Abstract
Clopidogrel and prasugrel belong to a thienopyridine class of oral antiplatelet drugs that, after having been metabolized in the liver, can inhibit platelet function by irreversibly antagonizing the P2Y12 receptor. Furthermore, thienopyridines influence numerous inflammatory conditions, but their effects on neutrophils have not been evaluated, despite the important role of these cells in inflammation. Therefore, we investigated the effect of prasugrel metabolites on neutrophils to further clarify the role of thienopyridines in inflammation. Interestingly, a prasugrel metabolite mixture, produced in vitro using rat liver microsomes, significantly inhibited N-formyl-methionyl-leucyl-phenylalanine (fMLP)- and platelet-activating factor (PAF)-induced neutrophil activation. More specifically, prasugrel metabolites inhibited neutrophil transmigration, CD16 surface expression, and neutrophil-platelet aggregation. Moreover, prasugrel metabolite pretreatment also significantly decreased fMLP- or PAF-induced extracellular-signal–regulated kinase phosphorylation as well as calcium mobilization. To determine the target of prasugrel in neutrophils, the role of both P2Y12 and P2Y13 receptors was studied using specific reversible antagonists, AR-C69931MX and MRS2211, respectively. Neither antagonist had any direct effect on the agonist-induced neutrophil functional responses. Our findings indicate that prasugrel metabolites may directly target neutrophils and inhibit their activation, suggesting a possible explanation for their anti-inflammatory effects previously observed. However, these metabolites do not act through either the P2Y12 or P2Y13 receptor in neutrophils.
Introduction
ADP-induced platelet activation is mediated by both the P2Y1 and P2Y12 receptors (Kahner et al., 2006; Murugappa and Kunapuli, 2006; Bhavaraju et al., 2010; Kim and Kunapuli, 2011), although the P2Y12 receptor also plays a crucial role when platelets are activated by other agonists. Antithrombotic drugs targeting the P2Y12 receptor, such as clopidogrel and prasugrel, have been successfully used to prevent thrombotic events (Bhavaraju et al., 2010; Kim and Kunapuli, 2011). Previous studies have shown that Gi signaling, mediated by the P2Y12 receptor, is dependent on the cholesterol-rich lipid rafts (Quinton et al., 2005) and that high-fat diet enhances platelet activation induced by other agonists (Nagy et al., 2011). In addition to chronic hypercholesterolemia, other pathologic conditions ranging from diabetes (Morel et al., 2012) to hypertension may increase P2Y12 receptor functions, and hence the risk of thrombosis. Moreover, polymorphisms in the P2Y12 gene that enhance receptor activity have been associated with increased risk of thrombotic events (Bura et al., 2006).
Despite their well known functions in hemostasis, platelets also play a role in the immune response and inflammation (Semple and Freedman, 2010; Semple et al., 2011) They store a number of chemokines, growth factors, and angiogenic factors that, upon stimulation, are released from their granules, activating other blood cells and the endothelium. Platelet granule release is potentiated by P2Y12 receptor stimulation (Dangelmaier et al., 2001; Garcia et al., 2010). The P2Y12 receptor gene variants correlate with pulmonary inflammation and asthma (Bunyavanich et al., 2012), and receptor deficiency abrogated dust mite–induced airway inflammation (Paruchuri et al., 2009), suggesting an important role for the P2Y12 receptor in pulmonary inflammation. Treatment with the P2Y12 receptor antagonist clopidogrel significantly attenuated lipopolysaccharide (LPS)-induced inflammatory responses and lung injury (Hagiwara et al., 2011). In addition, this drug also alleviated the severity of cardiac inflammation and post–myocardial infarction in a mouse myocardial infarction model (Liu et al., 2011). In contrast, two recent studies suggested that clopidogrel enhances inflammatory responses in rodent models of rheumatoid arthritis (Boilard et al., 2010; Garcia et al., 2011). The reasons for the proinflammatory effects of clopidogrel in this animal model compared with others is not known, although the use of exposed collagen in generating rheumatoid arthritis may activate unique inflammatory pathways that are regulated differently than those observed in other models of inflammation (Diehl et al., 2010; Hagiwara et al., 2011; Liu et al., 2011). Interestingly, previous studies suggest that the P2Y12 receptor may be expressed in cell types other than platelets, such as lymphocytes (Wang et al., 2004; Diehl et al., 2010), monocytes (Wang et al., 2004), and dendritic cells (Ben Addi et al., 2010), indicating that P2Y12 antagonists might also target the nonplatelet P2Y12 receptor.
Despite the well known role of neutrophils during the early stages of inflammation (Wagner and Roth, 1999) and their direct interaction with platelets (Weiss et al., 1998), the effect of P2Y12 antagonism on neutrophil activation has not been addressed. Therefore, we investigated whether P2Y12 is expressed in neutrophils and whether a P2Y12 receptor antagonist, prasugrel, could target these cells and alter their functions. Because prasugrel is a prodrug that needs to be metabolized in the liver to elicit its effects on the P2Y12 receptor (Kim and Kunapuli, 2011), to perform an in vitro study we used rat liver microsomes to generate the active metabolite of prasugrel (Cavin et al., 2001; Brandon et al., 2003). We also investigated possible in vivo effects of this drug in a LPS-induced model of systemic inflammation. Our results demonstrate that prasugrel metabolites inhibit neutrophil functional responses and that these effects are not mediated through P2Y12 or P2Y13 receptors, suggesting off-target effects of this drug on neutrophils.
Materials and Methods
Materials.
All reagents, analytical grade, were obtained from Thermo Fisher Scientific (Waltham, MA) unless stated otherwise. AR-C69931MX was a gift from AstraZeneca (Wilmington, DE), while MRS2211 was purchased from Tocris Bioscience (Minneapolis, MN). Prasugrel hydrochloride (Effient 10-mg tablets) was obtained from the Internal Pharmacy of Temple University (Philadelphia, PA). Rat liver microsomes were from Sigma-Aldrich (St. Louis, MO). Triton-X was also purchased from Sigma-Aldrich (St. Louis, MO). Ficoll-Paque was purchased from GE Healthcare Bio-Sciences AB (Uppsala, SE); phosphate-buffered saline (PBS) and Hanks’ balanced salt solution (HBSS) were purchased from Mediatech Inc. (Manassas, VA) and platelet-activating factor (PAF) from Calbiochem Corp. (San Diego, CA); N-formyl-methionyl-leucyl-phenylalanine (fMLP) was obtained from Sigma-Aldrich and leukotriene E4 (LTE4) from Cayman Chemical (Ann Arbor, MI). Antibodies against human CD41 (phycoerythrin [PE]-conjugated; clone HIP8), human CD16 (allophycocyanin [APC]-conjugated; clone eBioCD16), and mouse CD11b (PE-conjugated; clone M1/70) were obtained from eBioscience (San Diego, CA); phospho-extracellular-signal–regulated kinases (ERKs) 1/2 (Thr202/Tyr204) and total ERK2 antibodies were purchased from Cell Signaling Technology (Danvers, MA); DyLight 800 conjugated goat anti-rabbit IgG and DyLight 680 conjugated goat anti-mouse IgG were obtained from Thermo Fisher Scientific. Mouse-CD41 (fluorescein isothiocyanate [FITC]-conjugated; clone MWReg30) antibody and BD FACS lysing solution were obtained from BD Pharmingen (Franklin Lakes, NJ). Nitrocellulose membranes were purchased from Whatman Protran (Dassel, Germany), while Odyssey blocking buffer was from LI-COR Biosciences (Lincoln, NE) and fura-2 from Invitrogen (Grand Island, NY).
Human Platelet Isolation.
Human blood was obtained from healthy adult donors, following informed consent, in accordance with Institutional Review Board protocols at Temple University School of Medicine. Total blood was diluted with one-sixth volume of acid-citrate-dextrose (2.5 g of sodium citrate, 1.5 g of citric acid, and 2.0 g of glucose in 100 ml of deionized water). Platelet-rich plasma (PRP) was prepared by centrifugation of citrated blood at 230 × g for 20 minutes at room temperature. The PRP obtained was then centrifuged at 980 × g for 10 minutes at room temperature and the platelet pellet resuspended in Tyrode's buffer (138 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 5 mM glucose, and 10 mM HEPES; pH 7.4) containing 0.2 units/ml apyrase. Cells were counted using the Hemavet Multispecies Hematology System (Drew Scientific, Inc., Oxford, CT).
Human Neutrophil Isolation.
After removing PRP to isolate platelets, the original volume was restored by adding HBSS. Cells were then incubated in 6% dextran in 0.9% NaCl for 40 minutes at 25°C. The leukocyte-rich plasma was collected and centrifuged for 5 minutes at room temperature at 350 × g. The pellet containing cells was resuspended in 3 ml of HBSS–0.2% bovine serum albumin; cell suspension was added to 3 ml of Ficoll-Paque and centrifuged at 300 × g for 30 minutes at 25°C. Neutrophils were collected at the bottom of the tube. Red blood cell lysis was achieved by adding 7.5 ml of ice-cold 0.2% NaCl for 90 seconds, followed by 7.5 ml of 1.6% NaCl. Cells were resuspended in HBSS and counted using the Hemavet System. Only samples with a cell purity of 95% or higher were used for the experiments.
Platelet Aggregation.
Agonist-induced platelet aggregation was analyzed using a Chrono-Log (Havertown, PA) model 440-VS aggregometer with sample volumes of 0.5 ml in a cuvette holder, equipped with a thermostat at 37°C and set at constant stirring (900 rpm). Aggregometer output was recorded using a Kipp & Zonen type BD 12E flatbed chart recorder (Kipp & Zonen, Bohemia, NY) set at 0.2 mm/s. Platelets at 2 × 108 cells/ml concentration were preincubated with AR-C69931MX (100 nM and 1 μM), MRS2211 (10–50 μM), or prasugrel metabolites (2, 3, and 5 μM) for 2 minutes when appropriate, and aggregation was induced by addition of 2MeSADP (100 nM).
In Vitro Preparation of Prasugrel Metabolite Mixture.
Pooled male rat liver microsomes were adjusted to 2 mg of protein/ml in 100 nM potassium phosphate buffer, pH 7.4, and incubated with 2 mM NADPH, 5 mM glutathione, and 0.1 mM prasugrel dissolved in methanol (prodrug solution). As a control, the same concentration of prasugrel vehicle (methanol) was incubated with rat microsomes, NADPH, and glutathione in potassium buffer (all at the same concentration as prasugrel solution). Following incubation for 1 hour at 37°C under continuous stirring at 120 rpm and light protection, the solutions were aliquoted and stored at −20°C (Brandon et al., 2003).
Cell Viability.
Neutrophils (106 cells/tube in HBSS) were preincubated with prasugrel metabolites at different concentrations (5, 10, and 15 μM) or negative control for 2 minutes at 37°C to study cell viability after exposure to the mixture generated. Triton-X from Sigma-Aldrich (0.1% in PBS) was added as a positive control to permeabilize cell membrane. Cells were then incubated with propidium iodide (final concentration of 2 μg/ml) on ice for 5 minutes and analyzed on flow cytometry using a FACSCalibur analyzer (Becton Dickinson, USA), and data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR).
Chemotaxis Assay.
Chemotaxis was performed using a 96-well chemotaxis chamber (ChemoTx; Neuroprobe, Gaithersburg, MD) as previously described (Frevert et al., 1998). Briefly, the chemoattractants fMLP (10 μM), PAF (10 nM), or LTE4 (1 μM) were added to the well in the microplate (bottom chamber), while cells (1 × 105 cells/well) were seeded on the filter sites (top chamber). The chamber was then incubated for 1 hour at 37°C, 5% CO2. Cells were then removed from the top of the membrane and each well washed with HBSS to remove remaining cells. Plate and membrane were then centrifuged for 1 minute at 312 × g and the cell pellet resuspended. Neutrophils that had migrated to the bottom chamber were quantified by diluting in Turk’s dye and cells were counted as previously described (Cooper et al., 2010).
Platelet-Neutrophil Aggregate Formation.
We studied the formation of platelet-leukocyte aggregates by preincubating neutrophils (1 × 106 cells/tube) and platelets (1 × 106 cells/tube) with antibodies (diluted 1:50) against human CD41 (PE-conjugated) and CD16 (APC-conjugated) and either prasugrel or control for 2 minutes at 25°C. Aggregate formation was then induced by adding fMLP (10 μM), 2MeSADP (100 nM), or PAF (10 nM) and incubated for a further 20 minutes at 25°C. The reaction was stopped by adding 2% paraformaldehyde solution in PBS and samples were kept at 4°C until analysis (Rinder et al., 1991; Köhler et al., 2011).
In some cases, prasugrel was added to either the platelet or neutrophil preparation separately. To this end, both PRP and neutrophils were pretreated with either prasugrel (5 μM) or control for 5 minutes at 25°C. Following a washing step, platelets and neutrophils were resuspended in HEPES buffer (NaCl 150 mM, KCl 5 mM, MgSO4 1 mM, HEPES 10 mM) and added to tubes in equal volume. After addition of PE-conjugated anti-human CD41 and APC-conjugated anti-human CD16, samples were stimulated with fMLP (10 μM), PAF (10 nM), or LTE4 (1 μM) and incubated for 20 minutes at 25°C, then fixed using 2% paraformaldehyde and stored at 4°C prior to analysis. Flow cytometry was performed using a FACSCalibur analyzer, and data were analyzed with FlowJo software. Platelets and neutrophils were discriminated by forward and side light scatter and identified by their positive staining for PE-CD41 or APC-CD16, respectively. Events double positive for APC and PE identified platelet-neutrophil aggregates and were recorded as a percentage of a total of 10,000 gated neutrophils (Supplemental Fig. 1).
Western Blotting.
Neutrophils (1 × 107 cells/sample) were preincubated with 5 μM prasugrel for 2 minutes at 37°C, prior to stimulation with 10 μM fMLP or 10 nM PAF for 30 minutes at 37°C. The cells were centrifuged for 5 minutes at 10,000 × g at 4°C and lysed with lysis buffer (10 mM HEPES, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, and 0.2% NP40; pH 7.4) by vortexing them for 20 minutes at 4°C and microfuged for 5 minutes at 4°C at 10,000 × g. Sample buffer was added to supernatant, and samples were boiled for 5 minutes. Cell lysate was electrophoresed on 12% SDS-PAGE gels. Proteins were electrophoretically transferred to nitrocellulose membrane at 100 V for 1 hour. After completion of transfer, the membrane was incubated for 1 hour at room temperature with Odyssey blocking buffer. To determine ERK1/2 phosphorylation, the membrane was incubated simultaneously with phospho-ERK1/2 (Thr202/Tyr204) and total ERK2 antibodies (dilutions 1:1000 for both) in Odyssey blocking buffer with gentle agitation overnight at 4°C. Membrane was then washed before addition of secondary antibody conjugated to a fluorescent entity: DyLight 800 conjugated goat anti-rabbit IgG and DyLight 680 conjugated goat anti-mouse IgG (dilution 1:6000) in Odyssey blocking buffer with gentle agitation for 1 hour at room temperature. At the end of the incubation period, the membrane was washed, then dried, visualized, and analyzed on the Odyssey IR imaging system (LI-COR Biosciences).
Intracellular Calcium Release.
Neutrophils (6 × 106 cells/ml) were incubated with 1 μM fura-2 for 45 minutes at 37°C. Cells were then washed and resuspended in HBSS. Changes in fluorescence were measured using an Aminco-Bowman Series 2 luminescence spectrometer (Spectronic Instruments, USA) with a water-jacketed cuvette holder, set at a 37°C with constant stirring. Samples were analyzed using an excitation wavelength of 340 nm and an emission wavelength of 510 nm. After preincubation with either the negative control or prasugrel metabolites (2 minutes; 5 μM), calcium release was induced by addition of fMLP (10 μM) or PAF (10 nM). Fluorescence measurements were converted to calcium concentrations using the equation reported by Grynkiewicz et al. (1985) where Fmin and Fmax were determined with each respective platelet preparation.
Animals and Treatments.
Eight pathogen-free C57BL/6 male mice (weight, 25–30 g) were obtained from Taconic (Rockville, MA). The animals had unlimited access to food and water before treatment and were randomly assigned to one of the four groups considered (two animals per group): untreated, only prasugrel-treated, LPS-treated, and prasugrel- and LPS-treated mice. Prasugrel was orally administrated at a loading dose of 10 mg/kg and a maintenance dose of 3 mg/kg. LPS-treated animals received an i.p. dose of LPS (5 mg/kg). After 24 hours, mice were anesthetized and blood samples were collected by cardiac puncture (10:1 ratio of blood in 3.8% sodium citrate) for hematology studies that were performed using the Hemavet Multispecies Hematology System. The experimental protocol followed in this study was fully approved by the Institutional Animal Care and Use Committee of Temple University School of Medicine.
Platelet-Neutrophil Aggregate Formation and CD11b Expression.
Murine blood samples were incubated with FITC-conjugated anti-mouse CD11b and PE-conjugated anti-mouse CD41 or only anti-mouse CD11b for 10 minutes. Then BD FACS lysing solution was added to fix samples and lysate red blood cells. Samples were kept at 4°C up to analysis. Flow cytometry was performed on a FACSCalibur analyzer, and data were analyzed with FlowJo software.
Lung Histopathology and Myeloperoxidase Peroxidation.
Lungs were fixed in 10% formalin and embedded into paraffin blocks. Sections (5 μm) were cut on a microtome and stained with H&E. Slides were analyzed and compared based on cell infiltration. Part of the lungs were also homogenized and sonicated in cold PBS (pH 7.4). After centrifugation at 10,000 × g for 10 minutes at 4°C, myeloperoxidase (MPO) levels were detected using an MPO assay kit (Cayman Chemical).
Statistical Analysis.
Experiments were conducted in duplicate or triplicate and repeated at least three times. Data are reported as mean ± S.E.M. Statistical differences were analyzed by analysis of variance followed by Dunnett’s test or by Student’s t test, as appropriate. A P value of <0.05 was taken as significant.
Results
Prasugrel Metabolites Generated In Vitro Abolish Platelet Aggregation but Do Not Alter Neutrophil Viability.
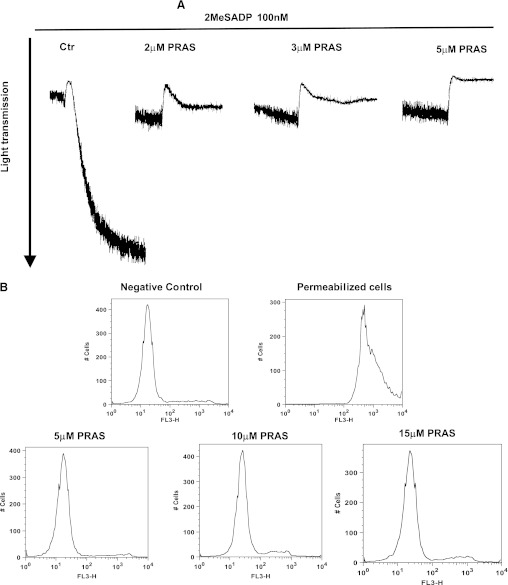
To evaluate the efficacy of the metabolite mixture generated, we tested it on the platelet P2Y12 receptor, by preincubating platelets with the metabolite mixture and then stimulating them with 2MeSADP (100 nM). Rat liver microsomes, enzymes, and methanol without prasugrel were added as a negative control. As shown in Fig. 1A, 2MeSADP-induced aggregation was dramatically reduced following treatment with the metabolite mixture in a concentration-dependent manner. An approximate concentration of 5 μM (based on the concentration of prasugrel originally added to a certain volume of microsomes) was able to abolish aggregation, but not shape change, as expected (negative control: 100% aggregation; prasugrel metabolites 2 μM: 7% ± 2%; prasugrel metabolites 3 μM: 4% ± 1%; prasugrel metabolites 5 μM: 0%). Hence, 5 μM prasugrel metabolite mixture was used for the experiments with neutrophils.
Fig. 1.
Prasugrel metabolites (PRAS) generated in vitro abolish platelet aggregation and do not alter neutrophil viability. (A) Human platelets were pretreated at 37°C with either PRAS (2, 3, and 5 μM) or negative control (Ctr; 5 μM) for 2 minutes and then stimulated with 2MeSADP (100 nM) as noted. All tracings are representative of at least three experiments from different donors. (B) Representative histograms showing propidium iodide exclusion in neutrophils untreated, permeabilized, and exposed to different concentrations of PRAS (5, 10, and 15 μM) for 2 minutes. Histograms are representative of at least three experiments from different donors.
The effect of these metabolites on neutrophil viability was determined using propidium iodide exclusion as an indicator of membrane integrity. Neutrophils were incubated with varying concentrations of metabolites (5, 10, and 15 μM) and compared with the negative control. In Fig. 1B, representative images of flow cytometry histograms indicate that when neutrophils were permeabilized, the geometric mean of fluorescence intensity (GMFI) was 852 ± 20, compared with 25 ± 3 for negative control cells. No difference was noted between negative control values and prasugrel metabolite–pretreated cells at all the concentrations analyzed (GMFI: 21 ± 7 for 5 μM, 29 ± 9 for 10 μM, and 32 ± 4 for 15 μM), indicating that prasugrel metabolites were not toxic for neutrophils.
Prasugrel Metabolites Significantly Inhibited Human Neutrophil Transmigration and CD16 Surface Expression.
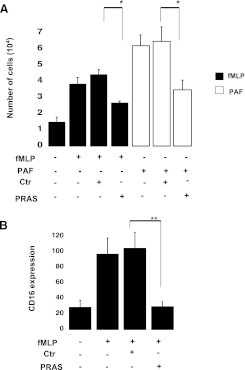
We analyzed the effect of these compounds on neutrophil chemotaxis in response to the chemoattractants fMLP (10 μM) or PAF (10 nM) for 1 hour as described in Materials and Methods. As expected, both fMLP and PAF caused significant transmigration of neutrophils (Fig. 2A). When the negative control mixture was incubated with the cells in the top chamber, transmigration in response to either fMLP or PAF was not affected. In contrast, neutrophils incubated with the prasugrel metabolite mixture had significantly decreased chemotaxis in response to either fMLP or PAF (P < 0.05) (Fig. 2A). Interestingly, when the prasugrel mixture was added with the chemoattractant (in the bottom chamber), no difference in cell migration was observed (number of cells transmigrated with fMLP alone: 3.9 ± 0.4 × 104; negative control: 4.9 ± 0.3 × 104; fMLP plus prasugrel metabolites: 5. 4 ± 0.3 × 104). These data suggest that neutrophils need to be preincubated with the drug before activation to be affected by the prasugrel mixture. Figure 2B shows flow cytometry data investigating fMLP-induced CD16 surface expression as an indication of neutrophil stimulation. A significant increase in CD16 surface expression was noted following fMLP exposure compared with unstimulated cells, as expected. Interestingly, a significant decrease (P ≤ 0.01) in GMFI was observed following preincubation (5 μM; 2 minutes) with the prasugrel metabolite mixture versus negative control samples, suggesting that prasugrel metabolites could also affect CD16 surface expression. These data clearly show that prasugrel metabolites affect neutrophil stimulation.
Fig. 2.
Prasugrel metabolites (PRAS) attenuate neutrophil transmigration and CD16 surface expression. (A) Effects of PRAS (5 μM) and the negative control (Ctr) on cell transmigration, when added directly to the cells with chemoattractant (fMLP, 10 μM; PAF, 10 nM). The number of neutrophils that transmigrated was measured after 1 hour of incubation at 37°C (n = 4, mean ± S.E.M.; *P ≤ 0.05, PRAS versus Ctr). (B) CD16 surface expression was measured following PRAS exposure (2 minutes; 5 μM) using flow cytometry (data shown as GMFI, mean ± S.E.M., n = 5; **P ≤ 0.01, PRAS versus Ctr).
Prasugrel Metabolite Preincubation Decreases ERK Phosphorylation in Agonist-Induced Neutrophil Activation.
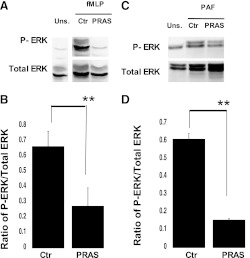
To evaluate the mechanism of action of prasugrel metabolites on neutrophil activation, we investigated whether intracellular signaling events were also altered. When cells were preincubated with prasugrel metabolites (5 μM; 2 minutes), fMLP-induced (10 μM) ERK phosphorylation was diminished (Fig. 3A). Quantitation of the results of Fig. 3A shows a significant decrease (P ≤ 0.01) of phosphorylated ERK when samples were preincubated with prasugrel metabolites (Fig. 3B). Furthermore, when cells were activated with PAF (10 nM), prasugrel metabolites also decreased PAF-induced ERK phosphorylation (P ≤ 0.05) (Fig. 3, C and D), suggesting that the effects of prasugrel metabolites on neutrophil signaling are agonist-independent.
Fig. 3.
Prasugrel metabolites (PRAS) inhibit agonist-induced ERK phosphorylation in neutrophils. Isolated human neutrophils were preincubated with PRAS or control (Ctr) for 2 minutes and stimulated with fMLP (A) or PAF (C) for 30 minutes at 37°C. ERK phosphorylation was measured by Western blot analysis using phosphospecific antibodies against ERK (n = 3, mean ± S.E.M.; **P ≤ 0.01, PRAS versus Ctr). Unstimulated cells (Uns.) analysis was also included. Representative images (A and C) and ratio of arbitrary densitometry units of phosphorylated protein values compared with total proteins (B and D) are shown.
Prasugrel Metabolites Inhibit Agonist-Induced Calcium Mobilization.
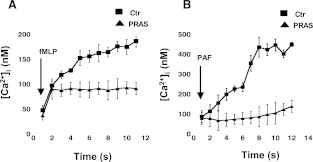
Because both fMLP and PAF cause increases in intracellular calcium in neutrophils, we analyzed the effects of pretreatment with prasugrel metabolites (5 μM; 2 minutes) on intracellular calcium levels following stimulation (Fig. 4). Representative curves in Fig. 4 show a significant decrease in both fMLP-induced (Fig. 4A) and PAF-induced (Fig. 4B) calcium mobilization, suggesting that prasugrel's effects on these cells are not agonist-specific.
Fig. 4.
Prasugrel metabolites (PRAS) decrease calcium mobilization following fMLP and PAF stimulation in neutrophils. Calcium mobilization was measured after pretreatment with either control (Ctr) or PRAS following stimulation with 10 μM fMLP (A) or 10 nM PAF (B). Figure shows the concentration of Ca2+ (nM) mobilized in time (seconds) (n = 4, mean ± S.E.M.; *P ≤ 0.05, PRAS versus Ctr).
Prasugrel Metabolites Reduce Neutrophil-Platelet Aggregate Formation.
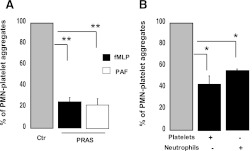
We further investigated the effect of the prasugrel mixture on neutrophil-platelet interaction by investigating neutrophil-platelet aggregate formation by flow cytometry. Neutrophils were gated based on CD16 expression and cell shape (Supplemental Fig. 1). Data were analyzed as a percentage of aggregates expressing both platelet and neutrophil receptor markers (CD41 and CD16, respectively). The percentage of aggregates for unstimulated neutrophils-platelets was 10.3 ± 3. The addition of fMLP or PAF resulted in increased percentages of aggregate formation (57 ± 5 and 37 ± 5 for fMLP- and PAF-activated cells, respectively). A significant decrease (P ≤ 0.01) in neutrophil-platelet aggregates was observed in samples preincubated with prasugrel metabolites (2 minutes; 5 μM) for both fMLP-stimulated (10 μM) and PAF-stimulated (10 nM) samples (Fig. 5A). There was a reduction from 100% (aggregates when cells were stimulated with agonist upon preincubation with negative control) to 25% ± 4% (fMLP) and 22% ± 6% (PAF). To ascertain whether neutrophils are a direct target for these metabolites or the effects observed are platelet-mediated, both cell types were incubated separately with the metabolite mixture prior to fMLP activation (Fig. 5B). As these metabolites affect platelet functions (Scott et al., 2009), it was not surprising to observe a decrease in aggregate formation when the metabolite mixture was preincubated with platelets alone (Fig. 5B). However, a significant decrease (P ≤ 0.05) in aggregate formation was noted (from 100% to 56% ± 1%) when only neutrophils were preincubated with the metabolites, suggesting a direct effect of the prasugrel metabolites on this cell type. Considering that CD16 expression was diminished after prasugrel metabolite exposure, we also investigated another neutrophil marker, CD11a, which was not altered by either stimulation or prasugrel metabolite exposure (Supplemental Fig. 2, A and B). Data show that when CD11a was used as neutrophil marker, the percentage of platelet-neutrophil aggregate formation was significantly inhibited (Supplemental Fig. 2C).
Fig. 5.
Prasugrel metabolites (PRAS) reduce neutrophil (PMN) and platelet interaction. Formation of platelet-neutrophil aggregates was determined using flow cytometry. (A) Both neutrophils and platelets were preincubated with PRAS (5 μM; 2 minutes) before stimulation with fMLP (10 μM) or PAF (10 nM) (n = 3, mean ± S.E.M.; **P ≤ 0.01, PRAS versus control [Ctr]). (B) Either neutrophils or platelets were preincubated with PRAS before being reconstituted. Aggregates were measured after stimulation with fMLP (n = 4, mean ± S.E.M.; *P ≤ 0.05, PRAS versus Ctr).
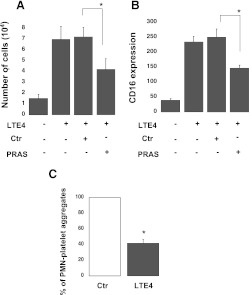
LTE4-Induced Neutrophil Activation Is Also Inhibited by Prasugrel Metabolites.
Previous studies have shown a decrease in LTE4-induced inflammation in P2Y12 knockout mice (Paruchuri et al., 2009). To determine whether LTE4 signaling in neutrophils is also altered in response to prasugrel metabolites,, we investigated the effect of prasugrel mixture pretreatment (5 μM; 2 minutes) (Fig. 6) when neutrophils were activated with LTE4 (1 μM). Similarly to the results obtained with other stimuli, cell transmigration and CD16 surface expression were significantly inhibited (P ≤ 0.05) by these metabolites compared with negative control (Fig. 6, A and B). Also, platelet and neutrophil interaction was decreased (P ≤ 0.05) (Fig. 6C).
Fig. 6.
LTE4-induced neutrophil activation is also inhibited by prasugrel metabolites (PRAS). (A) CD16 surface expression was determined by flow cytometry following pre-exposure to PRAS at a concentration of 5 μM for 2 minutes (n = 4, mean ± S.E.M.; *P ≤ 0.05, PRAS versus control [Ctr]) following stimulation with LTE4 (1 μM). (B) Neutrophil transmigration was evaluated after LTE4-induced (1 μM) activation (1 hour at 37°C) with cells pretreated with PRAS or negative Ctr (n = 3, mean ± S.E.M.; *P ≤ 0.05, PRAS versus Ctr). (C) Platelet-neutrophil aggregate formation was evaluated in the presence of PRAS (5 μM), following activation with LTE4 (1 μM) (n = 3, mean ± S.E.M.; *P ≤ 0.05, PRAS versus Ctr).
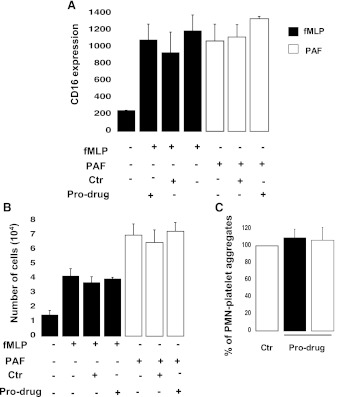
The Prodrug Form of Prasugrel Does Not Inhibit Neutrophil Functions.
To determine whether the observed inhibition of neutrophil activation was the result of unmetabolized forms of prasugrel, we preincubated the neutrophils with prasugrel prodrug in methanol (5 μM) (Fig. 7), then we stimulated the cells with either fMLP (10 μM) or PAF (10 nM). The negative control received the same amount of methanol. No changes were observed in CD16 expression (Fig. 7A) and cell transmigration (Fig. 7B) when neutrophils were pre-exposed to the prodrug compared with the control. Also, interaction with platelets was not affected, as the amount of aggregates formed was not significantly different from the control (Fig. 7C). These data suggest that the prodrug form of prasugrel requires further metabolization to affect neutrophil functions.
Fig. 7.
Neutrophil activation is not altered by prasugrel in the prodrug form. (A) CD16 surface expression was determined by flow cytometry following pre-exposure to prasugrel prodrug at a concentration of 5 μM for 2 minutes (n = 3, mean ± S.E.M.) following stimulation with either fMLP (10 μM) or PAF (10 nM). (B) Neutrophil transmigration was evaluated after fMLP-induced (10 μM) or PAF-induced (10 nM) activation (1 hour at 37°C) with cells incubated with prasugrel prodrug or negative control (Ctr) (n = 3, mean ± S.E.M.). (C) Platelet-neutrophil aggregate formation was evaluated in the presence of prasugrel prodrug (5 μM), following activation with fMLP (10 μM) or PAF (10 nM) (n = 3, mean ± S.E.M.).
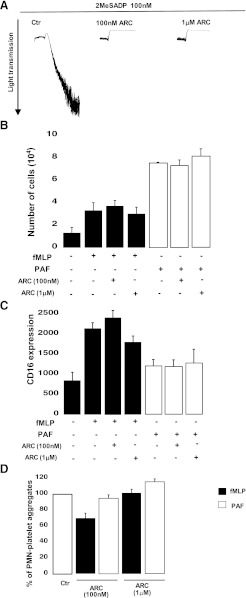
AR-C69931MX, a Reversible P2Y12 Antagonist, Did Not Inhibit fMLP-Induced Neutrophil Transmigration, CD16 Expression, and Neutrophil-Platelet Interaction.
The target of the active metabolite of prasugrel, the P2Y12 receptor, was predicted to be expressed in leukocytes (Diehl et al., 2010) and dendritic cells (Ben Addi et al., 2010). As our data indicate the possibility of a target for prasugrel metabolite on the neutrophil membrane, we determined whether the P2Y12 receptor is expressed in neutrophils and whether it is inhibited by the prasugrel metabolites. To test this possibility, we first analyzed P2Y12 receptor expression through Western blotting (Supplemental Fig. 3), and neutrophils did not seem to express this protein. Secondly, we used a reversible P2Y12 antagonist, AR-C69931MX (Kim and Kunapuli, 2011), which was first tested on platelets (Fig. 8A). This antagonist was able to abolish aggregation at both of the concentrations selected (100 nM and 1 μM). In contrast, when neutrophils were pretreated with AR-C69931MX (100 nM and 1 μM) prior to stimulation with either fMLP (10 μM) or PAF (10 nM), there was no effect of the antagonist on chemotaxis or CD16 expression (Fig. 8, B and C). These data suggest that the P2Y12 receptor is unlikely to be the target of prasugrel metabolites on neutrophils. Neutrophil-platelet interaction was also evaluated. Pretreatment of both neutrophils and platelets with AR-C69931MX (100 nM and 1 μM) prior to fMLP or PAF stimulation did not have any significant effect on neutrophil-platelet aggregate formation (Fig. 8D). We also tested aggregate formation when only neutrophils were preincubated with AR-C69931MX (1 μM) and found no difference in the percentage of fMLP-induced aggregate formation as compared with untreated control cells (48% ± 8% and 57% ± 5%, respectively). This antagonist did not influence neutrophil-platelet aggregate formation, further excluding the possibility of P2Y12 expression on neutrophils as the target of prasugrel metabolites.
Fig. 8.
Neutrophil chemotaxis and CD16 expression were not inhibited by AR-C69931MX (ARC), a reversible P2Y12 antagonist. (A) Human platelets were pretreated at 37°C with ARC (100 nM and 1 μM) for 2 minutes and then stimulated with 2MeSADP (100 nM). All tracings are representative of at least three experiments from different donors. (B) Neutrophils were incubated with either fMLP (10 μM) or PAF (10 nM) for 1 hour at 37°C, and transmigration was measured as number of cells counted in the bottom chamber. Cells were pretreated with ARC (100 nM and 1 μM) (n = 4, mean ± S.E.M.). (C) CD16 expression was determined in the presence or absence of ARC (100 nM and 1 μM) by flow cytometry (n = 4, mean ± S.E.M.). (D) Platelet-neutrophil aggregate formation induced by fMLP or PAF was evaluated in the presence or absence of ARC (100 nM and 1 μM) (n = 4, mean ± S.E.M.).
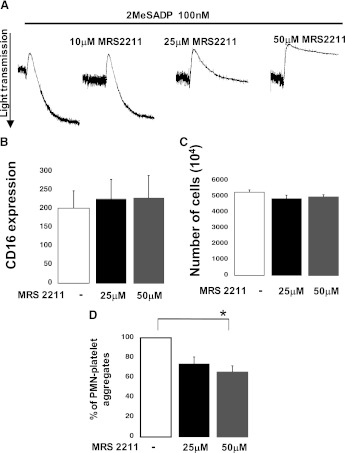
MRS2211, a Reversible P2Y13 Antagonist, Did Not Inhibit Neutrophil Functions.
P2Y13 receptor, another member of the P2Y family that shares a similar pharmacological profile with P2Y12, might be the target of prasugrel metabolites on the neutrophil surface. Therefore, we investigated this possibility by using a reversible P2Y13 antagonist, MRS2211 (Marteau et al., 2003; von Kugelgen, 2006), that we first tested on platelet aggregation. Figure 9A shows that 2MeSADP-induced platelet aggregation was not significantly inhibited when the antagonist was used at a concentration of 10 μM, which has been shown to be effective in other cell types (Marteau et al., 2003). However, aggregation was significantly inhibited when samples were preincubated with higher concentrations of MRS2211 (25 and 50 μM). As platelets are not known to express the P2Y13 receptor, the effects on aggregation at higher concentrations of MRS2211 are probably due to nonspecific effects on P2Y12 receptor. That considered, we investigated the effects of MRS2211 in a variety of neutrophil functional assays upon fMLP stimulation. No changes were observed in CD16 surface expression (Fig. 9B) and cell transmigration (Fig. 9C) in response to MRS2211 at either 25 or 50 μM, implying that the P2Y13 receptor is not likely the target of prasugrel metabolites in neutrophils. On the contrary, platelet-neutrophil aggregates were inhibited (P ≤ 0.05) when cells were preincubated with MRS2211 (Fig. 9D) at the highest concentration tested, probably due to its effect on platelets (shown in Fig. 7A) rather than any direct effect on neutrophils. To exclude this possibility, aggregate formation was also investigated when only neutrophils were pretreated with MRS2211 and then reconstituted with platelets. We noted that the percentage of fMLP-induced aggregates was 60 ± 7 when neutrophils were pretreated with MRS2211 (50 μM), compared with a percentage for untreated samples of 57 ± 5. These results confirmed that MRS2211 has no direct effects on neutrophil interaction with platelets and that the aggregate inhibition observed in Fig. 9D was platelet-mediated.
Fig. 9.
Neutrophil functions were not affected by MRS2211, a reversible P2Y13 antagonist. (A) Human platelets were pretreated at 37°C with MRS2211 (10, 25, and 50 μM) for 2 minutes and then stimulated with 2MeSADP (100 nM). All tracings are representative of at least three experiments from different donors. (B) CD16 surface expression was determined through flow cytometry in the absence or presence of MRS2211 at concentrations of 25 and 50 μM (n = 4, mean ± S.E.M.) following stimulation with fMLP (10 μM). (C) Neutrophil transmigration was evaluated in the absence or presence of the P2Y13 antagonist at different concentrations (n = 4, mean ± S.E.M.) after fMLP-induced (10 μM) activation. (D) Platelet-neutrophil aggregate formation in response to fMLP (10 μM) was evaluated in the presence of MRS2211 (25 and 50 μM) (n = 4, mean ± S.E.M.; *P ≤ 0.05, PRAS compared with negative control).
Prasugrel Treatments Decreased the Level of LPS-Induced Systemic Inflammation.
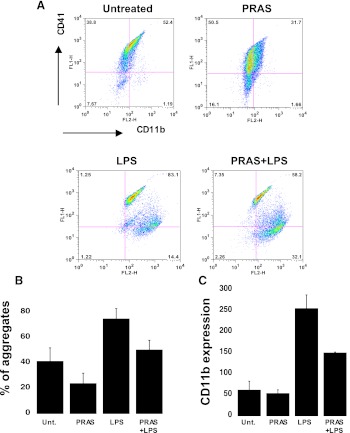
Mice were pretreated with prasugrel and then challenged with an i.p. dose of LPS for 24 hours (Fig. 10). An expected, circulating neutrophils and lymphocytes increased in response to LPS treatment (5 mg/kg), whereas no change was noted in red blood cells, suggesting that prasugrel treatment did not cause any bleeding (Supplemental Fig. 4). Neutrophil-platelet aggregate formation was investigated by flow cytometry. Neutrophils were gated, based on cell shape and CD11b expression. The percentage of aggregates positive for platelet (CD41) and neutrophil (CD11b) markers was analyzed, and aggregate formation was found to be increased in samples from LPS-treated mice as compared with untreated animals (Fig. 10, A and B). Interestingly, when LPS-treated animals were pretreated with prasugrel, the circulating aggregate levels appeared to be less than when they did not receive the drug (Fig. 10, A and B). However, aggregate levels were already decreased by the prasugrel alone, suggesting a protective role for this drug in platelet and neutrophil interaction. We also investigated CD11b expression in neutrophils during LPS-induced inflammation (Fig. 10C) through flow cytometry. The GMFI values indicate that CD11b expression was lower in LPS-challenged mice when pretreated with prasugrel. No change was noted between untreated and prasugrel pre-exposed animals (Fig. 10C).
Fig. 10.
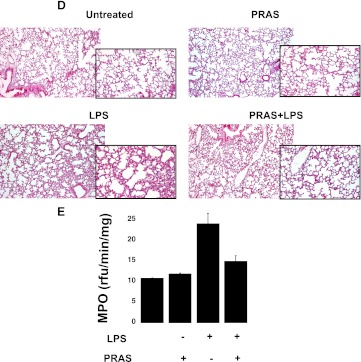
In vivo prasugrel treatment decreased LPS-induced systemic inflammation. (A) Representative dot plot images of percentage of neutrophil-platelet aggregates in blood samples of mice that were untreated, pre-exposed to prasugrel (PRAS), exposed to 24 hours of LPS-induced inflammation (LPS), and both LPS-challenged and prasugrel-treated (PRAS+LPS). Aggregates were identified as positive for both platelets (CD41, FITC-labeled) and neutrophils (CD11b, PE-labeled). (B) Graph showing the percentage of aggregate formation of the data represented in (A) (n = 2, mean ± S.E.M.). (C) CD11b expression indicated as GMFI values detected in gated neutrophils in whole-blood samples (n = 2, mean ± S.E.M.). (D) H&E staining of lung sections obtained from mice. Representative histomicrographs of lung sections from animals as outlined in (A) (magnification, 10× and 20×). (E) MPO analyses were performed in lung samples (n = 2, mean ± S.E.M.).
Considering that neutrophil infiltration in the lungs is an early event during inflammation (Nathan, 2006), we studied lung histopathology (Fig. 10D) and MPO activity (Fig. 10E). Representative images indicate that cell infiltration is diminished in LPS-treated mice when pre-exposed to prasugrel, compared with the LPS-treated animals (Fig. 10D). These data are confirmed by the analysis of MPO activity [fluorescence (rfu)/min/mg], which demonstrated a reduction in animals pretreated with prasugrel prior to administration of LPS (Fig. 10E).
Discussion
The thienopyridines, a class of antiplatelet drugs including clopidogrel and prasugrel, were designed to prevent platelet activation by antagonizing the P2Y12 receptor and thereby inhibiting ADP-induced platelet aggregation (Kim and Kunapuli, 2011). Recent studies show that the P2Y12 receptor is expressed not only in platelets and brain but also in other cell types, such as monocytes (Wang et al., 2004), dendritic cells (Ben Addi et al., 2010), and lymphocytes (Wang et al., 2004). As a result, these drugs could have wider effects on the immune system than expected. Recent studies have shown that these thienopyridines were able to affect inflammatory responses in a number of diseases (Paruchuri et al., 2009; Bhavaraju et al., 2010; Boilard et al., 2010; Garcia et al., 2011; Liu et al., 2011; Winning et al., 2011). However, despite the important role of neutrophils in inflammation (Nathan, 2006), these drugs have not been evaluated for their effect on neutrophil functions. Hence, the aim of this study was to understand whether prasugrel metabolites could influence neutrophil activation.
Prasugrel is orally administrated as a prodrug and requires processing in the liver to an active metabolite (also known as R-138727) that irreversibly inactivates the P2Y12 receptor (Scott et al., 2009). However, this active metabolite is highly unstable and is not available commercially to be used in in vitro experiments. Hence, to study in vitro effects of these metabolites, we developed a system of rat liver microsomes and enzymes to generate a mixture of prasugrel metabolites and used them on ex vivo human platelets and neutrophils. We used rat liver microsomes, because in the literature rat and human microsomes have been shown to have similar functions in metabolizing drugs (Cavin et al., 2001; Brandon et al., 2003), and also because thienopyridines have been successfully administrated in a variety of rat models (Garcia et al., 2011; Hagiwara et al., 2011). Previous studies on human plasma have shown that the metabolism of the prodrug results in vivo in the generation of other metabolites (Brandon et al., 2003; Farid et al., 2007); hence the mixture we prepared may contain other forms rather than the active one alone. After having been generated, this metabolite mixture was tested in a platelet aggregation assay to verify its efficacy. The metabolites produced were able to inhibit platelet aggregation, and the concentration of 5 μM was sufficient to completely abolish aggregation.
When tested on neutrophils, the prasugrel metabolite mixture inhibited several neutrophil functions, including transmigration and CD16 surface expression, indicating that prasugrel metabolites could inhibit neutrophil function directly. The ability of prasugrel metabolites to prevent neutrophil transmigration, which is an important event during the early stages of inflammation (Ley et al., 2007), by directly inhibiting neutrophil activation suggests a new feature for this drug. Previous studies showing that the P2Y12 receptor can be expressed by other cells of the immune system indicate that all the thienopyridine effects were not only platelet-mediated (Diehl et al., 2010); however, no previous publications have shown the direct effects of these drugs on neutrophils.
Our next step was to investigate whether these drugs could affect neutrophil-platelet aggregate formation, considering the importance of the interaction between platelets and neutrophils (Ley et al., 2007). Neutrophil-platelet interaction was inhibited following preincubation with prasugrel metabolites. However, to clarify whether these effects were mediated solely through direct interaction with platelets or whether neutrophils were also a direct target, we preincubated only neutrophils with the metabolite mixture prior to stimulation. Also in this case, the metabolites were able to decrease aggregate formation, suggesting for the first time that these drugs affect neutrophil-platelet interaction by directly inhibiting both cell types. These effects are not agonist-specific, as fMLP-, PAF-, and LTE4-induced activation was inhibited by prasugrel metabolites. It is important to notice that previous studies on human plasma following prasugrel intake have shown that other metabolites may be produced along with R-138727 (Farid et al., 2007). As a result, it is a possibility that one of these metabolites, and not the so far considered active form, is responsible for these effects on neutrophils.
Hence, we sought a possible target receptor on neutrophils that could interact with prasugrel metabolites. First, we evaluated P2Y12 receptor expression in neutrophils using Western blotting, as this receptor is the target of prasugrel in platelets and is known to be expressed in other cell types, such as leukocytes (Diehl et al., 2010) and dendritic cells (Ben Addi et al., 2010). Interestingly, we could not detect any receptor expression, suggesting that the receptor is either not expressed or is expressed below the level of detection. Therefore, we decided to study whether a selective reversible P2Y12 receptor antagonist, AR-C69931MX (Kim and Kunapuli, 2011), could inhibit neutrophil functions, but it did not elicit the same effects on neutrophils as the prasugrel metabolite mixture, indicating that the mechanism by which these metabolites alter neutrophil function is not through the P2Y12 receptor. Secondly, we considered the P2Y13 receptor, which is more ubiquitously expressed than the P2Y12 receptor (Marteau et al., 2003) but is pharmacologically very similar (Marteau et al., 2003; von Kugelgen, 2006; Kim and Kunapuli, 2011). Considering that no reliable antibody for this receptor is commercially available and mRNA studies do not always reflect protein expression, we selected a receptor antagonist previously studied in other cell types. The P2Y13 receptor antagonist was also unable to affect neutrophil activation, excluding this receptor as a possible target of prasugrel metabolites in neutrophils.
In order to understand the molecular mechanism behind prasugrel's effects, we investigated whether these metabolites affect intracellular signaling events in neutrophils. Both ERK phosphorylation and calcium mobilization induced by fMLP or PAF (Coelho et al., 2001; Hauser et al., 2001) were inhibited by prasugrel metabolites. It is possible that prasugrel metabolites antagonize fMLP, PAF, or LTE4 receptors and prevent neutrophil activation or that these metabolites inhibit Gi/o, the G protein responsible for ERK activation and calcium mobilization (Meshki et al., 2006). Alternatively, prasugrel metabolites could activate a different receptor on neutrophils, which elevates intracellular cyclic adenosine monophosphate (cAMP) levels and inhibits neutrophil functions. We believe that this is unlikely as prasugrel metabolites also failed to cause phosphorylation of vasodilator-stimulated phosphoprotein (downstream of cAMP signaling [Eckert and Jones, 2007]) in neutrophils (data not shown). Hence, further investigations are required to evaluate these possibilities.
To support our in vitro findings, we also investigated the effects of prasugrel in a LPS-induced model of systemic inflammation. Previous studies have demonstrated an anti-inflammatory effect of these antiplatelet drugs in different animal models (Hagiwara et al., 2011; Liu et al., 2011). In these studies, we specifically investigated the in vivo effect of prasugrel on neutrophil infiltration and in vivo interaction of neutrophils with platelets. Our data confirm our in vitro findings, indicating a lower cell infiltration and MPO activity in the lungs of prasugrel-treated animals compared with untreated ones. Also, the interaction between platelets and neutrophils was diminished when mice were exposed to the drug, suggesting a protective role for prasugrel during inflammation.
In conclusion, neutrophil activation could be directly inhibited by prasugrel metabolites, indicating that neutrophils are also a target for the metabolites of this antiplatelet drug. Some of the in vivo anti-inflammatory effects of the thienopyridines could be due to off-target effects of these metabolites on neutrophils. However, these metabolites do not act through either the P2Y12 or P2Y13 receptor in neutrophils.
Supplementary Material
Abbreviations
- APC
allophycocyanin
- cAMP, Cyclic adenosine monophosphate; ERK
extracellular-signal–regulated kinase
- FITC
fluorescein isothiocyanate
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- GMFI
geometric mean of fluorescence intensity
- HBSS
Hanks’ balanced salt solution
- LPS
lipopolysaccharide
- LTE4
leukotriene E4
- MPO
myeloperoxidase
- PAF
platelet-activating factor
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- PRP
platelet-rich plasma
Authorship Contributions
Participated in research design: Liverani, Kilpatrick, Kunapuli.
Conducted experiments: Liverani, Rico, Garcia.
Performed data analysis: Liverani, Kunapuli.
Wrote or contributed to the writing of the manuscript: Liverani, Kilpatrick, Kunapuli.
Footnotes
This research was supported by the National Institutes of Health [Grants K01-HL092586 (to A.E.G.), T32-HL07777 and K01-HL103197 (to M.C.R.), and HL60683 and HL93231 (to S.P.K.)].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Ben Addi A, Cammarata D, Conley PB, Boeynaems JM, Robaye B. (2010) Role of the P2Y12 receptor in the modulation of murine dendritic cell function by ADP. J Immunol 185:5900–5906 [DOI] [PubMed] [Google Scholar]
- Bhavaraju K, Mayanglambam A, Rao AK, Kunapuli SP. (2010) P2Y(12) antagonists as antiplatelet agents—recent developments. Curr Opin Drug Discov Devel 13:497–506 [PubMed] [Google Scholar]
- Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O'Donnell E, Farndale RW, Ware J, et al. (2010) Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327:580–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon EF, Raap CD, Meijerman I, Beijnen JH, Schellens JH. (2003) An update on in vitro test methods in human hepatic drug biotransformation research: pros and cons. Toxicol Appl Pharmacol 189:233–246 [DOI] [PubMed] [Google Scholar]
- Bunyavanich S, Boyce JA, Raby BA, Weiss ST. (2012) Gene-by-environment effect of house dust mite on purinergic receptor P2Y12 (P2RY12) and lung function in children with asthma. Clin Exp Allergy 42:229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bura A, Bachelot-Loza C, Ali FD, Aiach M, Gaussem P. (2006) Role of the P2Y12 gene polymorphism in platelet responsiveness to clopidogrel in healthy subjects. J Thromb Haemost 4:2096–2097 [DOI] [PubMed] [Google Scholar]
- Cavin C, Mace K, Offord EA, Schilter B. (2001) Protective effects of coffee diterpenes against aflatoxin B1-induced genotoxicity: mechanisms in rat and human cells. Food Chem Toxicol 39:549–556 [DOI] [PubMed] [Google Scholar]
- Coelho AL, De Freitas MS, Mariano-Oliveira A, Oliveira-Carvalho AL, Zingali RB, Barja-Fidalgo C. (2001) Interaction of disintegrins with human neutrophils induces cytoskeleton reorganization, focal adhesion kinase activation, and extracellular-regulated kinase-2 nuclear translocation, interfering with the chemotactic function. FASEB J 15:1643–1645 [DOI] [PubMed] [Google Scholar]
- Cooper D, Ilarregui JM, Pesoa SA, Croci DO, Perretti M, Rabinovich GA. (2010) Multiple functional targets of the immunoregulatory activity of galectin-1: control of immune cell trafficking, dendritic cell physiology, and T-cell fate. Methods Enzymol 480:199–244 [DOI] [PubMed] [Google Scholar]
- Dangelmaier C, Jin J, Smith JB, Kunapuli SP. (2001) Potentiation of thromboxane A2-induced platelet secretion by Gi signaling through the phosphoinositide-3 kinase pathway. Thromb Haemost 85:341–348 [PubMed] [Google Scholar]
- Diehl P, Olivier C, Halscheid C, Helbing T, Bode C, Moser M. (2010) Clopidogrel affects leukocyte dependent platelet aggregation by P2Y12 expressing leukocytes. Basic Res Cardiol 105:379–387 [DOI] [PubMed] [Google Scholar]
- Eckert RE, Jones SL. (2007) Regulation of VASP serine 157 phosphorylation in human neutrophils after stimulation by a chemoattractant. J Leukoc Biol 82:1311–1321 [DOI] [PubMed] [Google Scholar]
- Farid NA, Smith RL, Gillespie TA, Rash TJ, Blair PE, Kurihara A, Goldberg MJ. (2007) The disposition of prasugrel, a novel thienopyridine, in humans. Drug Metab Dispos 35:1096–1104 [DOI] [PubMed] [Google Scholar]
- Frevert CW, Wong VA, Goodman RB, Goodwin R, Martin TR. (1998) Rapid fluorescence-based measurement of neutrophil migration in vitro. J Immunol Methods 213:41–52 [DOI] [PubMed] [Google Scholar]
- Garcia A, Kim S, Bhavaraju K, Schoenwaelder SM, Kunapuli SP. (2010) Role of phosphoinositide 3-kinase beta in platelet aggregation and thromboxane A2 generation mediated by Gi signalling pathways. Biochem J 429:369–377 [DOI] [PubMed] [Google Scholar]
- Garcia AE, Mada SR, Rico MC, Dela Cadena RA, Kunapuli SP. (2011) Clopidogrel, a P2Y12 receptor antagonist, potentiates the inflammatory response in a rat model of peptidoglycan polysaccharide-induced arthritis. PLoS ONE 6:e26035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450 [PubMed] [Google Scholar]
- Hagiwara S, Iwasaka H, Hasegawa A, Oyama M, Imatomi R, Uchida T, Noguchi T. (2011) Adenosine diphosphate receptor antagonist clopidogrel sulfate attenuates LPS-induced systemic inflammation in a rat model. Shock 35:289–292 [DOI] [PubMed] [Google Scholar]
- Hauser CJ, Fekete Z, Adams JM, Garced M, Livingston DH, Deitch EA. (2001) PAF-mediated Ca2+ influx in human neutrophils occurs via store-operated mechanisms. J Leukoc Biol 69:63–68 [PubMed] [Google Scholar]
- Kahner BN, Shankar H, Murugappan S, Prasad GL, Kunapuli SP. (2006) Nucleotide receptor signaling in platelets. J Thromb Haemost 4:2317–2326 [DOI] [PubMed] [Google Scholar]
- Kim S, Kunapuli SP. (2011) P2Y12 receptor in platelet activation. Platelets 22:56–60 [DOI] [PubMed] [Google Scholar]
- Köhler D, Straub A, Weissmüller T, Faigle M, Bender S, Lehmann R, Wendel HP, Kurz J, Walter U, Zacharowski K, et al. (2011) Phosphorylation of vasodilator-stimulated phosphoprotein prevents platelet-neutrophil complex formation and dampens myocardial ischemia-reperfusion injury. Circulation 123:2579–2590 [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7:678–689 [DOI] [PubMed] [Google Scholar]
- Liu Y, Gao XM, Fang L, Jennings NL, Su Y, Q X, Samson AL, Kiriazis H, Wang XF, Shan L, Sturgeon SA, Medcalf RL, Jackson SP, Dart AM, Du XJ. (2011) Novel role of platelets in mediating inflammatory responses and ventricular rupture or remodeling following myocardial infarction. Arterioscler Thromb Vasc Biol 31:834–841 [DOI] [PubMed] [Google Scholar]
- Marteau F, Le Poul E, Communi D, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS. (2003) Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol 64:104–112 [DOI] [PubMed] [Google Scholar]
- Meshki J, Tuluc F, Bredetean O, Garcia A, Kunapuli SP. (2006) Signaling pathways downstream of P2 receptors in human neutrophils. Purinergic Signal 2:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel O, El Ghannudi S, Hess S, Reydel A, Crimizade U, Jesel L, Radulescu B, Wiesel ML, Gachet C, Ohlmann P. (2012) The extent of P2Y12 inhibition by clopidogrel in diabetes mellitus patients with acute coronary syndrome is not related to glycaemic control: roles of white blood cell count and body weight. Thromb Haemost 108:338–348 [DOI] [PubMed] [Google Scholar]
- Murugappa S, Kunapuli SP. (2006) The role of ADP receptors in platelet function. Front Biosci 11:1977–1986 [DOI] [PubMed] [Google Scholar]
- Nagy B, Jr, Jin J, Ashby B, Reilly MP, Kunapuli SP. (2011) Contribution of the P2Y12 receptor-mediated pathway to platelet hyperreactivity in hypercholesterolemia. J Thromb Haemost 9:810–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol 6:173–182 [DOI] [PubMed] [Google Scholar]
- Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA. (2009) Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med 206:2543–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinton TM, Kim S, Jin J, Kunapuli SP. (2005) Lipid rafts are required in Galpha(i) signaling downstream of the P2Y12 receptor during ADP-mediated platelet activation. J Thromb Haemost 3:1036–1041 [DOI] [PubMed] [Google Scholar]
- Rinder HM, Bonan JL, Rinder CS, Ault KA, Smith BR. (1991) Activated and unactivated platelet adhesion to monocytes and neutrophils. Blood 78:1760–1769 [PubMed] [Google Scholar]
- Scott DM, Norwood RM, Parra D. (2009) P2Y12 inhibitors in cardiovascular disease: focus on prasugrel. Ann Pharmacother 43:64–76 [DOI] [PubMed] [Google Scholar]
- Semple JW, Freedman J. (2010) Platelets and innate immunity. Cell Mol Life Sci 67:499–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple JW, Italiano JE, Jr, Freedman J. (2011) Platelets and the immune continuum. Nat Rev Immunol 11:264–274 [DOI] [PubMed] [Google Scholar]
- von Kügelgen I. (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110:415–432 [DOI] [PubMed] [Google Scholar]
- Wagner JG, Roth RA. (1999) Neutrophil migration during endotoxemia. J Leukoc Biol 66:10–24 [DOI] [PubMed] [Google Scholar]
- Wang L, Jacobsen SE, Bengtsson A, Erlinge D. (2004) P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DJ, Evanson OA, Fagliari JJ, Valberg S. (1998) Evaluation of platelet activation and platelet-neutrophil aggregates in Thoroughbreds undergoing near-maximal treadmill exercise. Am J Vet Res 59:393–396 [PubMed] [Google Scholar]
- Winning J, Claus RA, Pletz MW, Bauer M, Lösche W. (2011) Adenosine diphosphate receptor antagonist clopidogrel sulfate attenuates LPS-induced systemic inflammation in a rat model. Shock 36:317–, author reply 317–318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.