Abstract
This article is a report on a symposium sponsored by the American Society for Pharmacology and Experimental Therapeutics and held at the Experimental Biology 12 meeting in San Diego, CA. The presentations discussed the roles of a number of nuclear receptors in regulating glucose and lipid homeostasis, the pathophysiology of obesity-related disease states, and the promise associated with targeting their activities to treat these diseases. While many of these receptors (in particular, constitutive androstane receptor and pregnane X receptor) and their target enzymes have been thought of as regulators of drug and xenobiotic metabolism, this symposium highlighted the advances made in our understanding of the endogenous functions of these receptors. Similarly, as we gain a better understanding of the mechanisms underlying bile acid signaling pathways in the regulation of body weight and glucose homeostasis, we see the importance of using complementary approaches to elucidate this fascinating network of pathways. The observation that some receptors, like the farnesoid X receptor, can function in a tissue-specific manner via well defined mechanisms has important clinical implications, particularly in the treatment of liver diseases. Finally, the novel findings that agents that selectively activate estrogen receptor β can effectively inhibit weight gain in a high-fat diet model of obesity identifies a new role for this member of the steroid superfamily. Taken together, the significant findings reported during this symposium illustrate the promise associated with targeting a number of nuclear receptors for the development of new therapies to treat obesity and other metabolic disorders.
Introduction
Nuclear receptors are historically defined by virtue of their roles as endocrine or environmental sensors (Ai et al., 2009; Markov and Laudet, 2011; Merk and Schubert-Zsilavecz, 2012). As transcription factors that directly link their environments with key metabolic processes, they are attractive targets for therapeutic interventions. With respect to metabolic disorders that involve perturbations of glucose and lipid homeostasis, work performed in the past two decades has focused heavily on targeting the peroxisome proliferator-activated receptors (PPARs). While many PPAR agonists were proven to be clinically efficacious, their use became increasingly plagued by side effects. These events underscored the need to better understand how the metabolic processes that control lipid and glucose levels are regulated, with the goal of developing a new generation of nuclear receptor ligands to manage metabolic diseases. Toward this end, the nuclear receptors, constitutive androstane receptor (CAR), pregnane X receptor (PXR), farnesoid X receptor (FXR), and estrogen receptor β (ER-β) have emerged as attractive candidate receptors; bile-activated receptors, such as the membrane G protein–coupled receptor (GPCR) that is referred to as TGR5, are also being examined. While CAR, PXR, and FXR have been characterized as “xenobiotic” receptors, increasing evidence indicates that these receptors also play vital roles in responding to the presence of endogenous agonists. These receptors alter the expression levels of drug-metabolizing enzymes and transporters, thereby metabolically regulating the cellular levels of their cognate agonists. The targets of these finely tuned, catabolic feedback pathways are typically highly lipophillic substances that interact with and activate other receptors (i.e., the GPCR for bile acids, TGR5) and metabolic signaling pathways. The “xenobiotic” receptors and members of the “classical” steroid hormone receptor family (i.e., ER-β) are now understood to play important roles in the regulation of glucose and lipid dysfunction and have thus gained considerable attention. This symposium was organized to highlight new understandings of how these nuclear receptors mediate events associated with lipid dysfunction and obesity-related diseases, and how these mechanisms can be exploited to develop new therapies.
Endobiotic Functions of Xenobiotic Receptors and Xenobiotic Enzymes in Energy Metabolism
The PXR and CAR are two closely related and liver-enriched nuclear hormone receptors originally defined as xenobiotic receptors. Recently, an increasing body of evidence suggests that PXR and CAR also have endobiotic functions that impact not only glucose and lipid metabolismbut also the pathogenesis of metabolic diseases (Konno et al., 2008; Gao and Xie, 2010). Interestingly, the nuclear receptor target enzymes, such as the estrogen sulfotransferase, also play an important role in fat cell differentiation and energy metabolism.
CAR and PXR in Energy Metabolism.
In a recent study, we have uncovered an unexpected role of CAR in preventing obesity and alleviating type 2 diabetes (Gao et al., 2009). Using a high-fat diet-induced obesity model, we showed that treatment of wild-type mice with the CAR agonist 1,4-bis [2-(3,5-dichloropyridyloxy)] benzene (TCPOBOP) efficiently prevented the onset of obesity or reversed preinduced obesity. Treatment with TCPOBOP improved insulin sensitivity in both the high-fat diet-induced type 2 diabetic model and the ob/ob mice. In contrast, CAR null mice maintained on chow diet showed spontaneous insulin insensitivity, which could not be relieved by TCPOBOP treatment. The hepatic steatosis in high-fat diet-treated mice and ob/ob mice was markedly reduced by TCPOBOP treatment. The metabolic benefits of CAR activation may have resulted from the combined effect of inhibition of lipogenesis, very low density cholesterol secretion and export of triglycerides, and gluconeogenesis; increases in brown adipose tissue energy expenditure and peripheral fat mobilization may have also played a role. Similar effects of CAR activation in relieving high-fat diet and ob/ob models of steatosis and type 2 diabetes (Dong et al., 2009) and gestational obesity and diabetes (Masuyama and Hiramatsu, 2012a; Masuyama and Hiramatsu, 2012b) have been independently reported. These results have revealed an important metabolic function of CAR and may establish this “xenobiotic receptor” as a novel therapeutic target for the prevention and treatment of obesity and type 2 diabetes. The results of animal studies are consistent with the clinical observations that phenobarbital, a prototypical CAR activator, is known to decrease plasma glucose levels and improve insulin sensitivity in diabetic patients (Lahtela et al., 1985; Sotaniemi and Karvonen, 1989). The spontaneous insulin insensitivity in CAR null mice suggests an endogenous function of CAR, which may have been controlled by endogenous CAR ligand(s). Thus, an outstanding challenge is to identify endogenous CAR ligands that elicit the metabolic functions of CAR in vivo.
PXR is a sister xenobiotic receptor of CAR that shares many functions in xenobiotic regulation and related pathophysiology. Compared with CAR, the in vivo effects of PXR activation on type 2 diabetes are yet to be reported. Despite its ability to suppress gluconeogenesis (Kodama et al., 2004; Kodama et al., 2007), PXR activation is also known to cause hepatic steatosis (Zhou et al., 2006; Zhou et al., 2008; Cheng et al., 2012) and increase serum corticosteroid levels (Zhai et al., 2007), conditions known to be positively associated with insulin resistance. Based on these observations, it may not be a surprise that PXR activation may exert an adverse (rather than beneficial) effect on type 2 diabetes, at least in mouse models. Indeed, several known PXR-activating drugs, such as rifampicin, phenytoin, and cyclophosphamide, have been reported to induce hyperglycemia in patients (Luna and Feinglos, 2001). However, it remains to be determined whether PXR is the mediator for the drug-induced hyperglycemia. The effects of CAR and PXR on energy metabolism are summarized in Fig. 1A.
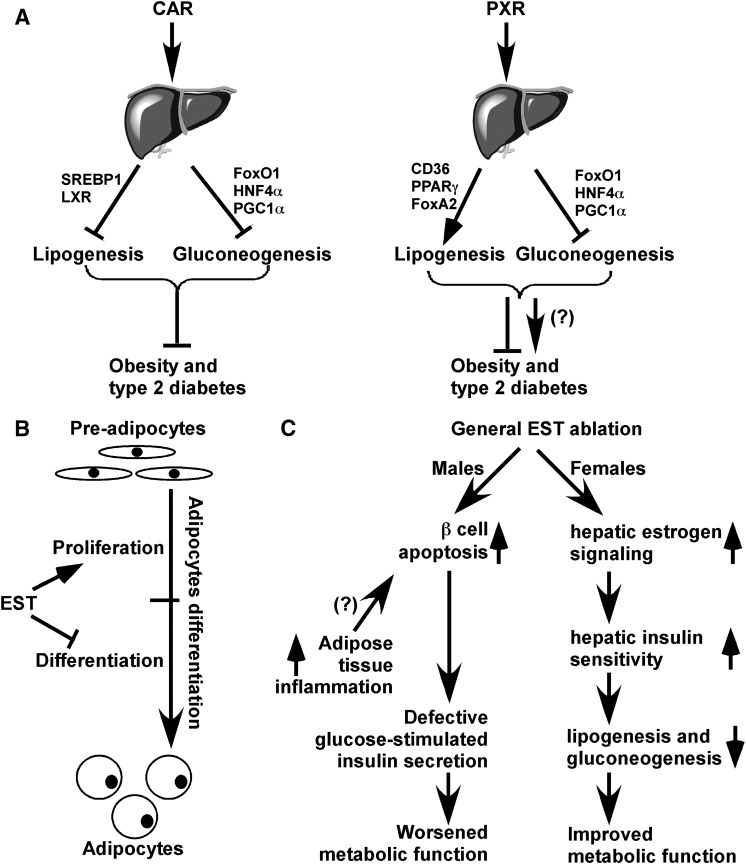
Fig. 1.
Endobiotic functions of xenobiotic receptors and xenobiotic enzymes in energy metabolism. (A) The roles of CAR and PXR on obesity and type 2 diabetes. Activation of CAR suppresses both hepatic gluconeogenesis and lipogenesis, likely mediated through its inhibitory effect on transcriptional factors such as FOXO1, HNF4α, PGC1α, LXR, and SREBP1. The in vivo benefit of CAR activation in relieving metabolic disease has been reported. In the liver, PXR has a similar inhibitory effect on gluconeogenesis. However, PXR activation may increase fatty acid influx and lipogenesis directly or by activating PPARγ and suppressing fatty acid oxidation through suppressing FoxA2. The in vivo significance of PXR in metabolic syndrome remains to be demonstrated. (B) The roles of EST on fat cell differentiation and (C) type 2 diabetes. In rodent cells, EST may inhibit adipocyte differentiation by sustained activation of ERK1/2 MAPK and inhibition of insulin signaling, leading to a failure of switch from clonal expansion to differentiation. The effect of EST on type 2 diabetes is gender-specific. Loss of EST improves and worsens metabolic functions in female and male mice, respectively.
Estrogen Sulfotransferase in Fat Cell Differentiation and Type 2 Diabetes.
The concept of endobiotic function of xenobiotic systems on energy metabolism can also be extended to xenobiotic enzymes, such as estrogen sulfotransferase (EST). EST is a phase II drug-metabolizing enzyme that is encoded by the SULT1E1 gene and known to catalyze the sulfoconjugation and deactivation of estrogens (Song et al., 1995). EST is highly expressed in the white adipose tissue of male mice, but the role of EST in the development and function of adipocytes remains largely unknown. We have previously reported on the transcriptional regulation of EST by the liver X receptor (LXR) (Gong et al., 2007) and glucocorticoid receptor (GR) (Gong et al., 2008) and examined the implications of this regulation in estrogen homeostasis and hormone-dependent breast cancer growth. In a more recent report, we showed that EST played an important role in adipocyte differentiation (Wada et al., 2011). EST is highly expressed in 3T3-L1 preadipocytes and primary mouse preadipocytes. The expression of EST was dramatically reduced in differentiated 3T3-L1 cells and mature primary adipocytes. Overexpression of EST in 3T3-L1 cells prevented adipocyte differentiation. In contrast, preadipocytes isolated from EST knockout mice exhibited enhanced differentiation. The inhibitory effect of EST on adipogenesis likely resulted from the sustained activation of the extracellular signal–regulated kinase (ERK)-1 and ERK2 mitogen-activated protein kinase (MAP) pathway and inhibition of insulin signaling, leading to a failure of switch from clonal expansion to differentiation. The enzymatic activity of EST was required for the inhibitory effect of EST on adipogenesis, because an enzyme-dead EST mutant failed to inhibit adipocyte differentiation. In vivo, overexpression of EST in the adipose tissue of female transgenic mice resulted in smaller adipocyte size. Taken together, our results suggest that EST functions as a negative regulator of adipogenesis.
In a more recent work, we showed that EST has a sex-specific effect on mouse models of type 2 diabetes (Gao et al., 2012). Specifically, loss of Est in female mice improved metabolic function in ob/ob mouse models of type 2 diabetes induced by dexamethasone and high-fat diet. The metabolic benefit of Est ablation included improved body composition, increased energy expenditure and insulin sensitivity, and decreased hepatic gluconeogenesis and lipogenesis. This metabolic benefit appeared to have resulted from decreased estrogen deprivation and increased estrogenic activity in the liver, whereas such benefit was abolished in ovariectomized mice. Interestingly, the effect was sex-specific, as Est ablation in ob/ob males exacerbated the diabetic phenotype, which was accounted for by a decrease in islet β cell mass and failure of glucose-stimulated insulin secretion in vivo. The loss of β cell mass in ob/ob male mice deficient of EST was associated with increased macrophage infiltration and inflammation in white adipose tissue. Our results revealed an essential role of EST in energy metabolism and the pathogenesis of type 2 diabetes. Inhibition of EST, at least in females, may represent a novel approach to manage type 2 diabetes. The effects of EST on fat cell differentiation and energy metabolism are summarized in Fig. 1, B and C, respectively.
In summary, the aforementioned examples support the notion that the “traditional” xenobiotic nuclear receptors and their target xenobiotic enzymes do have important roles in endobiotic metabolism, including energy metabolism. It is hoped that the endobiotic functions of the xenobiotic receptors and xenobiotic enzymes can be harnessed for the therapeutic management of metabolic diseases.
Bile Acid–Activated Receptors in Regulating Lipid and Glucose Metabolism
Bile Acids as Signaling Molecules.
Bile acids are signaling molecules and activate multiple cellular signaling pathways involving calcium mobilization, cyclic AMP synthesis, and protein kinase C translocation and activation. These molecules interact with the membrane GPCR named TGR5 (also known as GPBAR1) and with muscarinic and nuclear receptors, including the FXR, PXR, CAR, and the vitamin D receptor. This family of receptors is identified as a whole as bile acid–activated receptors (Nguyen and Bouscarel, 2008).
FXR is expressed in the liver, intestine, kidney, and adrenal glands, functioning as a bile acid sensor by regulating the expression of various transport proteins and biosynthetic enzymes crucial to the physiologic maintenance of bile acid homeostasis. TGR5, a member of the rhodopsin-like superfamily of GPCRs that transduces signals through G proteins (α–βγ subunits) is expressed in the ileum and colon. Both FXR and TGR5 play a role in regulating energy and glucose metabolism. TGR5 ligands decrease blood glucose levels and increase energy expenditure. FXR agonism reduces glucose plasma levels and triglycerides synthesis, induces insulin release, and ameliorates insulin signaling. However, FXR ligands increase the liver expression of the GR and stimulate gluconeogenic pathways in fasting. Because FXR deficiency ameliorates glucose tolerance in rodent model of diabetes, the role of this receptor in modulating glucose homeostasis requires further investigation.
Bile acids are amphipathic molecules synthesized in the liver following oxidation of cholesterol and stored in the gallbladder as the main constituent of bile. Chenodeoxycholic acid (CDCA) and cholic acid (CA) are the two primary bile acids in humans and are conjugated primarily to glycine and taurine (Fiorucci et al., 2009). The amphipathic chemical structure of bile acids is essential for the solubilization of dietary lipids. More than 95% of the bile acid pool is reabsorbed from the intestine, predominantly by an active sodium-dependent apical bile acid transporter in the terminal ileum and transported back to the liver bound primarily to albumin and to a lesser extent to lipoproteins (Keitel et al., 2008; Trauner et al., 2010). A limited pool of bile acids that is not reabsorbed in the small intestine undergoes dehydroxylation and deconjugation in the large intestine by bacterial enzymes, leading to the formation of the secondary bile acids: deoxycholic acid (DCA) from CA, and lithocholic acid (LCA) from CDCA (Keitel et al., 2008; Trauner et al., 2010). These bile acids are reabsorbed passively from the colon and return to the liver through the portal circulation to exert a feedback control on bile acid synthesis.
The liver plays a major role in maintaining plasma glucose homeostasis by controlling the balance between hepatic glucose uptake/utilization and hepatic glucose production (Fig. 2). This regulation undergoes dramatic adaptation in the fasting-feeding transition. In the fed state, the liver stores energy from glucose by synthesizing glycogen and fat. Insulin and glucose act in concert to promote the expression of genes orchestrating glucose utilization and fatty acid synthesis. Conversely, when plasma glucose concentrations decrease during fasting, the liver generates glucose via gluconeogenesis, a hepatic pathway regulated by the activity of two rate-limiting enzymes: glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK). The expression of these genes is tightly regulated at the transcriptional level by hormones controlling glucose homeostasis, with glucagon and glucocorticoids strongly promoting and insulin inhibiting hepatic gluconeogenesis via its suppression of both G6Pase and PEPCK expression levels. The expression of PEPCK and G6Pase is positively regulated in the fasting state by different transcription factors and coactivators, including the hepatic nuclear factor 4α (HNF4α), GR, the Forkhead box O1 (FOXO1), and peroxisome proliferator–activated receptor gamma coactivator 1 alpha (PGC1-α) (Yabaluri and Bashyam, 2010).
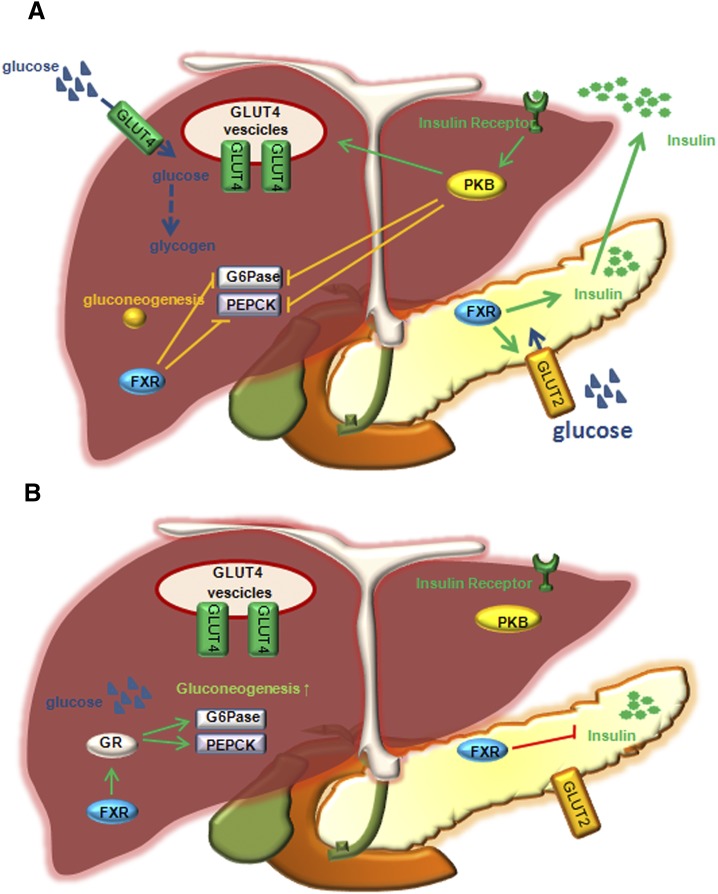
Fig. 2.
The role of FXR in regulation of glucose metabolism in mice. (A) In the fed state, insulin stimulates GLUT-4 recruitment to plasma membrane. Glucose taken up by GLUT-4 to hepatocytes stimulates glycogen synthesis. Insulin signaling activates PDZ-binding kinase, PBK, to phosphorylate and inhibit FOXO1, and results in inhibiting G6Pase and PEPCK expression and gluconeogenesis. Glucose is taken up by insulin-sensitive GLUT-2 to pancreatic β cells. FXR stimulates insulin gene transcription and secretion from β cells when glucose levels are high. (B) In the fasting state, glucagon stimulates gluconeogenesis by activating GR, which induces G6Pase and PEPCK. In addition, FXR activates GR (to stimulate G6Pase and PEPCK) and inhibits insulin gene transcription.
Bile acids exert an important role in regulating glucose metabolism in part via modulation of glycogen homeostasis in the liver. Bile acids stimulate the activity of glycogen synthase, an effect that would be instrumental to their ability to reduce glucose plasma levels (Fang et al., 2007). On the other hand, bile acids activate glycogen phosphorylase and the breakdown of glycogen to glucose-1P (Staels and Kuipers, 2007). Thus, it appears that by activating glycogen synthase and glycogen phosphorylase, bile acid-activated receptors exert an equivocal role in regulating liver glucose homeostasis.
The effects exerted by different bile acids on glycogen homeostasis are, at least partially, specific for each individual bile acid. Indeed, ursodeoxycholic acid (UDCA) activates glycogen phosphorylase in a dose-dependent manner with an EC50 of ∼9 μM and a maximum effect at concentrations greater or equal to 100 μM. Other bile acids, LCA, taurolithocholic acid (TLCA), and tauroursodeoxycholic acid (TUDCA), activate glycogen phosphorylase at significantly higher concentrations (∼100 μM), whereas TCA, CA, and glycoursodeoxycholic acid (GUDCA) have no effect. On the other hand, TCA and DCA (100 μM) activate glycogen synthase (Fang et al., 2007). This suggests that different bile acids differentially regulate glycogen synthesis and breakdown to maintain glycogen levels and glucose homeostasis.
FXR and Glucose Homeostasis.
FXR is a member of the nuclear receptor superfamily and is highly expressed in the liver, intestine, kidney, and adrenal glands (Fiorucci et al., 2009). The physiologic ligand of FXR is CDCA, which activates the receptor with an EC50 of 10 μM. FXR functions as a bile acid sensor in entero-hepatic tissues, regulating the expression of various transport proteins and biosynthetic enzymes crucial to the physiologic maintenance of bile acid homeostasis (Fiorucci et al., 2009). The link between FXR and glucose homeostasis has been suggested by several in vitro and in vivo studies. First of all, FXR gene expression is differentially regulated by insulin and glucose, with high concentrations of insulin negatively regulating its expression and glucose positively up-regulating it (Duran-Sandoval et al., 2004). However, insulin does not prevent the up-regulation of FXR expression by glucose. FXR is expressed in pancreatic β-cells and regulates insulin signaling (Fig. 2A). In βTC-6 cells, an insulin-secreting cell line derived from transgenic mice expressing the large T-antigen of simian virus 40 (SV40) in pancreatic β-cells, FXR induces expression of the glucose-regulated transcription factor KLF11 (Renga et al., 2010), which accounts for the effect of FXR on glucose-induced insulin gene transcription. In addition, FXR regulates insulin secretion by nongenomic effects by increasing AKT phosphorylation and translocation of glucose transporter-2 (GLUT-2) at the plasma membrane of β-cells, as well as GLUT-4 gene expression on hepatocytes (Shen et al., 2008), thus increasing glucose uptake by these cells (Fig. 2A). These FXR-mediated effects on insulin transcription and secretion occur only during conditions of high glucose concentrations (Renga et al., 2010).
Several animal studies have shown that FXR impacts insulin sensitivity, glycogen synthesis, and gluconeogenesis. Indeed, FXR-null mice are transiently hypoglycemic while fasted (Cariou et al., 2006; van Dijk et al., 2009) and exhibit delayed intestinal glucose absorption (van Dijk et al., 2009) and reduced hepatic glycogen content (Cariou et al., 2005; van Dijk et al., 2009). However, it is noteworthy that Fxr gene ablation in murine models of genetic (ob/ob) and diet-induced obesity improves hyperglycemia and glucose tolerance (Prawitt et al., 2011). By contrast, Fxr gene ablation in nondiabetic mice causes peripheral insulin resistance and impaired insulin signaling in adipose tissue and skeletal muscle (Cariou et al., 2006; Ma et al., 2006). In addition, the pharmacologic activation of FXR by GW4064 (a potent synthetic FXR agonist) and 6-ethyl-CDCA in murine models of diabetes results in a down-regulation of gluconeogenic genes in the liver (Ma et al., 2006; Zhang et al., 2006; Cipriani et al., 2010). These effects appear to be due, in part, to activation of SHP (small heterodimer partner), a canonical FXR target gene. Thus, in db/db diabetic mice feeding CA-induced SHP, the interaction of PGC1-α with GR, HNF4α, and FOXO1 was disrupted and gluconeogenesis ultimately decreased (Borgius et al., 2002; Cipriani et al., 2010). While results obtained following overexpression of SHP have indicated that SHP counter-regulated the activities of key nuclear receptors involved in gluconeogenesis, studies using Shp−/− mice have yielded contradictory results. Here, hepatic glucose production was found to be significantly increased in SHP+/+ but not in Shp−/− mice in response to fasting. Further, in contrast with that observed in diabetic mice, administration of GW-4064 has resulted in either a stimulation (Downes et al., 2003; Stayrook et al., 2005) or repression of PEPCK (Sinal et al., 2000; Yamagata et al., 2004; Ma et al., 2006) and may either decrease or have no impact on glucose levels (Stayrook et al., 2005; Ma et al., 2006; Zhang et al., 2006).
These discrepancies suggest that the FXR signaling in the liver depends on the activity of additional regulatory factors. Since the expression of FXR in the liver changes significantly during the fasting-to-refeeding transition (Duran-Sandoval et al., 2005), it is likely that the activity of FXR is modulated by the ability of hepatocytes to sense blood glucose levels. Thus, FXR agonism may elicit differential physiologic effects in fasted versus fed organisms. Indeed, we have shown that FXR activation exerts opposite effects during fasting or feeding conditions (Renga et al., 2012). Consequently, the activation of FXR down-regulates the expression of gluconeogenic genes Pepck and G6pase (Fig. 2A) in fed animals, but the opposite occurs in the fasting state (Fig. 2B).
In addition, our results demonstrated that, in the fasting state, the up-regulation of PEPCK and G6Pase requires the induction of another nuclear receptor: the GR, which is a positive regulator of gluconeogenesis (Fig. 2B) (Renga et al., 2012). During conditions of high energy demand, such as exercise, energy deprivation, or fasting, systemic glucocorticoid concentrations increase and their sensing by GR in the liver coordinates the activation of glucose mobilization from the liver via gluconeogenesis. Indeed, mice with conditional disruption of GR in hepatocytes exhibit profound hypoglycemia after prolonged fasting and are unable to up-regulate the expression of gluconeogenic enzymes, such as PEPCK.
Several studies have shown that a stronger activation of GR-dependent transcription occurs in various models of diabetes (i.e., Zucker diabetic fatty rats and db/db and ob/ob mice), whereas the down-regulation of GR, mRNA, and activity, via administration of a 11β-hydroxysteroid dehydrogenase type 1 inhibitor, reduces weight gain, hyperglycemia and insulin resistance in response to a high-fat diet in mice. The expression and activity of the GR is modulated by reciprocal interactions with other members of the nuclear hormone superfamily of regulatory factors, including the LXR. By performing a detailed characterization of the expression of the GR in mice lacking FXR, we have shown that the FXR signaling changes significantly during the fasting-refeeding transition. Noteworthy, in the fasting state, the up-regulation of PEPCK and G6Pase requires the induction of the GR, a positive regulator of gluconeogenesis. Thus, not only do Fxr−/− fasted mice have reduced liver expression levels of GR, but also the ablation of the GR by a GR siRNA in hepatocytes abrogates the effects of FXR agonism in vitro. Furthermore, mice harboring a disrupted FXR are refractory to GR activation in fasting, as demonstrated by the failure of dexamethasone treatment to increase either gluconeogenic gene expression or blood glucose levels in Fxr−/− mice. These results support a reciprocal regulation between the two receptors, indicating that intact FXR signaling is required to regulate gluconeogenic genes by GR agonists in fasted mice.
The indispensable role of the GR in mediating the effects of FXR on gluconeogenic genes has been corroborated by in vitro studies using a hepatoma cell line that was transfected with plasmids containing a small interference RNA against the GR. In these cells, the positive effect of FXR activation in terms of induction of PEPCK and G6Pase was modulated, thus supporting the role of the GR in mediating the regulatory effects of FXR on these genes. The identification of the GR as a new target of FXR was further confirmed by promoter analysis of both mouse and human GR promoters. These studies have revealed that the distal region of the GR promoter contains an ER-8 sequence that functions as an enhancer and mediates the transcription of GR in response to FXR activation under fasting (Renga et al., 2012).
In summary, the ability of FXR to regulate glucose homeostasis in the liver is largely dependent on blood glucose levels and the availability of co-regulatory factors, including the GR. This observation indicates that FXR plays only a supportive role in glucose homeostasis. Whether FXR would be a target in the treatment of diabetes is at the moment unclear, since FXR activation seems to promote gluconeogenesis in fasting conditions, and this effect may worsen glucose control.
TGR5 and Glucose Homeostasis.
The physiologic ligands for TGR5 are thought to be LCA and TLCA, which activate the receptor with an EC50 of 600 and 300 nM, respectively (Maruyama et al., 2002; Kawamata et al., 2003). TGR5 may play a potential role in type 2 diabetes, as suggested by a recent finding that oleanolic acid, a TGR5 agonist, lowered serum glucose and insulin levels and enhanced glucose tolerance in mice fed a high-fat diet (Sato et al., 2007). Moreover, the activation of TGR5 induced the production of glucagon-like peptide-1 (GLP-1) in an enteroendocrine cell line STC-1 (Katsuma et al., 2005). GLP1 belongs to the family of incretins, a group of gastrointestinal hormones secreted by intestinal entero-endocrine cells into the bloodstream within minutes after eating. The main physiologic role of incretins is to regulate insulin secretion in response to a meal (Baggio and Drucker, 2007). Confirming the data obtained in analyses of STC-1 cells, a recent study of mice that overexpressed TGR5 demonstrated that TGR5 overexpression induced intestinal GLP-1 release, improved hepatic and pancreatic function, and enhanced glucose tolerance in obese mice (Thomas et al., 2009). Despite the implication that TGR5 may be a therapeutic target to treat type 2 diabetes, common genetic variations within the TGR5 gene have been shown to be unrelated to the development of pre-diabetic phenotypes in a Caucasian population at increased risk for type 2 diabetes mellitus (Mussig et al., 2009).
TGR5 is a key factor in energy expenditure. Activation of TGR5 by secondary bile acids increases energy expenditure in brown adipose tissue, preventing obesity and resistance to insulin (Watanabe et al., 2006). This effect, which is FXR-independent but TGR5-dependent, has been explained by the ability of TGR5 to induce a cyclic-AMP–dependent thyroid hormone–activating enzyme type 2 iodothyronine deiodinase (D2), in thermogenic tissues (i.e., mouse brown fat and human skeletal muscle) via a TGR5-dependent manner. D2 subsequently converts thyroxine (T4) to tri-iodothyronine (T3). T3 is predicted to induce uncoupling protein (UCP) expression (Watanabe et al., 2006). UCP is known to dissipate the proton gradient in the electron transport chain. This pathway is thought to decrease the synthesis of ATP and, in this manner, increase energy expenditure; its relevance, however, has not been confirmed consistently and may be gender specific. Indeed, a further study on the responses of TGR5-null mice to a high-fat diet has shown that only female TGR5–/– mice on a high-fat diet gained more body weight than wild-type mice (reviewed in Fiorucci et al., 2009).
Despite observations of a potential role for TGR5 in regulating body weight and glucose homeostasis in mice, the therapeutic role of TGR5 as a drug target in obesity and diabetes is still not definitively proven. Oleanolic acid, a natural ligand of TGR5 isolated from Olea europaea, abolished the weight gain and insulin resistance in a high-fat diet model of obesity (Sato et al., 2007), but whether these effects are TGR5-dependent remains to be determined. In addition to oleanolic acid, other bile acids are also natural ligands for TGR5, including LCA, TLCA, DCA, CDCA, and CA (Fiorucci et al., 2009). However, these ligands are either toxic or not sufficiently safe. Among them, CDCA appears to be a promising ligand and has been applied in clinical practice (Fiorucci and Baldelli, 2009). However, high doses of CDCA can elevate serum levels of aspartate aminotransferase and alanine aminotransferase in patients due to liver damage.
Development of natural or semisynthetic TGR5 ligands may be a future direction to be undertaken for clinical trials. One group of the synthetic TGR5 ligands is the semi-synthetic steroidal TGR5 agonists, for example, 6α-ethyl-23(S)-methyl-cholic acid, which is a derivative of CDCA. The second group is the synthetic nonsteroidal TGR5 agonists, which may improve metabolic homeostasis, pancreatic insulin secretion, and inflammation (Fiorucci et al., 2009). In addition, the discovery of new compounds that can act as TGR5 agonists would be of high pharmacologic relevance. In this setting, we have recently shown that ciprofloxacin, an antibiotic, is a TGR5 agonist.
In summary, FXR and TGR5 are bile acid–regulated receptors and could be novel targets for regulating glucose and energy metabolism. Because FXR and TGR5 are expressed in different tissues and share common endogenous ligands, it is plausible that synthetic ligands could be developed to simultaneously target the two receptors in different tissues. These dual FXR/TGR5 ligands hold promise in the treatment of obesity and disorders of glucose homeostasis.
Tissue-Specific Functions of the FXR in the Liver and Intestine
The FXR not only plays an essential role in maintaining bile acid homeostasis but is also critical for liver and gastrointestinal functions, as indicated by observations that mice deficient in FXR develop cholestasis, hyperlipidemia, and liver tumors (Sinal et al., 2000; Chiang, 2004). The significant suppression of bile acid synthesis that occurs following activation of FXR involves reduction in the expression levels of genes encoding key bile-acid synthetic enzymes (e.g., CYP7A1 and CYP8B1) (Kim et al., 2007). FXR-mediated induction of SHP and intestinal fibroblast growth factor 19 (FGF19) in humans and FGF15 in mice has been shown to be responsible for this suppression (Goodwin et al., 2000; Inagaki et al., 2005). However, the exact contribution of the FXR/SHP and FXR/FGF15 pathways to this suppression and the associated cell-signaling pathway are unclear. By using novel genetically modified mice, we have shown that the intestinal FXR/FGF15 pathway was critical for suppressing both Cyp7a1 and Cyp8b1 gene expression, but the liver FXR/SHP pathway was important for suppressing Cyp8b1 gene expression and had a minor role in suppressing Cyp7a1 gene expression in mice. Furthermore, in vivo administration of FGF15 protein to mice led to a strong activation of ERK and, to a smaller degree, c-Jun N-terminal kinase (JNK) in the liver. In addition, deficiency of either the ERK or JNK pathway in mouse livers reduced the basal, but not the FGF15-mediated, suppression of Cyp7a1 and Cyp8b1 gene expression. However, deficiency of both ERK and JNK pathways prevented FGF15-mediated suppression of Cyp7a1 and Cyp8b1 gene expression (Kong et al., 2012). In conclusion, the current study clearly elucidates the underlying molecular mechanism of hepatic versus intestinal FXR in regulating the expression of genes critical for bile acid synthesis and hydrophobicity in the liver. These events are outlined in the schematic depicted in Fig. 3.
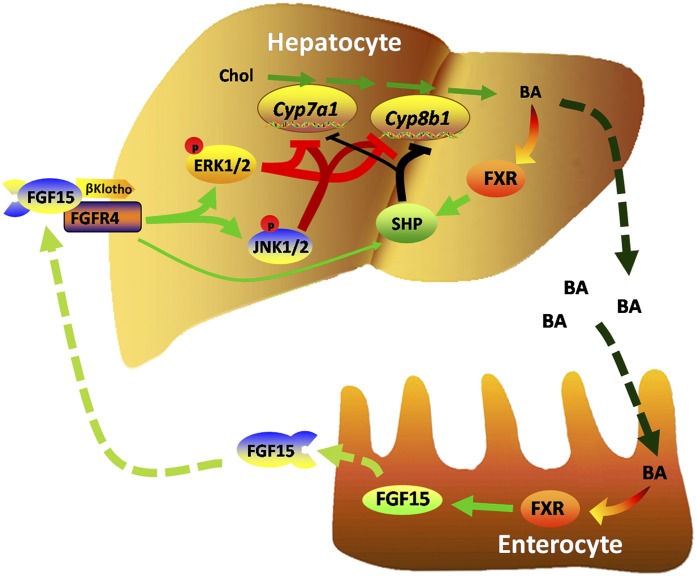
Fig. 3.
The role of hepatic and intestinal FXR in bile acid homeostasis. The presence of bile acids in intestinal enterocytes activates the FXR, which induces expression of FGF15. FGF15 binds and activates hepatic FGFR4, resulting in enhanced ERK and JNK signaling, which coordinates with FXR-induced SHP to repress the expression of CYP7A1 and CYP8B1.
Activation of FXR efficiently induces SHP transcription through head-to-tail chromatin looping (Li et al., 2010). As a unique nuclear receptor with only a ligand-binding domain but not a DNA-binding domain, SHP interacts with many transcription factors to inhibit their function. However, the regulation of SHP expression is not well understood. SHP is highly expressed in the liver, and previous studies have shown FXR highly induces SHP by binding to a FXR response element (FXRRE) in the promoter of the Nr0b2 gene, which encodes SHP. The FXR-SHP pathway is critical for maintaining bile acid and fatty acid homeostasis. An analysis of genome-wide FXR binding using chromatin immunoprecipitation (ChIP) coupled to massively parallel sequencing (ChIP-seq) (Thomas et al., 2010) identified a novel FXRRE in the 3′-enhancer region of the Shp gene. This downstream inverted repeat separated by one nucleotide is highly conserved throughout mammalian species. We hypothesized that this downstream FXRRE is functional and may mediate head-to-tail chromatin looping by interacting with the proximal promoter FXRRE to increase SHP transcription efficiency.
In the current study, a ChIP-quantitative PCR assay revealed that FXR strongly bound to this downstream FXRRE in mouse livers. The downstream FXRRE is important for FXR-mediated transcriptional activation revealed by luciferase gene transcription activation, as well as by deletion and site-directed mutagenesis. The chromatin conformation capture assay was used to detect chromatin looping, and the result confirmed the two FXRREs located in the Shp promoter and downstream enhancer interacted to form a head-to-tail chromatin loop. To date, the head-to-tail chromatin looping has not been reported in the liver. Our results suggest a mechanism by which activation of FXR efficiently induces SHP transcription through head-to-tail chromatin looping.
HNF4α is also a nuclear receptor critical for regulating liver development, differentiation, and function. The traditional paradigm suggests a linear activation of target gene transcription following direct binding of FXR to gene regulatory regions. However, our study showed that FXR activates gene transcription by cooperating with HNF4α to regulate gene transcriptional activation in the liver. Data obtained from the ChIP-seq of mouse livers showed that nearly 50% of FXR binding sites in the liver overlapped with HNF4α binding sites. Binding of HNF4α to shared target sites occurs upstream and in close proximity to FXR. Genes bound by both FXR and HNF4α are highly enriched in complement and coagulation cascades and drug metabolism, implying that these two factors co-regulate these pathways. Transcriptional and binding assays suggest HNF4α can moderately increase FXR transcriptional activity; however, results showed binding of HNF4α can be either dependent or independent of FXR activity at different shared binding sites. Co-immunoprecipitation assays revealed a direct FXR-HNF4α protein interaction that is dependent on FXR activity. Therefore, this study provides the first evidence of cooperative and independent interactions between FXR and HNF4α in regulating liver gene transcription in a genome-wide scale.
In summary, it is apparent that interactions among tissues, various intracellular signaling pathways, and transcription factors are important mechanisms by which FXR regulates liver and gastrointestinal function. This paradigm shift may provide a scientific basis for understanding liver biology as well as for designing novel therapies to treat liver and gastrointestinal diseases more effectively.
ER-β Selective Ligands as Novel Therapeutics for Obesity and Metabolic Diseases
Class I steroid hormone receptors, including the receptors for androgens and estrogens and their respective ligands, are critical regulators of lipid metabolism (Mauvais-Jarvis, 2011). The importance of this regulation is manifested in postmenopausal women and hypogonadal men confronting body weight gain, visceral and gluteal fat accumulation, and muscle and bone attrition (Brown et al., 2009). These hormone systems also regulate glucose homeostasis. Although testosterone is directly responsible for many of these actions in men, indirect effects via its aromatization to estradiol also contribute to its actions. Taken together, these observations implicate a pivotal role for estrogens in the maintenance of body composition in both men and women.
The physiologic effects of estrogens are mediated by two ERs, ER-α and ER-β (Matthews and Gustafsson, 2003). ER-α and ER-β are approximately 60% homologous in their ligand-binding domain and greater than 90% homologous in their DNA-binding domain, but share very minimal similarity in the N-terminal domain. Although the ligand-binding domains share only 60% sequence identity, their ligand-binding pockets are highly identical (Katzenellenbogen, 2011). With respect to amino acid composition, they differ by only two amino acids; the Leu-384 and Met-421 of ER-α are replaced by Met-336 and Ile-373, respectively, in ER-β. With respect to size, they differ by only 100Å. Here, the ER-α ligand-binding pocket is slightly larger than that of ER-β (i.e., 490 Å versus 390 Å). Despite the fact that these subtle differences are sufficient to develop isoform selective ligands that preferably bind to ER-α or ER-β, discriminating the overlapping but distinct physiologic actions of ER-α and ER-β continues to be a challenge.
Since the discovery of ER-β in 1996 by Gustafsson and colleagues, its contribution to normal physiology and pathologic transformation of tissues has been extensively studied (Gustafsson, 1997). Most of these studies recognized ER-β as a benevolent receptor with potential to prevent or treat several diseases including inflammation, cancer, neurologic diseases, and others (Harris, 2007). One of the areas with least clarity is the role of ER-β in obesity and metabolic diseases.
Knockout animal studies implicated a role for both ER-α and ER-β in the maintenance of body composition. Both isoforms are expressed in adipose tissue, indicating the potential for their ligands to elicit direct actions (Foryst-Ludwig and Kintscher, 2010). Earlier studies demonstrated that estradiol, through ER-α, reduced lipoprotein lipase gene expression and increased hormone sensitive lipase expression in adipose tissue, whereas AMP-activated kinase was increased in muscle (Palin et al., 2003; Rogers et al., 2009). Additionally, ER-αKO mice are obese, insulin resistant, and have de-regulated glucose tolerance. While the body weight and metabolism markers of regular rodent chow-fed ER-βKO mice were similar to those of wild-typemice, the high-fat diet–fed or ovariectomized ER-βKO mice gained body weight and accumulated adipose tissue to a greater extent than did wild-type mice (Foryst-Ludwig et al., 2008). These studies provide evidence for the antiobesity effects of ER-α and ER-β but suggest that their involvement might differ with the etiology of these diseases.
To address these issues, we synthesized a series of ER-β selective ligands isoquinolinones (Fig. 4A), displaying 10- to 100-fold selectively toward ER-β over ER-α (Yepuru et al., 2010). Although their binding to and transactivation of ER-β was similar to that of estradiol, they bound and activated ER-α at much lower potency and efficacy. In addition, these molecules did not cross-react with other receptors belonging to the nuclear receptor superfamily (all class I, PPAR-α, PPAR-γ, retinoid X receptor isoforms, and vitamin D receptor).
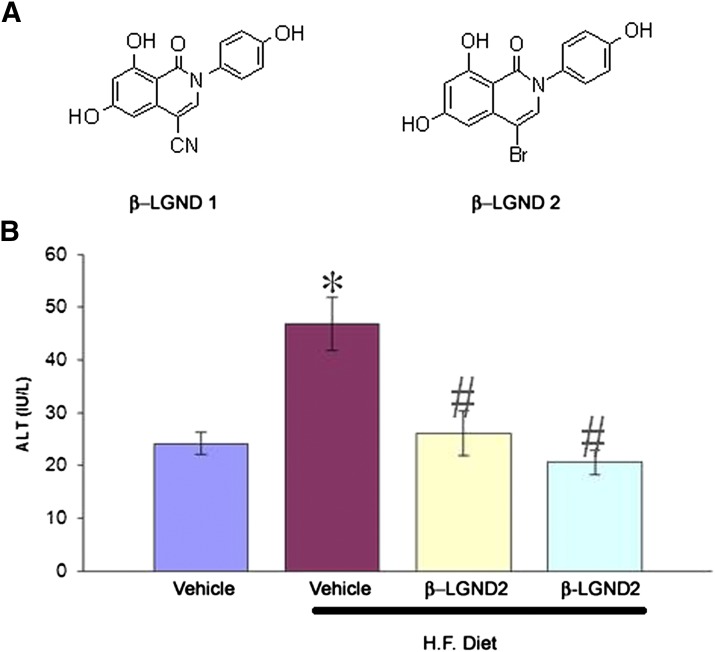
Fig. 4.
ER-β–selected agonists prevent high-fat diet–induced fatty liver. (A) The structure of ER-β selective agonists. (B) Effect of ER-β selective agonists on serum alanine aminotransferase (ALT) in animals fed a high-fat diet.
C57/BL6 mice fed with a high-fat diet were treated subcutaneously with vehicle or 30 mg/kg/day of two ER-β–selective ligands (Fig. 4) for 12 weeks. The body weights of mice fed with a high-fat diet and treated with an ER-β–selective agonist were significantly lower than those of vehicle-treated mice fed with a high-fat diet and were not different from the body weights of mice maintained on a normal diet (Yepuru et al., 2010). In addition to the body weight changes, we observed favorable changes in other biomarkers of metabolism, including cholesterol, leptin, and glucose tolerance. The effects were so dramatic that the body weights and metabolism profiles of the mice fed with a high-fat diet and treated with ER-β selective agonists were similar to those of mice maintained on normal diet. These results demonstrate the potential of ER-β and its ligands to combat obesity and metabolic diseases effectively.
Interestingly, ER-β–selective ligands did not reduce food consumption of these mice. This could be due to either the inability of ER-β to promote satiety or the failure of these ligands to cross the blood–brain barrier. ER-β–selective ligands’ inability to control food intake can likely be attributed to their site of action because they elicit their effect at the periphery or directly on adipose tissue instead of through the central nervous system. This distinguishes ER-β–selective ligands from most of the new chemical entities under development [e.g., Lorcarserin (Arena Pharmaceuticals), Qnexa (Vivus Inc.), and Contrave (Orexigen Ltd.)], which reduce body weight by suppressing appetite through targets in the central nervous system. Unfortunately, many anti-obesity drugs belonging to similar classes were eventually withdrawn from the market due to cardiovascular side effects (Connolly et al., 1997; Malgarini and Pimpinella, 2011).
Since long-term activation of ER-α could have unwarranted side effects (e.g., thromboembolism and breast and uterine cancers), we examined the effects of ER-β–selective agonists on the hypothalamus–pituitary–gonadal axis in males fed a high-fat diet and on uterine weight in females with ovariectomy-induced weight gain to ensure the absence of cross-reactivity with ER-α. The results conclusively demonstrated that the effects were not due to the cross reactivity with ER-α, as evidenced by the lack of hypothalamus–pituitary–gonadal axis activation and uterine weight increase (Yepuru et al., 2010).
GPCRs constitute the primary therapeutic target for many obesity drugs. Some of estradiol’s actions are also mediated by a GPCR, GPR-30 (Revankar et al., 2005). To further rule out the possibility that GPCRs could have played a role in the anti-obesity effect of ER-β–selective agonists, cross-reactivity against a panel of known GPCRs was evaluated. ER-β–selective ligands did not cross react with any of the tested GPCRs, indicating that these ligands and their anti-obesity effects are highly selective for ER-β (Yepuru et al., 2010).
Magnetic resonance imaging of mice in these obesity studies revealed that ER-β ligands not only reduced body fat but also increased the muscle mass (Yepuru et al., 2010). This observation is very unique to this class of anti-obesity drugs and has not been demonstrated as a function of ER-β or its ligands. Adipocytes and myocytes originate from the same mesenchymal stem cells, and their interaction has been implicated in the extent and nature of adipogenesis and myogenesis (Thanabalasundaram et al., 2012). In addition, they share competing signaling pathways, as in the case of PR domain containing 16, a protein that promotes adipose formation at the expense of muscle formation (Seale et al., 2008). Adipocytes store energy obtained from external sources, which is released during metabolic process for the utilization by muscle.
One of the models demonstrating the role of estrogens in body composition (decrease in fat mass and increase in muscle mass) is the aromatase knockout mice model. Although aromatase knockout mice have normal body weight initially, their adipose tissue levels significantly increase with age and with a concomitant decrease in muscle mass (Brown et al., 2009). These mice also demonstrate a decrease in their ambulatory potential. These results corroborate the assertion that estrogens have an effect on both adipose and muscle tissue. From our studies with ER-β selective agonists, we believe that ER-β is the mediator of these effects of estradiol by potentially increasing the metabolism rate, leading to release of energy from fat depots for muscle utilization.
To understand the mechanism involved in these effects, gene expression changes were measured in white and brown adipose tissue and muscle. ER-β ligands significantly increased UCP-1 in brown adipose tissue (Yepuru et al., 2010). UCP-1 is a mitochondrial protein that uncouples oxygen consumption and ATP synthesis to promote energy dissipation as heat (Ricquier, 2005). The expression of UCP-1 was also confirmed at protein level in brown adipose tissue.
Another gene that was up-regulated by ER-β selective ligands was Pgc-1 (Yepuru et al., 2010). PGC-1 was first identified as a binding partner of PPAR-γ in brown adipose tissue, and its primary function is oxidative metabolism and mitochondrial biogenesis in muscle. The skeletal phenotype of PGC-1KO mice is abnormal. Using in vitro studies, we demonstrated that PGC-1’s ability to coactivate PPAR-γ was impaired by ER-β and that this effect was dependent on the ability of ligands to bind ER-β. We speculate that ER-β might sequester PGC-1 away from PPAR-γ, thereby not only preventing the robust function of PPAR-γ but also increasing its own function. Since PGC-1 is a coactivator of estrogen-related receptors, proteins that share significant homology with ER-α and ER-β, there is a greater possibility that PGC-1 might be an ER-β coactivator. These results with UCP-1, PGC-1, and altered body composition all suggest that ER-β is a critical regulator of energy homeostasis. These hypotheses have to be tested in appropriate models.
Some of the most meaningful and robust gene expression changes observed in white adipose tissue following administration of ER-β selective agonists were that of Sterol Regulatory Element-Binding Protein (SREBP) and fatty acid synthase (Yepuru et al., 2010). SREBP is an important transcription factor that activates cholesterol-synthesizing genes. Small molecule inhibitors of SREBP are highly desirable due to their potential to treat atherosclerosis (Kamisuki et al., 2009). ER-β selective agonists markedly (6- to 7-fold) decreased SREBP in white adipose tissue of animals fed with a high-fat diet compared with vehicle-treated animals. This reduction in SREBP with concomitant decrease in fatty acid synthase (8-fold) could be an important pathway mediating ER-β’s effect on fat accumulation. Although early studies demonstrated highly identical results with estradiol on SREBP-1 and fatty acid synthase, isoform selectivity was demonstrated in this study with ER-β selective molecules, emphasizing the need to activate ER-β for these effects on lipogenic genes and proteins.
Obesity is associated with an increased risk of developing nonalcoholic fatty liver disease (NAFLD) and non-lcoholic steatohepatitis (NASH). NAFLD affects 10%–30% of the general US population and about 75%–90% of the morbidly obese population. Biochemically, patients with NASH demonstrate an increase in serum transaminases (aspartate aminotransferase and alanine aminotransferase), triglycerides, fatty acids, and insulin resistance. NASH progresses into fibrosis and cirrhosis with 5- and 10-year survival estimated as 67% and 59%, respectively. In this study, we demonstrated that animals fed a high-fat diet displayed the biochemical characteristics of NASH, and that these effects were all reversed by ER-β selective agonists (Fig. 4B).
These data suggest that there could potentially be another liver-specific therapeutic utility for ER-β ligands. Although not direct, indirect evidence demonstrates that estrogens through ER-α or ER-β might have favorable effects in the liver (Jones et al., 2006). An absence of aromatase in men leads to undesired hepatic accumulation of lipids, which could be reversed by estradiol (Jones et al., 2006). The hepatic lipid accumulation observed was characterized by increased expression of genes involved in lipid and fatty acid synthesis. The expression of these genes was also reversed by estradiol administration (Tian et al., 2012).
Our data are the first to demonstrate the potential benefits of ER-β selective ligands to treat obesity and metabolic diseases. We predict that the combined effects of an increase in UCP-1 in muscle, sequestering of PGC-1 by ER-β, and a marked decrease in SREBP and fatty acid synthase in white adipose tissue tilt the balance in favor of higher muscle mass, increased oxidative metabolism, and energy utilization and decreased fat accumulation; all contributing to promoting favorable changes in body composition and the lipid profile. Although studies with ER-βKO mice are next and needed to unequivocally prove that these effects were mediated by ER-β, we ruled out all of the potential proteins with which these ligands could have cross-reacted to explain the observed effects. From a basic mechanistic perspective, these data help support the idea that ER-β is a promising molecular target for the treatment of obesity and metabolic diseases and that ER-β plays an important role in mediating the effects of endogenous estrogens on body composition.
Summary and Future Directions
Within the nuclear receptor superfamily, the regulation of inflammation, lipid dysfunction, and obesity-related diseases was once thought to be dominated by the GR, thyroid hormone receptor, and PPARs; this focus has been substantially expanded to include several other nuclear receptors, such as FXR, PXR, CAR, and ER. This symposium focused on exploring our current understanding of the roles of nuclear receptors FXR, PXR, CAR, and ER in lipid, energy, and drug metabolism and in obesity-related disease, as well as the therapeutic potential for drugs targeting these receptors. The xenobiotic functions of PXR and CAR are well recognized, but their endobiotic functions in glucose and lipid metabolisms are somewhat unexpected. The role of PXR and CAR in lipid metabolism and atherosclerosis is controversial; both receptors appear to have pleiotropic effects on obesity and diabetes. It seems clear that there is an interaction between drug metabolism and lipid metabolism, and nuclear receptors provide a link for cross-talk between these two aspects of metabolism in the liver.
The role of FXR in regulation of bile acid and glucose metabolism is also controversial. Several recent studies have demonstrated that the liver FXR/SHP mechanism may not play a role in inhibiting bile acid synthesis; instead the intestine FXR/FGF15 to liver FGFR4/ERK1/2 signaling may be responsible for mediating inhibition of bile acid synthesis. FXR agonists have been shown to improve, worsen, or have no effect on hyperglycemia and insulin resistance in mice. The direct inhibition of gluconeogenesis and lipogenesis by the FXR/SHP pathway has been suggested but not proven. New data show that in the fed state, bile acid stimulation of glycogen synthesis may play a role in the control of blood glucose concentration. In the fasting state, FXR inhibits insulin secretion from pancreatic β cells but stimulates gluconeogenesis via activation of the GR. Recently, bile acid–activated TGR5 signaling has gained much attention as the major mechanism for control of glucose and energy metabolism and protection against hyperglycemia, diabetes and obesity. This is particularly relevant for developing bile acid–based drugs for treating chronic liver diseases, diabetes, and obesity.
The surprising new function of ERβ in obesity has been uncovered recently (Fig. 4). ER-β–selective agonists have been shown to increase energy metabolism and decrease lipogenesis, leading to improved lipid profiles and reduced weight in high-fat diet–induced obese mice. The emerging role of nuclear receptors in lipid, glucose, and energy metabolism has gained increasing attention. However, the underlying molecular mechanisms are not clear and remain to be elucidated. Different genetically modified mouse models are useful for uncovering novel functions of nuclear receptors. Screening of selective agonists for nuclear receptors and TGR5 would discover potential therapeutic drugs for treating liver diseases, diabetes, and obesity.
Abbreviations
- CA
cholic acid
- CAR
constitutive androstane receptor
- CDCA
chenodeoxycholic acid
- ChIP
chromatin immunoprecipitation
- DCA
deoxycholic acid
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinase
- EST
estrogen sulfotransferase
- D2
type 2 iodothyronine deiodinase
- FGF
fibroblast growth factor
- FOXO1
forkhead box protein O1
- FXR
farnesoid X receptor
- FXRRE
FXR response element
- G6Pase
glucose-6-phosphatase
- GLP-1
glucagon-like peptide-1
- GLUT
glucose transporter
- GPCR
G protein–coupled receptor
- GR
glucocorticoid receptor
- GUDCA
glycoursodeoxycholic acid
- HNF4α
hepatic nuclear factor 4α
- JNK
Jun N-terminal kinase
- LCA
lithocholic acid
- LXR
liver X receptor
- MAPK
mitogen-activated protein kinase
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PEPCK
phosphoenolpyruvate carboxykinase
- PGC1-α
peroxisome proliferator activated receptor gamma coactivator 1 alpha
- PPAR
peroxisome proliferator activated receptor
- PXR
pregnane X receptor
- SHP
small heterodimer partner
- SREBP
sterol regulatory element-binding protein
- T3
tri-iodothyronine
- T4
thyroxine
- TCA
taurocholic acid
- TCPOBOP
1,4-bis[2-(3,5-dichloropyridyloxy)] benzene
- TLCA
taurolithocholic acid
- TUDCA
tauroursodeoxycholic acid
- UCP
uncoupling protein
- UDCA
ursodeoxycholic acid
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Swanson, Wada, Xie, Renga, Zampella, Distrutti, Fiorucci, Kong, Thomas, Guo, Narayanan, Yepuru, Dalton, Chiang.
Footnotes
This work was supported in part by financial support from National Institutes of Health National Institute of Environmental Health [Grant ES019629] and National Institutes of Health National Institute of Diabetes and Digestive Diseases [Grants DK083952, DK081343, DK090036, DK44442, and DK58379].
References
- Ai N, Krasowski MD, Welsh WJ, Ekins S. (2009) Understanding nuclear receptors using computational methods. Drug Discov Today 14:486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
- Borgius LJ, Steffensen KR, Gustafsson JA, Treuter E. (2002) Glucocorticoid signaling is perturbed by the atypical orphan receptor and corepressor SHP. J Biol Chem 277:49761–49766 [DOI] [PubMed] [Google Scholar]
- Brown M, Ning J, Ferreira JA, Bogener JL, Lubahn DB. (2009) Estrogen receptor-alpha and -beta and aromatase knockout effects on lower limb muscle mass and contractile function in female mice. Am J Physiol Endocrinol Metab 296:E854–E861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk T, Grefhorst A, Bouchaert E, Fruchart JC, Gonzalez FJ, Kuipers F, Staels B. (2005) Transient impairment of the adaptive response to fasting in FXR-deficient mice. FEBS Lett 579:4076–4080 [DOI] [PubMed] [Google Scholar]
- Cariou B, van Harmelen K, Duran-Sandoval D, et al. (2006) The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem 281:11039–11049 [DOI] [PubMed] [Google Scholar]
- Cheng J, Krausz KW, Tanaka N, Gonzalez FJ. (2012) Chronic exposure to rifaximin causes hepatic steatosis in pregnane x receptor-humanized mice. Toxicol Sci 129:456–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JY. (2004) Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol 40:539–551 [DOI] [PubMed] [Google Scholar]
- Cipriani S, Mencarelli A, Palladino G, Fiorucci S. (2010) FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res 51:771–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. (1997) Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 337:581–588 [DOI] [PubMed] [Google Scholar]
- Dong B, Saha PK, Huang W, et al. (2009) Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc Natl Acad Sci USA 106:18831–18836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes M, Verdecia MA, Roecker AJ, et al. (2003) A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol Cell 11:1079–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Sandoval D, Cariou B, Percevault F, Hennuyer N, Grefhorst A, van Dijk TH, Gonzalez FJ, Fruchart JC, Kuipers F, Staels B. (2005) The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J Biol Chem 280:29971–29979 [DOI] [PubMed] [Google Scholar]
- Duran-Sandoval D, Mautino G, Martin G, Percevault F, Barbier O, Fruchart JC, Kuipers F, Staels B. (2004) Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes 53:890–898 [DOI] [PubMed] [Google Scholar]
- Fang Y, Studer E, Mitchell C, Grant S, Pandak WM, Hylemon PB, Dent P. (2007) Conjugated bile acids regulate hepatocyte glycogen synthase activity in vitro and in vivo via Galphai signaling. Mol Pharmacol 71:1122–1128 [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Baldelli F. (2009) Farnesoid X receptor agonists in biliary tract disease. Curr Opin Gastroenterol 25:252–259 [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Mencarelli A, Palladino G, Cipriani S. (2009) Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci 30:570–580 [DOI] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Clemenz M, Hohmann S, et al. (2008) Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet 4:e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Kintscher U. (2010) Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol 122:74–81 [DOI] [PubMed] [Google Scholar]
- Gao J, He J, Shi X, Stefanovic-Racic M, Xu M, O’Doherty RM, Garcia-Ocana A, Xie W. (2012) Sex-specific effect of estrogen sulfotransferase on mouse models of type 2 diabetes. Diabetes 61:1543–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, He J, Zhai Y, Wada T, Xie W. (2009) The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. J Biol Chem 284:25984–25992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Xie W. (2010) Pregnane X receptor and constitutive androstane receptor at the crossroads of drug metabolism and energy metabolism. Drug Metab Dispos 38:2091–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, Guo P, Zhai Y, Zhou J, Uppal H, Jarzynka MJ, Song WC, Cheng SY, Xie W. (2007) Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol Endocrinol 21:1781–1790 [DOI] [PubMed] [Google Scholar]
- Gong H, Jarzynka MJ, Cole TJ, et al. (2008) Glucocorticoids antagonize estrogens by glucocorticoid receptor-mediated activation of estrogen sulfotransferase. Cancer Res 68:7386–7393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, et al. (2000) A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6:517–526 [DOI] [PubMed] [Google Scholar]
- Gustafsson JA. (1997) Estrogen receptor beta—getting in on the action? Nat Med 3:493–494 [DOI] [PubMed] [Google Scholar]
- Harris HA. (2007) Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol 21:1–13 [DOI] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, et al. (2005) Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2:217–225 [DOI] [PubMed] [Google Scholar]
- Jones ME, Boon WC, Proietto J, Simpson ER. (2006) Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab 17:55–64 [DOI] [PubMed] [Google Scholar]
- Kamisuki S, Mao Q, Abu-Elheiga L, et al. (2009) A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chem Biol 16:882–892 [DOI] [PubMed] [Google Scholar]
- Katsuma S, Hirasawa A, Tsujimoto G. (2005) Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329:386–390 [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen JA. (2011) The 2010 Philip S. Portoghese Medicinal Chemistry Lectureship: addressing the “core issue” in the design of estrogen receptor ligands. J Med Chem 54:5271–5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata Y, Fujii R, Hosoya M, et al. (2003) A G protein-coupled receptor responsive to bile acids. J Biol Chem 278:9435–9440 [DOI] [PubMed] [Google Scholar]
- Keitel V, Kubitz R, Häussinger D. (2008) Endocrine and paracrine role of bile acids. World J Gastroenterol 14:5620–5629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, Gonzalez FJ. (2007) Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 48:2664–2672 [DOI] [PubMed] [Google Scholar]
- Kodama S, Koike C, Negishi M, Yamamoto Y. (2004) Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol 24:7931–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Moore R, Yamamoto Y, Negishi M. (2007) Human nuclear pregnane X receptor cross-talk with CREB to repress cAMP activation of the glucose-6-phosphatase gene. Biochem J 407:373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. (2012) Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 56:1034–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno Y, Negishi M, Kodama S. (2008) The roles of nuclear receptors CAR and PXR in hepatic energy metabolism. Drug Metab Pharmacokinet 23:8–13 [DOI] [PubMed] [Google Scholar]
- Lahtela JT, Arranto AJ, Sotaniemi EA. (1985) Enzyme inducers improve insulin sensitivity in non-insulin-dependent diabetic subjects. Diabetes 34:911–916 [DOI] [PubMed] [Google Scholar]
- Li G, Thomas AM, Hart SN, Zhong X, Wu D, Guo GL. (2010) Farnesoid X receptor activation mediates head-to-tail chromatin looping in the Nr0b2 gene encoding small heterodimer partner. Mol Endocrinol 24:1404–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Feinglos MN. (2001) Drug-induced hyperglycemia. JAMA 286:1945–1948 [DOI] [PubMed] [Google Scholar]
- Ma K, Saha PK, Chan L, Moore DD. (2006) Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 116:1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgarini RB, Pimpinella G. (2011) Phentermine plus topiramate in the treatment of obesity. Lancet 378:125–126, author reply 126–127 [DOI] [PubMed] [Google Scholar]
- Markov GV, Laudet V. (2011) Origin and evolution of the ligand-binding ability of nuclear receptors. Mol Cell Endocrinol 334:21–30 [DOI] [PubMed] [Google Scholar]
- Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. (2002) Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun 298:714–719 [DOI] [PubMed] [Google Scholar]
- Masuyama H, Hiramatsu Y. (2012a) Treatment with a constitutive androstane receptor ligand ameliorates the signs of preeclampsia in high-fat diet-induced obese pregnant mice. Mol Cell Endocrinol 348:120–127 [DOI] [PubMed] [Google Scholar]
- Masuyama H, Hiramatsu Y. (2012b) Treatment with constitutive androstane receptor ligand during pregnancy prevents insulin resistance in offspring from high-fat diet-induced obese pregnant mice. Am J Physiol Endocrinol Metab 303:E293–E300 [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. (2003) Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv 3:281–292 [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F. (2011) Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab 22:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merk D, Schubert-Zsilavecz M. (2012) Nuclear receptors as pharmaceutical targets: rise of FXR and rebirth of PPAR? Future Med Chem 4:587–588 [DOI] [PubMed] [Google Scholar]
- Müssig K, Staiger H, Machicao F, et al. (2009) Preliminary report: genetic variation within the GPBAR1 gene is not associated with metabolic traits in white subjects at an increased risk for type 2 diabetes mellitus. Metabolism 58:1809–1811 [DOI] [PubMed] [Google Scholar]
- Nguyen A, Bouscarel B. (2008) Bile acids and signal transduction: role in glucose homeostasis. Cell Signal 20:2180–2197 [DOI] [PubMed] [Google Scholar]
- Palin SL, McTernan PG, Anderson LA, Sturdee DW, Barnett AH, Kumar S. (2003) 17Beta-estradiol and anti-estrogen ICI:compound 182,780 regulate expression of lipoprotein lipase and hormone-sensitive lipase in isolated subcutaneous abdominal adipocytes. Metabolism 52:383–388 [DOI] [PubMed] [Google Scholar]
- Prawitt J, Abdelkarim M, Stroeve JH, et al. (2011) Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 60:1861–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renga B, Mencarelli A, D’Amore C, Cipriani S, Baldelli F, Zampella A, Distrutti E, Fiorucci S. (2012) Glucocorticoid receptor mediates the gluconeogenic activity of the farnesoid X receptor in the fasting condition. FASEB J 26:3021–3031 [DOI] [PubMed] [Google Scholar]
- Renga B, Mencarelli A, Vavassori P, Brancaleone V, Fiorucci S. (2010) The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta 1802:363–372 [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- Ricquier D. (2005) Respiration uncoupling and metabolism in the control of energy expenditure. Proc Nutr Soc 64:47–52 [DOI] [PubMed] [Google Scholar]
- Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. (2009) Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem Biophys Res Commun 382:646–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Genet C, Strehle A, Thomas C, Lobstein A, Wagner A, Mioskowski C, Auwerx J, Saladin R. (2007) Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun 362:793–798 [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, et al. (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Zhang Y, Ding H, Wang X, Chen L, Jiang H, Shen X. (2008) Farnesoid X receptor induces GLUT4 expression through FXR response element in the GLUT4 promoter. Cell Physiol Biochem 22:1–14 [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. (2000) Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731–744 [DOI] [PubMed] [Google Scholar]
- Song WC, Moore R, McLachlan JA, Negishi M. (1995) Molecular characterization of a testis-specific estrogen sulfotransferase and aberrant liver expression in obese and diabetogenic C57BL/KsJ-db/db mice. Endocrinology 136:2477–2484 [DOI] [PubMed] [Google Scholar]
- Sotaniemi EA, Karvonen I. (1989) Glucose tolerance and insulin response to glucose load before and after enzyme inducing therapy in subjects with glucose intolerance and patients with NIDDM having hyperinsulinemia or relative insulin deficiency. Diabetes Res 11:131–139 [PubMed] [Google Scholar]
- Staels B, Kuipers F. (2007) Bile acid sequestrants and the treatment of type 2 diabetes mellitus. Drugs 67:1383–1392 [DOI] [PubMed] [Google Scholar]
- Stayrook KR, Bramlett KS, Savkur RS, Ficorilli J, Cook T, Christe ME, Michael LF, Burris TP. (2005) Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology 146:984–991 [DOI] [PubMed] [Google Scholar]
- Thanabalasundaram G, Arumalla N, Tailor HD, Khan WS. (2012) Regulation of differentiation of mesenchymal stem cells into musculoskeletal cells. Curr Stem Cell Res Ther 7:95–102 [DOI] [PubMed] [Google Scholar]
- Thomas AM, Hart SN, Kong B, Fang J, Zhong XB, Guo GL. (2010) Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology 51:1410–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Gioiello A, Noriega L, et al. (2009) TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GX, Sun Y, Pang CJ, Tan AH, Gao Y, Zhang HY, Yang XB, Li ZX, Mo ZN. (2012) Oestradiol is a protective factor for non-alcoholic fatty liver disease in healthy men. Obes Rev 13:381–387 [DOI] [PubMed] [Google Scholar]
- Trauner M, Claudel T, Fickert P, Moustafa T, Wagner M. (2010) Bile acids as regulators of hepatic lipid and glucose metabolism. Dig Dis 28:220–224 [DOI] [PubMed] [Google Scholar]
- van Dijk TH, Grefhorst A, Oosterveer MH, Bloks VW, Staels B, Reijngoud DJ, Kuipers F. (2009) An increased flux through the glucose 6-phosphate pool in enterocytes delays glucose absorption in Fxr-/- mice. J Biol Chem 284:10315–10323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Ihunnah CA, Gao J, Chai X, Zeng S, Philips BJ, Rubin JP, Marra KG, Xie W. (2011) Estrogen sulfotransferase inhibits adipocyte differentiation. Mol Endocrinol 25:1612–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Mataki C, et al. (2006) Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439:484–489 [DOI] [PubMed] [Google Scholar]
- Yabaluri N, Bashyam MD. (2010) Hormonal regulation of gluconeogenic gene transcription in the liver. J Biosci 35:473–484 [DOI] [PubMed] [Google Scholar]
- Yamagata K, Daitoku H, Shimamoto Y, Matsuzaki H, Hirota K, Ishida J, Fukamizu A. (2004) Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem 279:23158–23165 [DOI] [PubMed] [Google Scholar]
- Yepuru M, Eswaraka J, Kearbey JD, Barrett CM, Raghow S, Veverka KA, Miller DD, Dalton JT, Narayanan R. (2010) Estrogen receptor-beta-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem 285:31292–31303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Pai HV, Zhou J, Amico JA, Vollmer RR, Xie W. (2007) Activation of pregnane X receptor disrupts glucocorticoid and mineralocorticoid homeostasis. Mol Endocrinol 21:138–147 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. (2006) Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA 103:1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Febbraio M, Wada T, et al. (2008) Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology 134:556–567 [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W. (2006) A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem 281:15013–15020 [DOI] [PMC free article] [PubMed] [Google Scholar]