Abstract
Acetaminophen (APAP)-induced liver injury is the leading cause of acute liver failure in many countries. This study determined the extent of liver protein sulfhydryl depletion not only in whole liver homogenate but also in the zonal pattern of sulfhydryl depletion within the liver lobule. A single oral gavage dose of 150 or 300 mg/kg APAP in B6C3F1 mice produced increased serum alanine aminotransferase levels, liver necrosis, and glutathione depletion in a dose-dependent manner. Free protein sulfhydryls were measured in liver protein homogenates by labeling with maleimide linked to a near infrared fluorescent dye followed by SDS-polyacrylamide gel electrophoresis. Global protein sulfhydryl levels were decreased significantly (48.4%) starting at 1 hour after the APAP dose and maintained at this reduced level through 24 hours. To visualize the specific hepatocytes that had reduced protein sulfhydryl levels, frozen liver sections were labeled with maleimide linked to horseradish peroxidase. The centrilobular areas exhibited dramatic decreases in free protein sulfhydryls while the periportal regions were essentially spared. These protein sulfhydryl-depleted regions correlated with areas exhibiting histopathologic injury and APAP binding to protein. The majority of protein sulfhydryl depletion was due to reversible oxidation since the global- and lobule-specific effects were essentially reversed when the samples were reduced with tris(2-carboxyethy)phosphine before maleimide labeling. These temporal and zonal pattern changes in protein sulfhydryl oxidation shed new light on the importance that changes in protein redox status might play in the pathogenesis of APAP hepatotoxicity.
Introduction
Because of concerns about the relatively high incidence of acetaminophen (APAP)-induced hepatotoxicity compared with other drugs, a U.S. Food and Drug Administration (FDA) Advisory Panel has recommended lowering the daily therapeutic dose of APAP (U.S. FDA, 2009). APAP is a safe analgesic/antipyretic drug at therapeutic dosages; however, when people unknowingly consume multiple products containing APAP, exceeding the maximum therapeutic dose, it can cause fatal acute liver failure. APAP presents a unique situation in overdose because liver failure and possibly death do not occur until days after the exposure. If caught early enough, typically within 12 hours, N-acetyl cysteine (NAC) is a highly effective antidote. NAC is believed to work via increasing glutathione (GSH) levels, which are dramatically decreased after APAP overdose. However, NAC may also work by providing reducing equivalents to the liver and modifying or reversing oxidative damage caused by reactive oxygen and nitrogen species (ROS/RNS) (Rafeiro et al., 1994; Bessems and Vermeulen, 2001; James et al., 2003; Han et al., 2006).
Because APAP remains the leading cause of acute liver failure in the Western world (Ryder and Beckingham, 2001; Lee, 2004), a large body of research has been conducted to understand the mechanisms behind the pathogenesis and identify potential therapeutic interventions for overdose that have a wider temporal window of efficacy. It is well established that the formation of the highly reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) is an initial step in the development of APAP-induced liver toxicity. At nonhepatotoxic doses, NAPQI is detoxified by conjugation with GSH (Bessems and Vermeulen, 2001; Hinson et al., 2010). In overdose situations, NAPQI leads to depletion of hepatic GSH, which allows excess NAPQI to covalently bind cellular macromolecules such as protein. These events are followed by oxidative stress and mitochondrial damage, both of which have been thought to be the major mechanisms for APAP-induced liver injury. APAP overdose causes the disruption of mitochondrial function, which further promotes oxidative stress and triggers the mitochondrial permeability transition (MPT). The MPT is followed by loss of mitochondrial membrane potential and cell death (Masubuchi et al., 2005; Hanawa et al., 2008; Burke et al., 2010).
Although these are the key events in APAP hepatotoxicity, there are likely other unidentified factors that play a role in the molecular pathogenesis. GSH is the major antioxidant in cells that is important in combating cellular ROS that can damage cellular macromolecules. Because GSH is depleted by NAPQI in APAP-induced hepatotoxicity, a major mechanism of peroxide detoxification is compromised. The APAP-induced GSH depletion may be expected to lead to dramatically altered redox status, which likely plays both direct and indirect roles in the pathogenesis of APAP overdose. One such potential effect is the oxidation of protein sulfhydryls, with subsequent altered enzymatic function.
There are conflicting reports about the extent of protein sulfhydryl depletion during APAP hepatotoxicity. One report found significant global protein sulfhydryl depletion during APAP hepatotoxicity (Tirmenstein and Nelson, 1990), whereas others identified only select protein sulfhydryl alterations (Gupta et al., 1997; Andringa et al., 2008), and still another found no significant change in protein sulfhydryl status (Smith and Mitchell, 1985). All these studies assessed global protein sulfhydryl status, so the changes, or lack thereof, do not provide insight into the regions of the liver that may be affected. Most importantly, any changes that occur might be diluted or offset by hepatocytes exhibiting none or diminished changes, respectively. This localization is important for a hepatotoxicant such as APAP because it causes hepatocellular injury within the centrilobular region of the lobule and essentially spares the periportal regions.
Exemplifying this situation, previously we found that protein glutathionylation was dramatically altered after APAP-induced hepatotoxicity in mice; however, the changes followed unique temporal and zonal pattern changes, with glutathionylation increasing in some hepatocytes, decreasing in others, and remaining constant in the remainder (Yang et al., 2012). Restricting the analysis to global glutathionylation levels would not have revealed these unique patterns or the importance these changes are likely to play in specific hepatocytes within the lobule.
Therefore, in this study, we assessed both global- and lobule-specific changes in protein sulfhydryls during APAP hepatotoxicity in mice. We found that APAP induced significant protein sulfhydryl depletion when assessed globally, and the changes were dramatic and clear when they were measured in specific hepatocytes using a histochemical approach in frozen liver sections. These results highlight the importance that protein sulfhydryl changes are likely to play during APAP hepatotoxicity and may provide new insight into understanding how some antidotes can provide protection even when administered well after the overdose.
Materials and Methods
Chemicals.
Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Protein separation equipment and reagents including electrophoresis, precast polyacrylamide gels, premixed buffers, loading buffer, and protein standard were purchased from Bio-Rad Laboratories (Hercules, CA). Phosphate-buffered saline (PBS) was purchased from Invitrogen (Carlsbad, CA).
Animals.
Six- to seven-week-old male B6C3F1 mice, provided by the U.S. Food and Drug Administration’s National Center for Toxicological Research (NCTR) breeding colony, were used for the study. Animal care was performed in accordance with the National Research Council’s “Guide for the Care and Use of Laboratory Animals” and was authorized by the NCTR Institutional Animal Care and Use Committee. After a 7-day acclimation period, the mice were weight ranked then randomized to experimental groups to ensure that the mean group weights did not differ significantly from each other. The mice were housed individually in polycarbonate cages using hardwood chip bedding. During the study, the room temperatures remained within 19°–23°C, and fluorescent lighting was provided on a 12 hours on/12 hours off cycle. Filtered tap water was provided ad libitum, and NIH-41 irradiated diet was provided ad libitum except during designated periods of fasting. Mice were fasted overnight for at least 12 hours before a single oral (gavage) dose of APAP (suspended in 0.5% methylcellulose and using a dose volume of 10 ml/kg body weight), with feed provided to the animals 4 hours after dosing. There were 3 to 4 mice in the 0.5% methylcellulose control groups, and four to five mice in the APAP low-dose (150 mg/kg) and high-dose (300 mg/kg) groups.
Clinical Pathology/Histopathology.
Approximately 1, 3, 6, or 24 hours after dosing, the mice were anesthetized with carbon dioxide, blood was withdrawn via cardiac puncture, and the mice were then euthanized by carbon dioxide asphyxiation. Blood in the serum separator tube was allowed to clot for 30 to 60 minutes at room temperature, and was centrifuged at 1000g for approximately 10 minutes; the serum was analyzed on an automated clinical chemistry analyzer (Alfa Wassermann ALERA, West Caldwell, NJ) to assess levels of alanine aminotransferase (ALT). The liver was immediately removed and weighed, and tissue samples were collected. Several tissue samples were cut from the left lateral lobe of the liver, wrapped in aluminum foil, frozen within 3 minutes of removal in an isopentane/dry ice slurry, and stored at −80°C for subsequent preparation of frozen liver sections. Additionally, tissue samples from the right lateral lobe of the liver were fixed in neutral buffered 10% formalin for approximately 48 hours and then routinely processed and examined by a board-certified veterinary pathologist. The remainder of the liver was wrapped in aluminum foil, frozen in an isopentane/dry ice slurry, and stored at −80°C for subsequent preparation of liver homogenates for GSH/GSSG analysis and SDS-polyacrylamide gel (PAGE).
GSH/GSSG Analysis.
The detection of GSH/GSSG has been previously described in detail elsewhere (Yang et al., 2012).
SDS-PAGE.
The protein sulfhydryl groups in total liver protein were detected by SDS-polyacrylamide gel with the following modifications. We homogenized 30 μg of frozen liver in lysis buffer [20 mM Tris HCl (pH 7.5), 137 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA] with fresh protease inhibitor cocktail using a FastPrep instrument (MP Biomedicals, Solon, OH). After centrifugation for 15 minutes at 13,000g at 4°C, the supernatant was kept at −80°C until use. The protein concentration was determined by the Bradford method using Bio-Rad Protein Assay reagent and bovine serum albumin (BSA) as a standard. Labeling of free protein sulfhydryls with fluorescently labeled maleimide (IRDye 800CW Maleimide; Li-Cor, Lincoln, NE) was performed concurrently for time-matched control and treated groups. Equal amounts (5 μg) of liver lysates were incubated with 2 mg/ml maleimide in the dark at room temperature for 1 hour, and then the excess unreacted maleimide was inactivated by adding 8 mM reduced glutathione for 30 minutes. To determine whether the oxidized sulfhydryls could be reduced back to free sulfhydryls (i.e., reversible oxidation), the protein sample was reduced with 5 mM tris(2-carboxyethy)phosphine (TCEP) for 30 minutes and desalted by spin columns (Thermo Fisher Scientific, West Palm Beach, FL) before maleimide labeling. The labeled proteins were loaded and separated on a 12% SDS-polyacrylamide gel. After electrophoresis, the gels were scanned with an Odyssey infrared scanner (Li-Cor), and the signals were quantified with the scanner’s software. Duplicate gels were stained with Coomassie Blue (Bio-Rad Laboratories) to ensure equal loading of samples.
Histochemical Analysis of Liver Free Protein Sulfhydryls and Immunohistochemical Analysis of APAP Covalent Binding to Protein.
From each animal, flash-frozen livers were embedded in optimal cutting temperature (OCT) medium. Six-micron-thick serial sections were cut from each block to facilitate the comparison of the lobule pattern of free protein sulfhydryl levels, APAP reactive metabolite binding, and morphologic changes. One section was stained with hematoxylin and eosin (H&E) for histopathologic examination by light microscopy. (The histopathologic gradings presented in the results were taken from the formalin-fixed sections because these sections had better preserved morphology.) The purpose of the H&E stained frozen section was for direct comparison of regional morphologic changes with sulfhydryl levels and APAP binding. One section was stained with maleimide linked to horseradish peroxidase (maleimide-HRP), and the remaining section was immunohistochemically stained with an anti-APAP antibody (Yang et al., 2012).
For histochemical detection of free protein sulfhydryl levels, frozen sections were fixed in ice-cold methanol for 3 minutes and washed in PBS for OCT removal. Endogenous peroxidase was inhibited by incubation with freshly prepared 3% hydrogen peroxide with 0.1% sodium azide for 10 minutes at room temperature. To detect free sulfhydryl groups, the sections were incubated with 8 μg/ml maleimide-HRP for 1 hour at room temperature. After washing in PBS, color was developed with diaminobenzidine, counterstained with hematoxylin, and mounted with Permount (Thermo Fisher Scientific, Pittsburgh, PA). To assess the reversibility of the oxidized sulfhydryls, serial sections were incubated with TCEP (1:100 in PBS) for 30 minutes at room temperature, washed in PBS, and then incubated with maleimide-HRP. All sections were examined by light microscopy (BX40, Olympus, Japan).
Statistical Analysis.
All statistical analyses were performed using SigmaPlot version 11.0 for Windows (Systat Software Inc., San Jose, CA). Data were analyzed by a one-way analysis of variance (ANOVA) followed by a Student-Neuman-Keuls post hoc test. The level of significance was defined as the 0.05 level of probability. ALT data were log-transformed before statistical analysis.
Results
Effect of APAP on Liver Injury and GSH Homeostasis.
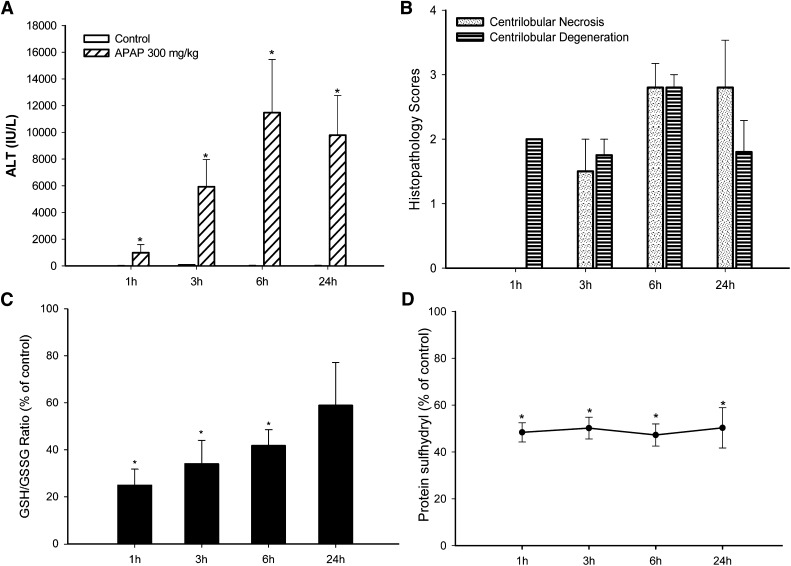
In this study, mice were administered a single dose of APAP (150 or 300 mg/kg, oral gavage) and sacrificed at 1, 3, 6, or 24 hours after the APAP administration. Consistent with previous observations, APAP treatment caused hepatocellular injury, as indicated by a statistically significant increase of serum ALT and histopathologic changes. Data for the control and 300 mg/kg dosed groups are shown in Fig. 1. The time course for APAP-induced increases of ALT is shown in Fig. 1A. Statistically significant increases of ALT started as early as 1 hour after dosing, with maximal ALT reached at 6 and 24 hours. Histopathologic changes corroborated the ALT results. Centrilobular hepatocellular degeneration was observed at 1, 3, 6, and 24 hours, and centrilobular hepatocellular necrosis was observed at 3, 6, and 24 hours after dosing (Fig. 1B). Hepatic GSH levels were maximally depleted by 1 hour, with some increase at 3 hours, and recovered to levels comparable with that of control animals at 6 and 24 hours (Table 1). The increase of GSSG levels started at 3 hours, with a dramatic increase at 6 hours (Table 1). The ratio of GSH to GSSG is an indicator of the oxidative state of the liver. The high dose of APAP (300 mg/kg) induced a profound decrease in the hepatic GSH/GSSG ratio at 1 hour, which remained decreased at 3 and 6 hours (Fig. 1C), suggesting a more oxidative environment. At 24 hours, the GSH/GSSG ratios were still lower than those of the controls, but the decrease was not statistically significant.
Fig. 1.
Time course for the effect of APAP on serum ALT, histopathology score, hepatic GSH/GSSG ratio, and protein sulfhydryl depletion (as measured by SDS-PAGE). APAP (300 mg/kg) was administered by oral gavage, then the blood and liver samples were collected at the indicated times. (A) APAP statistically significantly increased serum ALT values, indicative of hepatocellular injury. Liver histopathology lesions were scored on a 5-point scale. (B) All time-matched control animals had scores of zero. (C) Hepatic GSH and GSSG levels were measured by UPLC-MS, and the GSH/GSSG ratio was used as an indicator of oxidative stress. The GSH/GSSG ratio was statistically significantly decreased compared with control levels from 1 to 6 hours. The GSH/GSSG ratio was still decreased at 24 hours, but the decrease was not statistically significant. (D) Protein thiols were labeled with maleimide-IRDye, scanned, and quantified with a near-infrared scanner. The same samples were resolved on a duplicate gel followed by Coomassie Blue staining, and the protein sulfhydryl levels were normalized to the Coomassie Blue staining. APAP at 300 mg/kg produced a profound decrease of protein thiols at 1 hour through 24 hours. *ALT, GSH/GSSG ratio, and protein thiols of 300 mg/kg group were statistically significantly decreased (P < 0.05) compared with the control group. Histopathology scores were not statistically analyzed. Values are mean ± S.E.M.
TABLE 1.
Hepatic GSH and GSSG levels after APAP exposure
Mice (n = 3–5) were administered a single oral gavage dose of 0.5% methylcellulose vehicle (control) or APAP. Hepatic GSH and GSSG levels were measured by UPLC-MS. APAP at 300 mg/kg produced a profound decrease of GSH at 1 hour, with recovery starting to occur within 3 hours although levels were still decreased compared with controls.
| Group | GSH |
GSSG |
||||||
|---|---|---|---|---|---|---|---|---|
| 1 h | 3 h | 6 h | 24 h | 1 h | 3 h | 6 h | 24 h | |
| μmol/g tissue | μmol/g tissue | |||||||
| Control | 5.0 ± (0.8) | 4.8 ± (0.3) | 6.2 ± (0.7) | 7.8 ± (0.2) | 0.8 ± (0.1) | 0.5 ± (0.1) | 0.6 ± (0.0) | 0.6 ± (0.1) |
| 150 mg/kg | 4.2 ± (0.6) | 4.4 ± (0.2) | 4.4 ± (0.3) | 6.5 ± (1.0) | 0.8 ± (0.2) | 0.7 ± (0.0) | 0.7 ± (0.0) | 0.6 ± (0.0) |
| 300 mg/kg | 0.9 ± (0.3)a | 2.7 ± (0.6)a | 5.4 ± (1.0) | 7.2 ± (1.4) | 0.5 ± (0.1) | 0.8 ± (0.2) | 1.2 ± (0.2)b | 1.1 ± (0.3) |
APAP, acetaminophen; GSH, reduced glutathione; GSSG, oxidized glutathione; UPLC-MS, ultraperformance liquid chromatography–mass spectrometry.
GSH level of 300 mg/kg group was statistically significantly decreased (P < 0.05) compared with the control and 150 mg/kg groups.
GSSG level of 300 mg/kg group was statistically significantly increased (P < 0.05) compared with the control and 150 mg/kg groups. Values are mean ± (S.E.M.).
Decreased Global Hepatic Free Protein Sulfhydryls after APAP Exposure.
In addition to GSH depletion, a decrease of protein sulfhydryl groups was observed after APAP administration. In this study, the detection and quantification of the hepatic protein sulfhydryls are dependent on the alkylation between free sulfhydryls and fluorescence-tagged maleimide. Maleimide is a sulfhydryl-specific molecular probe, which reacts and labels free sulfhydryl groups in protein. Global protein thiol levels were measured by maleimide labeling followed by SDS-PAGE and image quantification with an Odyssey near-infrared scanner. Coomassie Blue staining of separate polyacrylamide gels loaded with the same samples confirmed that protein loading was similar across all samples and that the loss of free protein sulfhydryl groups was not an artifact of protein loading (data not shown). After a 300 mg/kg dose of APAP, statistically significant decreases were observed in global free protein sulfhydryl levels at all time points (Fig. 1D). At 1 hour, the protein sulfhydryl levels were 48.4% of controls. These levels remained depressed at the 3-, 6-, and 24-hour time points.
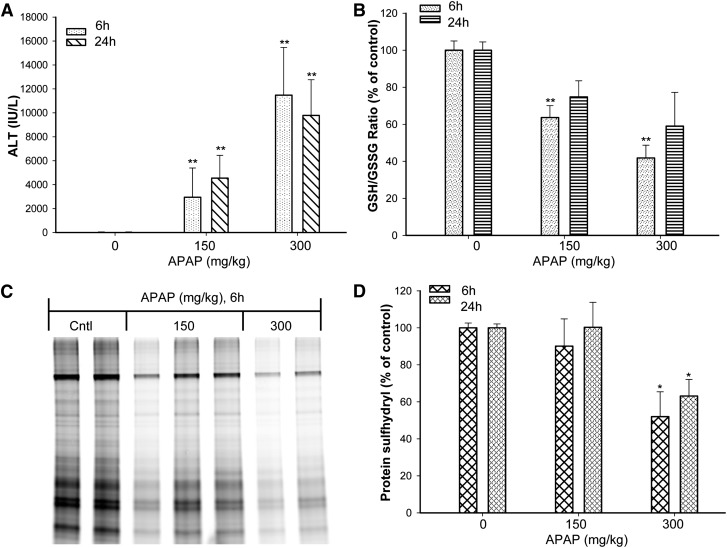
A dose–response relationship was evaluated. APAP exposure induced a dose-dependent increase in serum ALT activities (Fig. 2A). The low dose of APAP (150 mg/kg) appeared to maintain a lower level of GSH compared with controls, but the difference was not statistically significant (Table 1). In contrast, the high dose (300 mg/kg) induced a profound decrease in GSH at 1 hour, with levels starting to increase at 3 hours and recovery within 6 hours (Table 1). At 6 hours, both the low- and high-dose groups had statistically significantly decreased GSH/GSSG ratios, indicating that both treatments created a more oxidative environment (Fig. 2B).
Fig. 2.
Dose response for the effect of APAP on serum ALT, hepatic GSH/GSSG ratio, and protein sulfhydryl depletion. Mice (n = 3–5) were administered a single oral gavage dose of 0.5% methylcellulose vehicle (control) or APAP (150 or 300 mg/kg). (A) APAP increased serum ALT values in a dose-dependent manner. (B) Hepatic GSH and GSSG levels were measured by UPLC-MS, and the GSH/GSSG ratio was used as an indicator of oxidative stress. The GSH/GSSG ratio of the low- and high-dose animals was decreased in a dose-dependent manner compared with time-matched controls. Total liver protein was isolated, and protein thiols were labeled with maleimide-IRdye before loading on the gel. (C) One representative gel of 6-hour samples is shown. (D) The depletion of protein thiols was quantified with a near-infrared scanner. The same samples were resolved on a duplicate gel followed by Coomassie Blue staining, and the protein sulfhydryl levels were normalized to the Coomassie Blue staining. *ALT, GSH/GSSG ratio, and protein thiols of the 300 mg/kg group were statistically significantly decreased (P < 0.05) compared with the control and 150 mg/kg groups. **ALT and GSH/GSSG ratio of the 150 and 300 mg/kg groups were statistically significantly decreased (P < 0.05) compared with the control group. Values are mean ± S.E.M.
Figure 2C shows the protein sulfhydryl depletion in liver homogenates of APAP-treated animals. Figure 2, C and D shows the levels of protein sulfhydryl from animals treated with different doses of APAP. In the control animals, a wide range of hepatic protein sulfhydryl groups were labeled with the fluorescent probe (Fig. 2C). Statistically significant decreases were observed in protein thiol levels after APAP (300 mg/kg) administration (Fig. 2D). At 6 hours, some low-dose animals (150 mg/kg) exhibited decreases compared with the controls (Fig. 2C); however, the group mean loss of protein sulfhydryls was not statistically significant. At 24 hours, only the 300 mg/kg APAP-treated animals exhibited a statistically significant group mean decrease of protein sulfhydryls compared with the controls (Fig. 2D).
Lobule Zonation of APAP Adducts in Relation to Reduced Protein Sulfhydryls and Histopathologic Injury.
SDS-PAGE experiments provided useful information on the overall quantification of reduced protein sulfhydryls; however, they could not be used to determine the pattern of protein sulfhydryl loss within the hepatic lobule. This is important for a compound like APAP because it induces a specific centrilobular pattern of morphologic effects and essentially spares the periportal regions. Additionally, the protein’s surrounding environment plays an important role in protein sulfhydryl oxidation. To determine whether a certain subset of cells or region of the lobule was preferentially affected, histochemical analysis was conducted to assess the pattern of protein sulfhydryl changes using HRP-conjugated maleimide. In serial sections, the pattern of APAP adduct binding to macromolecules within the lobule was determined immunohistochemically using an anti-APAP antibody. This allowed a direct comparison between the regions of the lobule that had morphologic changes (H&E stain), free protein sulfhydryl changes (maleimide labeling), and APAP adducts (anti-APAP antibody).
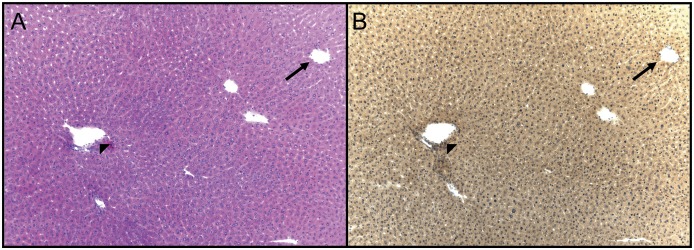
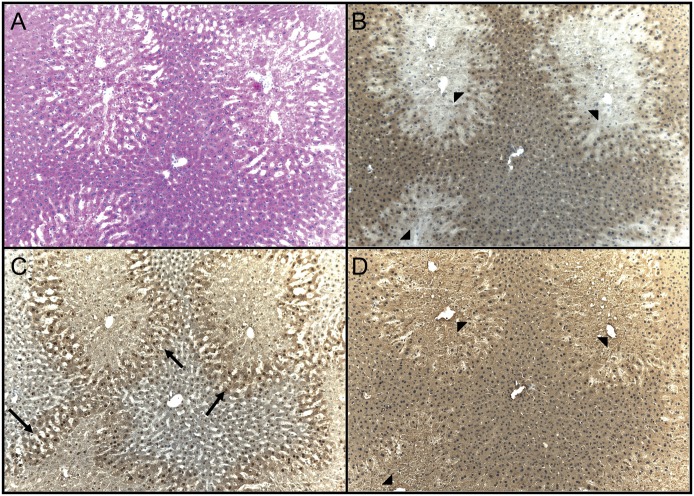
Light microscopic images of animals treated with control or 300 mg/kg APAP, Figs. 3, A and B, and 4, A–D, respectively, are provided. In control livers, normal morphology is seen with routine staining (Fig. 3A), and the level of reduced free-protein sulfhydryls was uniform throughout the lobule (Fig. 3B). At 1 hour after the high dose of APAP (300 mg/kg), a mild decrease of reduced protein sulfhydryls was observed throughout the central region hepatocytes (data not shown). At 3 hours, clear morphologic changes within the centrilobular hepatocytes were observed at the high dose of APAP (Fig. 4A), and dramatic decreases in protein sulfhydryls were observed within the centrilobular hepatocytes (Fig. 4B, arrowheads). The loss of protein sulfhydryls did not occur in the periportal areas of the livers of the APAP-treated mice. Figure 4C is a serial section stained for APAP-protein adducts (Fig. 4C, arrows) and shows that all cells that contained APAP protein adducts also had decreased levels of protein sulfhydryls (Fig. 4D). At the 6- and 24-hour time points, a similar pattern of protein sulfhydryl change was observed in the APAP high-dose animals (data not shown for 6 hours; 24-hour time point shown in Fig. 5). In contrast to the 300 mg/kg APAP group, the APAP low-dose (150 mg/kg) livers only exhibited the decrease of protein sulfhydryls at 24 hours. And the loss of protein sulfhydryls was not as dramatic as compared with the high dose (Fig. 5, B vs. E). These free-protein sulfhydryl-depleted regions correlated with areas exhibiting necrotic changes (Fig. 5, D vs. E) and APAP adducts (data not shown).
Fig. 3.
Histochemical detection of protein sulfhydryls in vehicle control (0.5% methylcellulose) mouse liver. Two serial sections were cut to facilitate the comparison of protein thiols with liver morphology. The slides were treated as follows: H&E stain (A) and histochemical stain using maleimide-HRP (B). All histochemically stained slides were counterstained with hematoxylin. The level of protein thiols was uniform throughout the liver lobule. The arrow indicates the centrilobular vein; the arrowhead indicates the portal triad. Original field magnification, 100×.
Fig. 4.
Histochemical detection of protein sulfhydryls and immunohistochemical detection of APAP adduction of cellular macromolecules in mouse liver 3 hours after treatment with an oral dose of 300 mg/kg APAP. Four serial sections were cut to facilitate the comparison of protein thiol levels with liver morphology, APAP adduction of protein, and reversibility of sulfhydryl oxidation. The slides were treated as follows: H&E stain (A), histochemical stain using maleimide-HRP (B), immunohistochemical stain using an anti-APAP antibody (C), and reduction of reversibly-oxidized protein sulfhydryls with TCEP followed by histochemical stain using maleimide-HRP (D). All histochemically and immunohistochemically stained slides were counterstained with hematoxylin. The level of protein thiol was dramatically reduced in the centrilobular regions (B, arrowheads), and this correlated with areas having APAP adducts (C, arrows). The oxidized sulfhydryls could be reduced back to free sulfhydryls using TCEP (D, arrowheads). Original field magnification, 100×.
Fig. 5.
Histochemical detection of protein sulfhydryls and immunohistochemical detection of APAP adduction of cellular macromolecules in mouse liver 24 hours after treatment with an oral dose of 150 or 300 mg/kg APAP. Three serial sections were cut to facilitate the comparison of protein thiol levels with liver morphology. The slides were treated as follows: H&E stain (A and D), histochemical stain using maleimide-HRP (B and E), and reduction of reversibly-oxidized protein sulfhydryls with TCEP followed by histochemical stain using maleimide-HRP (C and F).The histochemically stained slides were counterstained with hematoxylin. The level of protein thiol was dramatically reduced in the centrilobular regions (B and E, arrows), and this correlated with areas having hepatocellular injury (A and D, arrowheads). The oxidized sulfhydryls could be reduced back to free sulfhydryls using TCEP (C and F, arrows). Original field magnification, 100×.
Protein Sulfhydryl Oxidation Is Reversible.
Further experiments were conducted to determine the reversibility of the observed decrease of protein sulfhydryls. TCEP is a strong sulfhydryl-reducing agent that has previously been reported to reverse various protein oxidative modifications such as disulfide and mixed-disulfide bonds. To assess the reversibility of the sulfhydryl oxidative changes induced by APAP, liver homogenates or liver sections were first reduced with TCEP before maleimide labeling. The reducing treatment essentially eliminated the protein sulfhydryl depletion in the centrilobular hepatocytes (Figs. 4D, 5C, and 5F). In the SDS-PAGE experiment, a statistically significant decrease (∼50%) of protein sulfhydryl groups was observed at 1, 3, 6, and 24 hours after the high dose of APAP (300 mg/kg). Preincubation with TCEP fully (∼98%) restored the loss of protein sulfhydryls (Fig. 6). These experiments suggest that reversible oxidation accounts for the majority of protein sulfhydryl depletion after APAP. It should be pointed out that maleimide could have reacted with free GSH (or GSSG after reduction to GSH by TCEP) in the frozen sections and may have contributed to the signal that was observed. However, because the sections were fixed with methanol followed by extensive washing with PBS and peroxidase quenching water solution, it is unlikely that the small molecular weight GSH or GSSG molecules would have been retained in the sections. In addition, the results from the SDS-PAGE analysis lend support that the changes observed in the frozen liver sections are due to protein sulfhydryl changes and not due to changes in the level of GSH or GSSG.
Fig. 6.
The effect of APAP on protein sulfhydryls in mouse liver and the restoration of reversibly oxidized protein sulfhydryls. Mice (n = 3–5) were administered a single oral gavage dose of 0.5% methylcellulose vehicle (Cntl), APAP low dose (150 mg/kg), or APAP high dose (300 mg/kg) and total liver protein (24 hours) was isolated and resolved on a 12% SDS-PAGE gel. Protein thiols were labeled with maleimide-IRDye before loading on the gel. (A) The high-dose samples had decreased protein thiols. (B) The same samples were reduced with TCEP, desalted, and then labeled with maleimide-IRDye before loading on the gel. (C) The same samples were resolved on a duplicate gel followed by Coomassie Blue staining to confirm equal loading of protein between samples. The levels of protein sulfhydryl loss were quantified with a near-infrared scanner. (D) These results show that APAP induces protein sulfhydryl oxidation and the majority of the oxidation is reversible with a thiol-reducing agent (TCEP). *Protein thiols of 300 mg/kg group were statistically significantly decreased (P < 0.05) compared with the control and TCEP-treated samples.
Discussion
The objective of this study was to quantify and localize the decrease in protein sulfhydryls during APAP-induced liver injury. Our data demonstrate a statistically significant decrease of protein sulfhydryl groups within the centrilobular hepatocytes starting as early as 1 hour after the APAP dose, which remained decreased through 24 hours (Fig. 1). Oxidation of the free protein sulfhydryls occurred in the same regions of cells that contained APAP-protein adducts and developed necrotic changes (Figs. 4 and 5). Interestingly, the majority of protein sulfhydryl depletion was due to reversible oxidation because the global and lobule-specific effects were essentially reversed with TCEP treatment before maleimide labeling (Figs. 4, 5, and 6).
In a previous publication, we demonstrated the colocalization of protein glutathionylation, APAP-protein adducts, and centrilobular necrosis starting as early as 1 hour after the APAP dose and continuing through 24 hours (Yang et al., 2012). However, in contrast to the current results, the pattern of glutathionylation exhibited a complex pattern of changes over time, with glutathionylation increasing in centrilobular hepatocytes at early time points and then decreasing within the most affected centrilobular hepatocytes at later time points, while remaining elevated in hepatocytes on the periphery of the lesion. Therefore, glutathionylation could account for some of the loss of free sulfhydryls observed in the current study; however, there are likely additional sulfhydryl oxidative changes that likely occurred, such as disulfide formation. Because TCEP essentially reversed the oxidative changes, the oxidation appears to consist mostly of sulfhydryl changes that could theoretically be reversed by the affected cells once a more reducing environment is restored.
Because GSH is the main reducing equivalent in the cell and the ratio of GSH/GSSG is an indicator of the oxidative state of the cell, it is apparent from the GSH and GSH/GSSG analyses (Figs. 1 and 2) that at early time points, and even at 24 hours, the liver is in a more oxidative state after a hepatotoxic dose of APAP. Because APAP predominantly affects centrilobular hepatocytes, it is likely that the most affected centrilobular hepatocytes experience an even greater, or at least a more prolonged, oxidative environment since the GSH and GSH/GSSG analyses used whole-liver homogenates. This oxidative environment could not only lead to protein sulfhydryl oxidation but would prevent the oxidative changes from being reversed until the oxidative environment was reversed.
It is interesting to speculate that the therapeutic agent NAC may either directly or indirectly prevent and/or reverse protein sulfhydryl oxidation after a hepatotoxic dose of APAP. NAC provides therapeutic benefits when administered hours after the APAP dose in both mice (Salminen et al., 1998) and humans (Bessems and Vermeulen, 2001). It is generally believed that NAC provides therapeutic benefit by restoring GSH levels and preventing further damage from NAPQI. However, especially when NAC is administered hours after the APAP dose when parent drug levels are reduced so it is likely that preventing covalent binding by NAC is limited (Saito et al., 2010), it is possible that NAC provides protection by reversing oxidative protein changes on critical sulfhydryl-containing enzymes, which regulate important cellular pathways during the development of APAP-induced liver toxicity.
GSH plays a key role in the detoxification of xenobiotics in cells, and it is generally accepted that cells maintain high levels of reduced GSH to keep proteins in the reduced state (Jaeschke, 1990; Han et al., 2006). It is likely that GSH depletion and the ensuing oxidizing environment caused by NAPQI could lead to the loss of protein sulfhydryls. In a previous study (Tirmenstein and Nelson, 1990), the oxidation of protein thiols was shown to be 15 times the amount of loss from direct NAPQI conjugation. In this study, we established that APAP exposure resulted in decrease of 48.4% of the protein sulfhydryls as early as 1 hour after the APAP high dose when assessed in whole-liver homogenate via SDS-PAGE. Our data also support that the majority of the oxidative sulfhydryl changes were not due to NAPQI conjugation because the oxidized protein sulfhydryls could be reduced to free sulfhydryls using TCEP. It has been shown that APAP-induced oxidant stress starts immediately after GSH depletion (Bajt et al., 2004), which occurs within the first hour after exposure, as was observed in our study. Once GSH is depleted, protein sulfhydryls may act as an additional redox buffer.
It is possible that the oxidative protein sulfhydryl changes induced by APAP ultimately lead to cellular damage, as the protein sulfhydryl groups are important targets of oxidative stress, where protein oxidation can act as a cellular redox switch to modulate protein function, particularly those involving cell death (Han et al., 2006). ROS can cause the S-hydroxylation of protein sulfhydryls to the slightly oxidized state, sulfenic acid, which is reversible. Sulfenic acid can be further oxidized to sulfinic and sulfonic acid, which are irreversible modifications. Protein sulfhydryls may also enter other reactions such as the formation of reversible disulfide bridges, either internally or with different proteins and S-glutathionylated protein. Protein sulfhydryl modifications can also be induced by nitric oxide via the formation of peroxynitrite, a reactive nitrogen species (RNS). APAP has been shown to induce the formation of both ROS and RNS (Hinson et al., 2010), which is consistent with the oxidation of protein sulfhydryls observed our study.
Recent reports have suggested that peroxynitrite is an important mediator in APAP-induced hepatotoxicity. It has been shown that peroxynitrite is predominantly produced in mitochondria (Cover et al., 2005), which is the same organelle contributing to superoxide formation. Interestingly, oxidative stress can be generated through sulfhydryl modification of mitochondrial complex I (Tompkins et al., 2006). Therefore, it is possible that ROS/RNS stress could result in the modification of critical sulfhydryls in mitochondrial proteins, leading to further oxidative stress and eventually mitochondrial dysfunction. Indeed, several mitochondrial proteins, such as aldehyde dehydrogenase, are known to contain free sulfhydryl groups within their catalytic sites and are inactivated through S-nitrosylation (Moon et al., 2007; Andringa et al., 2008). Using an ischemia-reperfusion liver model, it was reported that ATP synthesis was markedly inhibited with the S-nitrosylation of mitochondrial proteins such as 3-ketoacyl-CoA thiolase, and that pretreatment with a peroxynitrite scavenger restored enzyme activity and ATP synthesis (Moon et al., 2008). In addition, several key components of the mitochondrial respiration chain have altered function after their glutathionylation status has been changed (Beer et al., 2004). Protein sulfhydryl oxidation has been shown to alter calcium homeostasis and MPT (Bellomo and Orrenius, 1985; Petronilli et al., 1994). Further studies are necessary to identify the thiol-oxidized proteins that may contribute to the MPT and cell death. The specific protein targets can be purified by biotin-conjugated maleimide and identified by mass spectrometric analysis (Song et al., 2010). We anticipate that the identification of specific proteins will be important to developing novel therapeutic targets that may restore protein sulfhydryl and prevent APAP-induced liver toxicity.
Peroxynitrite is both an oxidizing and nitrating agent, which leads to the formation of nitrotyrosine. Previous studies have indicated that nitrotyrosine staining takes place in the same cells that contain APAP adducts and developing necrotic death (Hinson et al., 1998; Knight et al., 2001). Surprisingly, only one primarily nitrated protein, manganese superoxide dismutase, was reported as inactivated during APAP toxicity. It is possible that other protein modifications (e.g., S-nitrosylation and sulfhydryl oxidation) caused by peroxynitrite play important roles in APAP-induced hepatotoxicity.
In conclusion, APAP overdose results in a more oxidizing environment based on GSH and GSH/GSSG levels and decreases the level of protein sulfhydryls in mouse liver. The centrilobular areas exhibited dramatic decreases in protein thiols while the periportal regions were essentially spared. Interestingly, the majority of the oxidation was reversible with a thiol-reducing agent. The changes in protein sulfhydryl oxidation may act as the sensor for oxidants such as ROS and RNS, and the loss of protein sulfhydryls may cause the mitochondrial dysfunction that is observed during APAP-induced liver toxicity.
Abbreviations
- ALT
alanine aminotransferase
- APAP
acetaminophen
- BSA
bovine serum albumin
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- HRP
horseradish peroxidase
- MPT
mitochondrial permeability transition
- NAC
N-acetyl cysteine
- NAPQI
N-acetyl-p-benzoquinoneimine
- NCTR
National Center for Toxicological Research
- OCT
optimal cutting temperature
- PBS
phosphate-buffered saline
- ROS/RNS
reactive oxygen/nitrogen species
- TCEP
Tris(2-carboxyethyl)phosphine
- UPLC-MS
ultraperformance liquid chromatography–mass spectrometry
Authorship Contributions
Participated in research design: Yang, Shi, Roberts, Hinson, Salminen.
Conducted experiments: Yang, Greenhaw, Muskhelishvili, Davis.
Performed data analysis: Yang, Salminen.
Wrote or contributed to the writing of the manuscript: Yang, Salminen.
Footnotes
This work was supported by the Research Participation Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration (to X.Y.); in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK081406] (to D.W.R.); by the Arkansas Children’s Hospital Research Institute and the Arkansas Biosciences Institute, the major research component of the Tobacco Settlement Proceeds Act of 2000; and by a grant from the National Institutes of Health [Grant R01-DK079008] (to J.A.H.).
Part owners of Acetaminophen Toxicity Diagnostics, LLC, a company working to develop a medical device for diagnosis of acetaminophen-induced liver injury.
Completed work while at the Division of Systems Biology, National Center for Toxicological Research.
The opinions expressed in this manuscript do not reflect the official positions or policies of the U.S. Food and Drug Administration.
References
- Andringa KK, Bajt ML, Jaeschke H, Bailey SM. (2008) Mitochondrial protein thiol modifications in acetaminophen hepatotoxicity: effect on HMG-CoA synthase. Toxicol Lett 177:188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. (2004) Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci 80:343–349 [DOI] [PubMed] [Google Scholar]
- Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, Murphy MP. (2004) Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. J Biol Chem 279:47939–47951 [DOI] [PubMed] [Google Scholar]
- Bellomo G, Orrenius S. (1985) Altered thiol and calcium homeostasis in oxidative hepatocellular injury. Hepatology 5:876–882 [DOI] [PubMed] [Google Scholar]
- Bessems JGM, Vermeulen NPE. (2001) Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 31:55–138 [DOI] [PubMed] [Google Scholar]
- Burke AS, MacMillan-Crow LA, Hinson JA. (2010) Reactive nitrogen species in acetaminophen-induced mitochondrial damage and toxicity in mouse hepatocytes. Chem Res Toxicol 23:1286–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. (2005) Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther 315:879–887 [DOI] [PubMed] [Google Scholar]
- Gupta S, Rogers LK, Taylor SK, Smith CV. (1997) Inhibition of carbamyl phosphate synthetase-I and glutamine synthetase by hepatotoxic doses of acetaminophen in mice. Toxicol Appl Pharmacol 146:317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Hanawa N, Saberi B, Kaplowitz N. (2006) Mechanisms of liver injury. III. Role of glutathione redox status in liver injury. Am J Physiol Gastrointest Liver Physiol 291:G1–G7 [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. (2008) Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem 283:13565–13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Pike SL, Pumford NR, Mayeux PR. (1998) Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem Res Toxicol 11:604–607 [DOI] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW, James LP. (2010) Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol 196:369–405 [DOI] [PMC free article] [PubMed]
- Jaeschke H. (1990) Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther 255:935–941 [PubMed] [Google Scholar]
- James LP, McCullough SS, Lamps LW, Hinson JA. (2003) Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci 75:458–467 [DOI] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. (2001) Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol Sci 62:212–220 [DOI] [PubMed] [Google Scholar]
- Lee WM. (2004) Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology 40:6–9 [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Suda C, Horie T. (2005) Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol 42:110–116 [DOI] [PubMed] [Google Scholar]
- Moon K-H, Abdelmegeed MA, Song B-J. (2007) Inactivation of cytosolic aldehyde dehydrogenase via S-nitrosylation in ethanol-exposed rat liver. FEBS Lett 581:3967–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, Conrads TP, Veenstra TD, Song BJ, Pacher P. (2008) Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology 135:1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronilli V, Costantini P, Scorrano L, Colonna R, Passamonti S, Bernardi P. (1994) The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J Biol Chem 269:16638–16642 [PubMed] [Google Scholar]
- Rafeiro E, Barr SG, Harrison JJ, Racz WJ. (1994) Effects of N-acetylcysteine and dithiothreitol on glutathione and protein thiol replenishment during acetaminophen-induced toxicity in isolated mouse hepatocytes. Toxicology 93:209–224 [DOI] [PubMed] [Google Scholar]
- Ryder SD, Beckingham IJ. (2001) ABC of diseases of liver, pancreas, and biliary system. Other causes of parenchymal liver disease. BMJ 322:290–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. (2010) Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology 51:246–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen WF, Jr, Voellmy R, Roberts SM. (1998) Effect of N-acetylcysteine on heat shock protein induction by acetaminophen in mouse liver. J Pharmacol Exp Ther 286:519–524 [PubMed] [Google Scholar]
- Smith CV, Mitchell JR. (1985) Acetaminophen hepatotoxicity in vivo is not accompanied by oxidant stress. Biochem Biophys Res Commun 133:329–336 [DOI] [PubMed] [Google Scholar]
- Song BJ, Suh SK, Moon KH. (2010) A simple method to systematically study oxidatively modified proteins in biological samples and its applications. Methods Enzymol 473:251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. (1990) Acetaminophen-induced oxidation of protein thiols. Contribution of impaired thiol-metabolizing enzymes and the breakdown of adenine nucleotides. J Biol Chem 265:3059–3065 [PubMed] [Google Scholar]
- Tompkins AJ, Burwell LS, Digerness SB, Zaragoza C, Holman WL, Brookes PS. (2006) Mitochondrial dysfunction in cardiac ischemia-reperfusion injury: ROS from complex I, without inhibition. Biochim Biophys Acta 1762:223–231 [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Association (2009) Joint Meeting of the Drug Safety and Risk Management Advisory Committee with the Anesthetic and Life Support Drugs Advisory Committee and the Nonprescription Drugs Advisory Committee; 2009 June 29–30; Adelphi, MD. [Google Scholar]
- Yang X, Greenhaw J, Ali A, Shi Q, Roberts DW, Hinson JA, Muskhelishvili L, Beger R, Pence LM, Ando Y, et al. (2012) Changes in mouse liver protein glutathionylation after acetaminophen exposure. J Pharmacol Exp Ther 340:360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]