Abstract
Enantioselective hydrolysis of oral racemic methylphenidate (dl-MPH) by carboxylesterase 1 (CES1) limits the absolute bioavailability of the pharmacologically active d-MPH isomer to approximately 30% and that of the inactive l-MPH to only 1–2%. Coadministration of dl-MPH with ethanol results in elevated d-MPH plasma concentrations accompanied by CES1-mediated enantioselective transesterification of l-MPH to l-ethylphenidate (EPH). The present study tested the hypothesis that administration of the pure isomer dexmethylphenidate (d-MPH) will overcome the influence of ethanol on d-MPH absorption by eliminating competitive CES1-mediated presystemic metabolism of l-MPH to l-EPH. Twenty-four healthy volunteers received dl-MPH (0.3 mg/kg) or d-MPH (0.15 mg/kg), with or without ethanol (0.6 g/kg). During the absorption phase of dl-MPH, concomitant ethanol significantly elevated d-MPH plasma concentrations (44–99%; P < 0.005). Furthermore, immediately following the ethanol drink the subjective effects of “high,” “good,” “like,” “stimulated,” and overall “effect” were significantly potentiated (P ≤ 0.01). Plasma l-EPH concentrations exceeded those of l-MPH. Ethanol combined with pure d-MPH did not elevate plasma d-MPH concentrations during the absorption phase, and the ethanol-induced potentiation of subjective effects was delayed relative to dl-MPH-ethanol. These findings are consistent with l-MPH competitively inhibiting presystemic CES1 metabolism of d-MPH. Ethanol increased the d-MPH area under the curve (AUC)0-inf by 21% following dl-MPH (P < 0.001) and 14% for d-MPH (P = 0.001). In men receiving d-MPH-ethanol, the d-MPH absorption partial AUC0.5–2 hours was 2.1 times greater and the time to maximum concentration (Tmax) occurred 1.1 hours earlier than in women, consistent with an increased rate of d-MPH absorption reducing hepatic extraction. More rapid absorption of d-MPH carries implications for increased abuse liability.
Introduction
Adult attention-deficit/hyperactivity disorder (ADHD) patients treated with methylphenidate (MPH) (Ritalin; Novartis Pharmaceuticals, Summit, NJ) commonly use or misuse ethanol (Levin and Kleber, 1995; Darredeau et al., 2007). In addition, the diversion and abuse of MPH has been on the rise (Scharman et al., 2007), and many, if not most, MPH abusers report coabuse with ethanol (Teter et al., 2003; Barrett et al., 2005; Darredeau et al., 2007; Novak et al., 2007; Wilens et al., 2008). In a previous drug interaction study using normal volunteers, ethanol was found to significantly increase positive subjective responses to dl-MPH (Patrick et al., 2007), consistent with the illicit popularity of this drug combination.
The influence of ethanol on dl-MPH pharmacokinetics (Patrick et al., 2007) includes (1) an increase in the rate of d-MPH absorption that has implications for heightened abuse liability (Volkow and Swanson, 2003; Spencer et al., 2006); (2) an increase in the mean maximum plasma concentration (Cmax) of d-MPH in which threshold brain concentrations associated with reinforcing effects may be reached (Volkow and Swanson 2003); and (3) an increase in the overall plasma exposure to d-MPH. In addition to these ethanol-mediated influences on the psychoactive d-MPH isomer (Srinivas et al., 1992; Aoyama et al., 1994), the inactive l-MPH isomer (Markowitz and Patrick, 2008) serves as the enantioselective transesterification substrate (Patrick et al., 2007; Zhu et al., 2011) for carboxylesterase 1 (CES1); l-MPH combines with ethanol to yield the inactive metabolite l-ethylphenidate (EPH; Fig. 1) (Patrick et al., 2005; Williard et al., 2007). This biotransformation product can serve as a biomarker for concomitant dl-MPH-ethanol exposure (Markowitz et al., 1999) analogous to the detection of the transesterification metabolite cocaethylene (ethylcocaine) evidencing cocaine-ethanol coabuse (Herbst et al., 2011).
Fig. 1.
CES1-mediated enantioselective deesterification of dl-MPH to the primary metabolite ritalinic acid and to the enantioselective transesterification product l-EPH with concomitant ethanol (from Patrick et al., 2007).
Over 80% of an oral dose of dl-MPH can be recovered in the urine (Redalieu et al., 1982) as the inactive (Patrick et al., 1981) hydrolysis metabolite ritalinic acid (Fig. 1). Approximately 1% is excreted unchanged (LeVasseur et al., 2008). Both the hydrolysis of dl-MPH to ritalinic acid and the transesterification with ethanol to yield l-EPH are primarily catalyzed by hepatic CES1 (Bourland et al., 1997; Sun et al., 2004; Zhu et al., 2008; 2009). Presystemic hydrolysis of oral dl-MPH results in the relatively low oral bioavailability of 25% for the therapeutic d-MPH isomer (Srinivas et al., 1992; Aoyama et al., 1994). However, due to the pronounced enantioselectivity of CES1 only 2–5% (Srinivas et al., 1993; Modi et al., 2000) or less (Patrick, et al., 2007) of the inactive l-MPH isomer (Markowitz and Patrick 2008) reaches the systemic circulation, except in the circumstance of a CES1 null allele (Patrick et al., 2007; LeVasseur et al., 2008; Zhu et al., 2008).
The single isomer d-MPH became a treatment option for ADHD in 2002. Head-to-head efficacy comparisons of immediate-release d-MPH versus dl-MPH in ADHD children using twice-daily regimens with d-MPH administered at half the milligram per kilogram dose of dl-MPH found comparable efficacy over the course of a typical school day (Wigal et al., 2004). The present study extends the pharmacological comparisons of d-MPH to dl-MPH as it pertains to differential interactions with concomitant ethanol. These results are discussed in the context of abuse liability, pharmacokinetic-pharmacodynamic correlations, and adult ADHD drug individualization.
We explored the hypothesis that the ethanol-induced increase in d-MPH bioavailability following dl-MPH administration will be significantly reduced upon substituting enantiopure d-MPH for dl-MPH, the reasoning being that removal from the formulation of the more rapidly metabolized CES1 substrate l-MPH will avoid competitive inhibition of CES1 during d-MPH absorption. In the following normal volunteer study, 12 men and 12 women were administered dl-MPH (0.3 mg/kg) or d-MPH (0.15 mg/kg) with or without ethanol (0.6 g/kg) 0.5 hours later in a randomized, four-way crossover design. Serial blood samples were drawn for enantiospecific pharmacokinetic analysis of plasma MPH and EPH and for blood ethanol determinations. Periodic subjective effects used visual analog scale (VAS) questionnaires and hemodynamic effects were recorded.
Materials and Methods
Research Subjects.
Each subject provided a written informed consent approved by the Medical University of South Carolina’s Office of Research Integrity. The study was conducted in the Clinical and Translational Research Center located at the Medical University of South Carolina. The study population consisted of 24 normal volunteers (12 men, 12 women) aged 21–42 years who were healthy as assessed by medical history, physical examination, 12-lead electrocardiogram, and routine laboratory tests. All subjects were within 15% of ideal body weight, were nonsmokers, and were asked to abstain from the use of caffeine containing beverages beginning at 7:30 PM the evening before and continuing through each active study day.
Study Design.
All subjects were admitted to the clinic on the morning of each active study day. One hour prior to dosing, the subjects received a light breakfast of a plain bagel (36 g total: fat 1 g, carbohydrate 29 g, protein 6 g) with cream cheese (30 g total: fat 9.2 g, protein 4.4 g, carbohydrate 0.03 g) and skim milk (240 ml total: fat 9.2 g, carbohydrate 11.5 g, protein 8.4 g) finished within 15 minutes. Then an indwelling venous catheter was placed in each subject’s arm for serial blood sampling. MPH was administered with 240 ml of water. Ethanol or nonethanol orange juice drinks were consumed 0.5 hours after MPH dosing. The ethanol drink was administered as 0.6 g/kg ethanol (0.66 ml/kg 95% ethanol) in 180 ml of orange juice and 60 ml of soda water, with water added to give a total volume of 450 ml. On alcohol-free treatments, the alcohol volume was replaced with water.
The drinks were consumed over 15 minutes at 30 ml/min. Subjects received a standard lunch 3.5 hours after MPH dosing. Lunch consisted of a turkey sandwich on whole wheat bread (2 slices bread, 3 slices turkey), 31 g (1 1/8 oz. bag) of baked potato chips (Baked Lay’s), 120 g of canned fruit in light syrup (Dole mixed fruit cups), and 360 ml of water. A standardized dinner was provided 10.5 hours after dosing. There was at least a 6-day washout period between treatment regimens and negative urine drug screens and urine pregnancy results (women) were obtained at the beginning of each active study session.
An open label, randomized, crossover study design was employed. Four treatment schedules were used: dl-MPH with or without ethanol (treatments A and B) using oral immediate-release dl-MPH HCl (0.3 mg/kg) administered as 10- and 5-mg tablets (Ritalin), and the 5-mg tablets cut to the nearest 2.5 mg using a tablet cutter as appropriate; d-MPH with or without ethanol, using oral immediate-release d-MPH hydrochloride (0.15 mg/kg) administered as 5- and 2.5-mg tablets (Focalin; Novartis Pharmaceuticals, Summit, NJ), and the 2.5-mg tablets cut to the nearest 1.25 mg using a tablet cutter as appropriate.
A total of 12 blood samples was taken over each active study period from the indwelling venous catheter. These blood collection times correspond to 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, and 12 hours after MPH dosing. Blood collection tubes (Vacutainers; Becton Dickinson, Rutherford, NJ) were previously stored in an ice bath and contained sodium oxalate to minimize postsampling MPH and EPH hydrolysis. Heparin-treated stoppered tubes (2 ml) were used to collect whole blood for blood ethanol analysis by the hospital clinical laboratory per standard procedures. Venous catheter lines were flushed of residual heparin solution prior to sampling. Samples were promptly centrifuged at 4°C for 5 minutes, and the plasma was immediately aspirated into separate labeled polypropylene vials and stored at −70°C until analysis.
Vital Signs.
Blood pressure, heart rate, temperature, and respiratory rate were obtained at the screening visit and recorded at the beginning and end of each of the three active sessions. Blood pressure and heart rate were periodically recorded at 0, 0.75, 1.75, 2.75, 3.75, 4.75, 5.75, and 12 hours after MPH dosing.
Visual Analog Scales.
A nine question drug subjective effects questionnaire used visual analog subscales for the following questions: (1) do you feel any drug effect?; (2) how high are you?; (3) do the drugs have any good effects?; (4) do the drugs have any bad effects?; (5) do you like the drugs?; (6) do you feel depressed; (7) how anxious are you?; (8) how stimulated do you feel?; and (9) how intoxicated do you feel?. A questionnaire was administered before (baseline) dosing with MPH and repeated at 0.75, 1.25, 2.25, 3.25, 4.25, 5.25, and 11 hours after MPH dosing. The subscales allowed rating of the degree to which the subject was experiencing each effect by making a vertical mark on a graduated (0–10) solid line ranging in drug effect from “not at all” (0) to “extremely” (10).
Recovery Period.
Following each study period, subjects remained at the study site until blood ethanol concentrations were below 10 mg% (mg/dl) as measured by a breathalyzer test.
d-MPH, l-MPH, d-EPH, and l-EPH Plasma Analysis.
Plasma analyses were conducted at MEDTOX Laboratories (St. Paul, MN) using liquid chromatography-tandem mass spectrometry and a vancomycin-based chiral stationary phase Chirobiotic V column (50 × 2.1 mm) from Advanced Separation Technologies (Whippany, NJ). A deuterated internal standard provided analytical control, with a range of spiked plasma calibrators run in parallel. The lower limit of quantitation was 0.05 ng/ml for each isomer (see Patrick et al., 2007 for details).
Pharmacokinetic Analysis.
Pharmacokinetic parameters were calculated by standard methods (Rowland and Towzer, 1989). The noncompartmental, analysis of enantiospecific MPH and EPH plasma concentrations was performed using WinNonlin v 5.1 (Pharsight, Cary, NC).
Statistical Analysis.
The mean and the least square geometric means of the two test treatments (d-MPH) and the reference (dl-MPH) were calculated for the Cmax and AUC. Ratios of the test geometric means to the reference, as well as the 90% confidence intervals about the reference, were determined. Comparisons between the male and female pharmacokinetic parameters were made using the Student's t test assuming equal variance. Correlations between parameters for individuals were assessed by linear regression analysis (Instat 3.01, GraphPad Software, San Diego, CA). The primary endpoint variables were compared using analysis of variance (ANOVA) with Treatment (A, B, C, D) as a between (repeated measures) factor and sex as a between subjects factor using the Latin square design to take into account sequence (carryover) effect as described by Winer (1962). The level of significance was set at P = 0.05.
Results
Human Subjects.
Twenty-four normal volunteers [12 men aged 22–30 years: mean (± S.D.) 25.8 (2.4), weight 82.2 (11.1) kg, all Caucasian; and 12 women aged 21–42 years: mean 26.9 (4.5), weight 59.6 (6.8) kg, 11 Caucasian, 1 Asian] completed the entire protocol. Treatment emergent sinus tachycardia resulted in one subject being removed from the study. Another subject withdraw due to a headache, nausea, and vomiting. Dropped subjects were replaced. Nine occurrences of headaches were reported and were treated with ibuprofen (400 mg administered at the earliest 3.5 hours following the MPH dose; thus after d-MPH Tmax and after time to peak subjective effects). No subject had any clinically significant findings on post-study “exit” laboratory tests.
Influence of Ethanol on d-MPH Pharmacokinetics: dl-MPH versus d-MPH.
Fig. 2A profiles the mean plasma concentration time course for the d-MPH isomer following oral dl-MPH (0.3 mg/kg; reference) with or without ethanol (0.6 g/kg); and Fig. 2B profiles the mean d-MPH plasma concentration following oral d-MPH (0.15 mg/kg; test) with or without ethanol (0.6 g/kg) in 24 normal volunteers. The corresponding pharmacokinetic parameters are reported in Table 1, whereas the statistical comparisons are given in Table 2. Ethanol elevated the mean d-MPH Cmax and area under the curve zero to infinity (AUC0-inf) values when dosing with dl-MPH, as well as the Cmax and AUC0-inf values when dosing with the pure isomer d-MPH. In the dl-MPH treatment, ethanol increased the mean Cmax (CV%) and AUC0-inf (CV%) values from 10.1 (31) ng/ml and 52.1 ng•h/ml (29), to 12.0 (22) ng/ml and 62.8 (26) ng•h/ml, respectively. The corresponding increases in d-MPH Cmax and AUC0-inf values by ethanol for the pure d-MPH treatments were 10.7 (21) ng/ml and 53.7 (22) ng•h/ml without ethanol, increasing to 12.4 (23) ng/ml and 61.8 (25) ng•h/ml with ethanol.
Fig. 2.
(A) mean d-MPH plasma concentrations (± S.D.) after dosing with dl-MPH (0.3 mg/kg) alone or dosing with dl-MPH (0.3 mg/kg) followed by ethanol (0.6 g/kg) 0.5 hour later; (B) mean d-MPH plasma concentrations after dosing with d-MPH alone or dosing with d-MPH (0.15 mg/kg) followed by ethanol (0.6 g/kg;) 0.5 hour later.
TABLE 1.
Pharmacokinetic parameters for subjects receiving dl-MPH or d-MPH with or without ethanol
Subjects received either dl-MPH (0.3 mg/kg) or d-MPH (0.15 mg/kg) with or without ethanol (0.6 g/kg) 0.5 hour later.
| Component | Parameter | dl-MPH Ethanol | dl-MPH | d-MPH Ethanol | d-MPH |
|---|---|---|---|---|---|
| Mean Values | CV% in parentheses | ||||
| d-MPH | K (h−1) | 0.247 (18) | 0.248 (20) | 0.259 (15) | 0.254 (23) |
| d-MPH | T1/2 (hours) | 2.9 (19) | 2.9 (19) | 2.7 (17) | 2.8 (20) |
| d-MPH | Cmax (ng/ml) | 12.0 (22) | 10.1 (31) | 12.4 (23) | 10.7 (21) |
| d-MPH | Tmax (hours) | 2.2 (34) | 2.4 (47) | 2.1 (48) | 2.0 (40) |
| d-MPH | AUClast (ng•h/ml) | 58.0 (24) | 47.9 (29) | 57.5 (24) | 50.1 (22) |
| d-MPH | AUC0-inf (ng•h/ml) | 62.8 (26) | 52.1 (29) | 61.8 (25) | 53.7 (22) |
| d-MPH | AUC0.5–2 (ng•h/ml) | 11.8 (36) | 8.5 (56) | 11.7 (47) | 11.1 (34) |
| l-MPH | K (h−1) | 0.477 (36) | 0.393 (41) | a | a |
| l-MPH | T1/2 (hours) | 1.7 (37) | 2.1 (41) | a | a |
| l-MPH | Cmax (ng/ml) | 0.32 (89) | 0.18 (86) | a | a |
| l-MPH | Tmax (hours) | 1.7 (41) | 1.8 (49) | a | a |
| l-MPH | AUClast (ng•h/ml) | 0.86 (86) | 0.45 (121) | a | a |
| l-MPH | AUC0-inf (ng•h/ml) | 1.08 (68) | 0.77 (73) | a | a |
| l-EPH | K (h−1) | 072 (23) | a | a | a |
| l-EPH | T1/2 (hours) | 1.0 (28) | a | a | a |
| l-EPH | Cmax (ng/ml) | 0.53 (81) | a | a | a |
| l-EPH | Tmax (hours) | 1.9 (91) | a | a | a |
| l-EPH | AUClast (ng•h/ml) | 1.19 (65) | a | a | a |
| l-EPH | AUC0-inf (ng•h/ml) | 1.61 (66) | a | a | a |
| Ethanol | Cmax (mg%) | 58.2 (25) | a | 58.7 (19) | a |
| Ethanol | Tmax (hours) | 1.8 (17) | a | 1.9 (20) | a |
| Ethanol | AUClast (mg%•h/ml) | 158 (34) | a | 158 (29) | a |
a, All plasma assay for all subjects below limit of detection; d-MPH arithmetic mean (SD%), least square geometric mean (90% CI), and geometric mean ratio of pharmacokinetic parameters for the 24 subjects.
TABLE 2.
Statistical comparisons between treatment groups by GeoMean ratio
| Parameter | Statistical Test | dl-MPH + Ethanol to dl-MPH | d-MPH + Ethanol to d-MPH | dl-MPH + Ethanol to d-MPH + Ethanol | dl-MPH to d-MPH |
|---|---|---|---|---|---|
| K d-MPH | GeoMean Ratio | 1.00 | 1.03 | 0.95 | 0.98 |
| 90% CI | 0.92–1.08 | 0.95–1.11 | 0.88–1.03 | 0.91–1.06 | |
| P value | 0.928 | 0.582 | 0.283 | 0.667 | |
| Cmax d-MPH | GeoMean Ratio | 1.22 | 1.15 | 0.97 | 0.91 |
| 90% CI | 1.13–1.32 | 1.06–1.24 | 0.90–1.04 | 0.84–0.98 | |
| P value | <.001 | 0.005 | 0.462 | 0.041 | |
| AUC0-inf | GeoMean Ratio | 1.21 | 1.14 | 1.01 | 0.95 |
| 90% CI | 1.14–1.30 | 1.07–1.22 | 0.94–1.08 | 0.89–1.01 | |
| P value | <0.001 | 0.001 | 0.846 | 0.193 | |
| AUC(0.5–2 hours) d-MPH | GeoMean Ratio | 1.72 | 0.95 | 1.09 | 0.6 |
| 90% CI | 1.32–2.24 | 0.73–1.24 | 0.84–1.42 | 0.46–0.79 | |
| P value | 0.001 | 0.75 | 0.57 | 0.002 | |
| K l-MPH | GeoMean Ratio | 1.26 | |||
| 90% CI | 1.08–1.48 | ||||
| P value | 0.018 | ||||
| Cmax l-MPH | GeoMean Ratio | 1.78 | |||
| 90% CI | 1.50–2.11 | ||||
| P value | <0.001 | ||||
| AUC0-inf l-MPH | GeoMean Ratio | 1.5 | |||
| 90% CI | 1.18–1.91 | ||||
| P value | 0.009 | ||||
| Cmax Ethanol | GeoMean Ratio | 0.99 | |||
| 90% CI | 0.91–1.06 | ||||
| P value | 0.744 | ||||
| AUC0–12 hours Ethanol | GeoMean Ratio | 0.99 | |||
| 90% CI | 0.90–1.08 | ||||
| P value | 0.795 |
Table 2 shows the pharmacokinetic statistical comparisons of the treatments. For the two dl-MPH treatments, ethanol significantly increased the Cmax by 22% [i.e., geometric mean (GeoMean) ratio 1.22], the AUC0-inf by 21%, and the MPH absorption phase partial AUC0.5–2 hours 72% relative to dosing with dl-MPH alone. In the pure isomer d-MPH treatments, ethanol significantly increased the Cmax by 15% and the AUC0-inf by 14% compared with d-MPH alone, but ethanol did not significantly influence the AUC0.5–2 hours.
In the comparison of dl-MPH versus d-MPH alone, or dl-MPH combined with ethanol versus d-MPH combined with ethanol, the 90% confidence interval for the GeoMean ratio for both Cmax and AUC0-inf demonstrated bioequivalence (i.e., CIs within 80–125; Table 2) (Metzler CM, 1991). In the dl-MPH-ethanol treatment, ethanol not only significantly elevated d-MPH (Fig. 2A) plasma concentrations but also l-MPH (Fig. 3) plasma concentrations during the absorption phases of the drug isomers and ethanol (Fig. 4). Compared with dl-MPH alone, ethanol increased the mean d-MPH plasma concentrations at 1 hour by 99%, (P < 0.000), 1.5 hours by 57% (P = 0.001), and 2.0 hours by 44%. The corresponding d-MPH partial AUC0.5–2 hours increased 72% (P = 0.001) during d-MPH absorption (Table 2). In contrast, ethanol did not significantly alter d-MPH early exposure when dosing with the pure d-MPH isomer; partial AUC0.5–2 hours decreased −5% (P = 0.75).
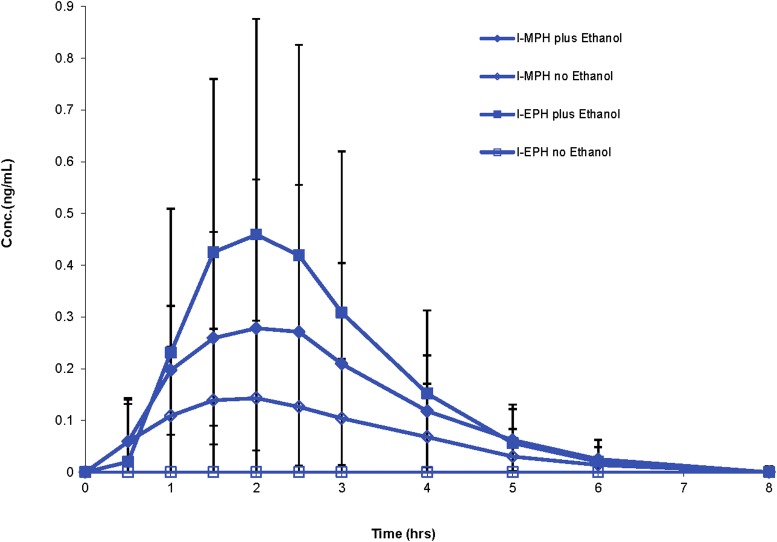
Fig. 3.
Mean l-MPH and l-EPH plasma concentrations (± S.D.) after dosing with dl-MPH (0.3 mg/kg) alone or dosing with dl-MPH (0.3 mg/kg) followed by ethanol (0.6 g/kg) 0.5 hour later.
Fig. 4.
There was significantly greater ethanol exposure (AUC0–12) in men than in women (± S.D.). In the dl-MPH-ethanol treatment the AUC was 31% higher for men (P < 0.01) and in the d-MPH-ethanol treatment the AUC was 34% higher for men (P < 0.01).
Ethanol elevated the mean Cmax (CV%) for l-MPH nearly twofold, with Cmax concentrations rising from 0.18 (86) ng/ml without ethanol to 0.32 (86) ng/ml with ethanol. The lower limit of detection for l-MPH (0.05 ng/ml) was reached at ∼5 hours postdosing. No l-MPH was detected in the d-MPH treatments.
Sex Differences in d-MPH, l-MPH, and Ethanol Pharmacokinetics.
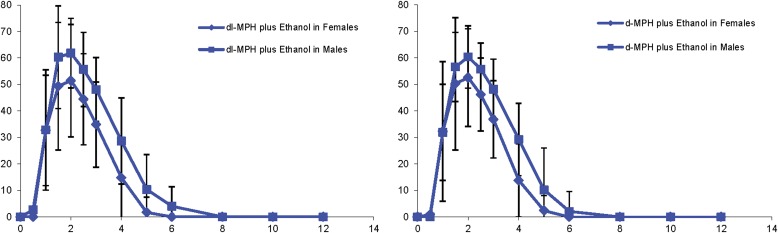
Although a trend was found for men having a greater overall exposure (AUC0-inf) to d-MPH, the AUC0.5–2 hours for d-MPH was significantly greater for men compared with women in the respective d-MPH-ethanol and d-MPH alone treatments: 14.5 versus 6.9 ng·h/ml; (P < 0.005) and 12.5 versus 7.4 ng·h/ml (P < 0.05). For the dl-MPH-ethanol and dl-MPH without ethanol treatments the corresponding values were 12.8 versus 8.9 (P = 0.17) and 9.4 versus 7.3 (P = 0.14), respectively. The Tmax values for men were less than for women in all four treatments, reaching significance again for the dl-MPH only, d-MPH-ethanol, and d-MPH alone treatments: 3 hours versus 1.9 hours (P < 0.05); 2.6 hours versus 1.6 hours (P = 0.01), and 2.3 hours versus 1.7 hours (P < 0.05), respectively (Fig. 5).
Fig. 5.
Sex-based differences in d-MPH plasma concentrations (± S.D.). The absorption phase partial AUC0.5–2 hours for the d-MPH-ethanol treatment (lower left) revealed the most prominent sex dimorphism, where d-MPH exposure in men was over twice that of the women (P < 0.05).
The Tmax for ethanol was 1.3 hours for both men and women. However, the overall exposure to ethanol was 31% and 34% greater in men than women in the dl-MPH-ethanol and d-MPH-ethanol treatments, respectively (P < 0.01). The mean Cmax and AUC0–12 values (S.D.) were 63.2 (15) mg% and 184 (23) ng•ml/h, respectively, in men compared with 53.9 (26) mg% and 133 (33) ng•ml/h for women (Fig. 4).
Unlike the time course for d-MPH in which d-MPH exposure was greater in men than women, the l-MPH isomer exposure was significantly greater in women than men in the racemic MPH treatments (P < 0.05), especially when dl-MPH was combined with ethanol, e.g., at post-dl-MPH dosing times 1 hour and 1.5 hours where plasma concentrations of l-MPH were nearly twice that in men compared with women. For this treatment, the AUC of l-MPH for women was approximately twice that for men (P < 0.05), with mean Cmax values of 0.3 ng/ml and 0.17 ng/ml, respectively (Fig. 6).
Fig. 6.
l-MPH exposure (± S.D.) was significantly greater in women than in men (P < 0.05).
Enantioselective Transesterification of l-MPH to l-EPH.
Metabolic transesterification of MPH to EPH was enantioselective in the formation of l-EPH (Fig. 1) and was only detected in the dl-MPH-ethanol treatments. l-EPH reached a mean Cmax of 0.45 ng/ml with a Tmax of 2 hours (Fig. 3). No significant sex difference was found in the extent of l-EPH formation. The highest individual concentration of plasma l-EPH was 1.28 ng/ml. No d-EPH was detected in any sample owing to the very limited extent to which d-MPH serves as a transesterification substrate and our lower limit of detection being 0.05 ng/ml of plasma (see Zhu et al., 2011 for d-EPH findings using increased sensitivity).
Subjective Effects.
Table 3 summarizes the maximum VAS subscale responses from “any drug effect” in general, as well as those more directly serving as surrogates for abuse liability, i.e., the positive subjective effects of “high,” “good,” “like,” and “stimulated.” These maximum effects occurred either at 0.75 hour following MPH or more frequently at 1.25 hours post-MPH. Other than “intoxicated,” the negative effects were generally below the effect scale of 1. In the dl-MPH-ethanol treatment, immediately following the consumption of the ethanol drink (0.75 hour after dl-MPH dosing), study subjects reported significantly increased “drug effect” and positive subjective effects compared with receiving dl-MPH alone. In the pure isomer d-MPH-ethanol treatment only the subscale of “high” reached significance (P = 0.044) at this early time when compared with d-MPH alone. However, the VAS questionnaire administered 0.5 hour later (1.25 hours following MPH dosing) revealed that the positive subjective effects produced by the d-MPH-ethanol combination had then become more prominent for the d-MPH-ethanol treatment compared with the dl-MPH-ethanol treatment.
TABLE 3.
VAS treatment comparisons
Overall “effect” and positive subjective effects of racemic dl-MPH compared with enantiopure d-MPH as influenced by ethanol within the drug absorption phase: 0.75 hour and 1.25 hours following MPH (T = 0) with or without ethanol (0.6 g/kg; consumed at a constant rate from 0.5–0.75 hour). Baseline values were subtracted from postdosing VAS ratings. All maximal effects occurred either at 0.75 hour post-MPH dosing or more frequently at 1.25 hours post-MPH.
| Subscale | Comparison | Ratio | P Value | Lower_CI | Upper_CI |
|---|---|---|---|---|---|
| Effect 0.75 hour | A − B | 2.46 | 0.000 | 1.69 | 3.58 |
| Effect 0.75 hour | C − D | 1.42 | 0.114 | 0.99 | 2.05 |
| High 0.75 hour | A − B | 2.11 | 0.005 | 1.38 | 3.23 |
| High 0.75 hour | C − D | 1.60 | 0.044 | 1.09 | 2.34 |
| Good 0.75 hour | A − B | 2.26 | 0.001 | 1.56 | 3.27 |
| Good 0.75 hour | C − D | 1.37 | 0.128 | 0.97 | 1.94 |
| Like 0.75 hour | A − B | 2.07 | 0.008 | 1.33 | 3.22 |
| Like 0.75 hour | C − D | 1.54 | 0.092 | 1.01 | 2.35 |
| Stimulated 0.75 hour | A − B | 1.73 | 0.035 | 1.13 | 2.64 |
| Stimulated 0.75 hour | C − D | 1.42 | 0.166 | 0.94 | 2.14 |
| Effect 1.25 hours | A − B | 1.39 | 0.064 | 1.04 | 1.86 |
| Effect 1.25 hours | C − D | 1.86 | 0.001 | 1.38 | 2.50 |
| High 1.25 hours | A − B | 2.40 | 0.003 | 1.51 | 3.82 |
| High 1.25 hours | C − D | 3.05 | 0.000 | 1.92 | 4.85 |
| Good 1.25 hours | A − B | 1.61 | 0.039 | 1.11 | 2.36 |
| Good 1.25 hours | C − D | 1.87 | 0.008 | 1.28 | 2.74 |
| Like 1.25 hours | A − B | 1.44 | 0.102 | 1.00 | 2.08 |
| Like 1.25 hours | C − D | 1.30 | 0.248 | 0.89 | 1.91 |
| Stimulated 1.25 hours | A − B | 1.24 | 0.354 | 0.84 | 1.84 |
| Stimulated 1.25 hours | C − D | 1.88 | 0.010 | 1.26 | 2.81 |
A, dl-MPH + ethanol; B, dl-MPH alone; C, d-MPH + ethanol; D, d-MPH alone.
The baseline values did not differ significantly from the 10 hour questionnaires, and baseline values were subtracted from the postdosing responses. There were no statistically different sex differences in subjective effects, although there was a trend toward greater effects in men than in women.
Hemodynamic Effects.
Concomitant ethanol significantly elevated heart rates in both the dl-MPH and d-MPH drug combination treatments (Fig. 7). There was a trend toward a greater effect on heart rate for the dl-MPH-ethanol treatment than for the d-MPH-ethanol treatment. The greatest time point increase in heart rate for any treatment was at the 0.75 hour reading for the dl-MPH-ethanol treatment (14 beats/min), the time corresponding to completion of the ethanol drink. The mean diastolic and systolic pressures were elevated more in the two ethanol combination treatments than in the MPH only treatments but these difference did not reach statistical significance.
Fig. 7.
Ethanol significantly elevated heart rate when combined with dl-MPH (Ritalin) or d-MPH (Focalin) with trends toward greater heart rates during the dl-MPH-ethanol treatment than for the d-MPH-ethanol treatment.
Discussion
Ethanol 0.5 hour following a dose of the racemic drug dl-MPH significantly increased early exposure to d-MPH. The absorption phase partial (see Chen et al., 2010) AUC0.5–2 hours for d-MPH was elevated by 72% compared with dosing with dl-MPH alone (P < 0.005; Fig. 2; Table 2). Contrasting these findings, ethanol did not significantly influence the AUC0.5–2 hours for the pure d-MPH form (−5%; P = 0.311). However, after the absorption phase of MPH and ethanol, ethanol significantly elevated d-MPH exposure for both enantiopure and racemic MPH (Fig. 2; Table 2).
The time interval of 0.5–2 hours was of special interest in this study because it brackets the time from when subjects first began ethanol consumption until the approximate end of the absorption phases for d-MPH (Fig. 2), l-MPH (Fig. 3), and ethanol (Fig. 4). In the dl-MPH-ethanol treatment, ethanol significantly increased the rate at which d-MPH and l-MPH reached the systemic circulation. The most rapid rise in plasma d-MPH occurred between the 0.5 and 1 hour sampling times where at 1 hour the mean d-MPH concentration had increased to twice that of the treatment receiving dl-MPH alone (P < 0.005).
Within the 0.5-1.0 hour period, one VAS questionnaire was administered (0.75 hour following dl-MPH; Table 3). The responses to the VAS subscales “any drug effect” and the positive subjective effects “high,” “good,” “like,” and “stimulated” were all significantly greater at 0.75 hour (immediately after ethanol consumption) for the racemic dl-MPH-ethanol combination compared with dl-MPH given alone. With the pure d-MPH-ethanol treatment, only “high” was significantly greater than for d-MPH alone (P = 0.044). Importantly, however, the mean peak positive subjective effect VAS responses to either dl-MPH or d-MPH were all significantly increased by ethanol, most frequently occurring at the subsequent 1.25 hour post-MPH VAS questionnaire. By that time, ethanol potentiated positive subjective responses to the pure d-MPH to a greater extent than for the racemate (Table 3).
Correlations between an increase in d-MPH absorption rate and enhanced positive subjective effects have served as surrogates for abuse liability. Increasing the rate of d-MPH absorption (Kollins et al., 1998; Spencer et al., 2012) and the subsequent rate of delivery to the central nervous system (Swanson and Volkow, 2003; Volkow and Swanson, 2003; Stoops et al., 2004; Spencer et al., 2006) strongly influence stimulant abuse liability. The present findings extend these pharmacokinetic-pharmacodynamic relationships to the early d-MPH exposure period following concomitant dl-MPH and ethanol while contributing to an understanding of the special reward value and high incidence of MPH-ethanol coabuse (Darredeau et al., 2007; Novak et al., 2007; Wilens et al., 2008).
The mean d-MPH partial AUC0.5–2 hours for men was greater than for women in all treatments and reached statistical significance for the two pure d-isomer treatments. This early d-MPH exposure period for the d-MPH ethanol combination and for d-MPH alone was 110% and 71% greater in men than women, respectively (Fig. 5). Also a significantly longer d-MPH Tmax occurred in men compared with women (see Results). Contrasting the sex differences in d-MPH exposure, the AUC for l-MPH (Fig. 6) was significantly greater in women than men. Regardless of sex, the plasma l-MPH concentrations remained an order of magnitude less than the corresponding d-MPH concentrations. There was a tendency toward greater positive subjective effects in men than in women, although these subscales did not reach statistical significance. An earlier MPH-ethanol study found women to experience a greater “stimulated” response than men. However, those results included data from ethanol-then-dl-MPH treatments and dosing was delayed 1.5 hours relative to breakfast to reduce food effects (Patrick et al., 2007).
The mechanism by which ethanol prominently influences the absorption phase for d-MPH in the dl-MPH treatment is consistent with the l-MPH component competitively inhibiting CES1 as the enzyme enantioselectively transesterifies l-MPH to l-EPH (Fig. 3; Patrick et al., 2007; Zhu et al., 2011). First-pass hepatic metabolism of l-MPH is so extensive that, in effect, the racemic drug is biocatalytically resolved before reaching the systemic circulation. Thus, following dl-MPH absorption, the elimination phase of d-MPH-ethanol interactions with CES1 becomes more comparable to that of the enantiopure d-MPH formulation. Thereafter, CES1 reverts to the more limited transesterification of d-MPH to d-EPH competing with hydrolysis (Zhu et al., 2011). In support of this competitive inhibition mechanism, human liver incubations of dl-MPH and ethanol in the presence or absence of the CES1 substrate cocaine demonstrated that cocaine significantly reduced both the rate of MPH deesterification to ritalinic acid as well as the rate of MPH transesterification to EPH (Koehm et al., 2010).
Ethanol also elevated plasma concentrations of the inactive enantiomer l-MPH, although these values did not reach l-EPH concentrations (Fig. 3). In an analogous fashion, ethanol has been reported to elevate cocaine exposure (Perez-Reyes, 1994; Farre et al., 1997) while serving as a transesterification substrate yielding CES1-mediated cocaethylene in humans (Herbst et al., 2011) and in other species (Roberts et al., 1993; 1995; Hedaya and Pan, 1996; Parker and Laizure, 2010).
The significant elevation of heart rate upon combining ethanol with either dl-MPH or d-MPH (Fig. 7) is consistent with the resulting increase in exposure to the pressor agent d-MPH. In addition, this cardiovascular response may be attributable to additive catecholaminergic influences of MPH and ethanol. The dose of ethanol used in the present study has been reported to elevate heart rate by 5.7 beats/min (Spaak et al., 2008). Similarly, combining ethanol with cocaine has been shown to significantly elevate heart rate in humans compared with cocaine alone (Herbst et al., 2011).
The pharmacokinetics of the dl-MPH and d-MPH treatments without ethanol provide evidence (Table 2) of d-MPH (0.15 mg/kg) bioequivalence to dl-MPH (0.3 mg/kg), with the caveat that the d-MPH tablets were not designed to be cut to the nearest one-half as was conducted in the present study. These results are consistent with extent of absorption comparisons between immediate-release d-MPH and modified-release MPH formulations (Tuerck et al., 2007).
Acknowledgments
The authors thank Paul J. Nietert for assistance in statistical analyses.
Abbreviations
- ADHD
attention-deficit/hyperactivity-disorder
- AUC
area under the curve
- AUC0-inf
area under the curve zero to infinity
- CES1
carboxylesterase 1
- Cmax
maximum plasma concentration
- EPH
ethylphenidate
- K
elimination rate constant
- MPH
methylphenidate
- Tmax
time to maximum concentration
- VAS
visual analog scale
Authorship contributions:
Participated in research design: Patrick, Straughn.
Conducted experiments: Bernstein, Malcolm, Patrick.
Contributed new reagents or analytic tools: Patrick.
Performed data analysis: Straughn, Reeves, Bell, Anderson, Patrick.
Wrote or contributed to the writing of the manuscript: Patrick, Straughn, Bell.
Footnotes
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant RO1AA016707]; the Medical University of South Carolina’s Clinical & Translational Research Center with support from the National Institutes of Health [Grant MO1RR01070-18]; and the National Institutes of Health National Center for Research Resources Southeastern Pre-doctoral Training in Clinical Research [Grant 1T32 RR023258].
Parts of this study were presented in Master's thesis (Reeves O III. Ethanol Interactions with dl-Methylphenidate versus d-Methylphenidate in Humans. Charleston, Medical University of South Carolina, 2011) and presented in abstract form [Reeves O, Straughn A, Bell G, Bernstein H, Malcolm R, and Patrick K (2011) Ethanol elevates plasma dexmethylphenidate and dl-methylphenidate concentrations and potentiates subjective effects. J Clin Pharmacol 51:1332] at the 40th Annual Meeting of the American College of Clinical Pharmacology; 2011 Sept 10–12, 2011; Chicago, IL.
References
- Aoyama T, Sasaki T, Kotaki H, Sawada Y, Sudoh Y, Honda Y, Iga T. (1994) Pharmacokinetics and pharmacodynamics of (+)-threo-methylphenidate enantiomer in patients with hypersomnia. Clin Pharmacol Ther 55:270–276 [DOI] [PubMed] [Google Scholar]
- Barrett SP, Darredeau C, Bordy LE, Pihl RO. (2005) Characteristics of methylphenidate misuse in a university student sample. Can J Psychiatry 50:457–461 [DOI] [PubMed] [Google Scholar]
- Bourland JA, Martin DK, Mayersohn M. (1997) Carboxylesterase-mediated transesterification of meperidine (Demerol) and methylphenidate (Ritalin) in the presence of [2H6]ethanol: preliminary in vitro findings using a rat liver preparation. J Pharm Sci 86:1494–1496 [DOI] [PubMed] [Google Scholar]
- Chen ML, Shah VP, Ganes D, Midha KK, Caro J, Nambiar P, Rocci ML, Jr, Thombre AG, Abrahamsson B, Conner D, et al. (2010) Challenges and opportunities in establishing scientific and regulatory standards for assuring therapeutic equivalence of modified-release products: workshop summary report. Eur J Pharm Sci 40:148–153 [DOI] [PubMed] [Google Scholar]
- Darredeau C, Barrett SP, Jardin B, Pihl RO. (2007) Patterns and predictors of medication compliance, diversion, and misuse in adult prescribed methylphenidate users. Hum Psychopharmacol 22:529–536 [DOI] [PubMed] [Google Scholar]
- Farré M, de la Torre R, González ML, Terán MT, Roset PN, Menoyo E, Camí J. (1997) Cocaine and alcohol interactions in humans: neuroendocrine effects and cocaethylene metabolism. J Pharmacol Exp Ther 283:164–176 [PubMed] [Google Scholar]
- Hedaya MA, Pan WJ. (1996) Cocaine and alcohol interactions in naive and alcohol-pretreated rats. Drug Metab Dispos 24:807–812 [PubMed] [Google Scholar]
- Herbst ED, Harris DS, Everhart ET, Mendelson J, Jacob P, Jones RT. (2011) Cocaethylene formation following ethanol and cocaine administration by different routes. Exp Clin Psychopharmacol 19:95–104 [DOI] [PubMed] [Google Scholar]
- Koehm M, Kauert GF, Toennes SW. (2010) Influence of ethanol on the pharmacokinetics of methylphenidate’s metabolites ritalinic acid and ethylphenidate. Arzneimittelforschung 60:238–244 [DOI] [PubMed] [Google Scholar]
- Kollins SH, Rush CR, Pazzaglia PJ, Ali JA. (1998) Comparison of acute behavioral effects of sustained-release and immediate-release methylphenidate. Exp Clin Psychopharmacol 6:367–374 [DOI] [PubMed] [Google Scholar]
- LeVasseur NL, Zhu HJ, Markowitz JS, DeVane CL, Patrick KS. (2008) Enantiospecific gas chromatographic-mass spectrometric analysis of urinary methylphenidate: implications for phenotyping. J Chromatogr B Analyt Technol Biomed Life Sci 862:140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Kleber HD. (1995) Attention-deficit hyperactivity disorder and substance abuse: relationships and implications for treatment. Harv Rev Psychiatry 2:246–258 [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Logan BK, Diamond F, Patrick KS. (1999) Detection of the novel metabolite ethylphenidate after methylphenidate overdose with alcohol coingestion. J Clin Psychopharmacol 19:362–366 [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Patrick KS. (2008) Differential pharmacokinetics and pharmacodynamics of methylphenidate enantiomers: does chirality matter? J Clin Psychopharmacol 28(3, Suppl 2)S54–S61 [DOI] [PubMed] [Google Scholar]
- Metzler CM. (1991) Sample sizes for bioequivalence studies. Stat Med 10:961–969, discussion 969–970 [DOI] [PubMed] [Google Scholar]
- Modi NB, Wang B, Noveck RJ, Gupta SK. (2000) Dose-proportional and stereospecific pharmacokinetics of methylphenidate delivered using an osmotic, controlled-release oral delivery system. J Clin Pharmacol 40:1141–1149 [PubMed] [Google Scholar]
- Novak SP, Kroutil LA, Williams RL, Van Brunt DL. (2007) The nonmedical use of prescription ADHD medications: results from a national Internet panel. Subst Abuse Treat Prev Policy 2:32–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RB, Laizure SC. (2010) The effect of ethanol on oral cocaine pharmacokinetics reveals an unrecognized class of ethanol-mediated drug interactions. Drug Metab Dispos 38:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick KS, Kilts CD, Breese GR. (1981) Synthesis and pharmacology of hydroxylated metabolites of methylphenidate. J Med Chem 24:1237–1240 [DOI] [PubMed] [Google Scholar]
- Patrick KS, Straughn AB, Minhinnett RR, Yeatts SD, Herrin AE, DeVane CL, Malcolm R, Janis GC, Markowitz JS. (2007) Influence of ethanol and gender on methylphenidate pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 81:346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick KS, Williard RL, VanWert AL, Dowd JJ, Oatis JE, Jr, Middaugh LD. (2005) Synthesis and pharmacology of ethylphenidate enantiomers: the human transesterification metabolite of methylphenidate and ethanol. J Med Chem 48:2876–2881 [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M. (1994) The order of drug administration: its effects on the interaction between cocaine and ethanol. Life Sci 55:541–550 [DOI] [PubMed] [Google Scholar]
- Redalieu E, Bartlett MF, Waldes LM, Darrow WR, Egger H, Wagner WE. (1982) A study of methylphenidate in man with respect to its major metabolite. Drug Metab Dispos 10:708–709 [PubMed] [Google Scholar]
- Roberts SM, Harbison RD, James RC. (1993) Inhibition by ethanol of the metabolism of cocaine to benzoylecgonine and ecgonine methyl ester in mouse and human liver. Drug Metab Dispos 21:537–541 [PubMed] [Google Scholar]
- Roberts SM, Phillips DL, Tebbett IR. (1995) Increased blood and brain cocaine concentrations with ethanol cotreatment in mice. Drug Metab Dispos 23:664–666 [PubMed] [Google Scholar]
- Rowland M, Tozer T. (1989) Clinical Pharmacokinetics: Concepts and Applications, Lea and Febiger, London [Google Scholar]
- Scharman EJ, Erdman AR, Cobaugh DJ, Olson KR, Woolf AD, Caravati EM, Chyka PA, Booze LL, Manoguerra AS, Nelson LS, et al. American Association of Poison Control Centers (2007) Methylphenidate poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 45:737–752 [DOI] [PubMed] [Google Scholar]
- Spaak J, Merlocco AC, Soleas GJ, Tomlinson G, Morris BL, Picton P, Notarius CF, Chan CT, Floras JS. (2008) Dose-related effects of red wine and alcohol on hemodynamics, sympathetic nerve activity, and arterial diameter. Am J Physiol Heart Circ Physiol 294:H605–H612 [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Ciccone PE, Madras BK, Dougherty DD, Bonab AA, Livni E, Parasrampuria DA, Fischman AJ. (2006) PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry 163:387–395 [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Martin JM, Moorehead TM, Mirto T, Clarke A, Batchelder H, Faraone SV. (2012) Importance of pharmacokinetic profile and timing of coadministration of short- and long-acting formulations of methylphenidate on patterns of subjective responses and abuse potential. Postgrad Med 124:166–173 [DOI] [PubMed] [Google Scholar]
- Srinivas NR, Hubbard JW, Korchinski ED, Midha KK. (1993) Enantioselective pharmacokinetics of dl-threo-methylphenidate in humans. Pharm Res 10:14–21 [DOI] [PubMed] [Google Scholar]
- Srinivas NR, Hubbard JW, Quinn D, Midha KK. (1992) Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther 52:561–568 [DOI] [PubMed] [Google Scholar]
- Stoops WW, Glaser PE, Fillmore MT, Rush CR. (2004) Reinforcing, subject-rated, performance and physiological effects of methylphenidate and d-amphetamine in stimulant abusing humans. J Psychopharmacol 18:534–543 [DOI] [PubMed] [Google Scholar]
- Sun Z, Murry DJ, Sanghani SP, Davis WI, Kedishvili NY, Zou Q, Hurley TD, Bosron WF. (2004) Methylphenidate is stereoselectively hydrolyzed by human carboxylesterase CES1A1. J Pharmacol Exp Ther 310:469–476 [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. (2003) Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev 27:615–621 [DOI] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, Boyd CJ, Guthrie SK. (2003) Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy 23:609–617 [DOI] [PubMed] [Google Scholar]
- Tuerck D, Wang Y, Maboudian M, Wang Y, Sedek G, Pommier F, Appel-Dingemanse S. (2007) Similar bioavailability of dexmethylphenidate extended (bimodal) release, dexmethyl-phenidate immediate release and racemic methylphenidate extended (bimodal) release formulations in man. Int J Clin Pharmacol Ther 45:662–668 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. (2003) Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry 160:1909–1918 [DOI] [PubMed] [Google Scholar]
- Wigal S, Swanson JM, Feifel D, Sangal RB, Elia J, Casat CD, Zeldis JB, Conners CK. (2004) A double-blind, placebo-controlled trial of dexmethylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 43:1406–1414 [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S. (2008) Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry 47:21–31 [DOI] [PubMed] [Google Scholar]
- Williard RL, Middaugh LD, Zhu HJ, Patrick KS. (2007) Methylphenidate and its ethanol transesterification metabolite ethylphenidate: brain disposition, monoamine transporters and motor activity. Behav Pharmacol 18:39–51 [DOI] [PubMed] [Google Scholar]
- Winer B. (1962) Statistical Principles in Experimental Design, 1st ed, McGraw-Hill, New York [Google Scholar]
- Zhu HJ, Appel DI, Jiang Y, Markowitz JS. (2009) Age- and sex-related expression and activity of carboxylesterase 1 and 2 in mouse and human liver. Drug Metab Dispos 37:1819–1825 [DOI] [PubMed] [Google Scholar]
- Zhu HJ, Patrick KS, Markowitz JS. (2011) Enantiospecific determination of DL-methylphenidate and DL-ethylphenidate in plasma by liquid chromatography-tandem mass spectrometry: application to human ethanol interactions. J Chromatogr B Analyt Technol Biomed Life Sci 879:783–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HJ, Patrick KS, Yuan HJ, Wang JS, Donovan JL, DeVane CL, Malcolm R, Johnson JA, Youngblood GL, Sweet DH, et al. (2008) Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet 82:1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]