Abstract
Dopaminergic neurons of the ventral tegmental area are important components of brain pathways related to addiction. Prolonged exposure of these neurons to moderate concentrations of dopamine (DA) decreases their sensitivity to inhibition by DA, a process called DA-inhibition reversal (DIR). DIR is mediated by phospholipase C and conventional subtype of protein kinase C (cPKC) through concurrent stimulation of D2 and D1-like DA receptors, or by D2 stimulation concurrent with activation of 5-HT2 or neurotensin receptors. In the present study, we further characterized this phenomenon by use of extracellular recordings in brain slices to examine whether DIR is linked to G protein-coupled receptor kinase-2 (GRK2) or dynamin by assessing DIR in the presence of antagonists of these enzymes. DIR was blocked by β-ARK1 inhibitor, which inhibits GRK2, and by dynasore, which blocks dynamin. Reversal of inhibition by D2 agonist quinpirole was produced by serotonin (50 µM) and by neurotensin (5–10 nM). Serotonin-induced or neurotensin-induced reversal was blocked by β-ARK1 inhibitor, dynasore, or cPKC antagonist 5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4c]carbazole-12-propanenitrile (Gö6976). This further characterization of DIR indicates that cPKC, GRK2, and dynamin play important roles in the desensitization of D2 receptors. As drugs of abuse produce persistent increases in DA concentration in the ventral tegmental area, reduction of D2 receptor sensitivity as a result of drug abuse may be a critical factor in the processes of addiction.
Introduction
Dopaminergic (DAergic) neurons in the ventral tegmental area (VTA) project to several regions of the mesocorticolimbic system including the nucleus accumbens, prefrontal cortex, and amygdala (Koob, 2003; Oades and Halliday, 1987). Increases in DAergic neurotransmission, which are caused by salient and motivational stimuli, are important for reward and reinforcement by numerous drugs of abuse (Di Chiara and Imperato, 1988; Wise, 1996; Mirenowicz and Schultz, 1996). Prolonged increases in dopamine (DA) concentrations in the VTA may affect the excitability of DAergic neurons of the VTA and may produce long-term changes in neurotransmission; for example, elevated DA can increase glutamatergic receptor expression in the prefrontal cortex (Gao and Wolf, 2008; Sun et al., 2008).
Five classes of DA receptors have been identified: two “D1-like” receptors (D1 and D5) and three “D2-like” receptors (D2, D3, and D4) (Sibley et al., 1993; Neve et al., 2004; Ciliax et al., 2000; Khan et al., 2000). The DAergic neurons of the VTA possess high densities of D2 (Bouthenet et al., 1991; Sesack et al., 1994) and D5 receptors (Ciliax et al., 2000; Khan et al., 2000) but low levels of D3 receptors (Bouthenet et al., 1991; Diaz et al., 1995; Gurevich and Joyce, 1999). The D1 and D4 receptors are quite sparse or are not detectable in the DAergic VTA neurons (Meador-Woodruff et al., 1992; Mengod et al., 1992; Rivera et al., 2008). However, the D1 receptors appear to be on presynaptic glutamatergic terminals projecting to the region, not on the DAergic VTA neurons themselves (Caillé et al., 1996).
The putative dopaminergic (pDAergic) VTA neurons fire action potentials spontaneously in vivo (Bunney et al., 1973) and in vitro (Brodie and Dunwiddie, 1987). This spontaneous firing is inhibited by the action of DA at D2 autoreceptors (Lacey et al., 1987; Brodie et al., 1990). However, we have demonstrated that prolonged application of DA results in a time- and concentration-dependent decrease in the magnitude of DA-induced inhibition, a phenomenon that we termed dopamine-inhibition reversal (DIR) (Nimitvilai and Brodie, 2010). This DIR is mediated by concurrent stimulation of D2 and D1-like receptors, requires 10 to 40 minutes to develop, and persists for up to 90 minutes (Nimitvilai and Brodie, 2010). Activation of the D1/D5 receptor linked to phosphatidylinositide (PI) accumulation, but not those that cause adenylyl cyclase (AC) activation, produced a decrease in sensitivity of the D2 receptor to its agonist (Nimitvilai et al., 2012c). DIR also requires the activation of phospholipase C (PLC) and conventional protein kinase C (cPKC), without involvement of AC, cAMP, or protein kinase A (Nimitvilai et al., 2012a). Recently, we have demonstrated that some (e.g., 5-HT2 and neurotensin) but not all (e.g., α1-adrenergic and group I metabotropic glutamate) Gq-coupled receptors that stimulate the PLC and PKC pathway can mediate the reversal of D2 agonist inhibition (Nimitvilai et al., 2012c).
An involvement of PKC in the phosphorylation and internalization of D2 receptors has been reported in many systems such as HEK293 cells, and striatal and hippocampal neurons (Namkung and Sibley, 2004; Bofill-Cardona et al., 2000; Thibault et al., 2011). Phosphorylation and internalization of D2 receptors may contribute to the reversal of DA inhibition found in the pDAergic VTA neurons. In the present study, therefore, we extended our investigation of DIR to examine elements shown to be involved in the phosphorylation and internalization of the D2 receptors. We also examined whether activation of either serotonin or neurotensin receptors requires similar phosphorylation and internalization processes to mediate the reversal of D2 agonist-induced inhibition.
Materials and Methods
Animals.
Male Fischer 344 (F344; adult rats, 4–6 weeks old, 90–150 g) used in these studies were obtained from Harlan Sprague-Dawley (Indianapolis, IN). All rats were treated in strict accordance with the National Institutes of Heath Guide for the Care and Use of Laboratory Animals, and all experimental methods were approved by the Animal Care Committee of the University of Illinois at Chicago.
Preparation of Brain Slices.
Brain slices containing the VTA were prepared from the subject animals as previously described elsewhere (Brodie et al., 1999a). Briefly, after brief isoflurane anesthesia and rapid removal of the brain, the tissue was blocked coronally to contain the VTA and substantia nigra; the cerebral cortices and a portion of the dorsal mesencephalon were removed. The tissue block was mounted in the Vibratome and submerged in chilled cutting solution to cut the coronal sections (400 μm thick). An individual slice was placed onto a mesh platform in the recording chamber and was totally submerged in artificial cerebrospinal fluid (aCSF) maintained at a flow rate of 2 ml/min; the temperature in the recording chamber was kept at 35°C. The composition of the aCSF in these experiments was (in mM): NaCl 126, KCl 2.5, NaH2PO4 1.24, CaCl2 2.4, MgSO4 1.3, NaHCO3 26, and glucose 11. The composition of the cutting solution was (in mM): KCl 2.5, CaCl2 2.4, MgSO4 1.3, NaHCO3 26, glucose 11, and sucrose 220. Both solutions were saturated with 95% O2/5% CO2 (pH = 7.4). Equilibration time of at least 1 hour was allowed after placement of tissue in the recording chamber before the electrodes were placed in the tissue.
Cell Identification.
The VTA was clearly visible in the fresh tissue as a gray area medial to the darker substantia nigra, and separated from the nigra by white matter. Recording electrodes were placed in the VTA under visual control. Putative DAergic (pDAergic) neurons have been shown to have distinctive electrophysiologic characteristics (Grace and Bunney, 1984; Lacey et al., 1989). We studied only those neurons that were anatomically located within the VTA and that conformed to the criteria for pDAergic neurons established in the literature and in this laboratory (Lacey et al., 1989; Mueller and Brodie, 1989). These criteria include broad action potentials (2.5 msec or greater, measured as the width of the biphasic or triphasic waveform at the baseline), slow spontaneous firing rate (0.5–5.0 Hz), and a regular interspike interval. The cells were not tested with opiate agonists as has been done by other groups to further characterize and categorize VTA neurons (Margolis et al., 2006; Chieng et al., 2011).
Additional characterization, such as determining the projection target of the cells we were studying (Margolis et al., 2008), would have been difficult as we have used extracellular recording to ensure high-quality, long-duration recordings. The long-duration, low-frequency action potentials that characterized the cells from which we recorded are associated with DA-sensitive, DA-containing neurons projecting to the nucleus accumbens, and DA sensitivity also is associated with DA VTA neurons projecting to the prefrontal cortex (Margolis et al., 2008). One consequence of differential initial sensitivity to DA inhibition among groups of neurons projecting to different brain areas (Margolis et al., 2008; Lammel et al., 2008) would be different amounts of DIR (Nimitvilai and Brodie, 2010), resulting in a greater relative change in neurons more sensitive to DA inhibition.
Drug Administration.
Drugs were added either to the aCSF or to the microelectrode filling solution (0.9% NaCl). Application of drugs to the aCSF by means of a calibrated infusion pump from stock solutions 100 to 1000 times the desired final concentrations was performed in such a way as to permit the drug solution to mix completely with aCSF before this mixture reached the recording chamber. Final concentrations were calculated from the aCSF flow rate, pump infusion rate, and concentration of drug stock solution. The small volume chamber (∼300 μl) used in these studies permitted the rapid application and washout of drug solutions. Typically drugs reach equilibrium in the tissue after 2 to 3 minutes of application.
When drugs were added to the microelectrode filling solution (0.9% NaCl), a concentration about 10 times greater than that which would have been used in the extracellular medium was needed. In all of our previous studies in which agonists and antagonists were delivered via the recording pipette (Nimitvilai et al., 2012b), the effective concentration of drugs were 10-fold higher than the effective concentration used in the extracellular medium. The concentrations of drugs used in the present study were likewise 10-fold higher than the concentrations reported in the literature for selective action. To allow time for the drug to diffuse from the pipette to the cell, the effects of bath-applied drugs were tested no less than 20 minutes after initiating the recording; this pipette-application method has produced comparable results to the administration of drugs through the extracellular medium in the cases in which both methods were tested (data not shown), with the advantage of more localized application and reduced expense. Such local delivery of drugs through recording pipettes has been used by our laboratory and others (Pesavento et al., 2000; Nimitvilai et al., 2012a). One disadvantage of this method is that the exact concentration of drug received by the neurons from which we recorded is unknown.
DA hydrochloride, quinpirole, serotonin (5-HT), neurotensin, and most of the salts used to prepare the extracellular media were purchased from Sigma (St. Louis, MO). Gö6976 (5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4c]carbozole-12-propanenitrile) and dynasore were purchased from Tocris (Ellisville, MO). β-ARK1 inhibitor (methyl 5-[2-(5-nitro-2-furyl)vinyl]-2-furoate) was purchased from Calbiochem (Gibbstown, NJ). MiTMAB (tetradecyltrimethlammonium bromide) was purchased from Abcam (Cambridge, MA).
Extracellular Recording.
Extracellular recording was chosen for these studies as this method permits the recordings to be of long duration and allows us to assess the effects of extended exposure (>60 minutes) to drugs. The limitation of only measuring spontaneous action potential frequency (rather than membrane potential or other electrophysiologic parameters) is counterbalanced by the advantage of being able to determine the time course of drug actions and interactions without disrupting the internal milieu. Extracellular recording electrodes were made from 1.5 mm diameter glass tubing with filament and were filled with 0.9% NaCl. Tip resistance of the microelectrodes ranged from 2 to 5 MΩ. A Fintronics amplifier was used in conjunction with an IBM PC-based data-acquisition system (ADInstruments Inc., Colorado Springs, CO). Offline analysis was used to calculate, display, and store the frequency of firing in 1-minute intervals.
Additional software was used to calculate the firing rate over 5-second intervals. The firing rate, which was determined before and during drug application, was calculated over 1-minute intervals before administration of drugs and during the drug effect. The peak drug-induced changes in firing rate were expressed as the percentage change from the control firing rate according to this formula: ((FRD − FRC) / FRC) × 100, where FRD is the firing rate during the peak drug effect and FRC is the control firing rate. The change in firing rate thus is expressed as a percentage of the initial firing rate, which controls for small changes in firing rate that may occur over time. This formula was used to calculate both excitatory and inhibitory drug effects. Peak excitation produced by the drug (e.g., DA) was defined as the peak increase in firing rate over the predrug baseline. Inhibition was defined as the lowest firing rate below the predrug baseline. Inhibition reversal was identified as a statistically significant reduction in the inhibition.
Data Collection.
For comparison of the time course of effects on firing rate, the data were normalized and averaged. Firing rates over 1-minute intervals were calculated and normalized to the 1-minute interval immediately before DA administration. These normalized data were averaged by synchronizing the data to the DA administration period, and graphs of the averaged data were made.
Statistical Analysis.
Averaged numerical values were expressed as the mean ± the standard error of the mean (S.E.M.). Mean response graphs are shown as the relative change in the firing rate normalized to the inhibition observed in the first 5-minute interval; in these cases, the mean percentage inhibition as a function of the baseline firing rate is indicated in the text. The effect of inhibitors alone on the firing rate was assessed using a paired t test. To address the question of whether there is a change in the magnitude of inhibition by DA agonists over time, the differences among firing rates during the long drug administration intervals in these studies were assessed with one-way repeated measures analysis of variance (ANOVA); degrees of freedom and statistical error terms are shown as subscripts to F in the text (Kenakin, 1987). Comparisons of degree of reversal of inhibition were not made, as there are a variety of factors that may contribute to different degrees of reversal, including the concentration of the agonist (Nimitvilai and Brodie, 2010). Statistical analyses were performed with OriginPro 8.5 (OriginLab Corp., Northampton, MA).
Results
VTA Neuron Characteristics.
A total of 121 VTA neurons were examined. Their firing rate in a normal extracellular medium ranged from 0.6 to 4.87 Hz, with a mean of 2.24 ± 0.08 Hz. All neurons had regular firing rates and were inhibited by DA agonists. Sensitivity to DA (0.5–5.0 μM) was initially assessed by administering the agonist for 5 minutes, and then washing it out until the firing rate recovered to at least 70% of the baseline firing rate; quinpirole (25–150 nM) was administered for 5 minutes, and the concentration was increased if inhibition greater than 50% was not achieved. The concentrations of agonist were adjusted for each neuron so that inhibition exceeded 50%, as inhibition that was less than 50% was not reliably reversed (Nimitvilai and Brodie, 2010). This method of adjusting the concentration of DAergic agonist controlled for differences in sensitivity between neurons but also sometimes resulted in the mean concentrations of DA or quinpirole slightly differing among groups. Overall, for pDAergic VTA neurons from adult rats, the concentration of DA used was 5.66 ± 0.67 µM (n = 31), which produced a mean change in firing rate of −67.55 ± 2.28% after 5 minutes of exposure; the concentration of quinpirole used was 84.19 ± 5.99 nM (n = 80), which produced a mean change in firing rate of −64.65 ± 1.59% after 5 minutes of exposure. There were no statistically significant differences in the concentration of DAergic agonists or in the percentage inhibition among the groups (Table 1; one-way ANOVA, P > 0.05).
TABLE 1.
Firing rates of VTA neurons: effects of inhibitors and quinpirole
| Figures | Change by inhibitors | Pre-quinpirole or DA baseline FR (Hz) | Percentage change by DA or quinpirole at 5 min |
|---|---|---|---|
| Figure 1 | |||
| Control (no inhibitor) | — | 2.07 ± 0.17 | −72.78 ± 4.83 |
| β-ARK1 inhibitor | −2.71 ± 3.25 | 2.45 ± 0.27 | −64.47 ± 3.42 |
| Dynasore | −2.51 ± 2.49 | 2.35 ± 0.24 | −62.99 ± 3.0 |
| Figure 2 | |||
| Control (no inhibitor) | — | 2.55 ± 0.34 | −68.27 ± 4.94 |
| Neurotensin (10 nM) | — | 3.63 ± 0.38 | −65.5 ± 4.16 |
| Neurotensin (5 nM) | — | 3.15 ± 0.58 | −61.65 ± 6.2 |
| β-ARK1 inhibitor | −7.75 ± 3.53 | 1.8 ± 0.45 | −68.21 ± 6.85 |
| Gö6976 | 0.88 ± 3.0 | 1.38 ± 0.22 | −64.54 ± 6.24 |
| Dynasore | −6.79 ± 3.9 | 1.75 ± 0.15 | −64.31 ± 6.35 |
| Figure 3 | |||
| Serotonin | — | 2.53 ± 0.21 | −64.02 ± 5.38 |
| β-ARK1 inhibitor | −2.95 ± 1.94 | 2.23 ± 0.32 | −73.68 ± 5.93 |
| Gö6976 | −9.04 ± 3.63 | 1.41 ± 0.28 | −58.3 ± 2.75 |
| Dynasore | −2.03 ± 2.63 | 2.59 ± 0.3 | −62.26 ± 6.57 |
β-ARK1 inhibitor, methyl 5-[2-(5-nitro-2-furyl)vinyl]-2-furoate; DA, dopamine; FR, firing rate; Gö6976 (5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4c]carbozole-12-propanenitrile); VTA, ventral tegmental area.
In the absence of DA transporter blockers, DA produces inhibitory effects at concentrations ranging from 0.5 to 100 μM, although in dissociated DA VTA neurons, concentrations as low as 50 nM can completely inhibit spontaneous action potential firing (Brodie et al., 1999b). Cells that did not return to at least 70% of their pre-DA firing rate during this washout were not used; 8 out of 129 cells did not return to 70% of their preagonist baseline firing rate. Table 1 also lists the effects on spontaneous firing rate of the various inhibitors used in the experiments to be described; none of the inhibitors produced a statistically significant change in the firing rate (paired t test, P > 0.05). One benefit of the extracellular recording method used in these studies is that long-duration recordings can be made reliably; the average recording duration was 95.58 ± 0.66 minutes, with a range of 90 to 105 minutes.
Dopamine Inhibition Reversal Did Not Occur When Either G Protein-Coupled Receptor Kinase-2 or Dynamin GTPase Was Suppressed.
Time-dependent reversal of DA inhibition occurs with moderate concentrations of DA alone or the D2 agonist quinpirole in the presence of D1-like receptor agonist (Nimitvilai and Brodie, 2010; Nimitvilai et al., 2012a). This phenomenon is dependent on calcium and is mediated by activation of the PLC and cPKC pathway (Nimitvilai et al., 2012a). D1/D5 agonists linked to the PI/PLC but not the AC/cAMP pathway also induce the reversal of quinpirole-induced inhibition (Nimitvilai et al., 2012c). There is evidence that agonist-induced D2 receptor desensitization and internalization is dependent on G protein-coupled receptor kinase-2 (GRK2) and endocytotic GTPase dynamin (Ito et al., 1999; Iwata et al., 1999; Thibault et al., 2011).
In the present study, therefore, we examined whether DIR is inhibited by blockers of GRK2 or dynamin (Figs. 1 and 2). Figure 1, A–D, illustrates data from single neurons. For clarity, the pooled data in Fig. 2 are presented normalized to the firing rate 5 minutes after DA was superfused; increases in the relative firing rate indicate reversal of inhibition, and decreases in the relative firing rate indicate more inhibition with time. The selective inhibitor of GRK2 called β-ARK1 inhibitor (300 μM) (Iino et al., 2002), the dynamin inhibitor dynasore (800 μM) (Macia et al., 2006), or the dynamin inhibitor MiTMAB (400 μM) (Quan et al., 2007) was dissolved in saline. Saline alone or saline containing one of these drugs was used to fill the recording electrodes that were used to make extracellular recordings of single pDAergic VTA neurons. After initiating recording of pDAergic neurons and allowing the drug in the pipettes to act locally for at least 20 minutes, concentrations of DA were applied in the superfusate in a stepwise fashion, in which each concentration was added for 5 minutes and increased until inhibition of firing of 50% or greater was achieved; this concentration was applied for 40 minutes. As in our previous studies (Nimitvilai et al., 2012c; Nimitvilai and Brodie, 2010), DA alone (Figs. 1A and 2) produced an inhibition in firing rate at 5 minutes of 72.78% ± 4.83%, and this inhibition partially reversed with time so that at 40 minutes there was a statistically significant reduction in the DA-induced inhibition.
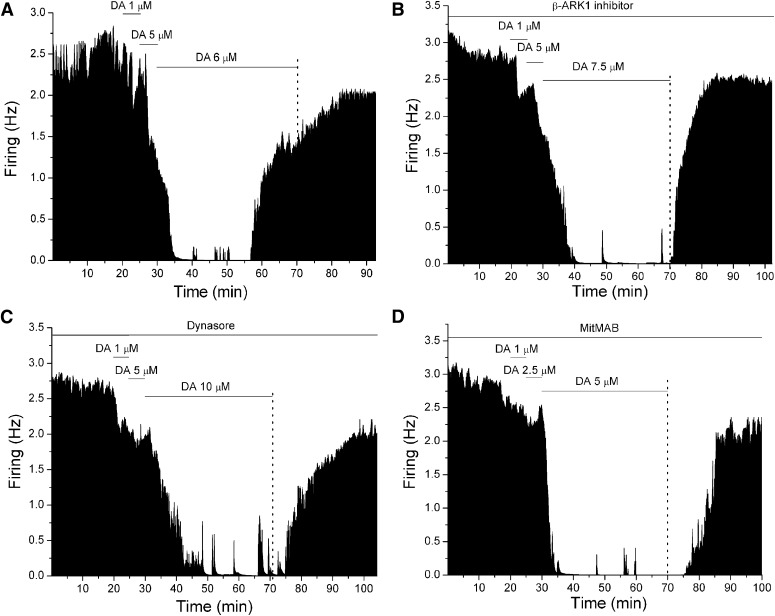
Fig. 1.
Mean ratemeter graphs of the effects of long-duration application of dopamine (DA) in the presence or absence of either G protein-coupled receptor kinase-2 (GRK2) inhibitor or dynamin inhibitor in single neurons. Vertical bars indicate the firing rate over 5-second intervals; the dashed vertical line indicates the end of DA administration for clarity. Horizontal bars indicate the duration of drug application (concentrations indicated above bar). (A) DA alone initially produced a decrease in the firing rate, which subsided over time in the continued presence of DA. (B) in the presence of 300 μM β-ARK1 inhibitor in the recording pipette, DA produced an inhibition in the firing rate, and this inhibition did not reverse with time. (C) in the presence of 800 µM dynasore in the recording pipette, DA produced an inhibition in the firing rate, with no reversal during DA administration. (D) in the presence of 400 μM MitMAB in the recording pipette, DA produced a statistically significant inhibition in the firing rate over the time course of administration, indicating blockade of reversal.
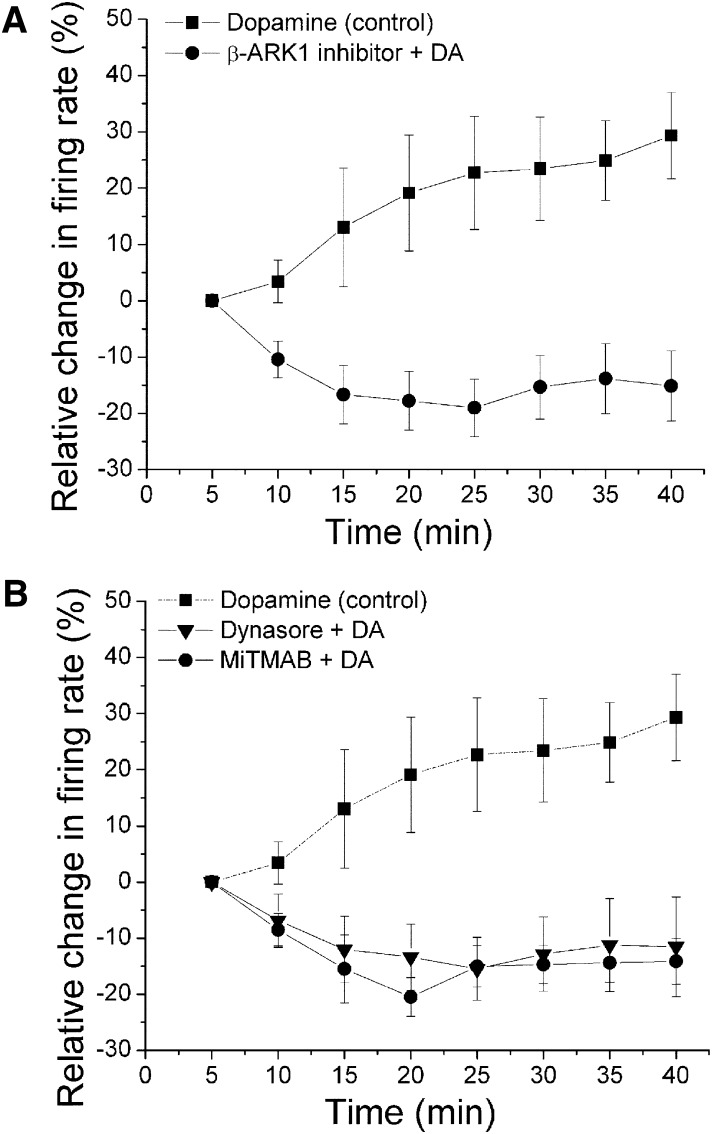
Fig. 2.
Effect of inhibitors of G protein-coupled receptor kinase-2 (GRK2) and dynamin on long-duration dopamine (DA) application. Relative change in the firing rate (mean ± S.E.M.) is plotted as a function of time. In experiments similar to those shown in Fig. 1, the effect of DA at each time point was normalized by subtracting the change in firing rate (%) at the 5-minute time point. (A) effect of β-ARK1 inhibitor on long-duration application of DA. A concentration of DA that produced more than 50% inhibition at 5-minute time point was applied for 40 minutes. Dopamine (▪, [DA] = 4.45 ± 1.25 μM, n = 10) alone initially inhibited the firing rate, and this inhibition statistically significantly reversed with time (one-way repeated measures ANOVA, F(7,63) = 5.96, P < 0.05). In the presence of β-ARK1 inhibitor (300 μM), no reversal of DA inhibition was observed; DA statistically significantly inhibited the firing rate over time (●, [DA] = 5.2 ± 1.2 μM, n = 9) (one-way repeated measures ANOVA, F(7,56) = 3.23, P < 0.05). (B) effect of dynamin inhibitors dynasore or MiTMAB on long-duration application of DA. The effect of DA alone (▪ and dashed line) from Fig. 2A is shown for comparison. In the presence of dynasore (800 μM) in the recording pipette, DA did not produce the inhibition reversal (▾, [DA] = 6.64 ± 1.58 μM, n = 7) (one-way repeated measures ANOVA, F(7,42) = 1.29, P > 0.05). In the presence of MiTMAB (400 μM) in the recording pipette, DA produced a statistically significant inhibition in the firing rate, with no reversal (●, [DA] = 7.5 ± 1.12 μM, n = 5) (one-way repeated measures ANOVA, F(7,28) = 5.46, P < 0.05).
In Fig. 2A, DIR is illustrated as a relative increase in firing rate (%) compared with the 5-minute time point (▪, [DA] = 4.45 ± 1.25 μM, n = 10) (one-way repeated measures ANOVA, F(7,63) = 5.96, P < 0.05). In the presence of β-ARK1 inhibitor (Figs. 1B and 2A), however, DA produced a statistically significant reduction in firing rate with no reversal (●, [DA] = 5.2 ± 1.2 μM, n = 9) (one-way repeated measures ANOVA, F(7,56) = 3.23, P < 0.05). In the presence of dynasore (Figs. 1C and 2B), no statistically significant reversal of DA inhibition was observed (▾, [DA] = 6.64 ± 1.58 μM, n = 7) (one-way repeated measures ANOVA, F(7,42) = 1.29, P > 0.05). Likewise, in the presence of dynamin inhibitor MiTMAB (Figs. 1D and 2B), DA produced a statistically significant inhibition in firing rate with no reversal (●, [DA] = 7.5 ± 1.12 μM, n = 5) (one-way repeated measures ANOVA, F(7,28) = 5.46, P < 0.05). These results suggest that DIR is mediated by GRK-2 phosphorylation and dynamin-dependent internalization of D2 receptors.
Activation of Neurotensin Receptors Reversed Quinpirole-Induced Inhibition through Conventional Protein Kinase C, G Protein-Coupled Receptor Kinase-2, and Dynamin-Dependent Processes.
We have demonstrated previously elsewhere that some, but not all, Gq-coupled receptors produce a decrease in sensitivity of D2 receptors to D2 agonist quinpirole (Nimitvilai et al., 2012c). Since activation of neurotensin receptors produces the reversal of quinpirole-induced inhibition, we explored whether this phenomenon is also mediated by GRK2- and dynamin-dependent processes (Fig. 3). The saline alone or saline containing either the GRK2-inhibitor β-ARK1 inhibitor (300 µM) or the dynamin-inhibitor dynasore (800 µM) was used to fill the recording pipettes, and these pipettes were used to measure changes in the firing rate of pDAergic VTA neurons over time. After obtaining the recording of pDAergic VTA neurons, the firing rate was measured for at least 20 minutes to allow the drug in the pipettes to act locally on the neurons. Then neurotensin (10 nM) was added to the superfusate for 15 minutes, producing an increase in the firing rate of 72.0% ± 29.2%; this new firing rate was used as a new baseline for measuring the effect of quinpirole over the 40-minute time course.
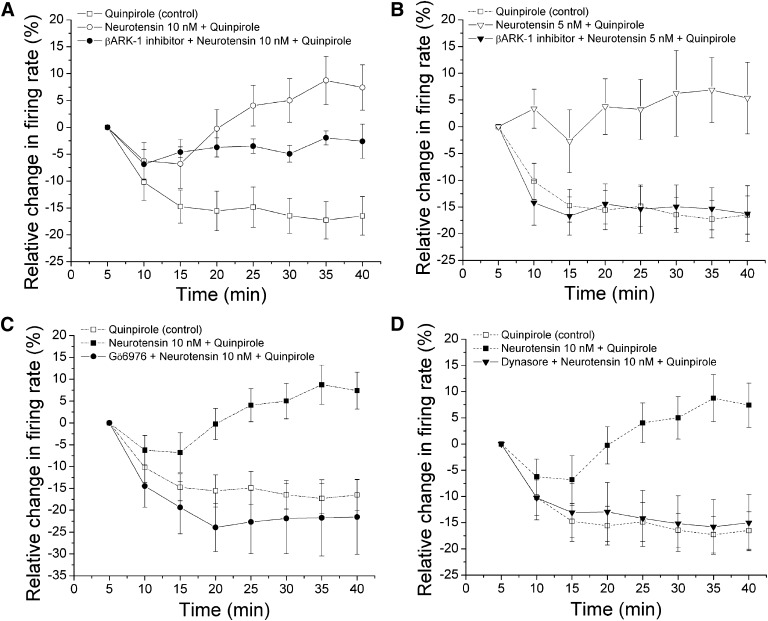
Fig. 3.
Effect of inhibitors of G protein-coupled receptor kinase-2 (GRK2), conventional protein kinase C (cPKC), and dynamin on long-duration application of neurotensin and quinpirole. Relative change in the firing rate (mean ± S.E.M.) is plotted as a function of time. (A) effect of β-ARK1 inhibitor on long-duration application of quinpirole and neurotensin (10 nM). Quinpirole (54 ± 8.5 nM) alone produced a sustained, statistically significant inhibition in the firing rate over the duration of quinpirole application (□, n = 10) (one-way repeated measures ANOVA, F(7,63) = 11.2, P < 0.05). With 10 nM neurotensin in the superfusate (○, n = 9), there was a statistically significant difference in the magnitude of quinpirole inhibition at the 25- and 40-minute time points compared with the 10-minute time point, indicating reversal of inhibition (one-way repeated measures ANOVA, F(7,35) = 4.0, P < 0.05). In the presence of 10 nM neurotensin in the superfusate and 300 μM β-ARK1 inhibitor in the recording pipette (●, n = 6), no reversal and no significant increase in inhibition in the firing rate produced by quinpirole were observed ([quinpirole] = 125 ± 8.26 nM) (one-way repeated measures ANOVA, F(7,35)= 3.33, P > 0.05). (B) effect of β-ARK1 inhibitor on long-duration application of quinpirole and neurotensin (5 nM). The effect of quinpirole alone (□ and dashed line) from A is shown for comparison. With 5 nM neurotensin in the superfusate (∇, n = 7), no significant inhibition produced by quinpirole (60.7 ± 9.2 nM) was observed (one-way repeated measures ANOVA, F(7,42) = 1.25, P > 0.05); there was a partial reversal in the magnitude of quinpirole inhibition over time. In the presence of 5 nM neurotensin in the superfusate and 300 μM β-ARK1 inhibitor in the recording pipette (▾, n = 8), quinpirole (45.83 ± 3.71 nM) produced a statistically significant inhibition in the firing rate over the time course (one-way repeated measures ANOVA, F(7,49)= 4.16, P < 0.05). (C) effect of Gö6976 on long-duration application of quinpirole and neurotensin (10 nM). The effect of quinpirole alone (□ and dashed line) and quinpirole in the presence of 10 nM neurotensin (▪ and dashed line) from A are shown for comparison. In the presence of 10 μM Gö6976 in the recording pipette and neurotensin in the superfusate (●, n = 5), no significant quinpirole inhibition reversal was observed ([quinpirole] = 64 ± 34.1 nM) (one-way repeated measures ANOVA, F(7,28)= 87.9, P < 0.05). (D) effect of dynasore on long-duration application of quinpirole and neurotensin. The effect of quinpirole alone (□ and dashed line) and quinpirole in the presence of 10 nM neurotensin (▪ and dashed line) from A are shown for comparison. In the presence of 10 nM neurotensin in the superfusate and 800 µM dynasore in the recording electrode (▾, n = 7), no reversal of quinpirole-induced inhibition was observed; quinpirole (75 ± 12.2 nM) produced a statistically significant inhibition in the firing rate over time (one-way repeated measures ANOVA, F(7,42) = 6.91, P < 0.05).
Concentrations of quinpirole were applied in a stepwise fashion, in which each concentration was added for 5 minutes and increased until inhibition of 50% or greater was achieved. This concentration of quinpirole was sustained for 40 minutes. As shown previously elsewhere (Nimitvilai et al., 2012c), in the presence of neurotensin (○, n = 9), quinpirole (100 ± 19.54 nM) produced a statistically significant inhibition in the firing rate with a maximum inhibition of −71.74% ± 5.85% at 10 minutes, and this inhibition partially reversed with time; there was a statistically significant difference between the last four time points compared with the 10-minute time point (one-way repeated measures ANOVA, F(7,35) = 4.0, P < 0.05). Without neurotensin, quinpirole (54 ± 8.5 nM) alone statistically significantly inhibited the firing rate, and this inhibition did not reverse with time (□, n = 10) (one-way repeated measures ANOVA, F(7,63) = 11.2, P < 0.05) (Fig. 3A). In control experiments, when neurotensin alone was applied for 60 minutes, the firing rate statistically significantly increased by 78.8 ± 20.7% within 15 minutes; there was no statistically significant change in the firing rate from 15 to 60 minutes of neurotensin administration (one-way repeated measures ANOVA, F(11,55) = 4.8, P < 0.05) (data not shown). This result indicates that the apparent reversal of inhibition was not due to a gradual increase in neurotensin-mediated excitation over time but more likely was due to reduction of the quinpirole-induced inhibition.
With β-ARK1 inhibitor (300 µM) in the recording pipettes, when neurotensin was applied in the superfusate (●, n = 6), no reversal of quinpirole inhibition was observed ([quinpirole] = 125 ± 8.26 nM); however, unlike under control conditions, quinpirole did not produce a statistically significant additional inhibition in the firing rate over time (one-way repeated measures ANOVA, F(7,35)= 3.33, P > 0.05) (Fig. 3A). As the effect of the β-ARK1 inhibitor could have been overwhelmed by the effect of the high neurotensin concentration, and as the β-ARK1 inhibitor has limited solubility, we tested whether a lower concentration of neurotensin could mediate the reversal of quinpirole inhibition, and whether the β-ARK1 inhibitor would be able to block quinpirole inhibition reversal induced by this lower neurotensin concentration.
Neurotensin (1 and 5 nM) was added to the superfusate for 15 minutes. Then concentrations of quinpirole that had produced at least 50% inhibition during the first 5 minutes were applied for 40 minutes with the continued administration of neurotensin. In the presence of 5 nM neurotensin, quinpirole (60.7 ± 9.2 nM, ∇, n = 7) inhibited the firing rate by 61.65% ± 6.2% at 5 minutes. Unlike the effect of quinpirole alone, quinpirole in the presence of 5 nM neurotensin did not significantly produce more inhibition over time; partial reversal of quinpirole inhibition was observed (one-way repeated measures ANOVA, F(7,42) = 1.25, P > 0.05) (Fig. 3B). Neurotensin (1 nM) did not mediate quinpirole inhibition reversal; there was a statistically significant increase in inhibition over the time course of quinpirole administration in the presence of 1 nM neurotensin (one-way repeated measures, F(7,21) = 5.03, P < 0.05) (data not shown). Thus, 5 nM neurotensin was used to test whether β-ARK1 inhibitor could block neurotensin-induced quinpirole inhibition reversal.
With β-ARK1 inhibitor (300 µM) in the recording pipettes and neurotensin (5 nM) in the superfusate, quinpirole (45.8 ± 3.7 nM, ▾, n = 8) produced a statistically significant inhibition in the firing rate with no reversal (one-way repeated measures ANOVA, F(7,49)= 4.2, P < 0.05), and this inhibition was similar to that produced by quinpirole alone (Fig. 3B). This result suggests that there is a competitive dose effect in the interaction between neurotensin and β-ARK1 inhibitor; greater activation of neurotensin receptors can overcome the interference by β-ARK1 inhibitor with the mechanism of reversal of quinpirole inhibition.
There is evidence that neurotensin activation of PKC can phosphorylate D2 receptors in HEK293 cells, resulting in phosphorylation and desensitization of the receptors (Thibault et al., 2011). Therefore, we examined whether inhibition of cPKC can interfere with neurotensin reversal of quinpirole-induced inhibition. In the presence of cPKC inhibitor Gö6976 (10 μM) in the recording pipette and neurotensin (10 nM) in the superfusate (●, n = 5), quinpirole statistically significantly inhibited the firing rate with no reversal ([quinpirole] = 64 ± 34.07 nM) (one-way repeated measures ANOVA, F(7,28)= 87.9, P < 0.05) (Fig. 3C), suggesting that neurotensin activation of cPKC may phosphorylate GRK2, which further phosphorylates and desensitizes the D2 receptors.
We then tested whether neurotensin reversal of quinpirole inhibition depends on a dynamin-dependent process. In the presence of neurotensin (10 nM) in the superfusate and dynasore (800 µM) in the recording electrode (▾, n = 7), no reversal of quinpirole-induced inhibition was observed; quinpirole (75 ± 12.2 nM) produced a statistically significant inhibition in the firing rate over time (one-way repeated measures ANOVA, F(7,42) = 6.91, P < 0.05) (Fig. 3D). This result suggests that desensitization of D2 receptors induced by the activation of neurotensin receptors is also dependent on a dynamin-dependent process, such as internalization of the receptor.
Activation of Serotonin Receptors Reversed Quinpirole-Induced Inhibition through Conventional Protein Kinase C, G Protein-Coupled Receptor Kinase-2, and Dynamin-Dependent Processes.
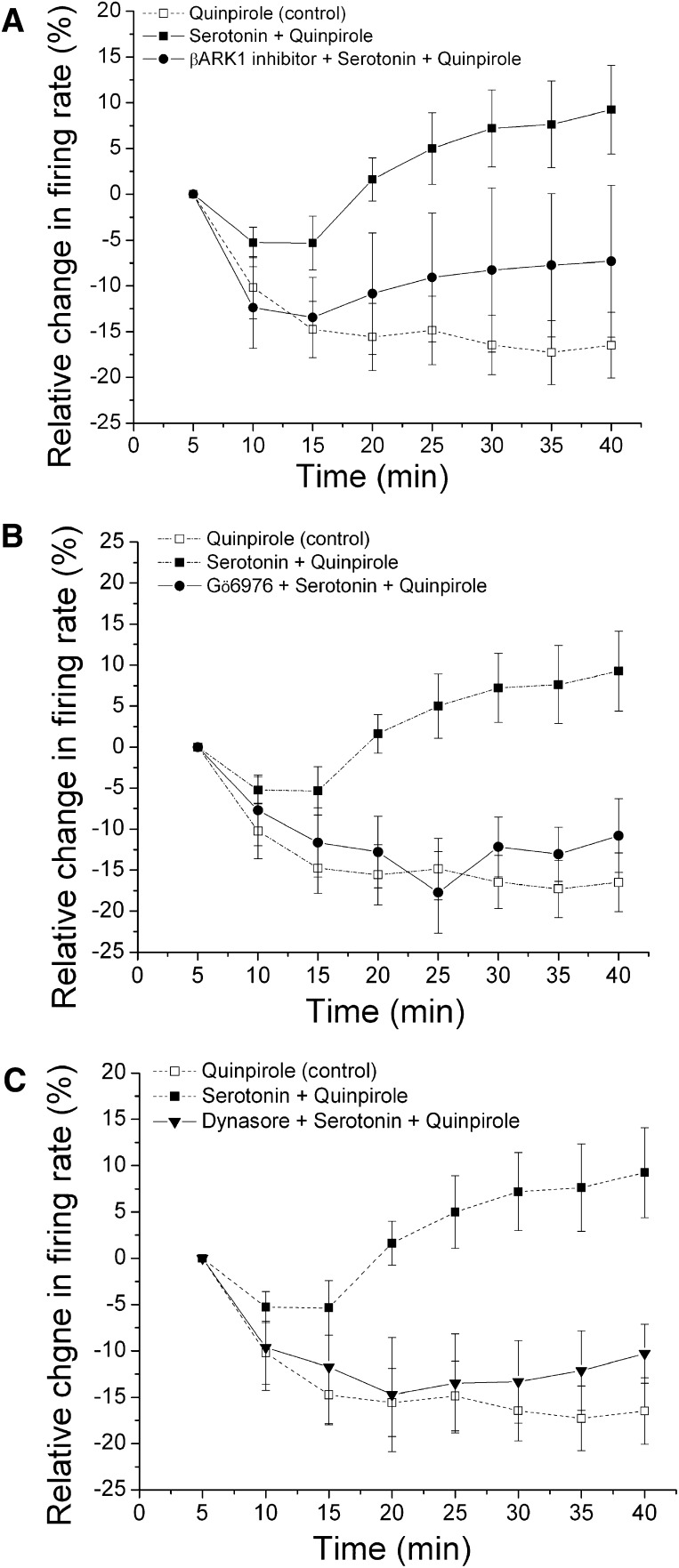
We also examined whether prolonged activation of the 5-HT receptors, which has been reported to reverse quinpirole inhibition (Nimitvilai et al., 2012c), depends on the phosphorylation and internalization induced by GRK2 and dynamin, respectively (Fig. 3). As in our previous report (Nimitvilai et al., 2012c), when 5-HT (50 µM) was added to the superfusate (▪, n = 9), quinpirole (115 ± 16.96 nM) produced an inhibition in the firing rate initially, with the maximum inhibition of 69.23% ± 5.85% at 10 minutes, an inhibition that partially reversed with time; there was a statistically significant difference between the firing rate at the last three time points and the firing rate at the 10-minute time point (one-way repeated measures ANOVA, F(7,56) = 5.18, P < 0.05) (Fig. 4A). Serotonin (50 μM) alone did not produce a statistically significant change in the firing rate over the 60-minute time course (one-way repeated measures ANOVA, F(11,33) = 2.3, P > 0.05) (data not shown).
Fig. 4.
Effect of inhibitors of G protein-coupled receptor kinase-2 (GRK2), conventional protein kinase C (cPKC), and dynamin on long-duration application of serotonin (5-HT) and quinpirole. Relative change in the firing rate (mean ± S.E.M.) is plotted as a function of time. (A) effect of β-ARK1 inhibitor on long-duration application of quinpirole and 5-HT. The effect of quinpirole alone (□ and dashed line) from Fig. 3A is shown for comparison. With 50 µM 5-HT in the superfusate (▪, n = 9), there was a statistically significant difference in the magnitude of quinpirole inhibition at the 30- and 40-minute time points compared with the 10-minute time point, indicating reversal of inhibition. In the presence of 50 µM 5-HT in the superfusate and 300 μM β-ARK1 inhibitor in the recording pipette (●, n = 7), no reversal and no significant inhibition in the firing rate produced by quinpirole were observed ([quinpirole] = 72.9 ± 8.0 nM) (one-way repeated measures ANOVA, F(7,42)= 1.49, P > 0.05). (B) effect of Gö6976 on long-duration application of quinpirole and serotonin. The effect of quinpirole alone (□ and dashed line) from Fig. 3A and quinpirole in the presence of 50 μM 5-HT (▪ and dashed line) from A are shown for comparison. In the presence of 10 μM Gö6976 in the recording pipette and 5-HT in the superfusate (●, n = 6), no significant quinpirole inhibition reversal was observed ([quinpirole] = 102.5 ± 25.3 nM) (one-way repeated measures ANOVA, F(7,35)= 92.8, P < 0.05). (C) effect of dynasore on long-duration application of quinpirole and 5-HT. The effect of quinpirole alone (□ and dashed line) from Fig. 3A and quinpirole in the presence of 5-HT (▪ and dashed line) from A are shown for comparison. In the presence of 50 µM 5-HT in the superfusate and 800 µM dynasore in the recording electrode (▾, n = 6), no reversal of quinpirole-induced inhibition was observed; quinpirole (125 ± 38.7 nM) produced a statistically significant inhibition in the firing rate over time (one-way repeated measures ANOVA, F(7,35) = 4.0, P < 0.05).
In the presence of 5-HT in the superfusate and β-ARK1 inhibitor (300 µM) in the recording electrode (●, n = 7), quinpirole (72.86 ± 8.01 nM) produced an inhibition in the firing rate, and this inhibition did not significantly reverse over the duration of the drug application (one-way repeated measures ANOVA, F(7,42) = 1.49, P > 0.05) (Fig. 4A).
We also tested whether blocking cPKC could interfere with the 5-HT reversal of quinpirole inhibition. A similar suppression of 5-HT reversal of quinpirole inhibition was observed when the cPKC inhibitor Gö6976 (10 µM) was present in the recording pipettes (●, n = 6); in this case, quinpirole (102.5 ± 25.3 nM) statistically significantly inhibited the firing rate over the duration of the drug application (one-way repeated measures ANOVA, F(7,35) = 2.8, P < 0.05) (Fig. 4B). These results suggest that 5-HT activation of cPKC may directly phosphorylate GRK2, and this activated GRK2 will further phosphorylate the D2 receptor.
When 5-HT was added in the superfusate with dynasore (800 µM) in the recording electrode (▾, n = 6), quinpirole (125 ± 38.7 nM) produced a statistically significant inhibition in the firing rate with no reversal (one-way repeated measures ANOVA, F(7,35) = 4.0, P < 0.05) (Fig. 4C). This result suggests that 5-HT reversal of quinpirole inhibition requires internalization of the D2 receptors, induced by the endocytic GTPase dynamin.
Discussion
We have previously reported a phenomenon of DIR that is induced by extended periods of exposure to moderate concentrations of DA; DIR persists for up to 90 minutes, and requires the concurrent stimulation of D1/D5 and D2 DA receptors (Nimitvilai and Brodie, 2010). We have also demonstrated that DIR is mediated by PLC and cPKC, and is dependent on both extracellular calcium influx and intracellular calcium release (Nimitvilai et al., 2012a). In addition, agonists of either 5-HT2 or neurotensin receptors, both of which are linked to PLC activation, produced a decrease in sensitivity of D2 receptor to the D2 agonist quinpirole (Nimitvilai et al., 2012c), suggesting that this phenomenon is mediated through the Gq/PLC/PKC pathway. In the present study, we extended our examination of the reversal of DA or quinpirole inhibition to show that enzymes involved in the phosphorylation of D2 receptors are required; phosphorylation as a result of DA, 5-HT, or neurotensin receptor stimulation is mediated by GRK2, and internalization is mediated by the endocytic GTPase dynamin. Of course, we are basing our interpretation on our pharmacologic results, but biochemical studies would be needed to confirm this interpretation of our findings. As we did not specifically examine the phosphorylation of D2 receptors, we cannot be certain that it is indeed the D2 receptor that is the phosphorylation target.
Functional efficiencies of G protein-coupled receptors (GPCRs) are not static; rather, they are dynamic and dependent on a memory of prior receptor activation (Hausdorff et al., 1990). Prolonged or repeated activation of a receptor results in a reduced response to a subsequent receptor stimulation, a process called desensitization. Desensitization can be homologous or heterologous; homologous desensitization is due to a decrease in response of a receptor as a result of binding its agonist, whereas heterologous desensitization is due to a decrease in response to a receptor as a result of agonist binding to a different receptor. We found that DIR of DAergic VTA neurons is neither homologous nor heterologous desensitization because it requires the concurrent stimulation of D2 and D1/D5 receptors (Nimitvilai and Brodie, 2010). To our knowledge, the D2 receptor is unique in that no other G protein-coupled receptor requires activation of two receptors to stimulate the processes necessary for desensitization of that receptor.
G protein-receptor kinases (GRKs) and β-arrestins are the two major cytoplasmic components responsible for desensitizing GPCR signaling (Premont et al., 1995; Sterne-Marr and Benovic, 1995). Binding of a ligand as well as the release of Gα and Gβγ induce the conformational change of the GPCR, resulting in a recruitment of GRK to the serine/threonine phosphorylation site of the intracellular loops or the COOH terminus of the receptor (Pitcher et al., 1998). GRK phosphorylation of the GPCR increases the affinity of that GPCR for β-arrestin. Once β-arrestin binds to GPCR, it prevents the reformation of functional GPCR so that a ligand cannot bind and activate the receptor. The β-arrestin also recruits clathrin and the adaptor protein AP2 to the phosphorylated GPCR, resulting in the formation of clathrin-coated vesicle. Dynamin GTPase then pinches off the clathrin-coated pit from the cell surface, internalizing the receptor (McMahon and Boucrot, 2011).
Dopamine receptors, like other GPCRs, can be regulated in a number of ways. Dopamine receptors contain phosphorylation sites for GRK, PKC, and Ca2+/calmodulin-dependent protein kinase II (CaMKII) on their third intracellular loop and their C-terminal region (Namkung and Sibley, 2004; Bofill-Cardona et al., 2000). Phosphorylation and desensitization of D2 receptors by PKC and CaMKII has also been studied in HEK293 cells, and striatal and hippocampal neurons (Rogue et al., 1990; Bofill-Cardona et al., 2000; Namkung and Sibley, 2004; Thibault et al., 2011). Second messenger-dependent protein kinases can either directly phosphorylate and desensitize the GPCR (Bofill-Cardona et al., 2000; Namkung and Sibley, 2004) or phosphorylate GRKs (Pronin and Benovic, 1997; Pronin et al., 1997; Chuang et al., 1996; Chuang et al., 1995; Winstel et al., 1996). Second-messenger protein kinase phosphorylation of GRK can either activate or inhibit GRK activity. For example, PKC phosphorylation can activate GRK2 (Chuang et al., 1995; Winstel et al., 1996), but inhibit GRK5 (Pronin and Benovic, 1997) activity in β-adrenergic receptors. Calmodulin inhibits GRK activity with a higher specificity of GRK5 (IC50 ∼50 nM) over GRK2 (IC50 ∼2 µM) (Chuang et al., 1996; Pronin et al., 1997). Phosphorylation and desensitization of DA D2 receptors by GRK2 and GRK5 have been reported (Ito et al., 1999).
At present, seven subtypes of GRKs (GRK1–7) have been identified. GRK1 and GRK7 are expressed in the photoreceptor rhodopsin, and GRK4 is found predominantly in the male germ line. GRK2, 3, and 6 are widely distributed in the rat brain, in which GRK2 is present in high levels in most brain areas and is also expressed in VTA neurons (Erdtmann-Vourliotis et al., 2001). In this study, we demonstrated that desensitization of the D2 receptor induced by DA, 5-HT, or neurotensin was not observed when GRK2 was suppressed, suggesting that GRK2 is involved in the desensitization of D2 receptors induced by DA, 5-HT, or neurotensin. We only used one antagonist (β-ARK1 inhibitor) to interfere with GRK2, as it is somewhat unique in having properties suitable for use with the methods used in other experiments in this study; more complex studies using other methods (e.g., siRNA or knockout of GRK2) would be needed to more definitively establish the importance of GRK2 in the desensitization mechanism. As cPKC is required to produce D2 desensitization (Nimitvilai et al., 2012a) and our present study shows that blocking of cPKC by Gö6976 also inhibited serotonin- or neurotensin-induced reversal of quinpirole inhibition, it is possible that activation of cPKC by either DA, 5-HT, or neurotensin results in its binding to and phosphorylation of GRK2.
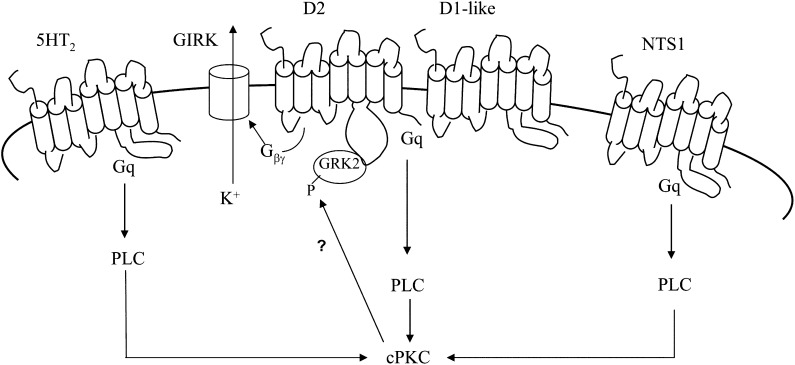
Of the seven subtypes of serotonin receptors, 5-HT2 is the G protein-coupled receptor that is linked to Gq, and 5-HT2 inhibitor ketanserin is able to block serotonin-induced quinpirole inhibition reversal (Nimitvilai et al., 2012c). For the neurotensin receptors, NTS1 is the likely subtype of the neurotensin receptor present in the VTA (Palacios and Kuhar, 1981; Quirion et al., 1985). Therefore, our model proposes that DA, 5-HT, or neurotensin activate a cPKC that binds to and phosphorylates GRK2, increasing the functional activity of GRK2 in DAergic VTA neurons (Fig. 5). The functional GRK2 may further phosphorylate the D2 receptor, resulting in the desensitization of the receptor. However, there is also evidence of the ability of GRK2 to constitutively attenuate D2 receptor function through a mechanism independent of receptor phosphorylation (Namkung et al., 2009).
Fig. 5.
Model of the action of different Gq-coupled receptors on conventional protein kinase C (cPKC) and D2 receptor sensitivity. The diagram illustrates a hypothetical interaction of 5-HT2, neurotensin (NTS1), and dopamine (D2, D1-like) receptors on cPKC in dopamine (DA) ventral tegmental area (VTA) neurons, based on the results of our study. The G-protein coupled inwardly rectifying potassium channel (GIRK) that is activated by Gβγ and that causes the hyperpolarization resulting in a decrease in the firing rate is shown. Activation of DA receptors (D2 plus D1/D5), serotonin 5-HT2, or neurotensin NTS1 receptors activates cPKC, which phosphorylates and stimulates G protein-coupled receptor kinase-2 (GRK2). The stimulated GRK2 then phosphorylates the D2 receptor, resulting in the desensitization of the receptor.
Whether GRK2 desensitization of D2 receptors in DAergic VTA neurons requires receptor phosphorylation is not known. It is unlikely that GRK5, which is also involved in phosphorylation and desensitization of D2 receptors in HEK293 and COS7 cells (Ito et al., 1999), is involved in DIR since it is strongly inhibited by both Ca2+/calmodulin and PKC (Pronin et al., 1997; Chuang et al., 1996), and overexpression of GRK5 fails to mediate desensitization of several GPCRs (Diviani et al., 1996; Rockman et al., 1996). In addition, GRK5 and GRK6, when coexpressed with D2 receptors in HEK293 cells, have no impact on agonist-induced D2 signaling (Namkung et al., 2009). Other possibilities, such as whether GRK3 or other proteins sensitive to β-ARK1 inhibitor are involved in the desensitization of the D2 receptor in DAergic VTA neurons, will be a subject for future study.
We also demonstrated that inhibition of dynamin GTPase suppressed DIR and the quinpirole inhibition reversal produced by either serotonin or neurotensin. These results suggest that once the D2 receptor has been desensitized, it will be internalized into the endosome. The endocytosed receptor may be dephosphorylated before returning to the cell surface, or it may be degraded to lysosomes. We have shown previously that the effect of DIR is long lasting, persisting for up to 90 minutes after washout of DA (Nimitvilai and Brodie, 2010). However, whether the reoccurrence of inhibition in the firing rate produced by D2 agonist is the result of the recycling of D2 receptors that had been initially desensitized or is the result of activation of newly synthesized D2 receptors is not known.
Exposure to drugs of abuse causes a sustained increase in DAergic neurotransmission in the reward system; desensitization of the inhibitory D2 autoreceptors in the VTA neurons through the PI/PLC/cPKC pathway may increase the excitability of these neurons. The present study further defines the mechanism of that desensitization induced by DA, 5-HT, or neurotensin, with the involvement of phosphorylation and internalization of D2 receptors. The action of drugs of abuse on DAergic VTA neurons to reduce D2 autoreceptor inhibition, resulting in an increase in DAergic neurotransmission in the reward/reinforcement system, may be a key event in the development of addiction. Understanding molecular mechanisms underlying the reversal of DA inhibition in the VTA may contribute to medication discovery for more effective treatment of addiction disorders.
Abbreviations
- AC
adenylyl cyclase
- aCSF
artificial cerebrospinal fluid
- β-ARK1 inhibitor
methyl 5-[2-(5-nitro-2-furyl)vinyl]-2-furoate
- cPKC
conventional protein kinase C
- DA
dopamine
- DAergic
dopaminergic
- Gö6976
5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4c]carbazole-12-propanenitrile
- DIR
dopamine-inhibition reversal
- GRK2
G protein-coupled receptor kinase-2
- 5-HT
serotonin
- MiTMAB
tetradecyltrimethlammonium bromide
- pDAergic
putative dopaminergic
- PI
phosphatidylinositide
- PKC
protein kinase C
- PLC
phospholipase C
- VTA
ventral tegmental area
Authorship contributions
Participated in research design: Nimitvilai, Brodie.
Conducted experiments: Nimitvilai, McElvain.
Performed data analysis: Nimitvilai, Brodie.
Wrote or contributed to the writing of the manuscript: Nimitvilai, Brodie.
Footnotes
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant AA05846 and AA09125].
References
- Bofill-Cardona E, Kudlacek O, Yang Q, Ahorn H, Freissmuth M, Nanoff C. (2000) Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J Biol Chem 275:32672–32680 [DOI] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. (1991) Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res 564:203–219 [DOI] [PubMed] [Google Scholar]
- Brodie MS, Dunwiddie TV. (1987) Cholecystokinin potentiates dopamine inhibition of mesencephalic dopamine neurons in vitro. Brain Res 425:106–113 [DOI] [PubMed] [Google Scholar]
- Brodie MS, McElvain MA, Bunney EB, Appel SB. (1999a) Pharmacological reduction of small conductance calcium-activated potassium current (SK) potentiates the excitatory effect of ethanol on ventral tegmental area dopamine neurons. J Pharmacol Exp Ther 290:325–333 [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. (1999b) Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res 23:1848–1852 [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res 508:65–69 [DOI] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. (1973) Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther 185:560–571 [PubMed] [Google Scholar]
- Caillé I, Dumartin B, Bloch B. (1996) Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res 730:17–31 [DOI] [PubMed] [Google Scholar]
- Chieng B, Azriel Y, Mohammadi S, Christie MJ. (2011) Distinct cellular properties of identified dopaminergic and GABAergic neurons in the mouse ventral tegmental area. J Physiol 589:3775–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang TT, LeVine H, 3rd, De Blasi A. (1995) Phosphorylation and activation of beta-adrenergic receptor kinase by protein kinase C. J Biol Chem 270:18660–18665 [DOI] [PubMed] [Google Scholar]
- Chuang TT, Paolucci L, De Blasi A. (1996) Inhibition of G protein-coupled receptor kinase subtypes by Ca2+/calmodulin. J Biol Chem 271:28691–28696 [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Nash N, Heilman C, Sunahara R, Hartney A, Tiberi M, Rye DB, Caron MG, Niznik HB, Levey AI. (2000) Dopamine D(5) receptor immunolocalization in rat and monkey brain. Synapse 37:125–145 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Lévesque D, Lammers CH, Griffon N, Martres MP, Schwartz JC, Sokoloff P. (1995) Phenotypical characterization of neurons expressing the dopamine D3 receptor in the rat brain. Neuroscience 65:731–745 [DOI] [PubMed] [Google Scholar]
- Diviani D, Lattion AL, Larbi N, Kunapuli P, Pronin A, Benovic JL, Cotecchia S. (1996) Effect of different G protein-coupled receptor kinases on phosphorylation and desensitization of the alpha1B-adrenergic receptor. J Biol Chem 271:5049–5058 [DOI] [PubMed] [Google Scholar]
- Erdtmann-Vourliotis M, Mayer P, Ammon S, Riechert U, Höllt V. (2001) Distribution of G-protein-coupled receptor kinase (GRK) isoforms 2, 3, 5 and 6 mRNA in the rat brain. Brain Res Mol Brain Res 95:129–137 [DOI] [PubMed] [Google Scholar]
- Gao C, Wolf ME. (2008) Dopamine receptors regulate NMDA receptor surface expression in prefrontal cortex neurons. J Neurochem 106:2489–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. (1984) The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4:2866–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN. (1999) Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology 20:60–80 [DOI] [PubMed] [Google Scholar]
- Hausdorff WP, Caron MG, Lefkowitz RJ. (1990) Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J 4:2881–2889 [PubMed] [Google Scholar]
- Iino M, Furugori T, Mori T, Moriyama S, Fukuzawa A, Shibano T. (2002) Rational design and evaluation of new lead compound structures for selective betaARK1 inhibitors. J Med Chem 45:2150–2159 [DOI] [PubMed] [Google Scholar]
- Ito K, Haga T, Lameh J, Sadée W. (1999) Sequestration of dopamine D2 receptors depends on coexpression of G-protein-coupled receptor kinases 2 or 5. Eur J Biochem 260:112–119 [DOI] [PubMed] [Google Scholar]
- Iwata K, Ito K, Fukuzaki A, Inaki K, Haga T. (1999) Dynamin and rab5 regulate GRK2-dependent internalization of dopamine D2 receptors. Eur J Biochem 263:596–602 [DOI] [PubMed] [Google Scholar]
- Kenakin TP. (1987) Analysis of dose-response data, in Pharmacologic Analysis of Drug-Receptor Interaction, pp 129–162, Raven Press, New York [Google Scholar]
- Khan ZU, Gutiérrez A, Martín R, Peñafiel A, Rivera A, de la Calle A. (2000) Dopamine D5 receptors of rat and human brain. Neuroscience 100:689–699 [DOI] [PubMed] [Google Scholar]
- Koob GF. (2003) Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27:232–243 [DOI] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. (1987) Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol 392:397–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. (1989) Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci 9:1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. (2008) Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57:760–773 [DOI] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10:839–850 [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. (2006) The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol 577:907–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. (2008) Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci 28:8908–8913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. (2011) Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12:517–533 [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Grandy DK, Damask SP, Civelli O, Watson SJ., Jr (1992) Distribution of D5 dopamine receptor mRNA in rat brain. Neurosci Lett 145:209–212 [DOI] [PubMed] [Google Scholar]
- Mengod G, Villaró MT, Landwehrmeyer GB, Martinez-Mir MI, Niznik HB, Sunahara RK, Seeman P, O’Dowd BF, Probst A, Palacios JM. (1992) Visualization of dopamine D1, D2 and D3 receptor mRNAs in human and rat brain. Neurochem Int 20 (Suppl):33S–43S [DOI] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. (1996) Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature 379:449–451 [DOI] [PubMed] [Google Scholar]
- Mueller AL, Brodie MS. (1989) Intracellular recording from putative dopamine-containing neurons in the ventral tegmental area of Tsai in a brain slice preparation. J Neurosci Methods 28:15–22 [DOI] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Urizar E, Javitch JA, Sibley DR. (2009) G protein-coupled receptor kinase-2 constitutively regulates D2 dopamine receptor expression and signaling independently of receptor phosphorylation. J Biol Chem 284:34103–34115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung Y, Sibley DR. (2004) Protein kinase C mediates phosphorylation, desensitization, and trafficking of the D2 dopamine receptor. J Biol Chem 279:49533–49541 [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. (2004) Dopamine receptor signaling. J Recept Signal Transduct Res 24:165–205 [DOI] [PubMed] [Google Scholar]
- Nimitvilai S, Arora DS, Brodie MS. (2012a) Reversal of dopamine inhibition of dopaminergic neurons of the ventral tegmental area is mediated by protein kinase C. Neuropsychopharmacology 37:543–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Arora DS, Brodie MS. (2012b) Reversal of dopamine inhibition of dopaminergic neurons of the ventral tegmental area is mediated by protein kinase C. Neuropsychopharmacology 37:543–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, Brodie MS. (2010) Reversal of prolonged dopamine inhibition of dopaminergic neurons of the ventral tegmental area. J Pharmacol Exp Ther 333:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimitvilai S, McElvain MA, Arora DS, Brodie MS. (2012c) Reversal of quinpirole inhibition of ventral tegmental area neurons is linked to the phosphatidylinositol system and is induced by agonists linked to G(q). J Neurophysiol 108:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. (1987) Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res 434:117–165 [DOI] [PubMed] [Google Scholar]
- Palacios JM, Kuhar MJ. (1981) Neurotensin receptors are located on dopamine-containing neurones in rat midbrain. Nature 294:587–589 [DOI] [PubMed] [Google Scholar]
- Pesavento E, Margotti E, Righi M, Cattaneo A, Domenici L. (2000) Blocking the NGF-TrkA interaction rescues the developmental loss of LTP in the rat visual cortex: role of the cholinergic system. Neuron 25:165–175 [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Freedman NJ, Lefkowitz RJ. (1998) G protein-coupled receptor kinases. Annu Rev Biochem 67:653–692 [DOI] [PubMed] [Google Scholar]
- Premont RT, Inglese J, Lefkowitz RJ. (1995) Protein kinases that phosphorylate activated G protein-coupled receptors. FASEB J 9:175–182 [DOI] [PubMed] [Google Scholar]
- Pronin AN, Benovic JL. (1997) Regulation of the G protein-coupled receptor kinase GRK5 by protein kinase C. J Biol Chem 272:3806–3812 [DOI] [PubMed] [Google Scholar]
- Pronin AN, Satpaev DK, Slepak VZ, Benovic JL. (1997) Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J Biol Chem 272:18273–18280 [DOI] [PubMed] [Google Scholar]
- Quan A, McGeachie AB, Keating DJ, et al. (2007) Myristyl trimethyl ammonium bromide and octadecyl trimethyl ammonium bromide are surface-active small molecule dynamin inhibitors that block endocytosis mediated by dynamin I or dynamin II. Mol Pharmacol 72:1425–1439 [DOI] [PubMed] [Google Scholar]
- Quirion R, Chiueh CC, Everist HD, Pert A. (1985) Comparative localization of neurotensin receptors on nigrostriatal and mesolimbic dopaminergic terminals. Brain Res 327:385–389 [DOI] [PubMed] [Google Scholar]
- Rivera A, Peñafiel A, Megías M, Agnati LF, López-Téllez JF, Gago B, Gutiérrez A, de la Calle A, Fuxe K. (2008) Cellular localization and distribution of dopamine D(4) receptors in the rat cerebral cortex and their relationship with the cortical dopaminergic and noradrenergic nerve terminal networks. Neuroscience 155:997–1010 [DOI] [PubMed] [Google Scholar]
- Rockman HA, Choi DJ, Rahman NU, Akhter SA, Lefkowitz RJ, Koch WJ. (1996) Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proc Natl Acad Sci USA 93:9954–9959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogue P, Zwiller J, Malviya AN, Vincendon G. (1990) Phosphorylation by protein kinase C modulates agonist binding to striatal dopamine D2 receptors. Biochem Int 22:575–582 [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. (1994) Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci 14:88–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ, Jr, Shen Y. (1993) Molecular neurobiology of dopaminergic receptors. Int Rev Neurobiol 35:391–415 [DOI] [PubMed] [Google Scholar]
- Sterne-Marr R, Benovic JL. (1995) Regulation of G protein-coupled receptors by receptor kinases and arrestins. Vitam Horm 51:193–234 [DOI] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. (2008) Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci 28:4216–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault D, Albert PR, Pineyro G, Trudeau LÉ. (2011) Neurotensin triggers dopamine D2 receptor desensitization through a protein kinase C and beta-arrestin1-dependent mechanism. J Biol Chem 286:9174–9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstel R, Freund S, Krasel C, Hoppe E, Lohse MJ. (1996) Protein kinase cross-talk: membrane targeting of the beta-adrenergic receptor kinase by protein kinase C. Proc Natl Acad Sci USA 93:2105–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. (1996) Neurobiology of addiction. Curr Opin Neurobiol 6:243–251 [DOI] [PubMed] [Google Scholar]