Abstract
Control of brain seizures after exposure to nerve agents is imperative for the prevention of brain damage and death. Animal models of nerve agent exposure make use of pretreatments, or medication administered within 1 minute after exposure, in order to prevent rapid death from peripheral toxic effects and respiratory failure, which then allows the testing of anticonvulsant compounds. However, in a real-case scenario of an unexpected attack with nerve agents, pretreatment would not be possible, and medical assistance may not be available immediately. To determine if control of seizures and survival are still possible without pretreatment or immediate pharmacologic intervention, we studied the anticonvulsant efficacy of the GluK1 (GluR5)/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahydroisoquinoline-3-carboxylic acid (LY293558) in rats that did not receive any treatment until 20 minutes after exposure to the nerve agent soman. We injected LY293558 intramuscularly, as this would be the most likely route of administration to humans. LY293558 (15 mg/kg), injected along with atropine and the oxime HI-6 at 20 minutes after soman exposure, stopped seizures and increased survival rate from 64% to 100%. LY293558 also prevented neuronal loss in the amygdala and hippocampus, and reduced neurodegeneration in a number of brain regions studied 7 days after soman exposure. Analysis of the LY293558 pharmacokinetics after intramuscular administration showed that this compound readily crosses the blood–brain barrier. There was good correspondence between the time course of seizure suppression by LY293558 and the brain levels of the compound.
Introduction
Nerve agents are lethal toxicants that are easy to transport and deploy, and for this reason they could be used in a terrorist attack with devastating consequences. As the primary action of nerve agents is the inhibition of acetylcholinesterase (AChE), the main causes of acute death after nerve agent exposure are cardiorespiratory depression and intense seizure activity [status epilepticus (SE)], which are due to elevated acetylcholine levels in the peripheral and central nervous system (for reviews see Bajgar, 2005; Collombet, 2011). If death is prevented but SE is not controlled in a timely manner, the ensuing brain damage from the intense seizures can produce long-term cognitive and other behavioral deficits (McDonough et al., 1986; Brown and Brix, 1998; Myhrer et al., 2005; Grauer et al., 2008). Research aimed at developing effective medical countermeasures to terminate seizure activity and prevent brain damage by nerve agent exposure has traditionally used models in which the animal is pretreated with an oxime or pyridostigmine. Usually, pretreatment is administered 30 minutes before nerve agent exposure, followed by atropine administration within 1 minute after exposure (e.g., Shih et al., 2003; Capacio et al., 2004; Joosen et al., 2009; Figueiredo et al., 2011). The reactivation of inhibited acetylcholinesterase by the oxime and the blockade of muscarinic receptors by atropine prevent acute death from cardiorespiratory depression and allow the subsequent testing of anticonvulsant compounds. What has to be considered, however, is that pretreatment is not possible in an unexpected emergency situation and, particularly, in an attack against civilians; furthermore, medical assistance is unlikely to be available within 1 minute after exposure.
In a previous study, we demonstrated that intraperitoneal injection of LY293558, a GluK1/AMPA receptor antagonist (Bleakman et al., 1996; note that the GluK1 subunit was formerly known as GluR5, see Collingridge et al., 2009 or Jane et al., 2009), terminated soman-induced seizures, produced 100% survival, and reduced brain damage in rats pretreated with the oxime HI-6 and injected with atropine 1 minute after exposure (Figueiredo et al., 2011). These promising results, along with already existing evidence from clinical studies on pain showing that LY293558 is well tolerated (Gilron et al., 2000; Sang et al., 2004; Tomillero and Moral, 2008), prompted us to continue our investigations on the antidotal efficacy of LY293558 against nerve agent exposure, and to build on the information required for approval of its use in humans in an emergency case. Using a drug administration protocol that is closer to a potential real-case scenario in humans, in the present study, we examined if LY293558 can still suppress soman-induced seizures and protect the brain from neuronal damage when administered to rats that have not received any pretreatment, and with atropine administration delayed to 20 minutes after exposure. We administered LY293558 via intramuscular injection, because this would be the most likely route of administration to humans in an emergency due to nerve agent exposure. We also determined the time course of elevation of LY293558 in the plasma and the brain after intramuscular injection to verify systemic absorption and determine whether exposure to LY293558 correlates with the time course of its anticonvulsant effects.
Materials and Methods
Animals.
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), weighing from 150 to 250 g at the start of the experiments, were individually housed in an environmentally controlled room (20–23°C, 12-hour light/dark cycle, lights on 06:00 AM), with food and water available ad libitum. The animal care and use programs at the US Army Medical Research Institute of Chemical Defense, the Uniformed Services University of the Health Sciences, and SRI International are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All animal experiments were conducted following the Guide for the Care and Use of Laboratory Animals by the Institute of Laboratory Animal Resources, National Research Council, and were in accordance with the guidelines of our institutions, after obtaining approval of the Institutional Animal Care and Use Committees (IACUC).
Soman Administration and Drug Treatment.
Soman (pinacoyl methylphosphonofluoridate) was obtained from Edgewood Chemical Biologic Center (Aberdeen Proving Ground, MD); the agent was diluted in cold saline and administered via a single subcutaneous injection (132 µg/kg, 1.2 × LD50). Twenty minutes after soman exposure, all rats received an i.m. injection of 2 mg/kg atropine sulfate (Sigma, St. Louis, MO), a muscarinic receptor antagonist, as well as an i.p. injection of 125 mg/kg HI-6 (Starks Associates, Buffalo, NY), a bispyridinium oxime that reactivates inhibited AChE primarily in the periphery (Joosen et al., 2011; Mercey et al., 2012) to control the peripheral effects of soman and prevent death from respiratory suppression. Rats that were treated only with atropine and HI-6 comprised the SOMAN group. Other rats were additionally treated with the GluK1/AMPA receptor antagonist LY293558 (kindly provided by Raptor Pharmaceutical Corp., Novato, CA), administered i.m. immediately after the atropine+HI-6 injection. In a previous study, injection of 50 mg/kg LY293558 suppressed seizures and produced a 100% survival rate in soman-exposed rats that were pretreated with HI-6 at 30 minutes prior to soman exposure and received atropine within 1 minute after exposure (Figueiredo et al., 2011). However, in soman-exposed rats that do not receive any pretreatment and receive delayed atropine administration, we have found that the same dose of LY293558 (i.e., 50 mg/kg) stops the seizures, but the animals become deeply sedated and die from respiratory depression within the next 4 hours. Therefore, in the present study, we administered only 15 mg/kg LY293558. A control group received saline instead of soman and was injected with atropine+HI-6.
Seizures were monitored behaviorally, and were classified according to the Racine scale (Racine, 1972) with minor modifications: Stage 0, no behavioral response; Stage 1, behavioral arrest; Stage 2, oral/facial movements, chewing, head nodding; Stage 3, unilateral/bilateral forelimb clonus without rearing, Straub tail, extended body posture; Stage 4, bilateral forelimb clonus plus rearing; Stage 5, rearing and falling; and Stage 6, full tonic seizures. A group of animals was implanted with electrodes for electroencephalographic (EEG) monitoring. Exposures to soman started 2 weeks after electrode implantation.
Electrode Implantation for EEG Recordings.
Rats were anesthetized with isoflurane using a gas anesthesia system (Kent Scientific, Torrington, CT). Five stainless steel, cortical screw electrodes were stereotaxically implanted using the following coordinates (after Paxinos and Watson, 2005): two frontal electrodes, 2.0 mm posterior from bregma and 2.5 mm lateral from the midline; two parietal electrodes, 5.0 mm posterior from bregma and 2.5 mm lateral from the midline; a cerebellar reference electrode implanted 1.0 mm posterior to lambda (see Fig. 2C). Each screw electrode (Plastics One Inc., Roanoke, VA) was placed in a plastic pedestal (Plastics One Inc., Roanoke, VA) and fixed to the skull with dental acrylic cement.
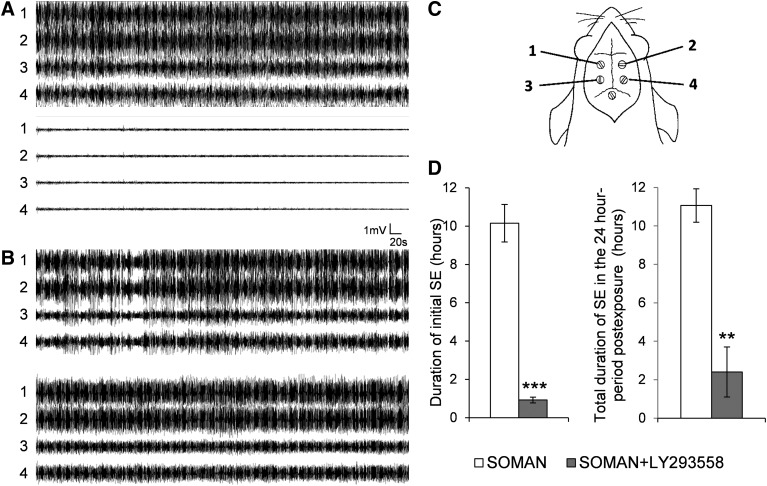
Fig. 2.
Administration of LY293558 (i.m.) stops soman-induced generalized seizures and reduces the total duration of status epilepticus (SE) in the 24-hour period after soman exposure. (A) Administration of 15 mg/kg LY293558 at 20 minutes after soman exposure completely blocked electrographic seizure activity within 40 minutes (bottom set of traces). (B) An example of seizures persisting in rats that did not receive LY293558. The numbers 1, 2, 3, and 4, in (A) and (B), refer to the electrodes/sites from where electrical activity was sampled, as shown diagrammatically in (C) (1, left frontal; 2, right frontal; 3, left parietal; 4, right parietal; 5, cerebellar reference electrode). (D) Left bar graph shows the duration of the initial SE (the SE that started within 10 minutes after soman exposure and was terminated by 15 mg/kg LY293558 in the SOMAN+LY293558 group, or spontaneously in the SOMAN group). Right bar graph shows the duration of SE throughout the 24-hour period after soman exposure. Sample size n = 5 for the SOMAN group, and n = 8 for the SOMAN+LY293558 group. **P < 0.01, ***P < 0.001 (unequal variance t test).
EEG Recordings and Analysis.
The rats with implanted electrodes for EEG recordings were placed in the EEG chamber and connected to the EEG system (Stellate, Montreal, Canada; 200-Hz sampling rate). Video-EEG recordings were performed in the freely moving rats. Recordings were visually analyzed offline with the filters set to 0.3 Hz for the low frequency filter, 60 Hz for the notch filter, and 70 Hz for the high-frequency filter, using the Harmonie Viewer 6.1e from Stellate (Montreal). EEG was recorded for 24 hours after soman injection. The termination of the soman-induced SE was defined as the disappearance of large amplitude, repetitive discharges (>1 Hz with at least double the amplitude of the background activity). If SE recurred at any time during the remainder of the 24-hour recording time, the duration of this seizure activity was included in the total duration of SE.
Fixation and Tissue Processing.
Seven days after soman exposure, rats were deeply anesthetized with pentobarbital (75–100 mg/kg, i.p.) and transcardially perfused with phosphate buffered saline (100 ml) followed by 4% paraformaldehyde (200 ml). The brains were removed and postfixed overnight at 4°C, then transferred to a solution of 30% sucrose in phosphate buffered saline for 72 hours, and frozen with dry ice before storage at −80°C until sectioning. A 1-in-5 series of sections from the rostral extent of the amygdala to the caudal extent of the entorhinal cortex was cut at 40 µm on a sliding microtome. One series of sections was mounted on slides (Superfrost Plus, Daigger, Vernon Hills, IL) for Nissl staining with cresyl violet. The adjacent series of sections were also mounted on slides for Fluoro-Jade C (FJC) staining. Analysis of neuronal loss from Nissl-stained sections was performed in the basolateral nucleus of the amygdala (BLA) and the CA1 hippocampal area along the rostrocaudal extent of the hippocampus. Analysis of neuronal degeneration from FJC-stained sections was performed in all the amygdalar nuclei, a neocortical area between coordinates −1.72 and −6.60 from bregma, extending from the retrosplenial cortex to the ectorhinal cortex in the medial to lateral direction (based on the atlas of Paxinos and Watson, 2005), the piriform cortex, the entorhinal cortex, and the CA1, CA3 and hilar areas of the ventral hippocampus; we studied the ventral hippocampus because it displays significantly more severe neurodegeneration after soman exposure than the dorsal hippocampus (Apland et al., 2010). All neuropathologic analysis was done in a blind fashion.
Stereological Quantification.
Design-based stereology was performed as described previously (Figueiredo et al., 2011). Briefly, BLA and CA1 regions were identified on slide-mounted sections and delineated for each slide of each animal, under a 2.5× objective, based on the atlas of Paxinos and Watson (2005). All sampling was done under a 63× oil immersion objective. Nissl-stained neurons were distinguished from glial cells by their larger size and pale nuclei surrounded by darkly stained cytoplasm containing Nissl bodies. The total number of Nissl-stained neurons was estimated using the optical fractionator probe, and, along with the coefficient of error (CE), were calculated using Stereo Investigator 9.0 (MicroBrightField, Williston, VT). The CE was calculated by the software according to Gunderson (m = 1; Gundersen et al., 1999) and Schmitz-Hof (second estimation; Schmitz and Hof, 2000) equations.
For Nissl-stained neurons in the BLA, a 1-in-5 series of sections was analyzed (7 sections on average for each rat). The counting frame was 35 × 35 µm, the counting grid was 190 × 190 µm, and the dissector height was 12 µm. Nuclei were counted when the cell body came into focus within the dissector, which was placed 2 µm below the section surface. Section thickness was measured at every counting site, and the average mounted section thickness was 20 µm. An average of 345 neurons per rat was counted, and the average CE was 0.05 for both the Gunderson and Schmitz-Hof equations. For Nissl-stained neurons in the CA1 area, a 1-in-10 series of sections was analyzed (8 sections on average for each rat). The counting frame was 20 × 20 µm, the counting grid was 250 × 250 µm, and the dissector height was 10 µm. Nuclei were counted when the cell body came into focus within the dissector, which was placed 2 µm below the section surface. Section thickness was measured at every counting site, and the average mounted section thickness was 17 µm. An average of 243 neurons per rat was counted, and the CE was 0.07 for Gunderson (m = 1) and 0.065 for Schmitz-Hof (2nd estimation) equation.
Fluoro-Jade C Staining.
FJC (Histo-Chem, Jefferson, AR) was used to identify irreversibly degenerating neurons. Mounted sections were treated as described in detail previously (Figueiredo et al., 2011), and then stained in a 0.0001% solution of FJC dissolved in 0.1% acetic acid. To determine the extent of neuronal degeneration, we used a series of adjacent Nissl-stained sections and traced the BLA, piriform cortex, entorhinal cortex, and the dorsal hippocampal subfields CA1, CA3, and hilus, as well as neocortex. The tracings from the Nissl-stained sections were superimposed on the FJC-stained sections, using the Stereo Investigator 9.0 (MicroBrightField, Williston, VT). The following rating system was used to score the extent of neuronal degeneration in each structure and substructures: 0 = no damage; 1 = minimal damage (1%–10%); 2 = mild damage (11%–25%); 3 = moderate damage (26%–45%); and 4 = severe damage (>45%). We have previously shown that qualitative assessment using this scale produces results that are in agreement with quantitative assessments (Qashu et al., 2010).
Statistical Analysis.
The group mean of the behavioral seizure scores (as seen in Fig. 1) was calculated using the highest behavioral seizure stage score observed within each 10-minute interval for each rat. The differences between treatment groups in seizure scores were tested for statistical significance, using analysis of variance (ANOVA). SE duration was compared between the SOMAN and the SOMAN+LY293558 group, using the unequal variance Student’s t test. ANOVA was used to compare the results from the stereologic estimations of the total number of neurons. The statistical values from these tests are presented as the mean ± S.E.M. Neurodegeneration scores were compared between groups for each structure separately using the Mann-Whitney U test; the statistical values are presented as median and the interquartile range (IQR, the difference between the 75th and the 25th percentiles). Differences were considered significant when P < 0.05. Sample sizes (n) refer to the number of animals.
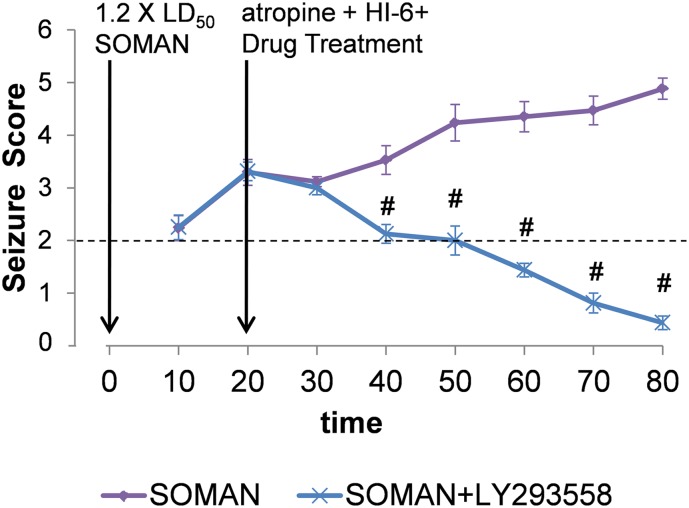
Fig. 1.
Time-course of behavioral seizure suppression by LY293558 (15 mg/kg) administered intramuscularly at 20 minutes after soman injection, along with atropine and HI-6. Data points are the group means ± S.E.M. (SOMAN, n = 18; SOMAN+LY293558, n = 18) of the maximum Racine scale scores, in blocks of 10 minutes. Vertical arrows indicate the time points of drug administration. #P < 0.05 in comparison with the SOMAN group (ANOVA, Bonferroni post-hoc test).
Pharmacokinetic Studies.
The plasma and brain concentrations of LY293558 were measured at the Biosciences Division of SRI International (Menlo Park, CA). Male Sprague-Dawley rats (2-month-old) were intramuscularly injected with 15 mg/kg of LY293558 in sterile water. The animals were observed for changes in behavioral activity. Blood (∼300 μl) was drawn at different time points after the injection (0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 24, and 48 hours) via the retro orbital sinus, under 60:40% CO2:O2 anesthesia. After collection of the last blood sample from a given rat, the brain was collected following euthanasia with an overdose of sodium pentobarbital. Brain sample-times were 0.25, 0.5, 1, 1.5, 8, and 48 hours after injection of LY293558. Control blood samples and brains were also collected from 3 untreated rats for baseline measurements. Blood samples (in tubes containing K3EDTA) were placed on ice after collection and processed to plasma within 30 minutes of collection by centrifugation at 2–8°C. Plasma samples were transferred to cryovials and stored frozen at −80°C (± 10°C) until analysis. Brain weight was measured for each animal prior to storage on dry ice. Brains were stored at −80°C (±10°C) until analysis. Drug levels were determined in collected plasma and brain samples using a bioanalytical method developed at SRI, as described later.
Measurements of LY293558 in Plasma and Brain.
Rat plasma and brain homogenates were mixed with acetonitrile to precipitate proteins, and were then clarified by centrifugation. Supernates were concentrated by centrifugal evaporation, and then reconstituted in 0.1% formic acid in water with 100 ng/ml methyl nicotinate (internal standard). Study samples were quantitated using a set of calibration standards prepared in blank matrix that were processed in parallel. For both matrices, quality control samples, at three concentrations, were run with study samples. Samples were analyzed by LC-MS/MS (Waters 2795 Alliance Integrated System HPLC and Micromass Quattro Ultima, Milford, MA) using a Luna C8(2) column (50 × 2.0 mm, 3 µm; Phenomex, Torrance, CA), eluted with a gradient of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile. The ionization was electrospray in the positive ion mode, and detection was by Multiple Reaction Monitoring with MRM transition of 280 to 137.8 and retention time of 3.9 minutes. The lower limit of quantitation (LLOQ) values of the assay were established at 5 ng/ml for plasma and 24.7 ng/g for brain.
Pharmacokinetic Data Analysis.
Means and standard deviations were calculated for the plasma and brain LY293558 concentrations at each time point. Pharmacokinetic analysis was performed using noncompartmental methods and the sparse modeling feature (WinNonlin Professional, Version 5.2, PharsightCorp, St. Louis, MO). Pharmacokinetic parameters measured included Cmax, time to reach maximal concentration, area under the plasma concentration-time curve to the last time-point, terminal elimination half-life. The ratios of brain to plasma concentration were also determined at each time point.
Results
Seizure Termination by LY293558.
The initiation of seizure activity became behaviorally evident within 10 minutes after soman injection, and behavioral seizure score of stage 3 or higher (behavioral SE) was reached within 15 minutes. From 60 rats that were exposed to soman, 9 rats did not develop behavioral SE and were not included in the study. While the SOMAN group received only atropine and HI-6 at 20 minutes after exposure, the SOMAN+LY293558 group also received an i.m. injection of 15 mg/kg LY293558, at the same time-point. Behavioral seizures were suppressed by LY293558 within 30 to 40 minutes after the injection (Fig. 1), while in the SOMAN-only rats that survived, SE stopped spontaneously within 7 to 10 hours. Survival rate in the SOMAN group was 64% (10 out of 28 rats died during SE), while in the SOMAN+LY293558 group survival rate was 100% (Table 1).
TABLE 1.
Efficacy of LY293558 (15 mg/kg) against soman-induced seizures and lethality, when administered along with atropine and HI-6, at 20 minutes after soman exposure
| SOMAN+ Atropine+ HI-6 | SOMAN+ Atropine+ HI-6+ LY293558 | |
|---|---|---|
| Latency to SE (min)a | 9.5 ± 2 | 9.85 ± 0.7 |
| Mortality rateb | 10/28 (35.7) | 0/18 (0) |
| Seizure controlc | 0/28 | 18/18 |
| Latency to stop SE (min)d | N/A | 36.9 ± 3.2 |
Data are mean ± S.E.M. and indicate the time elapsed between injection of soman and initiation of generalized seizures.
Data are number (%) of animals that died out of the total sample size.
Data are number of animals in which seizures were controlled by the administration of atropine+HI-6 or atropine+HI-6+LY293558 out of the total sample size.
Data are mean ± S.E.M. and indicate the time elapsed between injection of LY293558 and the suppression of behavioral seizures below stage 2.
In rats implanted with EEG electrodes, the duration of the electrographic SE (repetitive discharges appearing at a frequency of >1 Hz and with an amplitude at least double that of the background activity) was compared between a group of rats that received soman + atropine+HI-6 and a group that also received LY293558. The initial SE (the SE that was induced by soman and was terminated by LY293558, or terminated spontaneously in the SOMAN group) lasted for 10.15 ± 0.78 hours in the SOMAN group (n = 5) and 0.98 ± 0.15 hour in the SOMAN + LY293558 group (n = 8, Fig. 2). However, in some animals, SE returned at varying time points after cessation of the initial SE. Therefore, we continued EEG monitoring for 24 hours after soman exposure. SE did not reappear in the SOMAN-only rats. In 4 of the 8 rats that received LY293558, SE reoccurred between 12 and 22 hours after cessation of the initial SE and lasted about 3 hours. The total duration of SE within the 24-hour period after soman exposure was significantly shorter in LY293558-treated rats (2.4 ± 1.2 hours) compared with the rats that did not receive LY293558 (11.06 ± 0.87 hours, Fig. 2D, right bar graph).
Some qualitative observations of the EEG activity that are worth noting are as follows: 1) the appearance of repetitive high-frequency, large-amplitude EEG activity correlated well with stage 3 behavioral seizures; 2) during prolonged SE, as in the SOMAN group, it is the frequency of the repetitive discharges rather than their amplitude that subsides as SE approaches its point of cessation; and 3) when SE reoccurs, it is a “full-blown” SE that also subsides mainly in terms of frequency of the repetitive discharges, as it approaches the time point of termination.
Neuronal Loss and Degeneration, 7 Days after Soman Administration.
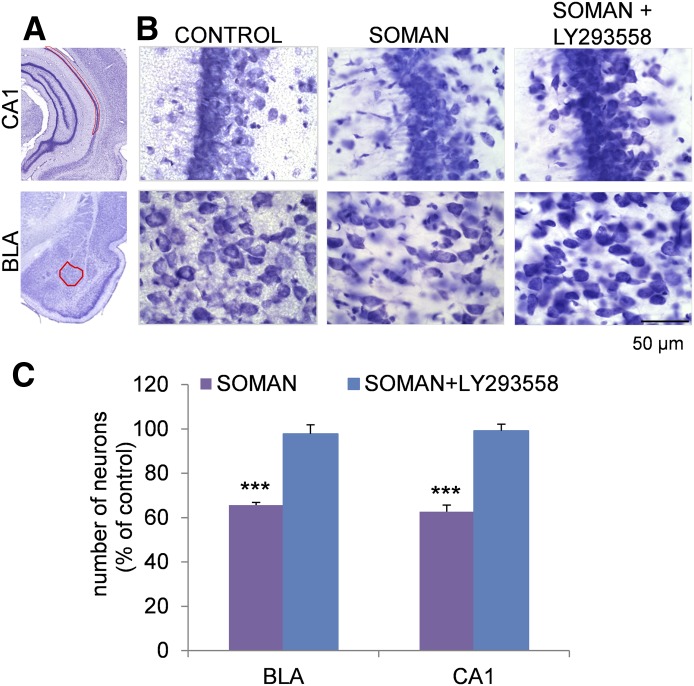
The neuroprotective efficacy of LY293558 was examined 7 days after exposure to soman. Estimation of the total number of neurons in the BLA and the CA1 hippocampal subfield, using an unbiased stereological method in Nissl-stained sections, showed that animals treated with LY293558 (n = 18) had a significantly higher total number of neurons in the BLA (113,433 ± 3,829) and CA1 area (552,260 ± 14,400) compared with the number of neurons in the SOMAN group (BLA, 76,184 ± 3,171; CA1, 348,986 ± 16,426; n = 18) (Fig. 3). The number of neurons in the BLA and CA1 area of the group that received LY293558 did not differ significantly from the number of neurons in the control group (BLA, 116,044 ± 3,112; CA1, 556,603 ± 14,559; n = 7). In contrast, the SOMAN group had a 35% neuronal loss in the BLA, and 38% neuronal loss in the CA1 area compared with the control animals (Fig. 3).
Fig. 3.
Administration of LY293558 (i.m.) protects against neuronal loss in the basolateral amygdala (BLA) and the CA1 hippocampal area, 7 days after soman-induced SE. (A) Panoramic photomicrographs of Nissl-stained sections of half hemispheres, outlining the amygdalar nucleus and the ventral hippocampal subfield where stereologic analysis was performed (red highlight). (B) Representative photomicrographs of Nissl-stained sections showing BLA and CA1 cells from a control rat that received saline in place of soman, a soman-exposed rat that did not receive anticonvulsant treatment, and a soman-exposed rat that received 15 mg/kg LY293558 at 20 minutes after soman exposure. Total magnification is 630× and scale bar is 50 µm. (C) Group data (mean ± S.E.M.; n = 18 for the SOMAN group and n = 18 for the SOMAN+LY293558 group) of stereologic estimation of the total number of Nissl-stained neurons in the BLA (left) and CA1 area (right), expressed as percent of the control. There was significant neuronal loss in the soman-exposed rats that did not receive anticovulsant treatment, whereas there was no neuronal loss in the soman-exposed rats that were treated with LY293558. *P < 0.05 in relation to the control group or the SOMAN+LY293558 group (ANOVA, LSD post hoc test).
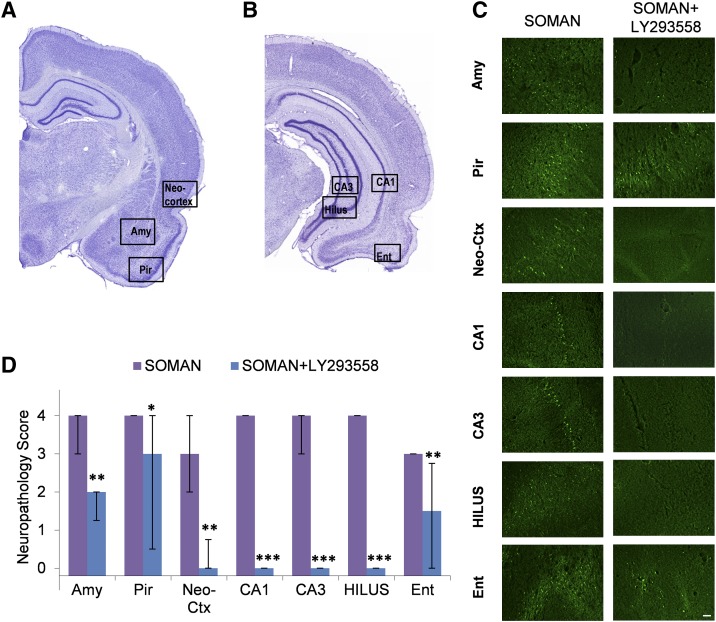
Neuronal degeneration (based on FJC staining) was significantly less extensive in the SOMAN+ LY293558 group compared with the SOMAN group, in all brain regions studied (Fig. 4). The neurodegeneration score for the amygdala was severe in the SOMAN group (median = 4, IQR = 3.00∼4.00) and mild in the SOMAN+LY293558 group (median = 2, IQR = 1.25∼2.00). Neuronal degeneration in the piriform cortex was severe in the SOMAN group (median = 4, IQR = 4.00∼4.00) and moderate in the SOMAN+LY293558 group (median = 3, IQR = 1.50∼4.00). In the neocortical sample from the temporal cortex, neuronal degeneration was moderate in the SOMAN group (median = 3, IQR = 2.00∼4.00) but virtually absent in the SOMAN+LY293558 group (median = 0, IQR = 0.00∼1.75). In the CA1, CA3, and hilar regions of the ventral hippocampus, neurodegeneration was severe in the SOMAN group (CA1, median = 4, IQR = 4∼4; CA3, median = 4, IQR=3∼4; hilus, median = 4, IQR=4∼4) and absent in the SOMAN+LY293559 group. In the entorhinal cortex, the neurodegeneration score was again significantly lower in the SOMAN+LY293558 group (median = 1.5, IQR = 0∼2.75) than in the SOMAN group (median = 3, IQR = 3∼3). The control group did not show any FJC-positive staining.
Fig. 4.
Administration of LY293558 (i.m.) protects against neuronal degeneration, 7 days after soman-induced SE. (A and B) Panoramic photomicrographs of Nissl-stained sections showing the brain regions from where the Fluoro-Jade C (FJC) photomicrographs shown in (C) were taken. (C) Representative photomicrographs of FJC-stained sections from the brain regions where neuronal degeneration was evaluated, for the SOMAN and the SOMAN+LY293558-treated groups. Total magnification is 100× . Scale bar is 50 μm. (D) Bar graph showing the median neuropathology score and interquartile range (n = 18 for the SOMAN group and n = 18 for the SOMAN+LY293558 group) for the amygdala (Amy), piriform cortex (Pir), entorhinal cortex (Ent), the CA1 and CA3 subfields of the ventral hippocampus, hilus, and neocortex (neo-Ctx). *P < 0.05, **P < 0.01, and ***P < 0.001 in comparison with the SOMAN group (Mann-Whitney U test).
Pharmacokinetics of LY293558.
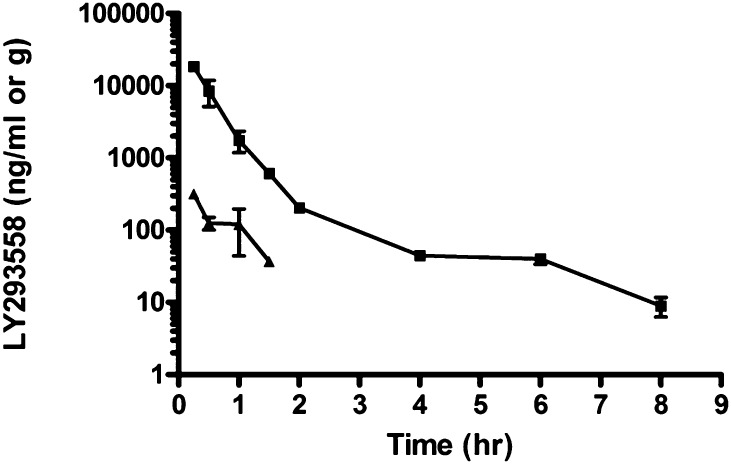
The only observable behavioral change in the rats that were injected with 15 mg/kg of LY293558 (i.m.) was slight hypoactivity. Figure 5 and Table 2 show the profile of drug concentrations in the plasma and brain. LY293558 was rapidly absorbed from an i.m. injection, with the highest concentration in both plasma and brain observed at the first time point of measurement (15 minutes after the injection). Drug concentrations then decreased with time after dose administration and were above the LLOQ of the assay for at least 8 hours in the plasma and 1.5 hours in the brain. After approximately a 2-hour distribution phase, the drug was eliminated from plasma with an elimination half-life of 1.5 hours. The Cmax was 18,433 ± 1472.9 ng/ml for plasma and 318.7 ± 23.9 for brain. Exposure to LY293558, based on values for the area under the plasma concentration-time curve to the last time-point, was 9396 ± 642.9 ng·hr/ml for plasma and 195.7 ± 16.8 hr.ng/g for brain. The mean brain to plasma ratio increased over time, ranging from 0.017 at 0.25 hour to 0.060 at 1.5 hours (see Table 2), suggesting that the rate of clearance of LY293558 from the brain may be slower than that from plasma.
Fig. 5.
Plasma (square) and brain (triangle) concentrations of LY293558 after i.m. injection of 15 mg/kg. For plasma samples: n = 6 at time points 0.25, 0.5 and 1 hour; n = 3 at time point 1.5 hours and later. For brain samples: n = 3 for all time points, except at 1 hour, where n = 6. Data points represent the mean and standard deviation.
TABLE 2.
Plasma and brain concentrations of LY293558 after i.m. administration at the dose of 15 mg/kg
Shown are the number of animals used at different time points, and the group mean and SD of the LY293558 concentrations. The ratio of the brain over plasma concentrations is shown on the last column.
| Time (hours) | Plasma ng/ml |
Brain ng/g |
Ratio B/P | ||||
|---|---|---|---|---|---|---|---|
| Number of Animals | Mean | SD | Number of Animals | Mean | SD | ||
| 0.25 | 6 | 18,435.0 | 1,472.9 | 3 | 318.7 | 23.9 | 0.017 |
| 0.5 | 6 | 8,453.3 | 3,341.5 | 3 | 125.3 | 24.6 | 0.015 |
| 1 | 6 | 1,758.3 | 570.7 | 6 | 119.8 | 76.1 | 0.068 |
| 1.5 | 3 | 609.7 | 61.1 | 3 | 36.5 | 3.5 | 0.060 |
| 2 | 3 | 204.7 | 27.8 | ||||
| 4 | 3 | 44.4 | 4.4 | ||||
| 6 | 3 | 40.0 | 6.2 | ||||
| 8 | 3 | 9.0 | 2.7 | <LLOQ | |||
| 24 | 3 | <LLOQ | |||||
| 48 | 3 | <LLOQ | <LLOQ | ||||
Discussion
A major symptom of nerve agent exposure is the development of intense seizures, which contribute significantly to the lethality of these agents. If death is prevented but brain seizures are not controlled effectively, the seizure-induced brain damage can have long-term consequences on cognitive and emotional functions (McDonough et al., 1986; Brown and Brix, 1998; Myhrer et al., 2005; Grauer et al., 2008). Therefore, a significant part of the research efforts aimed at developing medical countermeasures that will protect life and prevent or minimize the ensuing illnesses after exposure to nerve agents has been focused on finding safe and effective pharmacologic treatments that will control nerve agent–induced brain seizures. Muscarinic antagonists, benzodiazepines, and glutamate receptor antagonists have been tested, showing varying efficacies that depend on several factors, such as the timing of administration after exposure, dose, or drug treatment combinations. All of the animal models used in these experiments involve a pretreatment (Shih et al., 2003; Capacio et al., 2004; Joosen et al., 2009; Figueiredo et al., 2011), or, when no pretreatment is given, oximes and atropine are administered together within 1 minute after exposure (e.g., Moffett et al., 2011). Such protocols of drug administration increase survival rate and allow studies of other parameters but do not mimic a real-life situation in which exposure can be unexpected and pretreatment is not an option, and medical assistance may not be available within a minute after exposure. The present study is the first attempt to mimic such real-life situations by delaying all pharmacologic treatments to 20 minutes after exposure. In addition, the anticonvulsant treatment was given intramuscularly, which would be the most likely route of administration to humans.
As an anticonvulsant treatment, we used LY293558. This compound antagonizes AMPA receptors, and, from the different subtypes of kainate receptors, it selectively antagonizes those that contain the GluK1 subunit (Bleakman et al., 1996; Jane et al., 2009). The GluK1-containing kainate receptors appear to play an important role in seizure generation. Although they modulate both GABAergic and glutamatergic activity in different brain regions (Jane et al., 2009), in the amygdala, a brain region that plays a central role in seizure generation and propagation (Aroniadou-Anderjaska et al., 2008), the net effect of GluK1R activation is an increased excitability (Aroniadou-Anderjaska et al., 2012), and in the hippocampus, GluK1R antagonists block pilocarpine-induced seizures (Smolders et al., 2002). The reason why a glutamate receptor antagonist (i.e., LY293558) is effective against seizures induced by inhibition of AChE is that only the initiation and early phase of nerve agent–induced seizures are due to excessive acetylcholine and hyperstimulation of muscarinic receptors, while the glutamatergic system soon comes into play and is primarily responsible for the maintenance and reinforcement of seizure activity, as well as for the excitotoxic effects on brain neurons (McDonough and Shih, 1997; Shih et al., 2003).
We found that 15 mg/kg of LY293558, a dose that when given to control rats (for the pharmacokinetic studies) induced only slight hypoactivity, was sufficient to stop seizures, and produced 100% survival in rats that were also treated with atropine+HI-6. The importance of the dose of the anticonvulsant in relation to the timing of administration of drugs that control the peripheral cholinergic crisis deserves attention. Thus, in a previous study, 50 mg/kg of LY293558 injected into soman-exposed rats pretreated with HI-6 and given atropine at 1 minute after exposure, stopped seizures and prevented death (Figueiredo et al., 2011; lower concentrations of LY293558 were not tested in that study). However, as stated in the Materials and Methods section, the present study did not allow for the peripheral effects of soman to be counteracted with a pretreatment or with immediate drug administration after exposure; consequently, we had to reduce the dose of LY293558 because 50 mg/kg increased the mortality rate, apparently by contributing to cardiorespiratory suppression. In other words, when the peripheral effects of nerve agent exposure are not well under control with a pretreatment, care should be exercised so that the anticonvulsant is not administered at a dose that may worsen the cardiorespiratory problems. It is indeed noteworthy that 100 mg/kg of LY293558 given to normal rats (that are not exposed to anything toxic) produces only a transient sedation (Figueiredo et al., 2011), whereas 50 mg/kg of LY293558 can contribute to lethality of debilitated animals during cholinergic crisis. Thus, in deciding the appropriate dose of the anticonvulsant, one must take into consideration the timing and efficacy of coadministered medication that aims to prevent cardiorespiratory failure.
Although the anticonvulsant effects of LY293558 suggest that the compound penetrates the blood–brain barrier, the pharmacokinetics in blood and brain after systemic administration had not been reported for LY293558 or other decahydroisoquinolines. In the present study, we observed that after intramuscular injection, LY293558 was readily absorbed into the blood circulation and penetrated the brain with concentrations at the 1-hour time point that were close to 1/10 of the level seen in plasma at the same time. The time course of the effect of LY293558 on seizure suppression corresponded well with the increase in the concentration of this compound in the brain. The highest concentration in the brain was observed at 15 minutes after injection, whereas seizure suppression was evident within 10 minutes after injection, and suppression below behavioral stage 2 was achieved within 30 to 40 minutes. Since 15 minutes after injection was the first time point of measurement of LY293558 in the brain, it is possible that the levels were higher at earlier time points. Some of the LY292558 in the brain might be drug in the residual plasma that was not flushed from the brain. Assuming the residual plasma volume is 10.3 µl/g brain (Friden et al., 2010), about half of the drug measured in brain at the Cmax could be due to residual plasma, but the differential rate of clearance of LY293558 from brain versus plasma suggests that a fraction of the drug had passed through blood–brain barrier.
The cessation of the initial soman-induced SE that resulted from administration of LY293558, and the reduction of the total duration of SE within 24 hours after exposure were accompanied by protection against neuronal loss in the BLA and the CA1 hippocampal area, examined 7 days after soman exposure. Administration of LY293558 also reduced the number of irreversibly degenerating cells in all of the brain areas examined, namely the amygdala, piriform cortex, ventral hippocampus, entorhinal cortex, and a neocortical region, with the greatest protection observed in the hippocampus. Although the translation of the neuropathologic protection into protection against cognitive and emotional behavioral deficits remains to be investigated, it is reasonable to suggest that protection against brain damage may correlate with protection against behavioral dysfunction, as others have shown previously (McDonough et al., 1986; Myhrer et al., 2005). Nevertheless, in some animals, there were still degenerating neurons in the amygdala, piriform cortex, and entorhinal cortex. For complete neuronal protection to be achieved, perhaps an additional neuroprotectant should be coadministered with LY293558, and the seizures that re-emerge at later time points postexposure (i.e., during and after the first 24 hours) should also be monitored and controlled.
Acknowledgments
The views of the authors do not purport to reflect the position or policies of the Department of Defense or the US Army. The authors thank Dr. Cara Olsen for expert advice on the statistical analyses of the data.
Abbreviations
- AChE
acetylcholinesterase
- AMPA
(α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)
- BLA
basolateral amygdala
- CE
coefficient of error
- EEG
electroencephalogram
- HI-6
(1-(2-hydroxyiminomethylpyridinium)-3-(4-carbamoylpyridinium)-2-oxapropane dichloride
- FJC
Fluoro-Jade C
- LY293558
(3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahydroisoquinoline-3-carboxylic acid
- GluK1Rs
kainate receptors containing the GluK1 subunit
- SE
status epilepticus
Authorship Contributions
Participated in research design: Braga, Aroniadou-Anderjaska, Apland, Figueiredo, Qashu, Green.
Conducted experiments: Apland, Figueiredo, Swezey, Yang.
Performed data analysis: Figueiredo, Apland, Aroniadou-Anderjaska, Swezey, Yang, Green, Braga
Wrote or contributed to the writing of the manuscript: Aroniadou-Anderjaska, Figueiredo, Apland, Swezey, Green, Braga.
Footnotes
This work was supported by the CounterACT Program, National Institutes of Health, Office of the Director; and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant 5U01NS058162-07], and the Defense Threat Reduction Agency-Joint Science and Technology Office, Medical S&T Division [Grant CBM.NEURO.01.10.US.18 and CBM.NEURO.01.10.US.15].
References
- Apland JP, Figueiredo TH, Qashu F, Aroniadou-Anderjaska V, Souza AP, Braga MFM. (2010) Higher susceptibility of the ventral versus the dorsal hippocampus and the posteroventral versus anterodorsal amygdala to soman-induced neuropathology. Neurotoxicology 31:485–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Fritsch B, Qashu F, Braga MF. (2008) Pathology and pathophysiology of the amygdala in epileptogenesis and epilepsy. Epilepsy Res 78:102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska V, Pidoplichko V, Figueiredo TH, Almeida-Suhett CP, Prager EM, Braga MF. (2012). Presynaptic facilitation of glutamate release in the basolateral amygdala: a mechanism for the anxiogenic and seizurogenic function of GLUK1 receptors. Neuroscience 221:157–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgar J. (2005) Complex view on poisoning with nerve agents and organophosphates. Acta Med (Hradec Kralove) 48:3–21 [PubMed] [Google Scholar]
- Bleakman R, Schoepp DD, Ballyk B, Bufton H, Sharpe EF, Thomas K, Ornstein PL, Kamboj RK. (1996) Pharmacological discrimination of GluR5 and GluR6 kainate receptor subtypes by (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahyd roisdoquinoline-3 carboxylic-acid. Mol Pharmacol 49:581–585 [PubMed] [Google Scholar]
- Brown MA, Brix KA. (1998) Review of health consequences from high-, intermediate- and low-level exposure to organophosphorus nerve agents. J Appl Toxicol 18:393–408 [DOI] [PubMed] [Google Scholar]
- Capacio BR, Byers CE, Merk KA, Smith JR, McDonough JH. (2004) Pharmacokinetic studies of intramuscular midazolam in guinea pigs challenged with soman. Drug Chem Toxicol 27:95–110 [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. (2009) A nomenclature for ligand-gated ion channels. Neuropharmacology 56:2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombet JM. (2011) Nerve agent intoxication: recent neuropathophysiological findings and subsequent impact on medical management prospects. Toxicol Appl Pharmacol 255:229–241 [DOI] [PubMed] [Google Scholar]
- Figueiredo TH, Qashu F, Apland JP, Aroniadou-Anderjaska V, Souza AP, Braga MF. (2011) The GluK1 (GluR5) Kainate/alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist LY293558 reduces soman-induced seizures and neuropathology. J Pharmacol Exp Ther 336:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridén M, Ljungqvist H, Middleton B, Bredberg U, Hammarlund-Udenaes M. (2010) Improved measurement of drug exposure in the brain using drug-specific correction for residual blood. J Cereb Blood Flow Metab 30:150–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilron I, Max MB, Lee G, Booher SL, Sang CN, Chappell AS, Dionne RA. (2000) Effects of the 2-amino-3-hydroxy-5-methyl-4-isoxazole-proprionic acid/kainate antagonist LY293558 on spontaneous and evoked postoperative pain. Clin Pharmacol Ther 68:320–327 [DOI] [PubMed] [Google Scholar]
- Grauer E, Chapman S, Rabinovitz I, Raveh L, Weissman BA, Kadar T, Allon N. (2008) Single whole-body exposure to sarin vapor in rats: long-term neuronal and behavioral deficits. Toxicol Appl Pharmacol 227:265–274 [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kiêu K, Nielsen J (1999) The efficiency of systematic sampling in stereology—reconsidered. J Microsc 193:199–211 [DOI] [PubMed] [Google Scholar]
- Jane DE, Lodge D, Collingridge GL. (2009) Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology 56:90–113 [DOI] [PubMed] [Google Scholar]
- Joosen MJ, Jousma E, van den Boom TM, Kuijpers WC, Smit AB, Lucassen PJ, van Helden HPM. (2009) Long-term cognitive deficits accompanied by reduced neurogenesis after soman poisoning. Neurotoxicology 30:72–80 [DOI] [PubMed] [Google Scholar]
- Joosen MJ, van der Schans MJ, van Dijk CG, Kuijpers WC, Wortelboer HM, van Helden HP. (2011) Increasing oxime efficacy by blood-brain barrier modulation. Toxicol Lett 206:67–71 [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Shih TM. (1997) Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev 21:559–579 [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Smith RF, Smith CD. (1986) Behavioral correlates of soman-induced neuropathology: deficits in DRL acquisition. Neurobehav Toxicol Teratol 8:179–187 [PubMed] [Google Scholar]
- Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard PY. (2012) Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc Chem Res 45:756–766 [DOI] [PubMed] [Google Scholar]
- Moffett MC, Schultz MK, Schwartz JE, Stone MF, Lumley LA. (2011) Impaired auditory and contextual fear conditioning in soman-exposed rats. Pharmacol Biochem Behav 98:120–129 [DOI] [PubMed] [Google Scholar]
- Myhrer T, Andersen JM, Nguyen NH, Aas P. (2005) Soman-induced convulsions in rats terminated with pharmacological agents after 45 min: neuropathology and cognitive performance. Neurotoxicology 26:39–48 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2005) The Rat Brain in Stereotaxic Coordinates, Ed. 4th Elsevier, New York, NY [Google Scholar]
- Qashu F, Figueiredo TH, Aroniadou-Anderjaska V, Apland JP, Braga MF. (2010) Diazepam administration after prolonged status epilepticus reduces neurodegeneration in the amygdala but not in the hippocampus during epileptogenesis. Amino Acids 38:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294 [DOI] [PubMed] [Google Scholar]
- Sang CN, Ramadan NM, Wallihan RG, et al. (2004) LY293558, a novel AMPA/GluR5 antagonist, is efficacious and well-tolerated in acute migraine. Cephalalgia 24:596–602 [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. (2000) Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat 20:93–114 [DOI] [PubMed] [Google Scholar]
- Shih TM, Duniho SM, McDonough JH. (2003) Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol 188:69–80 [DOI] [PubMed] [Google Scholar]
- Smolders I, Bortolotto ZA, Clarke VR, et al. (2002) Antagonists of GLU(K5)-containing kainate receptors prevent pilocarpine-induced limbic seizures. Nat Neurosci 5:796–804 [DOI] [PubMed] [Google Scholar]
- Tomillero A, Moral MA. (2008) Gateways to clinical trials. Methods Find Exp Clin Pharmacol 30:643–672 [PubMed] [Google Scholar]