 7 h/day during their sleep period and were maintained on a 12-hour light/dark cycle. We have previously shown that this IH exposure drops arterial pO2 levels from ∼70 to 35 mm Hg while pCO2 levels remain unchanged (Snow et al., 2008). The Institutional Animal Care and Use Committee of the University of New Mexico School of Medicine reviewed and approved all animal protocols. All protocols conform to the National Institutes of Health guidelines for animal use.
Isolated Mesenteric Artery Preparation.
At the end of the sham or IH exposure period, rats were euthanized with sodium pentobarbital (150 mg/kg i.p.). Mesenteric arterial arcades were isolated and placed in physiologic salt solution (PSS; in mM: 129.8 NaCl, 5.4 KCl, 0.44 NaH2PO4, 0.83 MgSO4, 19.0 NaCO3, 1.8 CaCl2, and 5.5 glucose) at 4°C until used for contractile studies or protein analysis. Third-order mesenteric arteries with a pressurized inner diameter of 150–250 μm were dissected from the mesenteric arterial cascades and cleared of adipose and connective tissue. An artery segment was placed in a vessel chamber (Living Systems Instrumentation, Burlington, VT) and secured with silk thread on glass micropipettes. Unless noted, experiments were conducted in endothelium-intact arteries. Endothelial disruption was performed by physically rubbing the endothelium with moose mane in a subset of experiments. Vessels were pressurized to 60-mm Hg with a peristaltic pump (Living Systems Instrumentation) and superfused with PSS (bubbled with 21% O2/6% CO2/balance N2 and heated to 37°C) at a rate of 5 ml/min.
Constrictor Studies.
ET-1 constriction was assessed in isolated mesenteric arteries in the absence or presence of the following inhibitors: the nonselective PKC inhibitor Go6983 (1 μM; 3-[1-[3-(dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione; Biomol International; Farmingdale, NY), the classical PKC (cPKC) inhibitor Go6976 (1 μM; 5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile; Biomol International), the PKCδ inhibitor rottlerin (3 μM; 3′-[(8-cinnamoyl-5,7-dihydroxy-2,2-dimethyl-2H-1-benzopyran-6-yl)methyl]-2′,4′,6′-trihydroxy-5′-methylacetophenone; Biomol International), the PDK-1 inhibitor OSU-03012 (10 μM; 2-amino-N-[4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]phenyl]acetamide; Cayman Chemical, Ann Arbor, MI), and the phosphoinositide 3-kinase (PI3K) inhibitor LY294002 (10 μM; 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one; Cayman Chemical). The concentration used for each inhibitor was based on previous studies that established inhibition parameters for PKC isoform(s) (Martiny-Baron et al., 1993; Gschwendt et al., 1994, 1996; Zhu et al., 2004). All inhibitors were added to the PSS superfusate for 30 minutes prior to initiation of ET-1 concentration-response curves and remained in the superfusate throughout the contractile studies. For vehicle studies an equal volume of the appropriate vehicle was added to the superfusate. Baseline diameter was recorded, and then vessels were exposed to increasing concentrations of ET-1 (American Peptide, Sunnyvale, CA) from 10−10 to 10−7.5 M. The presence of active tone was verified by comparison with the maximal passive diameter in Ca2+-free PSS (in mM: 129.8 NaCl, 5.4 KCl, 0.44 NaH2PO4, 0.83 MgSO4, 19.0 NaCO3, 5.5 glucose, and 4.5 EGTA). Constriction in response to the PKC activator phorbol 12,13-dibutyrate (PDBu; Sigma-Aldrich, St. Louis, MO) in the absence or presence of OSU-03012 was also assessed in arteries from sham-treated rats to determine if the effects of OSU-03012 were mediated by nonspecific effects on PKC. Additionally, constriction in response to PDBu was assessed in the presence of Go6983 and rottlerin to compare the effects of OSU-03012 with general PKC and PKCδ inhibition, respectively. All data are expressed as percent constriction compared with baseline diameter.
PDK-1 Phosphorylation.
Phosphorylation of PDK-1 at the Ser241 residue was assessed in mesenteric arteries from sham- and IH-exposed rats. Arteries were isolated and placed in ice-cold lysis buffer (25 mM Tris-HCl [pH 7.6], 150 mM NaCl, 1% Nonidet P40, 1% sodium deoxycholate, 0.1% SDS; Thermo Scientific; Waltham, MA) containing 1× Halt phosphatase inhibitor cocktail (Thermo Scientific), 2.5 mM phenylmethanesulfonyl fluoride (Sigma-Aldrich), and 1× Complete protease inhibitor cocktail (Santa Cruz Biotechnology, Santa Cruz, CA). Tissues were homogenized and centrifuged for 10 minutes at 1500 × g at 4°C. Supernatants containing equal protein concentrations (15 μg; as assessed by a Pierce BCA Protein Assay Kit; Thermo Scientific) were added to 5× sample buffer, heated to 95°C for 5 minutes, separated on a 4%–15% gradient gel (Bio-Rad Laboratories, Hercules, CA), and then transferred to a polyvinylidene fluoride membrane. Phosphorylated and total PDK-1 protein (58–68 kDa) were analyzed following overnight incubation with phospho-PDK-1 Ser241 (1:250; Cell Signaling Technology, Danvers, MA) and PDK-1 (1:250; Cell Signaling Technology) primary antibodies. Proteins were visualized using a LI-COR Odyssey Infrared Imaging System (LI-COR, Lincoln, NE). Phosphorylated PDK-1 is expressed as a ratio of total PDK-1. Total PDK-1 levels were normalized to Coomassie Blue staining.
PKCδ/PDK-1 Immunoprecipitation.
Association of PKCδ and PDK-1 was evaluated using a Pierce Co-Immunoprecipitation Kit (Thermo Scientific). Mesenteric arterial arcades were isolated from sham- and IH-exposed rats, placed into 150 μl of lysis buffer (25 mM Tris, 150 mM NaCl, 1 mM EDTA, and 1% Nonidet P40; Thermo Scientific), homogenized, and centrifuged as described above. Following centrifugation, lysates (100 μg) were incubated overnight at 4°C in an immunoprecipitation column prepared with a PKCδ antibody (AbCam, Cambridge, MA) according to the manufacturer’s protocol. Samples were eluted and prepared for Western blotting as described above using 50-μg protein for each sample. Blots were incubated overnight with a PKCδ (1:250; Cell Signaling Technology) or PDK-1 (1:250; AbCam) primary antibody at 4°C. Data are expressed as the ratio of PDK-1 (58–68 kDa) to PKCδ (78 kDa). Total PKCδ levels were normalized to Coomassie Blue staining.
Blood Pressure Measurements.
Blood pressure was measured by radiotelemetry in a second group of rats exposed to sham or IH conditions. Telemetry transmitters (Data Sciences International, St. Paul, MN) were surgically implanted under isoflurane anesthesia with the catheter placed in the abdominal aorta via the femoral artery; rats were allowed to recover for at least 7 days. Baseline blood pressure was recorded for 3 days after the recovery period, and then sham or IH exposure was initiated as described above. The IH exposure was extended to 21 days to allow for pressure to reach a plateau, as previously described (Kanagy et al., 2001). After 21 days of sham or IH cycling, rats received either vehicle (transgenic dough diet; Bio-Serv, Frenchtown, NJ) or the PDK-1 inhibitor OSU-03012 (33 mg/day in dough diet [based on a previous study (Gao et al., 2008)]; Cayman Chemical) for 3 days with continued measurement of blood pressure and continued exposure to sham or IH conditions.
Statistical Analysis.
Concentration-response curves were analyzed by two-way repeated-measures analysis of variance, and Student-Newman-Keuls post hoc analysis was used to detect differences between groups. Western blot densitometry levels and blood pressure changes were compared by Student’s t tests. The blood pressure response over time was compared between sham and IH conditions by two-way analysis of variance. Data are expressed as mean ± S.E.M. A P value of <0.05 was considered statistically significant.
Results
ET-1-Mediated Constriction in Mesenteric Arteries.
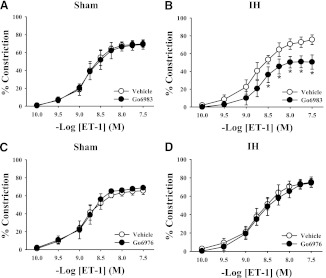
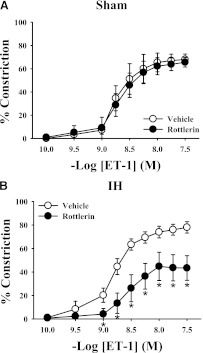
A comparison of ET-1 constriction under various conditions in mesenteric arteries from sham- and IH-exposed rats is shown in Table 1. ET-1–mediated vasoconstriction was greater in endothelium-intact mesenteric arteries from IH-exposed rats compared with arteries from sham-exposed rats (Fig. 1). Pan-PKC inhibition with the non-isoform-selective inhibitor Go6983 had no effect on ET-1–mediated vasoconstriction in mesenteric arteries from control rats (Fig. 2A) but attenuated constriction in arteries from IH-treated rats (Fig. 2B). In contrast, Go6976, a selective cPKC inhibitor, had no effect on ET-1–induced constriction in arteries from either sham or IH group rats (Fig. 2, C and D). These results confirm that PKC activation contributes to ET-1–mediated vasoconstriction following IH exposure and that the PKC isoform(s) involved is not a cPKC isoform. Much like the nonselective PKC inhibitor Go6983, the PKCδ inhibitor rottlerin attenuated ET-1–mediated constriction in arteries from IH-exposed rats but was without effect in arteries from sham group rats (Fig. 3). It has been demonstrated that rottlerin can inhibit PKCε, but ET-1–mediated constriction was unaffected by the PKCε inhibitor V1-2myr (data not shown). Taken together, these results suggest that PKCδ is involved in the heightened constriction in response to ET-1 following IH exposure.
TABLE 1.
Characteristics of concentration-response curves
| Group | −Log EC50 | Emax |
|---|---|---|
| Sham | 8.64 ± 0.09 | 70.1 ± 1.1 |
| IH | 8.70 ± 0.05 | 77.4 ± 0.7 * |
| Sham + Go6976 | 8.88 ± 0.10 | 69.2 ± 1.0 |
| IH + Go6976 | 8.68 ± 0.05 | 74.4 ± 2.2 |
| Sham + Go6983 | 8.80 ± 0.07 | 69.0 ± 2.2 |
| IH + Go6983 | 8.70 ± 0.11 | 49.1 ± 3.7 * # |
| Sham + rottlerin | 8.64 ± 0.08 | 65.7 ± 2.0 |
| IH + rottlerin | 8.45 ± 0.06 | 43.2 ± 4.6 * # |
| Sham + OSU-03012 | 8.41 ± 0.05 | 71.7 ± 1.72 |
| IH + OSU-03012 | 8.41 ± 0.15# | 57.2 ± 2.0 * # |
IH, intermittent hypoxia.
P < 0.05 vs. respective sham treatment
P < 0.05 vs. IH.
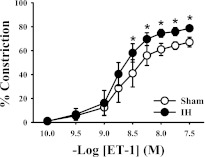
Fig. 1.
Exposing rats to 2 weeks of IH leads to increased constrictor sensitivity to ET-1 in endothelium-intact mesenteric arteries compared with arteries from sham-exposed controls. Values are expressed as percentage of constriction as compared with baseline lumen diameter. *P < 0.05 versus sham; n = 10/group.
Fig. 2.
Pan-PKC inhibition with Go6983 (1 μM) had no effect on ET-1-mediated vasoconstriction in mesenteric arteries from sham-exposed rats (A) but attenuated constriction in arteries from IH-exposed rats (B). Go6976 (1 μM), a selective cPKC inhibitor, did not affect ET-1–induced constriction in arteries from either sham (C) or IH (D) group rats. *P < 0.05 versus respective vehicle treatment; n = 5–6/group.
Fig. 3.
The PKCδ inhibitor rottlerin (3 μM) was without effect on the ET-1 constrictor response in arteries from sham group rats (A) but attenuated constriction in response to ET-1 in mesenteric arteries from IH-exposed rats (B). *P < 0.05 versus vehicle treatment; n = 5/group.
Effects of PDK-1 Inhibition on ET-1-Mediated Constriction.
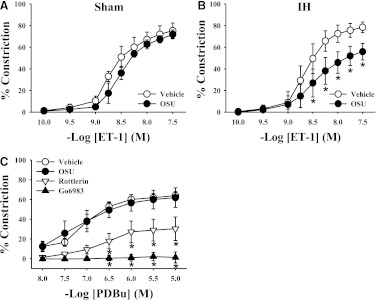
Similar to the effect of the PKC inhibitors rottlerin and Go6983, ET-1–mediated constriction in mesenteric arteries from IH-exposed rats was greatly reduced after exposure to the PDK-1 inhibitor OSU-03012 (Fig. 4). In contrast, OSU-03012 had no effect on ET-1–mediated constriction in arteries from sham-exposed rats, suggesting that ET-1 does not normally signal through PDK-1 to constrict mesenteric arteries. PKC-mediated vasoconstriction was measured in the absence and presence of Go6983, rottlerin, and OSU-03012 using the PKC activator PDBu in arteries from rats in the sham group to verify that PKCδ can contribute to constriction in control arteries if activated and that the effects of OSU-03012 were not due to nonspecific inhibition of PKC. As shown in Fig. 4C, OSU-03012 had no effect on PDBu-mediated vasoconstriction. Conversely, inhibition of PKCδ (rottlerin) greatly blunted PDBu-mediated constriction, whereas the nonselective PKC inhibitor (Go6983) completely blocked constriction. These results suggest that OSU-03012 does not attenuate ET-1 constriction through inhibition of PKCδ and that PKCδ can mediate vasoconstriction in mesenteric arteries from control rats if it is activated.
Fig. 4.
Mesenteric arteries were incubated with the PDK-1 inhibitor OSU-03012 (OSU; 10 μM), and constriction in response to ET-1 was assessed. As with PKCδ inhibition, ET-1–mediated vasoconstriction was unaffected by PDK-1 inhibition in arteries from sham group rats (A) but constriction was attenuated in arteries from IH-exposed rats (B). (C) Constriction mediated by the PKC activator PDBu in arteries from sham group rats was similar in the absence or presence of PDK-1 inhibition but was diminished by nonselective PKC inhibition with Go6983 (1 μM) or inhibition of PKCδ with rottlerin (3 μM). *P < 0.05 versus vehicle treatment; n = 6–10/group.
It is not clear whether augmented PDK-1/PKCδ signaling in arteries from IH-exposed rats is unique to ET-1 signaling. However, constriction in response to the α-adrenergic receptor agonist phenylephrine was similar in arteries from sham- and IH-exposed rats and was not differentially affected by administration of OSU-03012, rottlerin, or Go6983, inhibitors of PDK-1 and PKC, respectively (data not shown).
Effects of PI3K Inhibition on ET-1-Mediated Constriction.
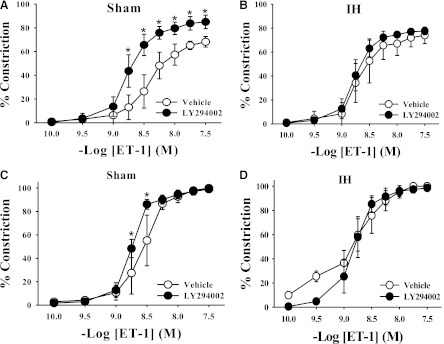
PI3K is a potential upstream regulator of PDK-1 activity; thus, the effect of PI3K inhibition on ET-1 vasoconstriction was assessed. The PI3K inhibitor LY294002 had no effect on ET-1-mediated constriction in mesenteric arteries from IH-exposed rats (Fig. 5B); however, PI3K inhibition augmented ET-1-mediated constriction in arteries from rats in the sham group (Fig. 5A). To determine whether the augmented ET-1 constriction in the presence of PI3K inhibition in arteries from sham group rats was due to an effect on some endothelial factor, ET-1 constriction was evaluated in the presence of LY294002 in endothelium-disrupted arteries. The augmented ET-1 response in arteries from sham group rats was largely abolished by endothelial disruption, with only a slight, although significant, difference between vehicle- and LY294002-treated arteries (Fig. 5C), suggesting that PI3K may both activate an endothelial pathway and have an additional inhibitory effect in vascular smooth muscle. PI3K inhibition had no effect in endothelium-disrupted arteries from IH-exposed rats (Fig. 5D). Overall, these data suggest that the contribution of PDK-1 to augmented ET-1 constrictor sensitivity in IH is independent of PI3K activity.
Fig. 5.
LY294002 (10 μM), an inhibitor of PI3K, augmented vasoconstriction in endothelium-intact mesenteric arteries from sham-exposed rats (A) but had no effect in arteries from IH-exposed rats (B). Treatment with LY294002 in endothelium-disrupted arteries diminished the augmented constrictor response in arteries in the sham group (C) and had no inhibitory effect in arteries in the IH group (D). *P < 0.05 versus respective vehicle group; n = 5/group.
PDK-1 Phosphorylation and Interaction with PKCδ.
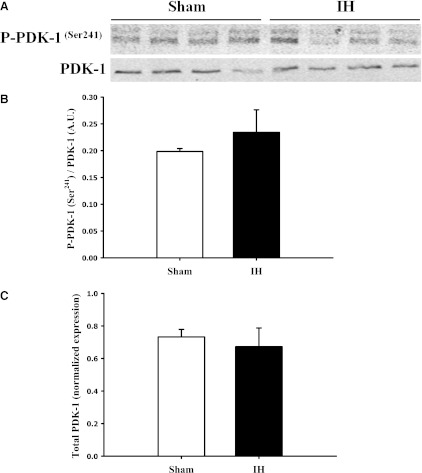
Phosphorylation of PDK-1 at the Ser241 residue was measured as one indication of PDK-1 activity. There were no detectable differences in phosphorylation levels between mesenteric arteries from sham and IH group rats (Fig. 6, A and B), indicating that phosphorylation of this residue is not differentially regulated by exposure to IH. ET-1 stimulation of arteries did not affect PDK-1 phosphorylation (data not shown). Total levels of PDK-1 were not altered by IH exposure (Fig. 6C).
Fig. 6.
Total and phosphorylated PDK-1 (58–68 kDa) was measured in mesenteric artery homogenates from sham and IH group rats. Representative images are shown in (A), and the average ratios of densitometry values of phosphorylated to total PDK-1 are shown in (B). When expressed as a ratio of total PDK-1 protein, PDK-1 phosphorylation at Ser241 was similar between the sham and IH groups. Total PDK-1 was normalized to Coomassie Blue staining and was not altered by IH exposure (C). The images shown include tissue from four rats per group and are representative of three replicates. A.U., arbitrary units.
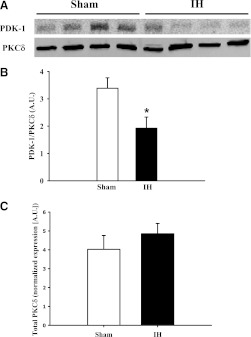
Interactions between PKCδ and PDK-1 in mesenteric arteries from sham and IH group rats were assessed by measuring coimmunoprecipitation of these proteins (Fig. 7) because the degree of their interaction can be used as an indirect indication of PKCδ activation—PKCδ phosphorylation by PDK-1 decreases its association with PDK-1 (Gao et al., 2001). PKCδ was immunoprecipitated from mesenteric artery lysates, and levels of PKCδ-bound PDK-1 were assessed. The ratio of PDK-1 to PKCδ was lower in arteries from IH-exposed rats compared with those from rats in the sham arm (Fig. 7, A and B), demonstrating that IH treatment results in decreased protein-protein interaction between PDK-1 and PKCδ and suggesting enhanced PDK-1 phosphorylation of PKCδ. Total PKCδ levels were similar between arteries from sham and IH group rats (Fig. 7C).
Fig. 7.
(A) Mesenteric artery homogenates from sham and IH group rats were immunoprecipitated with PKCδ (78 kDa) antibody and probed for PDK-1 (58–68 kDa). There was diminished interaction between PDK-1 and PKCδ in arteries from rats in the IH group compared with those from the sham group (B). Total PKCδ levels were normalized to Coomassie Blue staining and were not affected by exposure to IH (C). *P < 0.05 versus sham. The images shown include tissue from four rats per group and are representative of three replicates. A.U., arbitrary units.
PDK-1 Contribution to Blood Pressure.
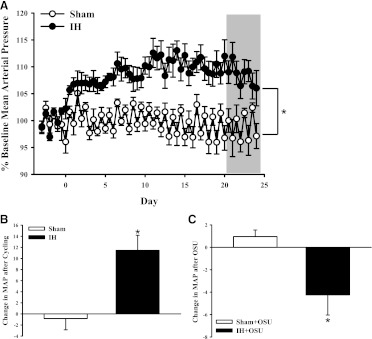
Blood pressure was measured continuously at baseline and throughout the sham or IH exposure period. Blood pressure was elevated after exposure to IH was initiated, but sham exposure had no effect on blood pressure (Fig. 8). Three days of treatment with the PDK-1 inhibitor OSU-03012 significantly decreased blood pressure in IH-exposed rats compared with pressure prior to beginning treatment with OSU-03012; however, PDK-1 inhibition did not affect blood pressure in sham-exposed rats. Treatment with OSU-03012 did not affect plasma concentrations of ET-1 in sham or IH group rats. These results provide evidence of a role for PDK-1 in the IH-induced increase in blood pressure.
Fig. 8.
Blood pressure was monitored by telemetry at baseline and throughout sham and IH exposure protocols (A). The gray box indicates the period of vehicle or OSU-03012 (OSU; 33 mg/day) treatment. The change in mean arterial pressure compared with baseline is shown in (B). Exposure to IH caused an increase in blood pressure, but sham conditions did not. (C) Treatment with the PDK-1 inhibitor OSU caused a decrease in blood pressure in IH- but not sham-exposed rats. *P < 0.05 versus sham; n = 4–6/group.
Discussion
The findings of this study demonstrate that PDK-1 and PKCδ contribute to the observed increase in ET-1 vasoconstrictor sensitivity in a rodent model of sleep apnea. Furthermore, PDK-1 contributes to the elevated blood pressure observed following chronic exposure to IH to mimic sleep apnea. These results provide the first evidence of a novel role for PDK-1 in blood pressure regulation and ET-1 constrictor sensitivity that is not present under normal conditions but is unmasked after exposure to IH.
In agreement with previous findings using endothelium-disrupted mesenteric arteries (Allahdadi et al., 2008b), this study demonstrates that augmented ET-1–mediated vasoconstriction is dependent on PKCδ in endothelium-intact mesenteric arteries. This PKCδ-dependent mechanism does not appear to be present in all vascular beds since isolated aortic segments from sham- and IH-exposed rats both show attenuated vasoconstriction in response to pan-PKC and cPKC inhibitors but PKCδ inhibition has no effect (Allahdadi et al., 2008b). Furthermore, inhibition of PDK-1, a kinase activator of PKCδ, with OSU-03012 attenuates ET-1–mediated vasoconstriction in arteries from IH- but not from sham-exposed rats.
Previous studies from our laboratory showed that 2-week exposure to IH increased blood pressure, with a corresponding augmentation of vasoconstrictor reactivity to ET-1 (Kanagy et al., 2001; Allahdadi et al., 2005). Circulating concentrations of ET-1 are increased following exposure to IH, and both increased ET-1 expression and increased blood pressure were attenuated by in vivo treatment with the antioxidant tempol, suggesting that the IH-induced increase in blood pressure is oxidative-stress–dependent (Troncoso Brindeiro et al., 2007). We also reported that the IH-induced increase in blood pressure is prevented or reversed by ETAR antagonists (Kanagy et al., 2001; Allahdadi et al., 2008a).
Our results indicate that PKC inhibitors that block PKCδ activity inhibit ET-1–mediated constriction in mesenteric arteries from IH-exposed rats, but those that do not inhibit this isoform are without effect. Therefore, even though rottlerin has non-PKCδ targets (Soltoff, 2007), the selective attenuation of ET-1 constriction in arteries from IH-exposed rats suggests that one or more targets of rottlerin—PKCδ or others—is activated by ET-1 only in IH arteries. Taken together with the finding that OSU-03012 attenuates ET-1 constrictor sensitivity only in arteries from IH-exposed rats, our results suggest that exposure to IH unmasks a novel ET-1 constrictor pathway including both PDK-1 and PKCδ that is not present under normal conditions.
Results of the current study highlight a novel role for PDK-1 in regulating cardiovascular function following exposure to IH. The majority of studies using the PDK-1 inhibitor OSU-03012 have focused on cancer therapy and the drug’s ability to block Akt signaling rather than using it as a tool to inhibit PKC signaling (Zhu et al., 2004; Gao et al., 2008). Thus, the current finding that PDK-1 can regulate constrictor sensitivity to ET-1 and blood pressure following exposure to IH is the first suggestion that this kinase may play a role in blood pressure regulation under some conditions.
The increase in blood pressure following IH exposure likely involves multiple pathways rather than simply being a result of increased PDK-1/PKCδ activity. Previous studies from our laboratory have demonstrated that inhibiting the ETAR (Allahdadi et al., 2008a) or scavenging of ROS (Troncoso Brindeiro et al., 2007) completely prevents or reverses the IH-induced increase in blood pressure, suggesting that pathways contributing to elevated blood pressure are downstream of these mediators. Additionally, PDK-1 has multiple targets, including Akt. It is possible that a 3-day treatment with the PDK-1 inhibitor OSU-03012 impairs Akt and nitric oxide (NO) signaling to mitigate blood pressure lowering.
As described above, PDK-1 can be activated by binding to phosphoinositides, such as phosphoinositol 3,4,5-trisphosphate (PIP3), generated by PI3K. However, PI3K inhibition with LY294002 did not affect ET-1-induced constriction in mesenteric arteries from IH group rats even though PI3K inhibition augmented vasoreactivity in endothelium-intact arteries from sham group rats. This augmented constriction in the sham group could be due to inhibition of PI3K/PDK-1/Akt activation of endothelial NO synthase (eNOS) (Dimmeler et al., 1999; Zeng et al., 2000; Xiao-Yun et al., 2009). This is supported by the observation that disrupting the endothelium greatly attenuated the effect of PI3K inhibition in arteries from sham group rats but has not been confirmed by direct measurement of NO production or eNOS activity. The lack of effect of LY294002 in the arteries from IH-exposed rats suggests that these arteries have lower basal NO production or some other form of endothelial dysfunction that may contribute to the hypercontractile state mediated by ET-1 and PKCδ, as shown in Fig. 3. This potential loss of a PI3K-dependent dilator effect following IH warrants further investigation.
PDK-1 can be activated by conformational changes following binding of lipid products such as PIP3 at the pleckstrin homology (PH) domain. However the lack of effect on ET-1 vasoreactivity by LY294002 in IH vessels suggests that the augmented PDK-1/PKCδ contribution to ET-1-induced constriction apparent after IH exposure is not caused by increased PIP3 production. Indeed, other studies demonstrated that lipid binding at the PH domain does not affect PKC phosphorylation levels (Bayascas et al., 2008) and PDK-1 phosphorylates PKC even when the PH domain is mutated (Sonnenburg et al., 2001). Our results are thus more consistent with PDK-1 relying on PI3K-generated PIP3 to activate Akt and eNOS but phosphorylating PKC independently of PIP3 binding to its PH domain.
Interestingly, our data suggest that IH exposure diminishes the physical association of PKCδ and PDK-1. Gao and colleagues (2001) demonstrated that PDK-1 preferentially binds unphosphorylated PKCβII over the phosphorylated form and concluded that PDK-1 not only phosphorylates the activation loop of PKCβII and upregulates PKCβII autophosphorylation but that it is the release from PDK-1 that primes PKCβII for autophosphorylation. Therefore, the decreased association of PDK-1 and PKCδ observed in mesenteric arteries from IH-exposed rats is consistent with the previous observation of increased PKCδ autophosphorylation (Allahdadi et al., 2008b), and thus increased PKCδ activation.
The mechanism by which IH exposure leads to dysregulation of PDK-1 and its downstream target PKCδ is not yet defined. We have previously shown that arteries from IH-exposed rats have increased levels of ROS (Troncoso Brindeiro et al., 2007) and ROS have been shown to activate PDK-1 (Prasad et al., 2000; Zou et al., 2003), raising the possibility that IH-induced elevations in ROS may contribute to the aberrant PDK-1 signaling in mesenteric arteries from IH-exposed rats. Block et al. (2008) showed that increased ROS in mesangial cells activate the tyrosine kinase Src, which phosphorylates PDK-1, increasing its activity. Tyrosine kinases such as Src have also been shown to activate PKCδ (Rybin et al., 2007) and may contribute to IH-mediated increases in PDK-1 and PKCδ activity, and future studies examining the effects of ROS on PDK-1 activity and phosphorylation of its target PKCδ may provide insight into the mechanism of IH-induced dysregulation of PDK-1 signaling. Of interest, constriction in response to phenylephrine is similar in arteries from sham and IH group rats, and vascular expression of PDK-1 and PKCδ is not increased by IH. Thus, it appears that IH augments ET-1 constriction by facilitating coupling of the ETAR to PKCδ in a PDK-1–dependent manner and that the blood-pressure–lowering effect of OSU-03012 in IH but not sham group rats, similar to our earlier findings with ETAR antagonists (Allahdadi et al., 2008a), is consistent with a selective effect of IH on ET-1 constriction.
In conclusion, our results indicate that exposure to IH increases constrictor sensitivity to ET-1 in a PDK-1- and PKCδ-dependent manner. This pathway is not active under normal conditions, as evidenced by the lack of effect of PDK-1 and PKCδ inhibitors in control conditions, but is unmasked by IH exposure. Although PDK-1 does not appear to regulate blood pressure under normal conditions, our results provide novel evidence that PDK-1 contributes to the IH-induced elevation in blood pressure. The intriguing role of PDK-1 in various pathologies such as cancer, diabetes, and insulin resistance, and now OSA-induced hypertension, suggests that this kinase plays a pivotal role in vascular smooth muscle cell biology. With a PDK-1 inhibitor already in clinical trials, PDK-1 may provide a novel therapeutic target for blood pressure regulation in OSA patients.
Abbreviations
- cPKC
classical protein kinase C
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin-1
- ETAR
endothelin-1 A receptor; Go6976, 5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile; Go6983, 3-[1-[3-(dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione
- IH
intermittent hypoxia; LY294002, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one
- NO
nitric oxide
- OSA
obstructive sleep apnea
- OSU-03012
2-amino-N-{4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phenyl}-acetamide
- PDBu
phorbol 12,13-dibutyrate
- PDK-1
phosphoinositide-dependent kinase-1
- PH
pleckstrin homology
- PI3K
phosphoinositide 3-kinase
- PIP3
phosphoinositol 3,4,5-trisphosphate
- PKC
protein kinase C
- PSS
physiological salt solution
- ROS
reactive oxygen species
Authorship Contributions
Participated in research design: Webster, Osmond, Walker, Kanagy.
Conducted experiments: Webster, Osmond, Paredes, DeLeon, Jackson-Weaver.
Performed data analysis: Webster, Osmond, Paredes, DeLeon, Jackson-Weaver.
Wrote or contributed to the writing of the manuscript: Webster, Osmond, Kanagy.
Footnotes
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL07736, HL95649, and HL82799] and the American Heart Association [Grant 12POST8690008].
References
- Allahdadi KJ, Cherng TW, Pai H, Silva AQ, Walker BR, Nelin LD, Kanagy NL. (2008a) Endothelin type A receptor antagonist normalizes blood pressure in rats exposed to eucapnic intermittent hypoxia. Am J Physiol Heart Circ Physiol 295:H434–H440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahdadi KJ, Duling LC, Walker BR, Kanagy NL. (2008b) Eucapnic intermittent hypoxia augments endothelin-1 vasoconstriction in rats: role of PKCdelta. Am J Physiol Heart Circ Physiol 294:H920–H927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahdadi KJ, Walker BR, Kanagy NL. (2005) Augmented endothelin vasoconstriction in intermittent hypoxia-induced hypertension. Hypertension 45:705–709 [DOI] [PubMed] [Google Scholar]
- Bayascas JR, Wullschleger S, Sakamoto K, García-Martínez JM, Clacher C, Komander D, van Aalten DM, Boini KM, Lang F, Lipina C, et al. (2008) Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Mol Cell Biol 28:3258–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block K, Eid A, Griendling KK, Lee DY, Wittrant Y, Gorin Y. (2008) Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem 283:24061–24076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley TD, Floras JS. (2009) Obstructive sleep apnoea and its cardiovascular consequences. Lancet 373:82–93 [DOI] [PubMed] [Google Scholar]
- Dematteis M, Julien C, Guillermet C, Sturm N, Lantuejoul S, Mallaret M, Lévy P, Gozal E. (2008) Intermittent hypoxia induces early functional cardiovascular remodeling in mice. Am J Respir Crit Care Med 177:227–235 [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605 [DOI] [PubMed] [Google Scholar]
- Gao M, Yeh PY, Lu YS, Hsu CH, Chen KF, Lee WC, Feng WC, Chen CS, Kuo ML, Cheng AL. (2008) OSU-03012, a novel celecoxib derivative, induces reactive oxygen species-related autophagy in hepatocellular carcinoma. Cancer Res 68:9348–9357 [DOI] [PubMed] [Google Scholar]
- Gao T, Toker A, Newton AC. (2001) The carboxyl terminus of protein kinase c provides a switch to regulate its interaction with the phosphoinositide-dependent kinase, PDK-1. J Biol Chem 276:19588–19596 [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. (1996) Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett 392:77–80 [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Müller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. (1994) Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun 199:93–98 [DOI] [PubMed] [Google Scholar]
- Kanagy NL, Walker BR, Nelin LD. (2001) Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension 37:511–515 [DOI] [PubMed] [Google Scholar]
- Kikkawa U, Matsuzaki H, Yamamoto T. (2002) Protein kinase C delta (PKC delta): activation mechanisms and functions. J Biochem 132:831–839 [DOI] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. (1998) Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281:2042–2045 [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marmé D, Schächtele C. (1993) Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem 268:9194–9197 [PubMed] [Google Scholar]
- Newton AC. (2009) Lipid activation of protein kinases. J Lipid Res 50 (Suppl):S266–S271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad N, Topping RS, Zhou D, Decker SJ. (2000) Oxidative stress and vanadate induce tyrosine phosphorylation of phosphoinositide-dependent kinase 1 (PDK1). Biochemistry 39:6929–6935 [DOI] [PubMed] [Google Scholar]
- Rybin VO, Guo J, Gertsberg Z, Elouardighi H, Steinberg SF. (2007) Protein kinase Cepsilon (PKCepsilon) and Src control PKCdelta activation loop phosphorylation in cardiomyocytes. J Biol Chem 282:23631–23638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, Walker BR, Resta TC. (2008) Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol 104:110–118 [DOI] [PubMed] [Google Scholar]
- Soltoff SP. (2007) Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci 28:453–458 [DOI] [PubMed] [Google Scholar]
- Sonnenburg ED, Gao T, Newton AC. (2001) The phosphoinositide-dependent kinase, PDK-1, phosphorylates conventional protein kinase C isozymes by a mechanism that is independent of phosphoinositide 3-kinase. J Biol Chem 276:45289–45297 [DOI] [PubMed] [Google Scholar]
- Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. (2007) Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol 293:H2971–H2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao-Yun X, Zhuo-Xiong C, Min-Xiang L, Xingxuan H, Schuchman EH, Feng L, Han-Song X, An-Hua L. (2009) Ceramide mediates inhibition of the AKT/eNOS signaling pathway by palmitate in human vascular endothelial cells. Med Sci Monit 15:BR254–BR261 [PubMed] [Google Scholar]
- Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ. (2000) Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101:1539–1545 [DOI] [PubMed] [Google Scholar]
- Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, Shaw YJ, Kulp SK, Chen CS. (2004) From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res 64:4309–4318 [DOI] [PubMed] [Google Scholar]
- Zou MH, Hou XY, Shi CM, Kirkpatick S, Liu F, Goldman MH, Cohen RA. (2003) Activation of 5′-AMP-activated kinase is mediated through c-Src and phosphoinositide 3-kinase activity during hypoxia-reoxygenation of bovine aortic endothelial cells. Role of peroxynitrite. J Biol Chem 278:34003–34010 [DOI] [PubMed] [Google Scholar]