Abstract
Despite aggressive treatment with radiation and chemotherapy, recurrence of glioblastoma multiforme (GBM) is inevitable. The objective of this study was to show that the blood-brain barrier (BBB), through a combination of tight junctions and active efflux transporters in the brain microvasculature, can significantly restrict delivery of molecularly targeted agents to invasive glioma cells. Transgenic mice lacking P-glycoprotein (P-gp) and breast cancer resistance protein (Bcrp) were used to study efflux of erlotinib at the BBB. A U87 rat xenograft model of GBM was used to investigate the regional distribution of erlotinib to the tumor, and brain regions surrounding the tumor. The effect of concurrent administration of elacridar on regional tumor distribution of erlotinib was evaluated. We show that erlotinib transport across an intact BBB is significantly restricted due to P-gp- and Bcrp-mediated efflux transport. We then show that the BBB is sufficiently intact in areas of brain adjacent to the tumor core to significantly restrict erlotinib delivery. Inhibition of P-gp and Bcrp by the dual inhibitor elacridar dramatically increased erlotinib delivery to the tumor core, rim, and normal brain. These results provide conclusive evidence of the impact that active efflux at the BBB has on the delivery of molecularly targeted therapy to different tumor regions in glioma. These data also support the possibility that the repeated failure of clinical trials of new drugs for gliomas may be in part due to a failure to achieve effective concentrations in invasive tumor cells that reside behind an intact BBB.
Introduction
Glioblastoma multiforme (GBM) is an invasive tumor and should be regarded as a disease of the entire brain (Berens and Giese, 1999; Agarwal et al., 2011b). Despite recent advances in surgery, radiotherapy, and chemotherapy, there is no effective treatment of GBM, and progression of the disease is almost always the cause of death. The median survival of patients with GBM is only 16–19 months (Grossman et al., 2010). A fatal characteristic of GBM is the invasive growth of tumor cells into normal brain tissue surrounding the main tumor mass, making complete surgical resection not feasible. Tumor invasion in GBM has been reported as early as 1938, when Hans-Joachim Scherer described the diffuse invasion of GBM by defining secondary patterns that reflected growth of tumors in neighboring brain tissue (Scherer, 1938). In 1961, Matsukado showed that more than 50% of untreated brain tumors spread into the contralateral hemisphere (Matsukado et al., 1961). Even radical surgical approaches, such as complete removal of the tumor-bearing hemisphere (hemispherectomy), do not prevent recurrence, and have been discontinued (Dandy, 1928; Bell and Karnosh, 1949). Therefore, further progress in treating this disease requires that the invasive glioma cells, which can reside centimeters away from the main tumor mass, are effectively treated (Agarwal et al., 2011b).
Advances in our understanding of the molecular pathogenesis of GBM have led to the development of many promising small-molecule tyrosine kinase inhibitors (TKIs) that inhibit critical signaling pathways essential for growth and development of the tumor. However, clinical trials evaluating these molecularly targeted TKIs in GBM have mostly resulted in disappointing failures (Huang et al., 2009). Among the various hypotheses developed to explain the failure of promising molecularly targeted agents in GBM, one that has often been overlooked is impaired delivery of drugs to their intracellular targets. The central nervous system (CNS) is protected by the blood-brain barrier (BBB), which restricts the passage of most small and large molecules into the brain (Pardridge, 2005). ATP-binding cassette (ABC) transporters, such as P-glycoprotein (P-gp, Abcb1) and the breast cancer resistance protein (Bcrp, Abcg2), constitute a vital component of this barrier that restricts CNS drug penetration by actively effluxing drugs out of the brain (Schinkel and Jonker, 2003). It has been shown that these two “gatekeepers” restrict the entry of several molecularly targeted and other anticancer agents into the brain (Chen et al., 2009; Agarwal et al., 2010; Agarwal et al., 2011a,c). Certainly, restricted drug delivery to the target can help explain the inefficacy of otherwise potent molecularly targeted agents in GBM (Agarwal et al., 2011b).
Several recent studies have suggested that drug delivery to the tumor is not restricted in GBM. These studies concluded that the BBB is overcome by residual damage from radiotherapy, and/or by the pathologic infiltrative characteristics of GBM that compromise the functional integrity of the BBB (Hofer and Frei, 2007). These conclusions were based on the finding that drug concentrations in the tumor (resected tissue) were several-fold greater than plasma concentrations. However, these tissues are frequently contaminated with residual blood, which increases measured drug concentrations in the tumor. Therefore, drug concentration measurements in resected brain tissues can be misleading and should be cautiously interpreted. Moreover, a significant finding in all of the previously mentioned studies is that drug concentrations in areas distant from the tumor core (non–contrast-enhancing regions) were severalfold lower than that in the tumor core (contrast-enhancing areas) (Fine et al., 2006; Rosso et al., 2009; Pitz et al., 2011). This suggests that although the BBB may be disrupted at or near the tumor core, it most certainly is intact near the growing edge of the tumor, a region where invasive tumor cells may reside. An intact BBB along with its functional efflux transport systems in such regions of the brain can significantly impede drug delivery to the invasive tumor, which, in almost all cases, is more important to treat after resection of the primary tumor.
The objective of this study was to show that 1) the blood-brain barrier can be heterogeneously disrupted in GBMs and can be intact in areas away from the primary tumor, and 2) an intact BBB in such areas, along with its functional efflux transport systems, can significantly restrict delivery of molecularly targeted agents to invasive gliomas. Using a xenograft model of GBM, we show that the BBB restricts delivery of the molecularly targeted agent erlotinib to the brain and brain tumor. Furthermore, we show that pharmacological inhibition of P-gp- and Bcrp-mediated active efflux at the BBB results in a significant increase in drug concentration in the brain, even in the tumor core where the BBB is thought to be disrupted.
Materials and Methods
Chemicals.
Erlotinib hydrochloride and AG1478 were procured from LC Laboratories (Woburn, MA). Elacridar (GF120918) was obtained from Toronto Research Chemicals (Toronto, Ontario, Canada). Ammonium formate and acetonitrile were high-pressure liquid chromatography (HPLC) grade and were obtained from Sigma-Aldrich (St. Louis, MO).
Steady-State Brain Distribution of Erlotinib in Friend Leukemia Virus Strain B (FVB) Mice.
Steady-state brain distribution of erlotinib was examined in male Friend leukemia virus strain B wild-type, Mdr1a/b−/−, Bcrp1−/−, and Mdr1a/b−/−Bcrp1−/− mice (n = 4 per genotype) from Taconic Farms Inc. (Hudson, NY). All animals were 8–10 weeks old at the time of the experiment. Animals were maintained under temperature-controlled conditions with a 12-hour light/dark cycle and unlimited access to food and water. All studies were carried out in accordance with the guidelines set by the Principles of Laboratory Animal Care (National Institutes of Health) and were approved by The Institutional Animal Care and Use Committee of the University of Minnesota.
Erlotinib was infused to steady-state using Alzet osmotic minipumps (Durect Corporation, Cupertino, CA). A 15-mg/ml solution of erlotinib in dimethylsulfoxide was filled in the minipumps (model 1003D), and the pumps were equilibrated by soaking them overnight (12 hours) in a sterile saline solution at 37°C. The pump operated at a flow rate of 1 µl/h, yielding a constant rate infusion of 15 µg/h (0.6 mg/h/kg). Mice were anesthetized using 5% isoflurane (Boynton Health Service Pharmacy, Minneapolis, MN) and were maintained under anesthesia using 2% isoflurane in oxygen. The abdominal cavity was shaved and cleaned and a small midline incision was made in the lower abdomen under the rib cage. An incision was made in the peritoneal wall directly beneath the cutaneous incision, and the primed pump was inserted into the peritoneal cavity. The musculoperitoneal layer was closed with sterile absorbable sutures and the skin incision was closed using sterile wound clips. The animals were allowed to recover on a heated pad. Erlotinib half-life in mice has been reported to be approximately 1 hour (Marchetti et al., 2008), so an infusion lasting 48 hours was considered to be sufficient to attain steady state in both the brain and plasma. The animals were euthanized 48 hours post surgery using a carbon dioxide chamber. Blood was collected by cardiac puncture and the whole brain was harvested. Plasma was obtained by centrifuging the blood sample at 3500 rpm for 10 minutes. Plasma and brain specimens were stored at −80°C until analysis by liquid chromatography mass spectrometry LCMS-MS.
Brain concentrations were corrected for drug present in residual blood in the brain using the correction factor described by Dai et al. (2003). Briefly, Dai and coworkers showed that brain space for plasma in the brain vasculature is 1.4% of an FVB mouse brain. Using the measured plasma concentration, the amount of drug in the brain vasculature was determined and the amount of drug in the brain was corrected for drug present in the vasculature using the following equation:
Glioblastoma Cells.
Human glioblastoma U87 cells transfected with wild-type epidermal growth factor receptor were grown in a short-term cell culture (7–14 days) at 37°C with 5% carbon dioxide in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, and 1% antibiotic/antimycotic (Invitrogen, Carlsbad, CA). Immediately before inoculation, cells were harvested and suspended in phosphate-buffered saline to a concentration of 5 × 104 cells/μl.
Orthotopic Xenograft Model.
All experiments in the xenograft model were performed on a protocol approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Animals were fed a standard rodent diet, and were maintained in a pathogen-free environment. Athymic male nude rats (Rowett Nude; Charles River Laboratories, Wilmington, MA), 6–7 weeks of age, were obtained from Charles River Laboratories and housed in the Biologic Resources Unit of the Cleveland Clinic. Rats were anesthetized by an intraperitoneal injection of ketamine and xylazine and inoculated with U87 cells (2.5 × 105 cells in a total volume of 5 μl) by injection into the right frontal region using a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The tumor was allowed to grow for 3 weeks before being used for further studies.
Regional Tumor Distribution of Erlotinib.
Tumor-bearing rats were randomly assigned to one of the three groups (n = 6 per group): control (no erlotinib treatment), erlotinib treatment with perfusion, and erlotinib treatment without perfusion. The rats in the treatment groups received 20 mg/kg/day erlotinib (in 0.5% methylcellulose) by oral gavage for 5 days. All animals were sacrificed 30 minutes after the last erlotinib dose followed by collection of blood and brain tissue. Rats in the perfusion group underwent cardiac perfusion with saline prior to collection of brain. Brain tissue was visually dissected into three parts: the tumor, the brain tissue adjacent to the tumor, and the contralateral hemisphere. Plasma was separated by centrifuging the blood at 3500 rpm for 10 minutes. Tissue specimens were flash frozen in liquid nitrogen and stored at −80°C until further analysis. Tissue specimens from each group were analyzed for erlotinib concentrations.
Effect of Elacridar on Distribution of Erlotinib to the Tumor.
In a separate study, tumor-bearing rats were divided into three groups (n = 4 per group): control (no erlotinib treatment), erlotinib treatment, and treatment with erlotinib plus elacridar. The rats in the treatment groups received 20 mg/kg/day erlotinib (in 0.5% methylcellulose) by daily gavage for 3 days. Rats in the erlotinib plus elacridar group received an additional dose of 10 mg/kg elacridar by intravenous administration into the jugular vein 30 minutes before each erlotinib dose. Animals were sacrificed 30 minutes after the last erlotinib dose followed by collection of blood. All rats were then perfused with saline and brain tissue was harvested. Plasma and brain tissue were processed as described previously. All samples were stored at −80°C until further analysis.
Quantification of Erlotinib in Brain and Plasma by LCMS-MS.
The concentration of erlotinib in mouse plasma and brain homogenate was determined by HPLC coupled with mass spectrometry. Prior to analysis, frozen samples were thawed at room temperature. Brain samples were homogenized using 3 volumes of ice-cold 5% bovine serum albumin in phosphate-buffered saline using a tissue homogenizer (Fisher Scientific, Pittsburgh, PA). One hundred microliters of specimen plasma and brain homogenate was spiked with 50 ng of internal standard, tyrphostin (AG1478), and alkalinized by the addition of 100 µl of a pH 11 buffer (1 mM sodium hydroxide, 0.5 mM sodium bicarbonate). Samples were extracted by vigorous vortexing with 1 ml of ice-cold ethyl acetate followed by centrifugation at 7500 rpm for 15 minutes at 4°C. A volume of 750 µl of the organic layer was transferred to fresh polypropylene tubes and dried under nitrogen. Samples were reconstituted in 100 µl of mobile phase and transferred to glass autosampler vials. A volume of 10 µl was injected in the HPLC system using a temperature-controlled autosampling device maintained at 10°C. Chromatographic analysis was performed using an Agilent model 1200 separation system (Santa Clara, CA). Separation of analytes was achieved using an Agilent Eclipse XDB-C18 RRHT threaded column (4.6 mm ID × 50mm, 1.8 μm) fitted with an Agilent C18 guard column (4.6 mm ID × 12.5 mm, 5 μm) (Santa Clara, CA). The mobile phase was composed of acetonitrile: 20 mM ammonium formate (containing 0.1% formic acid), (45:55 v/v), and was delivered at a flow rate of 0.25 ml/min. The column effluent was monitored using a Thermo Finnigan TSQ Quantum 1.5 detector (San Jose, CA). The instrument was equipped with an electrospray interface, and controlled by the Xcalibur version 2.0.7 data system (Thermo Scientific, San Jose, CA). The samples were analyzed using an electrospray probe in the positive ionization mode operating at a spray voltage of 4500 V for both erlotinib and the internal standard. The spectrometer was programmed to allow the [MH]+ ion of erlotinib at mass-to-charge ratio (m/z) 395.14 and that of the internal standard at m/z 316.68 to pass through the first quadrupole (Q1) and into the collision cell (Q2). The collision energy was set at 14 V for erlotinib and 9 V for tyrphostin. The product ions for erlotinib (m/z 278.91) and the internal standard (m/z 300.9) were monitored through the third quadrupole (Q3). The scan width and scan time for monitoring the two product ions were 1.5 m/z and 0.5 seconds, respectively. The assay was sensitive over a range of 2.5 ng/ml to 1 µg/ml with the coefficient of variation being less than 15% over the entire range.
Statistical Analysis.
Comparisons between groups were made using Sigmaplot, version 11 (Systat Software, Inc., Point Richmond, CA). The statistical difference between the two groups was tested using the two-sample t test and significance was declared at P < 0.05. Multiple groups were compared by one-way analysis of variance with the Holm-Sidak post-hoc test for multiple comparisons at a significance level of P < 0.05.
Results
P-gp and Bcrp Mediated Efflux Restricts CNS Delivery of Erlotinib.
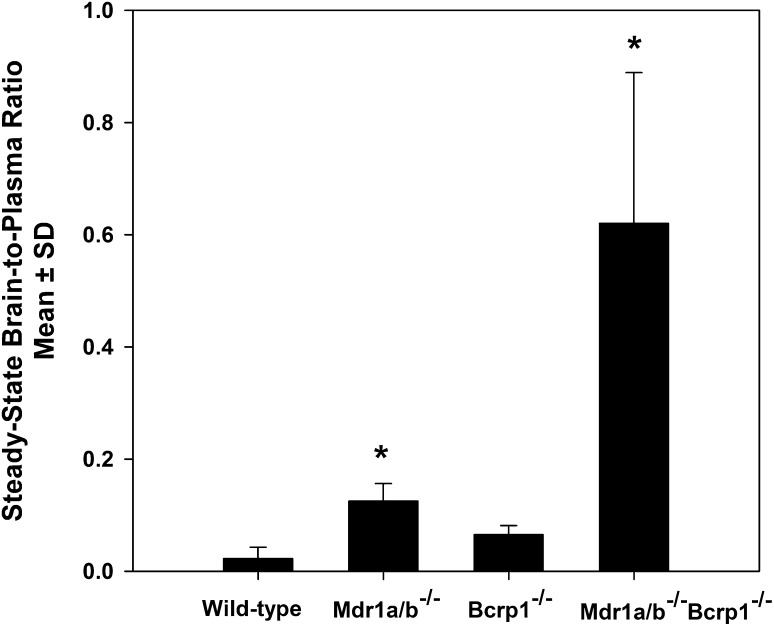
The influence of P-gp and Bcrp on the transport of erlotinib across the BBB was examined by studying its steady-state brain distribution in FVB mice. After a continuous intraperitoneal infusion lasting 48 hours, mean steady-state plasma concentrations of erlotinib ranged from 72 ± 42 ng/ml in the wild-type mice to 99 ± 22 ng/ml in the Mdr1a/b−/−Bcrp1−/− mice. Steady-state plasma concentrations were not significantly different from each other between the four mouse groups (Table 1). Mean steady-state brain concentration was 4.3 ± 5.5 ng/g in the wild-type, 12 ± 2.8 ng/g in the Mdr1a/b−/−, 4.9 ± 2.9 ng/g in the Bcrp1−/−, and 34 ± 17 ng/g in the Mdr1a/b−/−Bcrp1−/− mice. The corresponding steady-state brain-to-plasma ratio was 0.02 ± 0.02 in the wild-type mice and increased to 0.09 ± 0.06 (4.5-fold) in the Mdr1a/b−/−, 0.07 ± 0.02 in the Bcrp1−/− (3.5-fold), and 0.62 ± 0.27 (31-fold) in the Mdr1a/b−/−Bcrp1−/− mice (Fig. 1). This indicates that these two ABC transporters efflux erlotinib at the BBB, and their absence in the combined P-gp/Bcrp knockout mice results in a dramatic enhancement in brain distribution of erlotinib.
TABLE 1.
Steady-state plasma and brain concentrations of erlotinib in wild-type, Mdr1a/b−/−, Bcrp1−/−, and Mdr1a/b−/−Bcrp1−/− mice after a constant intraperitoneal infusion at a rate of 0.6 mg/h/kg
Data are presented as the mean ± S.D.
| Genotype | n | Plasma Concentration | Brain Concentration | Brain-to-Plasma Ratio |
|---|---|---|---|---|
| ng/ml | ng/g | |||
| FVB (wild-type) | 4 | 72 ± 41 | 4.3 ± 5.5a | 0.02 ± 0.02 |
| Bcrp1−/− | 4 | 71 ± 3 | 4.9 ± 2.9a | 0.07 ± 0.02 |
| Mdr1a/b−/− | 4 | 81 ± 44 | 12 ± 2.8a,b | 0.09 ± 0.06b |
| Mdr1a/b−/−Bcrp1−/− | 4 | 99 ± 21 | 34 ± 17b | 0.62 ± 0.27b |
P < 0.05 compared with corresponding plasma concentration.
P < 0.05 compared with wild-type group.
Fig. 1.
Steady-state brain distribution of erlotinib in FVB wild-type, Mdr1a/b−/−, Bcrp1−/−,and Mdr1a/b−/−Bcrp1−/− mice. Brain-to-plasma ratios after a 0.6-mg/h/kg intraperitoneal infusion for 48 hours. The values are presented as the mean ± S.D. *P < 0.05, compared with wild-type; n = 4 per mouse genotype.
Regional Brain Delivery of Erlotinib in GBM Model.
The impact of the BBB in restricting delivery of erlotinib to the tumor was investigated using the U87 rat xenograft model of GBM. Tumor-bearing rats were administered a daily dose of erlotinib, and concentrations in three different brain regions were determined. We first studied the effect of perfusion, prior to tissue collection, on the concentrations of erlotinib in the brain. Residual blood remaining in the brain vasculature can contribute to drug concentration measurements in the brain, resulting in significant error, especially for drugs that do not penetrate the BBB well. Erlotinib concentrations in all three brain areas decreased significantly when the brain was perfused with saline prior to harvesting (Figs. 2A and 2B). Drug concentrations were approximately 34% lower in the tumor core at 1.83 ± 0.54 µg/g, whereas that in the tumor rim and normal brain decreased by more than 50% to 0.39 ± 0.19 µg/g and 0.37 ± 0.22 µg/g, respectively (P < 0.05). These findings suggest that drug remaining in residual blood within the brain vasculature, within the gross tumor or brain, can contribute significantly to total brain concentrations, especially where there are low concentrations in the brain parenchyma, thereby introducing significant error to the overall results. All subsequent experiments were therefore conducted after perfusing the brain using saline to avoid overestimation of drug concentrations and misinterpretation of results.
Fig. 2.
Effect of perfusion on regional distribution of erlotinib. Erlotinib concentrations (A) and brain-to-plasma ratios (B) in the tumor core, rim, and normal brain were significantly lower in rats that were perfused with saline (gray bars) prior to collection of brain tissue compared with the nonperfused rats (black bars). This indicates that residual drug in brain vasculature can contribute significantly to total brain levels. Therefore, drug concentration measurements in brain tissues that are not perfused or corrected can be misleading and should be cautiously interpreted. The values are presented as the mean ± S.D. *P < 0.05, compared with tumor core; †P < 0.05, compared with nonperfused group; n = 6 per group.
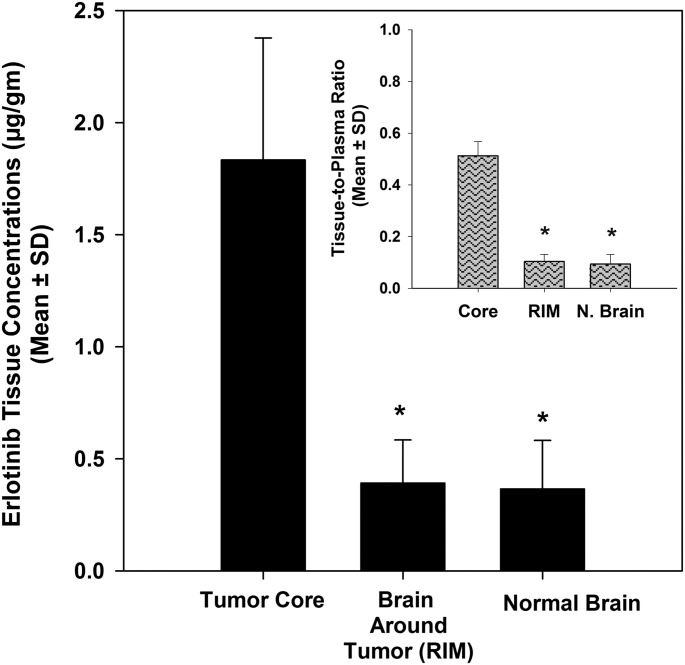
Comparison of erlotinib in the three brain regions showed significant heterogeneity in brain distribution of erlotinib. Erlotinib concentrations in the tumor core were 1.83 ± 0.54 µg/g and decreased significantly to 0.39 ± 0.19 µg/g (4.7-fold) in the brain around the tumor and to 0.37 ± 0.22 µg/g (5-fold) in the contralateral hemisphere (P < 0.05, Fig. 3). The tissue-to-plasma concentration ratio was 0.51 ± 0.06 in the tumor core and decreased significantly to 0.10 ± 0.03 in the tumor rim and 0.09 ± 0.03 in the normal contralateral hemisphere (P < 0.05). This indicates that although drug delivery is not completely restricted by the BBB in the core, the intact BBB definitely limits transport of erlotinib to sites both immediately adjacent (rim) and significantly distant from the tumor core (normal brain).
Fig. 3.
Regional distribution of erlotinib in the U87 rat xenograft model. Erlotinib concentrations in the tumor core were significantly greater than those in the brain around the tumor and normal brain (contralateral hemisphere). The corresponding brain-to-plasma ratios (inset) suggests that the BBB may be intact in areas away from the tumor, indicated by the restricted delivery of erlotinib to the brain around the tumor and the normal contralateral hemisphere. The values are presented as the mean ± S.D. *P < 0.05, compared with tumor core; n = 6.
Effect of Pharmacological Inhibition of P-gp and Bcrp on Regional Erlotinib Delivery.
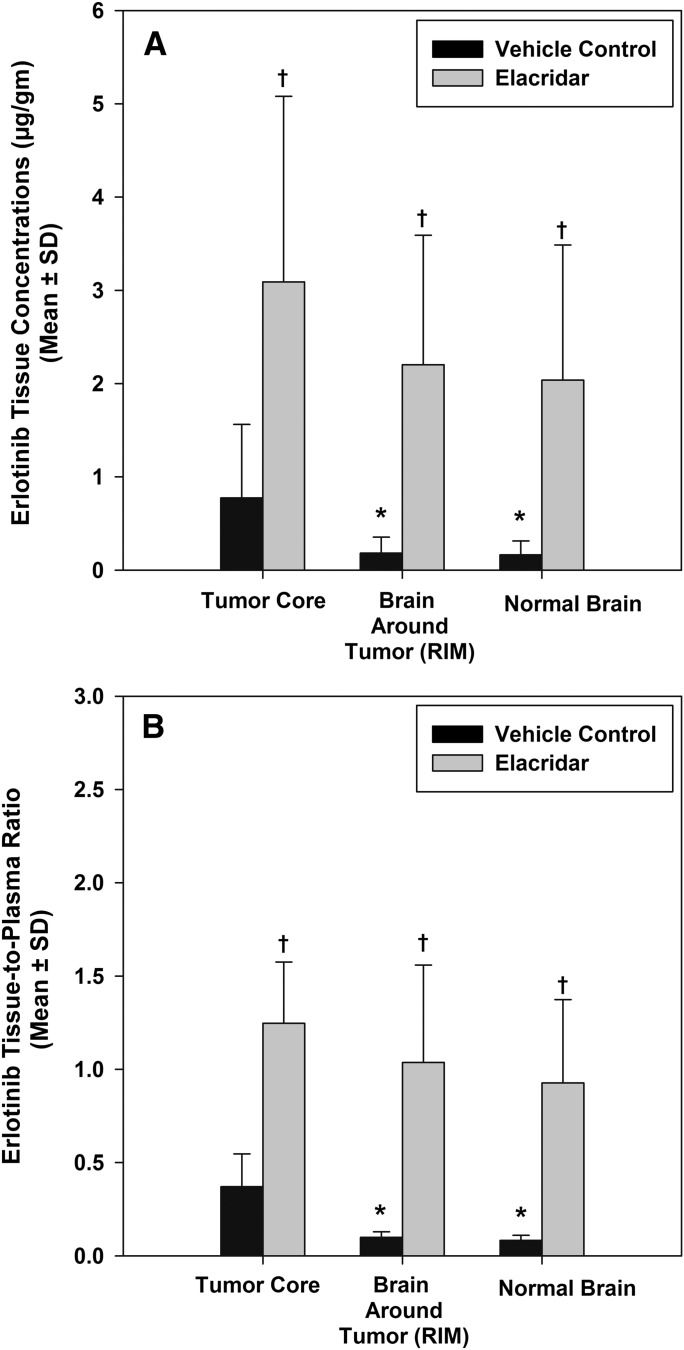
Inhibition of P-gp and Bcrp using small-molecule inhibitors is a possible strategy to enhance brain penetration of substrate drugs. We investigated this by using the dual P-gp/Bcrp inhibitor elacridar (GF120918) in combination with erlotinib. Erlotinib concentrations in all three brain regions increased significantly in rats that were administered elacridar concurrently with erlotinib, compared with those which were treated with erlotinib alone (Fig. 4A). Concentrations in the tumor core increased 4-fold while that in the brain around the tumor and normal brain increased more than 12-fold compared with the vehicle treated group (P < 0.05). In the group that received both elacridar and erlotinib, concentrations in the three brain regions were not statistically different from each other, with the tissue-to-plasma ratio reaching a value of ∼1 (Fig. 4B). This indicates that pharmacological inhibition of P-gp and Bcrp by elacridar dramatically enhances erlotinib delivery, especially to the tumor periphery and normal brain tissue, where the BBB is intact. Comparison of erlotinib levels in Fig. 4A and Figs. 2 and 3 reveals that tissue concentrations in this study were lower than the previous studies. It is possible that the lower tissue concentrations in this study can be related to differences in experiments as a result of differences in effect of tumor on the BBB. Importantly, the relative magnitude of the difference between the three regions remained the same (compare Fig. 4B with Fig. 2B).
Fig. 4.
Influence of elacridar on regional distribution of erlotinib. Erlotinib concentrations (A) in the tumor core, rim, and normal brain increased significantly in the elacridar-treated group (gray bars) compared with the control (black bars). The erlotinib brain-to-plasma ratio (B) increased significantly and was approximately 1 in all three brain regions, indicating that when these two transporters are inhibited, there is no restriction of the delivery of erlotinib to the brain. This indicates that concurrent administration of a modulator of drug transporters, such as elacridar, can be used as a strategy to enhance delivery of substrate chemotherapeutic agents to the brain. The values are presented as the mean ± S.D. *P < 0.05, compared with tumor core; †P < 0.05, compared with vehicle control; n = 4 per group.
Discussion
GBM is an invasive disease that affects the whole brain. The infiltrative nature of the disease makes treatment particularly challenging, because surgery does not remove tumor cells that have invaded into normal brain areas, and protective mechanisms such as the BBB shield and protect these invasive cells from chemotherapeutic agents (Agarwal et al., 2011b). Recent studies have questioned the role of the BBB in limiting delivery and thus efficacy of chemotherapy, based on findings that drug concentrations in the tumor (resected tumor tissue) were several-fold higher than that in plasma (Hofer and Frei, 2007; Blakeley et al., 2009; Pitz et al., 2011). However, these concentrations do not represent drug levels in other areas of the brain, especially regions where the BBB may be intact and capable of restricting drug delivery. Given that the target in question includes invasive tumor cells away from the site of surgical resection, drug concentrations in these invasive sites are of paramount importance. We have demonstrated that the BBB is intact in a rat xenograft model of GBM, especially in areas that are distant from the central tumor mass. This intact BBB restricts the delivery of the molecularly targeted agent erlotinib, a substrate for P-gp and Bcrp, to the brain and brain tumor. We show that erlotinib concentrations in sites away from the main tumor, such as the tumor rim and the contralateral normal hemisphere, are severalfold lower than that in the tumor core. Furthermore, we demonstrate that pharmacological inhibition of the two efflux transporters results in a dramatic increase in drug distribution to the entire brain. Finally, we have also demonstrated that measurement of drug levels obtained from clinical samples may be unreliable due to contamination by the drug in the tumor vasculature. Studies that use resected clinical tissue to demonstrate that a drug can cross the BBB should therefore be viewed with caution. Similarly, studies claiming that a drug crosses the BBB following evaluation of drug levels in the tumor mass only in a xenograft model, especially when saline perfusion has not been performed, should also be viewed with caution.
The brain distribution study in FVB mice shows that erlotinib is a substrate for both P-gp and Bcrp and that together the two transporters significantly restrict its brain penetration (Fig. 1). The impact of P-gp and Bcrp on brain penetration of erlotinib has been previously shown by several other studies (de Vries et al., 2010; Kodaira et al., 2010; Elmeliegy et al., 2011). Our results are consistent with these reports and show that erlotinib distribution to the brain increases dramatically when both P-gp and Bcrp are absent at the BBB (Fig. 1). This finding is similar to our previous studies with other TKIs where we reported that P-gp and BCRP cooperate at the BBB and compensate functionally for each other’s loss (Chen et al., 2009; Agarwal et al., 2010; Agarwal et al., 2011c). In a recent study, using a highly sensitive liquid chromatography-tandem mass spectrometry method to determine the quantitative expression of membrane transporters at the BBB of the three transgenic mouse genotypes, we showed that P-gp or Bcrp is completely absent at the BBB in the single P-gp–knockout and Bcrp-knockout mice and the combined P-gp/Bcrp–knockout mice, and that the loss of one transporter did not influence the expression of the other (Agarwal et al., 2012). This validation of the transgenic mouse model further supports the hypothesis that the greater than additive increase in brain penetration in the absence of P-gp and Bcrp is due to a simple functional compensation between the two transporters at the BBB, rather than an upregulation of a transporter.
We used the U87 rat xenograft model of GBM to show that the BBB is intact and restricts delivery of erlotinib to the brain, especially to areas away from the tumor core. Erlotinib concentrations in the brain around the tumor and the normal brain were up to 7-fold lower than that in plasma (Fig. 3). The tissue-to-plasma ratios of 0.14 in the normal brain and 0.17 in the rim were similar to the steady-state brain-to-plasma ratios in the FVB wild-type mice and indicate that the BBB may be fully intact in these areas. The tissue-to-plasma ratio of less than unity in the tumor core (0.52) suggests that the BBB is not completely disrupted even in the tumor core and restricts drug penetration to a small extent. Recently, there have been several studies that have investigated the impact of the blood-brain barrier in restricting drug delivery to the tumor (Fine et al., 2006; Hofer and Frei, 2007; Blakeley et al., 2009; Rosso et al., 2009; Pitz et al., 2011). Pitz and coworkers showed that concentrations of many anticancer drugs in contrast-enhancing areas of the tumor were generally higher than the corresponding plasma concentrations (Pitz et al., 2011). Likewise, tumor concentrations of paclitaxel and temozolomide have also been reported to be higher than that in plasma (Fine et al., 2006; Rosso et al., 2009). All these studies determined drug concentrations in resected tumor tissue and reported significantly high concentrations of chemotherapeutic agents therein, concluding that drug delivery to the tumor is not restricted in GBM. Many of these previous studies also report that drug concentrations in non–contrast-enhancing regions of the brain (areas distant from the tumor core) were severalfold lower than that in the contrast-enhancing tumor (Fine et al., 2006; Blakeley et al., 2009; Rosso et al., 2009; Pitz et al., 2011). These findings imply that although the pathologic characteristics of GBM compromise the integrity of the BBB in or near the tumor core, leading to higher drug concentrations there, the BBB most certainly is intact near the growing edge of the tumor. Invasive cells residing in such areas may remain shielded from chemotherapy and eventually give rise to the recurrent tumor. Ultimately, it is the areas of invasive tumor that are the critical issue in controlling this disease; the areas of enhancing tumor (tumor mass) often can be removed surgically. Thus, conclusions drawn on the basis of drug concentrations in a resected tumor can be highly misleading and do not represent the status of the BBB throughout the entire brain in GBM.
Many small-molecule anticancer TKIs are substrates for P-gp and Bcrp, and therefore they do not cross the BBB to a significant extent (Dai et al., 2003; Chen et al., 2009; Lagas et al., 2009; Polli et al., 2009; Agarwal et al., 2010; Agarwal et al., 2011c). Consequently, inhibition of these two transporters has been proposed as a possible strategy to enhance brain penetration of substrate drugs. Elacridar (GF120918) is a dual inhibitor of P-gp and Bcrp that was developed to reverse multidrug resistance seen in cancer (Hyafil et al., 1993). Several preclinical studies have shown that brain distribution of dual P-gp/BCRP substrates increases dramatically when elacridar is administered concomitantly (Breedveld et al., 2005; Chen et al., 2009; Lagas et al., 2009; Agarwal et al., 2010; Agarwal et al., 2011c). We therefore used elacridar to investigate its influence on delivery of erlotinib to the brain and brain tumor.
When elacridar was administered concurrently with erlotinib, concentrations in all three brain regions increased significantly compared with the vehicle-treated group (Fig. 4). In the elacridar-treated group, the tissue-to-plasma ratio in all three brain regions was approximately 1, indicating that when the two transporters are inhibited, there is no restriction to the transport of erlotinib across the BBB. The fact that elacridar increased erlotinib delivery to the tumor core as well suggests that the BBB is not completely disrupted, even in the tumor core, and has functional efflux transporters capable of restricting drug delivery. Thus, inhibition of P-gp and Bcrp can be an attractive strategy to enhance delivery of substrate drugs across the BBB and thereby improve their efficacy.
In conclusion, this study shows the impact of an intact BBB on the delivery of a molecularly targeted agent to the brain and brain tumor. We show that drug concentrations in the tumor core are not representative of those in the entire brain and should not be used as a guide for adequacy of drug delivery to the brain. Finally, we show that concurrent administration of a modulator of drug transporters, such as elacridar, can be used as a strategy to enhance delivery of substrate chemotherapeutic agents to the brain.
Several clinical trials evaluating the epidermal growth factor receptor inhibitor erlotinib have reported disappointing results (Peereboom et al., 2010; Prados et al., 2009; Reardon et al., 2010; Raizer et al., 2010). The fact that recurrence occurs in areas away from the margins of surgical resection is indicative that invasive glioma cells in these areas are not being effectively treated. Confusion over the status of the BBB in glioma has stemmed from reports that use the findings of high drug concentrations in resected tumor tissue to represent drug delivery to the entire brain. This study shows that studies of that type can be highly misleading since the BBB may not be disrupted in areas of the brain away from the tumor, and drug concentrations in these areas can be significantly lower. Therefore, successful targeting of the invasive glioma cells will require enhanced delivery of chemotherapeutic agents across an intact BBB to the entire brain.
Acknowledgments
We dedicate this manuscript to the memory of Juan Palomo.
Abbreviations
- ABC
ATP-binding cassette
- Abcb1/Mdr1
gene encoding the murine P-glycoprotein
- Abcg2/Bcrp1
gene encoding the murine breast cancer resistance protein
- BBB
blood-brain barrier
- Bcrp
breast cancer resistance protein
- CNS
central nervous system
- FVB
Friend leukemia virus strain B
- GBM
glioblastoma multiforme
- GF120918 (elacridar)
N-{4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethyl]-5-methoxy-9-oxo-10H-acridine-4-carboxamide}
- LC
liquid chromatography
- P-gp
P-glycoprotein
- MS
mass spectrometry
- TKI
tyrosine kinase inhibitor
Authorship Contributions
Participated in research design: Agarwal, Manchanda, Vogelbaum, Ohlfest, Elmquist.
Conducted experiments: Agarwal, Manchanda.
Contributed new reagents or analytic tools: Vogelbaum, Elmquist.
Performed data analysis: Agarwal, Vogelbaum, Elmquist.
Wrote or contributed to the writing of the manuscript: Agarwal, Vogelbaum, Ohlfest, Elmquist.
Footnotes
This work was supported by a grant from the National Institutes of Health National Cancer Institute (Grant CA138437) to W.F.E., M.A.V., and J.R.O. Funding for S.A. was provided by the Doctoral Dissertation Fellowship, University of Minnesota.
References
- Agarwal S, Hartz AM, Elmquist WF, Bauer B. (2011a) Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des 17:2793–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. (2010) Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther 334:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF. (2011b) Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med 13:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sane R, Ohlfest JR, Elmquist WF. (2011c) The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther 336:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Uchida Y, Mittapalli RK, Sane R, Terasaki T, Elmquist WF. (2012) Quantitative proteomics of transporter expression in brain capillary endothelial cells isolated from P-glycoprotein (P-gp), breast cancer resistance protein (Bcrp), and P-gp/Bcrp knockout mice. Drug Metab Dispos 40:1164–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E, Jr, Karnosh LJ. (1949) Cerebral hemispherectomy; report of a case 10 years after operation. J Neurosurg 6:285–293 [DOI] [PubMed] [Google Scholar]
- Berens ME, Giese A. (1999) “...those left behind.” Biology and oncology of invasive glioma cells. Neoplasia 1:208–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley JO, Olson J, Grossman SA, He X, Weingart J, Supko JG, New Approaches to Brain Tumor Therapy (NABTT) Consortium (2009) Effect of blood brain barrier permeability in recurrent high grade gliomas on the intratumoral pharmacokinetics of methotrexate: a microdialysis study. J Neurooncol 91:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, Schellens JH. (2005) The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res 65:2577–2582 [DOI] [PubMed] [Google Scholar]
- Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF. (2009) P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther 330:956–963 [DOI] [PubMed] [Google Scholar]
- Dai H, Marbach P, Lemaire M, Hayes M, Elmquist WF. (2003) Distribution of STI-571 to the brain is limited by P-glycoprotein-mediated efflux. J Pharmacol Exp Ther 304:1085–1092 [DOI] [PubMed] [Google Scholar]
- Dandy WE. (1928) Removal of right cerebral hemisphere for certain tumors with hemiplegia. J Am Med Assoc 90:823–825 [Google Scholar]
- de Vries NA, Buckle T, Zhao J, Beijnen JH, Schellens JH, van Tellingen O. (2012) Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drugs 30:443–449 [DOI] [PubMed] [Google Scholar]
- Elmeliegy MA, Carcaboso AM, Tagen M, Bai F, Stewart CF. (2011) Role of ATP-binding cassette and solute carrier transporters in erlotinib CNS penetration and intracellular accumulation. Clin Cancer Res 17:89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine RL, Chen J, Balmaceda C, et al. (2006) Randomized study of paclitaxel and tamoxifen deposition into human brain tumors: implications for the treatment of metastatic brain tumors. Clin Cancer Res 12:5770–5776 [DOI] [PubMed] [Google Scholar]
- Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J, NABTT CNS Consortium (2010) Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 16:2443–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer S, Frei K. (2007) Gefitinib concentrations in human glioblastoma tissue. J Neurooncol 82:175–176 [DOI] [PubMed] [Google Scholar]
- Huang TT, Sarkaria SM, Cloughesy TF, Mischel PS. (2009) Targeted therapy for malignant glioma patients: lessons learned and the road ahead. Neurotherapeutics 6:500–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil F, Vergely C, Du Vignaud P, Grand-Perret T. (1993) In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res 53:4595–4602 [PubMed] [Google Scholar]
- Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. (2010) Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J Pharmacol Exp Ther 333:788–796 [DOI] [PubMed] [Google Scholar]
- Lagas JS, van Waterschoot RA, van Tilburg VA, Hillebrand MJ, Lankheet N, Rosing H, Beijnen JH, Schinkel AH. (2009) Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clin Cancer Res 15:2344–2351 [DOI] [PubMed] [Google Scholar]
- Marchetti S, de Vries NA, Buckle T, Bolijn MJ, van Eijndhoven MA, Beijnen JH, Mazzanti R, van Tellingen O, Schellens JH. (2008) Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1-/-/Mdr1a/1b-/- (triple-knockout) and wild-type mice. Mol Cancer Ther 7:2280–2287 [DOI] [PubMed] [Google Scholar]
- Matsukado Y, MacCarty CS, Kernohan JW. (1961) The growth of glioblastoma multiforme (astrocytomas, grades 3 and 4) in neurosurgical practice. J Neurosurg 18:636–644 [DOI] [PubMed] [Google Scholar]
- Pardridge WM. (2005) The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peereboom DM, Shepard DR, Ahluwalia MS, et al. (2010) Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol 98:93–99 [DOI] [PubMed] [Google Scholar]
- Pitz MW, Desai A, Grossman SA, Blakeley JO. (2011) Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol 104:629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli JW, Olson KL, Chism JP, John-Williams LS, Yeager RL, Woodard SM, Otto V, Castellino S, Demby VE. (2009) An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-3-chloro-4-[(3-fluorobenzyl)oxy]phenyl-6-[5-([2-(methylsulfonyl)ethyl]aminomethyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab Dispos 37:439–442 [DOI] [PubMed] [Google Scholar]
- Prados MD, Chang SM, Butowski N, et al. (2009) Phase II study of erlotinib plus temozolomide during and after radiation therapy in patients with newly diagnosed glioblastoma multiforme or gliosarcoma. J Clin Oncol 27:579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizer JJ, Abrey LE, Lassman AB, et al. North American Brain Tumor Consortium (2010) A phase II trial of erlotinib in patients with recurrent malignant gliomas and nonprogressive glioblastoma multiforme postradiation therapy. Neuro-oncol 12:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Desjardins A, Vredenburgh JJ, et al. (2010) Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J Neurooncol 96:219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso L, Brock CS, Gallo JM, Saleem A, Price PM, Turkheimer FE, Aboagye EO. (2009) A new model for prediction of drug distribution in tumor and normal tissues: pharmacokinetics of temozolomide in glioma patients. Cancer Res 69:120–127 [DOI] [PubMed] [Google Scholar]
- Scherer H-J. (1938) Structural development in gliomas. Am J Cancer 34:334–351 [Google Scholar]
- Schinkel AH, Jonker JW. (2003) Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev 55:3–29 [DOI] [PubMed] [Google Scholar]