Abstract
Carboxylesterases (CES) are a well recognized, yet incompletely characterized family of proteins that catalyze neutral lipid hydrolysis. Some CES have well-defined roles in xenobiotic clearance, pharmacologic prodrug activation, and narcotic detoxification. In addition, emerging evidence suggests other CES may have roles in lipid metabolism. Humans have six CES genes, whereas mice have 20 Ces genes grouped into five isoenzyme classes. Perhaps due to the high sequence similarity shared by the mouse Ces genes, the tissue-specific distribution of expression for these enzymes has not been fully addressed. Therefore, we performed studies to provide a comprehensive tissue distribution analysis of mouse Ces mRNAs. These data demonstrated that while the mouse Ces family 1 is highly expressed in liver and family 2 in intestine, many Ces genes have a wide and unique tissue distribution defined by relative mRNA levels. Furthermore, evaluating Ces gene expression in response to pharmacologic activation of lipid- and xenobiotic-sensing nuclear hormone receptors showed differential regulation. Finally, specific shifts in Ces gene expression were seen in peritoneal macrophages following lipopolysaccharide treatment and in a steatotic liver model induced by high-fat feeding, two model systems relevant to disease. Overall these data show that each mouse Ces gene has its own distinctive tissue expression pattern and suggest that some CES may have tissue-specific roles in lipid metabolism and xenobiotic clearance.
Introduction
Carboxylesterases (CES) comprise a family of proteins that catalyze neutral lipid hydrolysis (Hosokawa et al., 2007; Williams et al., 2010; Holmes and Cox, 2011). Substrate specificity among CES is often broad, overlapping, or yet to be identified, leading to difficulty in performing comprehensive functional assays (Staudinger et al., 2010). Still, certain CES have long been recognized to play important roles in the biotransformation of ester- and amide-containing compounds to affect the detoxification and/or activation of a variety of xenobiotics, narcotics, and pharmacologic agents in liver and intestine (Satoh and Hosokawa, 1998, 2006). In addition, growing evidence suggests that some CES enzymes may contribute to aspects of lipid metabolism through triglyceride, cholesteryl ester, or retinyl ester hydrolysis (Schreiber et al., 2009; Parathath et al., 2011; Quiroga and Lehner, 2011; Ghosh, 2012).
The CES family has been organized into five isoenzyme classes based on sequence similarity and gene structure (Hosokawa et al., 2007; Holmes et al., 2010b; Williams et al., 2010; Holmes and Cox, 2011). The six human CES genes, including one pseudogene, reside on chromosome 16; and the 20 mouse Ces genes, also including one pseudogene, are located on chromosome 8. The large number of Ces genes in rodents is believed to have arisen by tandem duplication. Thus, the sequence similarities of mouse Ces mRNA species and protein products are quite high, particularly within an isoenzyme class, which complicates the selective detection of Ces members in vivo.
With the recent description of a standardized nomenclature for mammalian carboxylesterases (Holmes et al., 2010b) and the advent of technologies that allow for quantitative and specific detection of closely related mRNA species (Valasek and Repa, 2005), we undertook a comprehensive survey of Ces mRNA expression in the mouse. Our goals were to: first, determine the tissue distribution of mouse Ces mRNAs to identify organs beyond liver and intestine that express various Ces family members; second, evaluate the regulation of Ces gene expression by various nuclear hormone receptors (NHR) that serve as lipid and xenobiotic-sensing transcription factors, as well as pharmacologic targets (Chawla et al., 2001); and finally, to interrogate Ces expression in cell and organ systems relevant to disease, the macrophage, and the steatotic liver. This Ces expression survey reveals novel tissues and regulation of family members, suggesting potential role(s) for these enzymes in lipid metabolism, xenobiotic clearance in extrahepatic tissues, as well as in the pharmacologic response to NHR activation by synthetic ligands.
Materials and Methods
The liver X receptor (LXR) agonist, T0901317 [T1317, (Repa et al., 2000)], was purchased from Cayman Chemical (Ann Arbor, MI); pregnane X receptor (PXR) agonist, pregnenolone-16α-carbonitrile [PCN, (Jones et al., 2000)], and the constitutive androstane receptor (CAR) agonist, 1,4-Bis-[2-(3,5-dichloro-pyridyloxy)]benzene, 3,3′,5,5′-tetrachloro-1,4-bis(pyridyloxy)benzyne [TCPOBOP, (Tzameli et al., 2000)], were obtained from Sigma-Aldrich Chemical (St. Louis, MO). Ligands for the peroxisome proliferator-activated receptors [PPARα-GW7647, (Brown et al., 2001); PPARβ-GW0742, (Sznaidman et al., 2003); and PPARγ-GW7845, (Henke et al., 1998)] and FXR [GW4064, (Maloney et al., 2000)] were provided by Timothy M. Willson (GlaxoSmithKline, Research Triangle Park, NC). The retinoid X receptor (RXR) agonist LG268 (Mukherjee et al., 1997) was provided by Richard A. Heyman (Aragon Pharmaceuticals, San Diego, CA).
Animals and Treatments
Mice were housed in a temperature-controlled environment with 12-hour light/dark cycles (light: 6:00 AM to 6:00 PM) and allowed free access to water and a cereal-based rodent diet (#7001; Teklad Diets, Madison, WI). For establishing the tissue distribution of Ces mRNAs, three male C57Bl/6 mice were euthanized (at 10:00, 4 hours after lights on) by exsanguination under deep anesthesia, and tissues were harvested. For evaluating NHR regulation of Ces gene expression, male A129/SvJ mice received ligands by oral gavage, as this strain of mice has been previously used for similar studies where drug doses were established (Cha and Repa, 2007). Agonists were suspended in 1% w/v methylcellulose and 1% v/v Tween-80 (vehicle) to deliver the following quantities of ligand in each dose using 10 μl/g body weight: LG268, 30 mg/kg body weight (mpk); GW7647, 10 mpk; GW0742, 10 mpk; GW7845, 20 mpk; T1317, 30 mpk; GW4064, 100 mpk; PCN, 50 mpk; and TCPOBOP, 3 mpk. For gavage dosing, treatments were administered at the beginning of the dark cycle (at 6:00 PM) and again 12 hours later at the beginning of the light cycle (at 6:00 AM), at which point food was removed. After 4 hours (at 10:00), mice were euthanized, and tissues were harvested. For the high-fat diet study, male C57BL/6 mice were fed either high-fat diet containing 58 kcal% fat provided by soybean and coconut oils (#D12331; Research Diets, New Brunswick, NJ), or a cereal-based rodent diet (#7001, Teklad) for 16 weeks before livers were harvested at 10:00.
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Cell Culture
All cells were cultured in a 37°C humidified incubator with 5% CO2.
Cell Lines.
The adenoma-derived glucagonoma cell line αTC1-clone 9 [CRL-2350, (Powers et al., 1990)] was obtained from American Type Culture Collection (Manassas, VA). The insulin-secreting MIN6 cell line, passage #24 (Miyazaki et al., 1990), was kindly provided by Melanie Cobb (UT Southwestern). αTC1 cells were cultured in DMEM with 4 mM l-glutamine adjusted to contain 1.5 g/l sodium bicarbonate and 3 g/l glucose with 10% heat-inactivated dialyzed FBS, further supplemented with 15 mM HEPES, 0.1 mM nonessential amino acids, and 0.02% BSA. MIN6 cells were maintained in DMEM (4.5g/l glucose) with 2 mM l-glutamine, 1 mM sodium pyruvate, and 10% heat-inactivated FBS.
Islets.
Mouse pancreatic islets were prepared as previously described (Chuang et al., 2008). Briefly, the pancreata from three C57Bl/6 male mice were perfused and digested with liberase R1 (Roche, Indianapolis, IN). Islets were then isolated using Ficoll gradient centrifugation and hand-selection under a stereomicroscope for transfer to RPMI 1640 medium (11.1 mM glucose) supplemented with 10% (v/v) heat-inactivated FBS, 100 IU/ml penicillin, and 100 µg/ml streptomycin. Islets were allowed to recover overnight before RNA was isolated.
Macrophages.
Male A129/SvJ mice received an intraperitoneal injection of 1 ml 3%-thioglycollate (autoclaved and aged for 3 months) to elicit macrophages. Three days later, mice were euthanized and macrophages were withdrawn by sterile lavage using ice-cold saline. Cells were collected by gentle centrifugation and plated at 1 × 105 cells/cm2 in high-glucose DMEM containing 10% heat-inactivated FBS and 100 IU/ml penicillin per 100 μg/ml streptomycin. After 6 hours, cells were washed with sterile PBS and provided fresh media containing saline (vehicle, 0.1% v/v) or 100 ng/ml lipopolysaccharide (LPS, Sigma). Cells were harvested 4 hours later for RNA isolation.
Preparation of Samples for RNA Measurements
Mice were anesthetized and exsanguinated via the inferior vena cava. Small intestines were removed, flushed with ice-cold saline, and cut into three sections of equal length, which we denote as duodenum, jejunum, and ileum. The sections were slit lengthwise and the mucosae were obtained by gentle scraping. Intestinal mucosa, liver, adrenal glands, brain, epididymal white adipose tissue, intrascapular brown adipose tissue, kidneys, lung, skeletal muscle (quadriceps), and spleen samples were flash-frozen in liquid nitrogen and stored at –80°C. Total RNA was isolated from tissue samples, cultured cells, and islets using RNA STAT-60 (Tel-Test, Inc., Friendswood, TX) as previously described (Kurrasch et al., 2004). RNA concentration was determined by absorbance at 260 nm, and RNA quality by the 260/280 ratio.
Quantitative Real-Time PCR
Quantitative real-time PCR (qPCR) was performed using an Applied Biosystems 7900HT sequence detection system as described (Kurrasch et al., 2004; Valasek and Repa, 2005). Briefly, total RNA was treated with DNase I (RNase-free; Roche Molecular Biochemicals, Indianapolis, IN) and reverse-transcribed with random hexamers using SuperScript II (Invitrogen, Carlsbad, CA) to generate cDNA. Primers for each gene were designed using a variety of primer design algorithms: DLux (Invitrogen, https://orf.invitrogen.com/lux/); PrimerBlast (NCBI, http://www.ncbi.nlm.nih.gov/tools/primer-blast), and Integrated DNA Technology (http://www.idtdna.com/scitools/applications/RealTimePCR/default.aspx) to ensure that for each target: primers spanned an intron; primers did not anneal to unanticipated off-site cDNA or genomic sequences, and primers exhibited maximal 3′ mismatch for closely related CES family members to achieve target specificity. All primer pairs were validated by: analysis of template titration [acceptable primers exhibited slopes of –3.3 ± 0.1 for plot of Cq versus log[cDNA, ng], (Bustin et al., 2009)]; appearance of a single peak upon a graded temperature dissociation analysis; and the absence of PCR product upon omission of template cDNA. Of note, no discernible PCR product was generated using three different primer sets designed for Ces1b, using template cDNA from liver, intestine, kidney, and a universal RNA mixture. It is likely that Ces1b mRNA is expressed at levels too low to detect by qPCR or expressed in minor cell or tissue types. Finally, while multiple transcript isoforms, generated by alternative promoter usage and/or alternative splicing of exons, have been described for human CES genes, this is not yet the case for mouse Ces genes; thus, our qPCR primer designs are consistent with the current GenBank reference sources, and all primer sequences and gene annotations are provided in Supplemental Table 1.
Each qRT-PCR was analyzed in duplicate and contained in a final volume of 10 μl: 25 ng of cDNA, each primer at 150 nM, and 5 μl of 2× SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Results were evaluated by the comparative cycle number at threshold method (Schmittgen and Livak, 2008) using cyclophilin as the invariant reference gene (Dheda et al., 2004; Kosir et al., 2010).
Analysis of Data
Data are reported as means ± SEM for the number of animals per tissue and/or treatment group, as specified in each figure legend. GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA) was used to perform all statistical analyses. For the multi-agonist study, statistical differences were determined by one-way ANOVA with Dunnett’s post hoc analysis, which compares each treatment group to the vehicle-treated control group (significantly different groups denoted by asterisk: *, P < 0.05; **, P < 0.01; ***, P < 0.001). A Student’s t-test was used for comparison of only two groups.
Results
The Ces1 Family is Highly Expressed in Liver and the Ces2 Family in Intestine, But These and Other Ces Members Are Present in a Wide Variety of Tissues and Cells in the Mouse.
Numerous reports have appeared describing the distribution, regulation and function of various carboxylesterases in the mouse model (Poole et al., 2001; Holmes et al., 2009, 2010a; Xu et al., 2009; Staudinger et al., 2010; Zhang et al., 2012). However, with the confusion regarding nomenclature and ortholog assignment, and the nonselectivity of some oligonucleotide and antibody probes, the reliability of these findings has been uncertain. With the standardization of Ces nomenclature (Holmes et al., 2010b) and the target specificity available using quantitative real-time PCR (qPCR), we have now performed a comprehensive survey of Ces expression in the mouse. The utility of our qPCR method is readily apparent by the distinct expression patterns of Ces mRNAs, thus confirming the specificity of primers. In addition, the rank order of Ces mRNA levels in liver tissue observed in our studies is consistent with recently published findings for 11 Ces members using a branched-DNA signal amplification assay (Zhang et al., 2012). Hence, our qPCR method provides a reliable means to determine steady-state levels of Ces mRNA in the mouse.
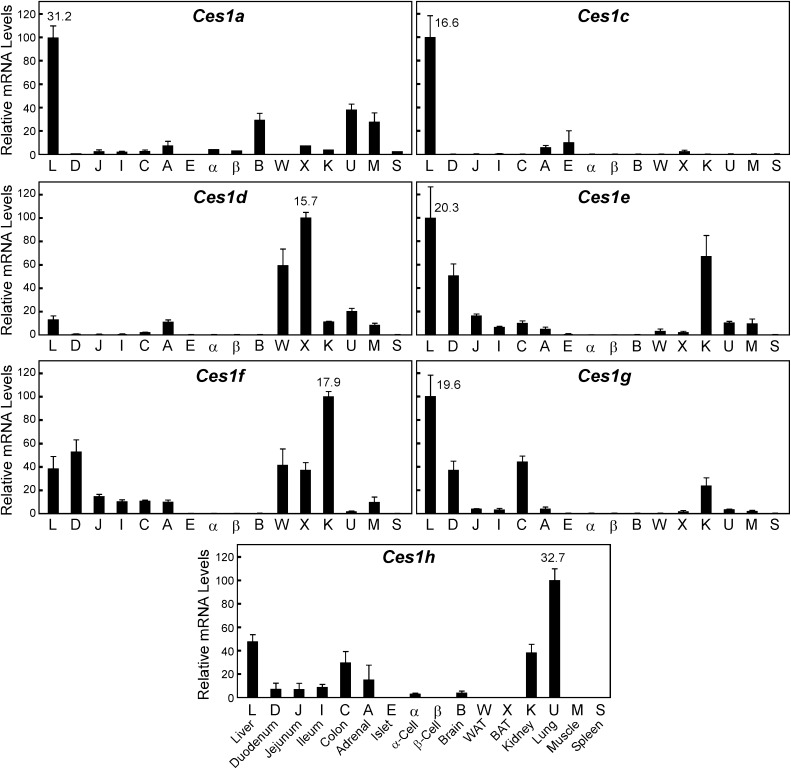
The mouse Ces1 family consists of eight genes Ces1a to Ces1h (Fig. 1) located in tandem on chromosome 8 (between coordinates 95,544,116 and 95,903,624). Each mouse Ces1 gene contains 13–14 exons and encodes a protein with a predicted mass of 62 kDa. The Ces1 family has been reported to be expressed predominantly in liver [reviewed in (Holmes et al., 2010b)], and indeed all detected Ces1 members were present in liver; however, we noted four important points. First, there was no detectable expression of Ces1b mRNA in any tissue tested, including liver, despite our efforts to identify primer sets and tissues that would yield a qPCR product by this method. Second, Ces1d mRNA was detectable in liver, but was far more abundant in brown (BAT, denoted as “X” in Fig. 1) and white adipose tissues (WAT, “W” in Fig. 1). Third, Ces1f mRNA levels were greatest in kidney, a tissue that expressed high levels of many Ces members. Finally, while the highest mRNA levels for Ces1h are found in mouse lung, it should be noted that the quantification cycle [Cq, (Bustin et al., 2009)] observed for this PCR product is nearly at the limit of detection. Thereby, this mRNA species was found only at very low levels in all mouse tissues in which it was expressed. Thus, in line with previous studies describing the Ces1 family members as the hepatic carboxylesterases, four of the seven detectable mRNAs (Ces1a, Ces1c, Ces1e, and Ces1g) were most highly expressed in liver compared with 15 other mouse tissues and cell types.
Fig. 1.
Tissue distribution of the carboxylesterases: family 1. The relative mRNA levels are depicted for mouse liver (L), duodenum (D), jejunum (J), ileum (I), colon (C), adrenal (A), islet (E), α-cell line (αTC1, α), and β-cell line (MIN6, β), brain (B), epididymal white adipose tissue (WAT, W), intrascapular brown adipose tissue (BAT, X), kidney (K), lung (U), muscle (M), and spleen (S). Tissues were obtained from adult (3–5 months of age) C57Bl/6 mice that were fed ad libitum a standard low-fat rodent diet. Individual mRNA values were calculated relative to cyclophilin, and the mean values were arithmetically adjusted to depict the highest-expressing tissue as a unit of 100. Values represent the means ±SEM of three independent samples for each tissue or cell line. Note that as these data are portrayed, comparisons can only be made between the different tissues for a single Ces mRNA family member, not between the various Ces mRNA species (see Fig. 4 for this comparison). The average cycle-at-threshold (Cq) value used for quantification is provided for the tissue showing the most abundant mRNA level for each Ces.
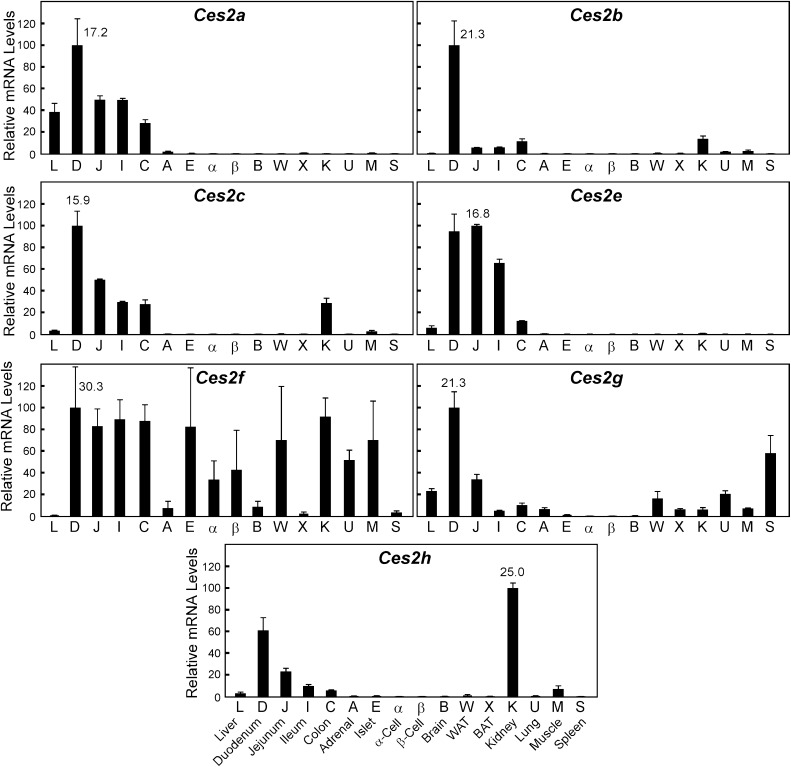
The mouse Ces2 family contains eight members (Ces2a–Ces2h) with one, Ces2d, identified as a pseudogene (excluded in our survey). In line with previous studies which have shown that family 2 encodes the intestinal carboxylesterases [reviewed in (Holmes et al., 2010b)], almost all the Ces2 members were most highly expressed in segments of the small intestine (Fig. 2). The only exception was Ces2h, which exhibited the highest mRNA levels in kidney, followed closely by the small intestine. For all Ces2 members, there was a cephalocaudal distribution of mRNA with highest levels in the proximal third (duodenum) of the small intestine, and decreasing amounts throughout the rest of the small bowel and into the colon. Of note, although Ces2 mRNA levels are highest in the intestine, there is also relatively high expression in other organs including liver (Ces2a, 2e), kidney (Ces2c), and spleen (Ces2g). Ces2f mRNA levels were extremely low in all tissues tested.
Fig. 2.
Tissue distribution of the carboxylesterases: family 2. Refer to the legend of Fig. 1 for details.
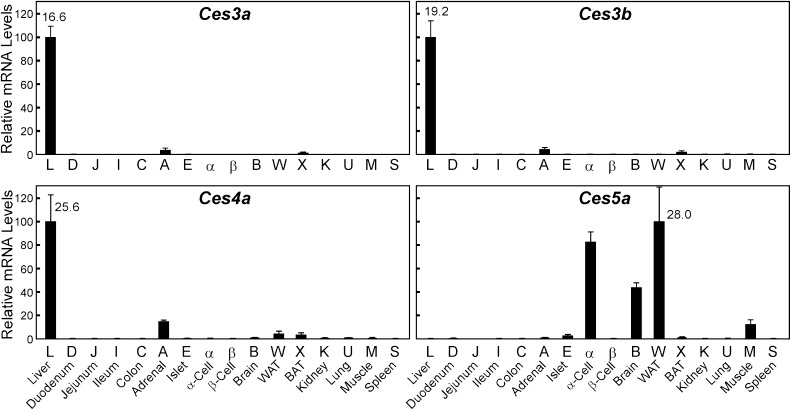
The mouse Ces3 family includes two genes, Ces3a and Ces3b, which are almost exclusively expressed in liver (Fig. 3). Families 4 and 5 have only one member each, Ces4a and Ces5a. Ces4a mRNA levels are highest in liver, and Ces5a expression is evident in WAT, brain, and the glucagonoma alpha-cell line (αTC1).
Fig. 3.
Tissue distribution of the carboxylesterases: families 3, 4, and 5. Refer to the legend of Fig.1 for details.
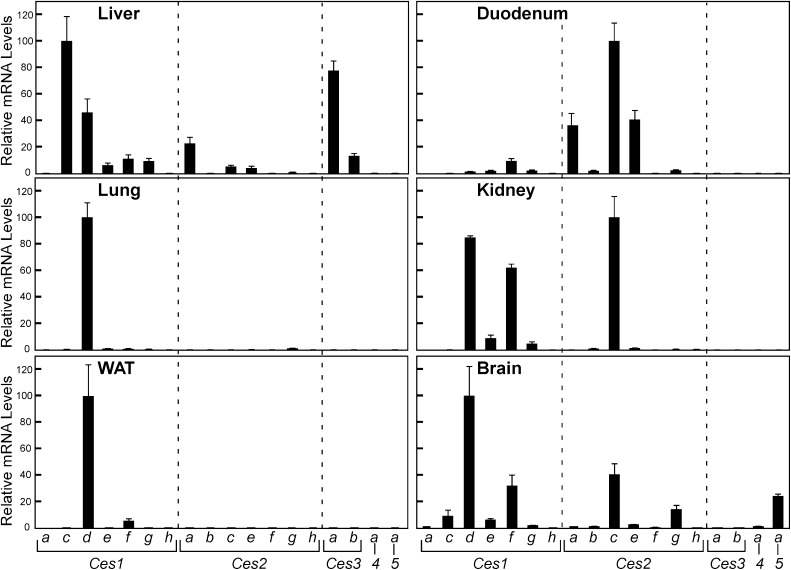
The Rank Order of Ces mRNA Levels within Tissues Reveals That Members Outside of Family 1 Are Likely to Play an Important Role in Liver, and Kidney and Brain Exhibit Unique Expression Patterns of Ces mRNA Species.
Our experimental strategy allowed for a rank order determination of mRNA levels for Ces members within a given tissue (Fig. 4). The PCR primers were designed to provide equivalent PCR amplification efficiency for all Ces targets and showed no product formation in the absence of template cDNA. Therefore, as the same samples were used for all Ces mRNA analyses, we could compare the relative expression of all 18 Ces members to one another in a given tissue or cell type. Viewing the data in this rank order format for liver reveals that in addition to the “liver” CES1 family of enzymes, CES2A, CES3A, and CES3B proteins may play some role in hepatic lipid metabolism and/or xenobiotic response. The “intestine” Ces2 family are by far the most abundant Ces members expressed in the duodenum (Ces2c > > Ces2a = Ces2e). In lung, Ces1d mRNA levels are so abundant (Cq = 17.2) that other Ces members (Ces1e, 1f, and 2g), which exhibit significant (Cq < 24) expression, and Ces1h, which is found nearly exclusively in this tissue, are barely evident. The kidney and brain express a unique complement of “liver” Ces1 (Ces1d and 1f) and “intestine” Ces2 (Ces2c) mRNA species, and brain is the only tissue among those we examined that exhibits appreciable relative levels of Ces5a mRNA, which encodes a secreted carboxylesterase member also known as cauxin, previously identified in urine and epididymal fluid (Ecroyd et al., 2006; Miyazaki et al., 2006). Our results for Ces5a mRNA distribution are consistent with those reported for ram tissues, in which expression was not observed in kidney (Ecroyd et al., 2006), and to our knowledge our findings are the first to demonstrate appreciable Ces5a mRNA expression in brain, which was not evaluated in previous studies. For all other mouse tissues tested, except cells of the endocrine pancreas, which do not appear to abundantly express any carboxylesterase mRNA, the rank order data resemble those of lung and white adipose tissue, with other CES members dwarfed by Ces1d expression (data not shown).
Fig. 4.
Comparative expression levels of mRNA for carboxylesterases of liver, duodenum, kidney, and lung. The relative mRNA levels of all Ces family members are provided for each tissue. Individual values were calculated relative to cyclophilin, and the means were arithmetically adjusted to depict the highest-expressed Ces mRNA species for each tissue as a unit of 100. Values represent the means ±SEM of three independent samples for each tissue.
Short-Term Administration of Synthetic Agonists for Nuclear Hormone Receptors Results in Differential Expression of Ces Family Members.
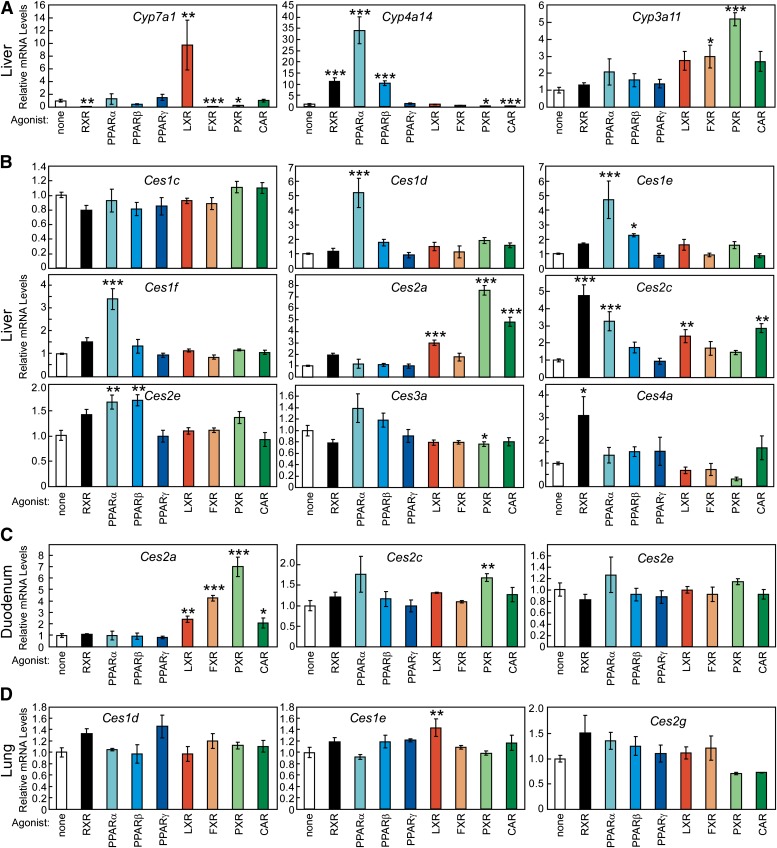
A subset of the NHR superfamily of ligand-activated transcription factors has been characterized as lipid or xenobiotic sensors (Chawla et al., 2001). For these NHRs (LXRs, FXR, PPARs, PXR and CAR), ligand-activation induces the expression of genes encoding transporters, enzymes, and binding proteins to modulate the intracellular levels for a receptor’s respective ligand. To test whether Ces mRNA expression is subject to this regulation, and perhaps reveal novel lipid metabolic pathway(s) affected by Ces gene products, we evaluated Ces expression in a variety of tissues from mice treated with NHR agonists. Potent synthetic agonists that have been developed for pharmacologic activation of specific NHRs were administered to mice by oral gavage twice, 16 and 4 hours prior to harvesting tissues, to allow sufficient time for direct gene activation or repression by NHRs. The efficacy of this dosing regimen was confirmed by the measurement of known hepatic target genes for these NHRs (Fig. 5A). Cholesterol 7α-hydroxylase (Cyp7a1) mRNA levels were increased 9.7-fold by an LXR agonist and decreased by ligands for RXR (to 1%), FXR (to 7%), and PXR (to 22%), changes consistent with previous reports (Repa et al., 2000). The PPAR target gene, cytochrome P450 4A14 [Cyp4a14, (Patsouris et al., 2006)] exhibited greater hepatic mRNA levels in mice treated with a PPARα agonist (GW7647, 34-fold), a PPARβ ligand (GW0742, 10.5-fold) and an RXR drug (LG268, 11.3-fold). Finally, administration of ligands for the xenobiotic receptors, PXR and CAR, resulted in enhanced expression of the drug-metabolizing enzyme, cytochrome P450 3A11 (5.2-fold for PXR, 2.7-fold for CAR). Thus, this relatively short-term (16 hours) dosing method delivered sufficient NHR agonists to affect gene transcription.
Fig. 5.
Regulation of carboxylesterase mRNA levels by nuclear hormone receptor agonists. Relative mRNA levels are depicted for adult (3–5 months of age) A129/SvJ mouse liver, duodenum, and lung. Tissues were harvested from mice that had received two doses of nuclear receptor agonist by oral gavage (16 and 4 hours before tissue collection). Each dose provided agonist of a given nuclear hormone receptor as follows: Vehicle (1% w/v methylcellulose, 1% v/v Tween-80), 30 mg/kg body weight (mpk) LG268 (RXR), 10 mpk GW7647 (PPARα), 10 mpk GW0742 (PPARβ), 20 mpk GW7845 (PPARγ), 30 mpk T1317 (LXR), 100 mpk GW4064 (FXR), 50 mpk PCN (PXR), and 3 mpk TCPOBOP (CAR). Individual values were calculated relative to cyclophilin and the mean values were arithmetically adjusted to depict the vehicle-treated group at a unit of 1. (A) Changes in the hepatic expression of selected cytochrome P450 (CYP) enzymes confirm the efficacy of this dosing regimen. Ces mRNA levels are shown for liver (B), duodenum (C), and lung (D). Values reflect the means ±SEM (n = 4). *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared with the vehicle-treated group (white bar), as determined by one-way ANOVA with Dunnett’s post hoc comparison.
In liver, we observed numerous changes in Ces mRNA levels following administration of NHR ligands to mice (Fig. 5B). PPARα activation resulted in increased expression of Ces1d (5.2-fold), Ces1e (4.7-fold), Ces1f (3.4-fold), Ces2c (3.3-fold), and Ces2e (1.7-fold). PPARβ activation significantly increased the expression of Ces1e (2.3-fold), and Ces2e (1.7-fold). Administration of agonists for LXR, PXR, and CAR all resulted in increased hepatic mRNA levels of Ces2a (3-, 7.6-, and 4.8-fold, respectively), while PXR activation significantly decreased expression of Ces3a (to 76%). Ces2c was the most responsive hepatic carboxylesterase to NHR activation, as its expression was increased by agonists for RXR (4.8-fold), PPARα (3.3-fold), LXR (2.4-fold), and CAR (2.9-fold). Ces4a expression was significantly increased upon RXR (3.1-fold) activation alone. Of note, the hepatic expression of Ces1d, Ces1e, and Ces1f due to NHR activation had very similar patterns (Fig. 5B), even though they have unique tissue distribution profiles (Fig. 1). In mucosa obtained from the proximal third of the small intestine (duodenum), the mRNA levels of two Ces members were altered by NHR activation (Fig. 5C). Duodenal Ces2a mRNA levels were increased by agonists for LXR (2.4-fold), FXR (4.3-fold), PXR (7.1-fold), and CAR (2.1-fold); whereas Ces2c was significantly upregulated by only PXR activation (1.6-fold). Finally, in the lung, Ces mRNA levels were largely unaffected by NHR ligand treatment, with only a modest, but significant, increase observed for Ces1e mRNA levels. It is of interest that certain genes such as Ces2c and Ces1d respond differently to NHR activation in different organs, suggesting there is tissue-specific regulation of these carboxylesterases.
Differential Expression of Ces in LPS-Activated Macrophages and in Steatotic Livers of High-fat Fed Mice.
We extended this Ces expression survey to cells and tissues associated with disease: the activated macrophage (atherosclerosis and inflammatory disease) and the steatotic liver (insulin resistance and the metabolic syndrome). As these disease-associated conditions have also been the subject of transcriptomic and lipidomic analyses (Joseph et al., 2003; Welch et al., 2003; Barish et al., 2005; Dennis et al., 2010; Fu et al., 2011), the concomitant regulation of Ces members may reveal pathways of lipid metabolism affected by these enzymes.
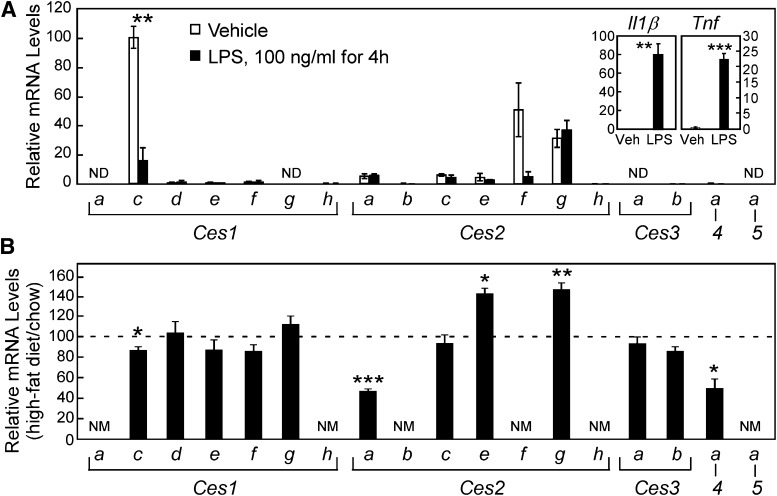
A carboxylesterase cloned from human monocytes (CES1) has been characterized and found to promote cholesteryl ester hydrolysis when overexpressed in macrophages or mice (Ghosh, 2000, 2012; Zhao et al., 2007). However, a functional ortholog of this enzyme in the mouse macrophage has not yet been identified [reviewed in (Quiroga and Lehner, 2011)]. More recently, human CES3 has also been implicated in cholesteryl ester hydrolysis in macrophages (Zhao et al., 2012), and the expression of this CES member appears to be dependent on the abundance of CES1. Again, however, it is unclear which mouse Ces member may impart this CES3 function in macrophages. Thus we evaluated the expression of all mouse Ces mRNAs in primary mouse macrophages (Fig. 6A). Thioglycollate-elicited peritoneal macrophages were harvested from mice and then treated in culture for 4 hours with either vehicle (saline) or LPS to activate an inflammatory response as demonstrated by an increase in mRNA levels for the cytokines, interleukin 1β and tumor necrosis factor (Tnf) (Fig. 6A, inset). Ces1c, Ces2f, and Ces2g were the most abundant carboxylesterase mRNA species in unstimulated mouse macrophages. After LPS treatment, a significant decrease in the expression of Ces1c and a nearly significant decline in Ces2f mRNA levels (P = 0.06) occurred, making Ces2g the most plentiful carboxylesterase in the activated macrophage. It should be noted that the mRNA level for the most abundant macrophage carboxylesterase, Ces1c (qPCR Cq=28.8) represents less than 1% of the mRNA level for Ces1c observed in liver (qPCR Cq= 16.6), which suggests a low abundance of these enzymes in the mouse macrophage. Future studies will be required to ascertain the functional contribution of mouse Ces members to macrophage lipid biology, in analogy to the reports regarding the roles of CES1 and CES3 in human macrophage cholesterol homeostasis (Ghosh, 2000, 2011; Zhao et al., 2007, 2012).
Fig. 6.
Comparative expression of carboxylesterases in thioglycollate-elicited mouse peritoneal macrophages and in the livers of high-fat diet fed mice. (A) The relative mRNA levels of Ces are depicted for thioglycollate-elicited mouse macrophages, treated in culture for 4 hours with saline (vehicle, 0.1% v/v, white bars) or 100 ng/ml LPS (black bars). Increased expression of interleukin-1β and Tnf mRNA demonstrate efficacy of LPS administration (inset). Results depict the mean ±SEM for three wells per treatment. All values were mathematically adjusted relative to the most abundant value (Ces1c, vehicle) set at 100%, so all bars in this graph can be compared. **, P < 0.01, *** P < 0.001 significant effect of LPS treatment by Student’s t-test. (B) Hepatic levels of Ces mRNA in mice fed for 16 weeks a high-fat diet relative to mice fed a low-fat control diet, which is represented by the hatched line, set at 100%. Expressed in this manner, comparisons cannot be made between CES members in this panel. Results depict the mean ± SEM for n = 6 mice per dietary group, *, P < 0.05; **, P < 0.01; ***, P < 0.001 significant effect of high-fat feeding as determined by Student’s t-test. ND, not detected. NM, not measured.
Finally, to complement our survey of Ces gene regulation by lipid-sensing transcription factors, we elected to evaluate Ces mRNA levels in a steatotic liver model induced by feeding a high-fat diet. Male C57Bl/6 mice were fed ad libitum a diet containing 58 kcal% fat (soybean and coconut oils) for 16 weeks. Compared with mice fed a standard chow diet, the mice fed high-fat had greater body weight (1.67-fold), increased liver weight (1.31-fold), and impaired glucose tolerance (increased fasting glucose, and more profound glucose excursion following an oral glucose challenge) (data not shown). The “liver” Ces1 family showed very little change in mRNA levels, except the most abundant member, Ces1c (see Fig. 4), for which mRNA levels were reduced about 20% by high-fat feeding. The steatotic livers of these high-fat fed mice also exhibited increased Ces2e and Ces2g mRNA, while Ces2a and Ces4a mRNA levels were decreased.
Discussion
To our knowledge this is the first comprehensive analysis of Ces expression in the mouse. This project was largely instigated by the recent report recommending a standardized nomenclature for Ces genes (Holmes et al., 2010b), and the fact that these genes repeatedly appear in microarray data, where it is often unclear which Ces member(s) is represented. We elected to perform this survey in mice, as these are widely used subjects in lipid and xenobiotic research, for which a large collection of genetic, dietary, and drug-induced models of lipid-related disease are available. In addition, the mouse has 20 Ces genes, the largest number of any mammalian species reported to date (Williams et al., 2010), thus making the selectivity and sensitivity of our qPCR method especially appropriate.
The first important and quite surprising finding was that these 20 Ces members exhibit very distinct expression patterns. It is thought that the 20 mouse Ces genes arose through tandem duplication, and indeed, they share remarkable sequence similarity and gene structure. They even reside in a cluster on mouse chromosome 8, which would suggest that they might share enhancer elements and regulatory sequences to dictate their expression levels, as seen for hepatic Ces1d, Ces1e, and Ces1f due to NHR activation (Fig. 5). However, while there are some general patterns (Ces1s enriched in liver, Ces2s abundant in intestine), each Ces member is expressed in a unique set of tissues (Figs. 1–4), and many show selective changes in gene expression by drug (Figs. 5 and 6A) or diet (Fig. 6B) treatment. These data provide valuable insight regarding potential xenobiotic activity of CES in organs other than the intestine and liver. Although major questions still remain about these enzymes, including the identification of substrates, which remains difficult, and the localization of activity, this report will inform future studies.
The second finding is that the mRNA levels of several Ces members are regulated by the lipid-sensing nuclear hormone receptors. The role of carboxylesterases in drug metabolism has been widely appreciated, and previous work from the Klasseen and Staudinger groups has convincingly shown that the xenobiotic nuclear hormone receptors PXR and CAR increase expression of Ces2a (previously Ces6) and Ces2c (Xu et al., 2009; Staudinger et al., 2010; Zhang et al., 2012). Our work confirms these findings (Fig. 5) and extends these analyses to reveal that NHRs traditionally associated with fatty acid and/or sterol regulation can likewise regulate the expression of Ces mRNAs.
Bile Acids/FXR.
A previous report suggested that modifying bile acid homeostasis in mice by depletion using cholestyramine or supplementation with sodium cholate increased hepatic levels of Ces1g mRNA [previously called ES-x, (Ellinghaus et al., 1998)]. Our specific, synthetic agonist, GW4064, for the bile acid nuclear receptor, FXR, did not recapitulate these findings in liver, nor were changes in Ces1g mRNA observed in mouse intestine (data not shown), suggesting that alternate bile acid–responsive, non-FXR mechanisms may be responsible for differential Ces1g expression.
Fatty Acids and Fibrates/PPARs.
Early studies in mice and rats demonstrated that certain drugs that enhanced hepatic peroxisome proliferation also caused an increase in microsomal carboxylesterase activity (Mentlein et al., 1986; Ashour et al., 1987; Hosokawa and Satoh, 1993; Parker et al., 1996). In particular, treating rodents with the PPARα ligands clofibrate and WY14,643, or perfluorinated fatty acids resulted in increased CES enzyme activity of five to six subclasses defined by substrate specificity. Most recently, two reports have demonstrated that fibrate treatment of C57/Bl6 mice for 5 days to 2 weeks does not upregulate hepatic Ces1d mRNA levels (Dolinsky et al., 2003; Zhang et al., 2012). These recent findings differ from our results showing that administration of the potent, specific PPARα agonist GW7647 to A129/SvJ mice is associated with increased expression of Ces1d, Ces1e, Ces1f, Ces2c and Ces2e mRNA in liver. These differing findings could be due to drug specificity (Thomas et al., 2002), duration of dosing, and/or mouse strain. (Although our studies were not designed and optimized for direct comparison of strains, the Cq relative to reference cyclophilin gene expression suggests that C57Bl/6 mice exhibit higher basal Ces1d mRNA levels than A129/SvJ, which may preclude them from exhibiting robust upregulation of this Ces family member.)
Oxysterols/LXR.
Treatment with ligands for the “sterol-sensing” LXRs resulted in increased expression of hepatic and intestinal Ces2a and Ces2c. In addition, a synthetic ligand for the common heterodimer partner, RXR, caused a robust increase in Ces2c and Ces4a mRNA levels. These results suggest either a combinatorial effect of RXR with other regulators (PPARα, LXR in the case of Ces2c), or a novel transcriptional mechanism involving RXR homodimers and/or other RXR heterodimer partners not interrogated in our experiments (such as RARs, VDR, or NGFIB). It should be noted that T1317, the LXR agonist used in these studies, has been shown to function as a potent ligand for human PXR (Xue et al., 2007), and to exhibit some activity toward mouse PXR and FXR (Repa et al., 2000; Mitro et al., 2007). While we did not detect a significant increase in the mRNA levels of Cyp3a11, the surrogate marker of PXR activation used to define drug specificity and potency in our studies (Fig. 5A), we cannot rule out the possibility that PXR mediates some of the effects of T1317 in regulating hepatic Ces2a expression.
The mammalian carboxylesterases have become enzymes of increasing interest, particularly in regard to potential activities as cholesteryl ester and/or triacylglyceride hydrolases (Quiroga and Lehner, 2012; Ghosh, 2012; Zechner et al., 2012). Recombinant mouse Ces1e (called Es22) enzyme shows robust retinyl ester hydrolysis activity and modest, albeit significant, activity as a cholesteryl ester hydrolase (Schreiber et al., 2009). It has been noted that several tissues exhibit triglyceride hydrolysis activity that cannot be accounted for by known lipases (e.g., ATGL, HSL, and others), thus supporting the existence of additional neutral lipases (Zechner et al., 2012). Mouse Ces1d (also called TGH or Ces3) has been identified as a triglyceride hydrolase (Dolinsky et al., 2001), and mice lacking this enzyme show dramatic changes in energy expenditure, plasma lipoprotein profiles, and hepatic steatosis (Wei et al., 2010). Thus a number of Ces members have been implicated in regulation of lipid hydrolysis; however, except for Ces1d, it is not clear if or which mouse Ces gene(s) may show similar activities. This report reveals many other Ces genes that may be involved in these processes, based solely on evidence from gene expression changes due to lipid-sensing transcription factors, and disease models. Acute activation of fatty acid-sensing transcription factors (RXRs, PPARs; see Fig. 5) results in increased hepatic RNA levels of Ces1members (1d, 1e, and 1f), although chronic high-fat feeding of mice does not result in altered expression of these genes in the steatotic liver (Fig. 6B). The Ces2 members exhibit both acute (Ces2a, Ces2c, and Ces2e) effects due to short-term administration of ligands to the lipid-sensing nuclear hormone receptors, and differential mRNA levels in the steatotic liver (Ces2e and Ces2g). Finally, two Ces genes exhibited altered expression in LPS-treated mouse macrophages (Ces1c and Ces2f), suggesting that these carboxylesterases may play a role in the lipid biology of this important cell type, as previously reported for other CES members in human macrophages (Ghosh, 2000, 2011; Zhao et al., 2007, 2012).
While it is clear that there are many posttranscriptional levels of regulation that limit the interpretation of mRNA expression data, this survey offers valuable information to guide future studies of the CES family of enzymes. Given the current lack of CES-selective substrates and/or antibodies for the 19 mouse enzymes, a comprehensive analysis of protein abundance or activity is not yet possible. Therefore, this description of mouse distribution and regulation of Ces mRNA expression provides a framework for future research into the pharmacologic response and extrahepatic xenobiotic clearance capacity of the highly similar, yet variably regulated carboxylesterases.
Supplementary Material
Acknowledgments
We thank Lilja Kjalarsdottir for providing RNA from mouse islets and Jen-Chieh Chuang (REATA Pharmaceuticals, Irving, TX) for providing liver RNA from chow- and HF-fed mice.
Abbreviations
- BAT
brown adipose tissue
- CAR
constitutive androstane receptor (NR1I3)
- CES
carboxylesterase
- Cq
real-time PCR cycle number at threshold used for quantification
- CYP
cytochrome P450
- FXR
farnesoid X receptor (NR1H4)
- IL-1β
interleukin-1β
- LPS
lipopolysaccharide
- LXR
liver X receptor (NR1H2 and H3)
- mpk
milligram per kilogram body weight
- NHR
nuclear hormone receptor
- PCN
pregnenolone-16α-carbonitrile
- PPAR
peroxisome proliferator-activated receptor (NR1C1, C2, and C3)
- PXR
pregnane X receptor (NR1I2)
- qPCR
quantitative real-time PCR
- RXR
retinoid X receptor (NR2B1, B2, and B3)
- TCPOBOP
1,4-Bis-[2-(3,5-dichloro-pyridyloxy)]benzene, 3,3′,5,5′-tetrachloro-1,4-bis(pyridyloxy)benzyne
- WAT
white adipose tissue
Authorship Contributions
Participated in research design: Jones, Repa.
Conducted experiments: Jones, Taylor, Tong.
Performed data analysis: Jones, Taylor, Tong.
Wrote or contributed to the writing of the manuscript: Jones, Repa.
Footnotes
This work was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01DK078592] to J.J.R. A.M.T. received predoctoral support from the Pharmacology Training Grant at UT Southwestern, National Institutes of Health [Grant T32 GM007062].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Ashour M-BA, Moody DE, Hammock BD. (1987) Apparent induction of microsomal carboxylesterase activities in tissues of clofibrate-fed mice and rats. Toxicol Appl Pharmacol 89:361–369 [DOI] [PubMed] [Google Scholar]
- Barish GD, Downes M, Alaynick WA, Yu RT, Ocampo CB, Bookout AL, Mangelsdorf DJ, Evans RM. (2005) A nuclear receptor atlas: macrophage activation. Mol Endocrinol 19:2466–2477 [DOI] [PubMed] [Google Scholar]
- Brown PJ, Stuart LW, Hurley KP, Lewis MC, Winegar DA, Wilson JG, Wilkison WO, Ittoop OR, Willson TM. (2001) Identification of a subtype selective human PPARalpha agonist through parallel-array synthesis. Bioorg Med Chem Lett 11:1225–1227 [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, et al. (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622 [DOI] [PubMed] [Google Scholar]
- Cha J-Y, Repa JJ. (2007) The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem 282:743–751 [DOI] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. (2001) Nuclear receptors and lipid physiology: opening the X-files. Science 294:1866–1870 [DOI] [PubMed] [Google Scholar]
- Chuang J-C, Cha J-Y, Garmey JC, Mirmira RG, Repa JJ. (2008) Research resource: nuclear hormone receptor expression in the endocrine pancreas. Mol Endocrinol 22:2353–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EA, Deems RA, Harkewicz R, et al. (2010) A mouse macrophage lipidome. J Biol Chem 285:39976–39985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A. (2004) Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 37:112–114, 116, 118–119 [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Gilham D, Hatch GM, Agellon LB, Lehner R, Vance DE. (2003) Regulation of triacylglycerol hydrolase expression by dietary fatty acids and peroxisomal proliferator-activated receptors. Biochim Biophys Acta 1635:20–28 [DOI] [PubMed] [Google Scholar]
- Dolinsky VW, Sipione S, Lehner R, Vance DE. (2001) The cloning and expression of a murine triacylglycerol hydrolase cDNA and the structure of its corresponding gene. Biochim Biophys Acta 1532:162–172 [DOI] [PubMed] [Google Scholar]
- Ecroyd H, Belghazi M, Dacheux J-L, Miyazaki M, Yamashita T, Gatti J-L. (2006) An epididymal form of cauxin, a carboxylesterase-like enzyme, is present and active in mammalian male reproductive fluids. Biol Reprod 74:439–447 [DOI] [PubMed] [Google Scholar]
- Ellinghaus P, Seedorf U, Assmann G. (1998) Cloning and sequencing of a novel murine liver carboxylesterase cDNA. Biochim Biophys Acta 1397:175–179 [DOI] [PubMed] [Google Scholar]
- Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. (2011) Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 473:528–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. (2000) Cholesteryl ester hydrolase in human monocyte/macrophage: cloning, sequencing, and expression of full-length cDNA. Physiol Genomics 2:1–8 [DOI] [PubMed] [Google Scholar]
- Ghosh S. (2011) Macrophage cholesterol homeostasis and metabolic diseases: critical role of cholesteryl ester mobilization. Expert Rev Cardiovasc Ther 9:329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S. (2012) Early steps in reverse cholesterol transport: cholesteryl ester hydrolase and other hydrolases. Curr Opin Endocrinol Diabetes Obes 19:136–141 [DOI] [PubMed] [Google Scholar]
- Henke BR, Blanchard SG, Brackeen MF, et al. (1998) N-(2-Benzoylphenyl)-L-tyrosine PPARgamma agonists. 1. Discovery of a novel series of potent antihyperglycemic and antihyperlipidemic agents. J Med Chem 41:5020–5036 [DOI] [PubMed] [Google Scholar]
- Holmes RS, Cox LA. (2011) Comparative structures and evolution of vertebrate carboxyl ester lipase (CEL) genes and proteins with a major role in reverse cholesterol transport. Cholesterol 2011:781643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes RS, Cox LA, VandeBerg JL. (2009) A new class of mammalian carboxylesterase CES6. Comp. Biochem. Physiol. Part D Genomics Proteomics 4:209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes RS, Cox LA, VandeBerg JL. (2010a) Mammalian carboxylesterase 3: comparative genomics and proteomics. Genetica 138:695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes RS, Wright MW, Laulederkind SJF, et al. (2010b) Recommended nomenclature for five mammalian carboxylesterase gene families: human, mouse, and rat genes and proteins. Mamm Genome 21:427–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa M, Furihata T, Yaginuma Y, Yamamoto N, Koyano N, Fujii A, Nagahara Y, Satoh T, Chiba K. (2007) Genomic structure and transcriptional regulation of the rat, mouse, and human carboxylesterase genes. Drug Metab Rev 39:1–15 [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Satoh T. (1993) Differences in the induction of carboxylesterase isozymes in rat liver microsomes by perfluorinated fatty acids. Xenobiotica 23:1125–1133 [DOI] [PubMed] [Google Scholar]
- Jones SA, Moore LB, Shenk JL, et al. (2000) The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol 14:27–39 [DOI] [PubMed] [Google Scholar]
- Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. (2003) Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 9:213–219 [DOI] [PubMed] [Google Scholar]
- Kosir R, Acimovic J, Golicnik M, Perse M, Majdic G, Fink M, Rozman D. (2010) Determination of reference genes for circadian studies in different tissues and mouse strains. BMC Mol Biol 11:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrasch DM, Huang J, Wilkie TM, Repa JJ. (2004) Quantitative real-time polymerase chain reaction measurement of regulators of G-protein signaling mRNA levels in mouse tissues. Methods Enzymol 389:3–15 [DOI] [PubMed] [Google Scholar]
- Maloney PR, Parks DJ, Haffner CD, et al. (2000) Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem 43:2971–2974 [DOI] [PubMed] [Google Scholar]
- Mentlein R, Lembke B, Vik H, Berge RK. (1986) Different induction of microsomal carboxylesterases, palmitoyl-CoA hydrolase and acyl-L-carnitine hydrolase in rat liver after treatment with clofibrate. Biochem Pharmacol 35:2727–2730 [DOI] [PubMed] [Google Scholar]
- Mitro N, Vargas L, Romeo R, Koder A, Saez E. (2007) T0901317 is a potent PXR ligand: implications for the biology ascribed to LXR. FEBS Lett 581:1721–1726 [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. (1990) Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127:126–132 [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Yamashita T, Hosokawa M, Taira H, Suzuki A. (2006) Species-, sex-, and age-dependent urinary excretion of cauxin, a mammalian carboxylesterase. Comp Biochem Physiol Part B Biochem Mol Biol 145:270–277 [DOI] [PubMed] [Google Scholar]
- Mukherjee R, Davies PJA, Crombie DL, et al. (1997) Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature 386:407–410 [DOI] [PubMed] [Google Scholar]
- Parathath S, Dogan S, Joaquin VA, Ghosh S, Guo L, Weibel GL, Rothblat GH, Harrison EH, Fisher EA. (2011) Rat carboxylesterase ES-4 enzyme functions as a major hepatic neutral cholesteryl ester hydrolase. J Biol Chem 286:39683–39692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AG, Pinot F, Grant DF, Spearow J, Hammock BD. (1996) Regulation of mouse liver microsomal esterases by clofibrate and sexual hormones. Biochem Pharmacol 51:677–685 [DOI] [PubMed] [Google Scholar]
- Patsouris D, Reddy JK, Müller M, Kersten S. (2006) Peroxisome proliferator-activated receptor α mediates the effects of high-fat diet on hepatic gene expression. Endocrinology 147:1508–1516 [DOI] [PubMed] [Google Scholar]
- Poole M, Bridgers K, Alexson SEH, Corton JC. (2001) Altered expression of the carboxylesterases ES-4 and ES-10 by peroxisome proliferator chemicals. Toxicology 165:109–119 [DOI] [PubMed] [Google Scholar]
- Powers AC, Efrat S, Mojsov S, Spector D, Habener JF, Hanahan D. (1990) Proglucagon processing similar to normal islets in pancreatic α-like cell line derived from transgenic mouse tumor. Diabetes 39:406–414 [DOI] [PubMed] [Google Scholar]
- Quiroga AD, Lehner R. (2011) Role of endoplasmic reticulum neutral lipid hydrolases. Trends Endocrinol Metab 22:218–225 [DOI] [PubMed] [Google Scholar]
- Quiroga AD, Lehner R. (2012) Liver triacylglycerol lipases. Biochim Biophys Acta 1821:762–769 [DOI] [PubMed] [Google Scholar]
- Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. (2000) Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524–1529 [DOI] [PubMed] [Google Scholar]
- Satoh T, Hosokawa M. (1998) The mammalian carboxylesterases: from molecules to functions. Annu Rev Pharmacol Toxicol 38:257–288 [DOI] [PubMed] [Google Scholar]
- Satoh T, Hosokawa M. (2006) Structure, function and regulation of carboxylesterases. Chem Biol Interact 162:195–211 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108 [DOI] [PubMed] [Google Scholar]
- Schreiber R, Taschler U, Wolinski H, et al. (2009) Esterase 22 and beta-glucuronidase hydrolyze retinoids in mouse liver. J Lipid Res 50:2514–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Xu C, Cui YJ, Klaassen CD. (2010) Nuclear receptor-mediated regulation of carboxylesterase expression and activity. Expert Opin Drug Metab Toxicol 6:261–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznaidman ML, Haffner CD, Maloney PR, et al. (2003) Novel selective small molecule agonists for peroxisome proliferator-activated receptor δ (PPARdelta)—synthesis and biological activity. Bioorg Med Chem Lett 13:1517–1521 [DOI] [PubMed] [Google Scholar]
- Thomas JW, Bramlett KS, Montrose C, et al. (2002) A chemical switch regulates fibrate specificity for PPARα versus LXR. J Biol Chem 278:2403–2410 [DOI] [PubMed] [Google Scholar]
- Tzameli I, Pissios P, Schuetz EG, Moore DD. (2000) The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol 20:2951–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valasek MA, Repa JJ. (2005) The power of real-time PCR. Adv Physiol Educ 29:151–159 [DOI] [PubMed] [Google Scholar]
- Wei E, Ben Ali Y, Lyon J, Wang H, Nelson R, Dolinsky VW, Dyck JRB, Mitchell G, Korbutt GS, Lehner R. (2010) Loss of TGH/Ces3 in mice decreases blood lipids, improves glucose tolerance, and increases energy expenditure. Cell Metab 11:183–193 [DOI] [PubMed] [Google Scholar]
- Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. (2003) PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-γ target genes in macrophages. Proc Natl Acad Sci USA 100:6712–6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ET, Wang H, Wrighton SA, Qian Y-W, Perkins EJ. (2010) Genomic analysis of the carboxylesterases: identification and classification of novel forms. Mol Phylogenet Evol 57:23–34 [DOI] [PubMed] [Google Scholar]
- Xu C, Wang X, Staudinger JL. (2009) Regulation of tissue-specific carboxylesterase expression by pregnane x receptor and constitutive androstane receptor. Drug Metab Dispos 37:1539–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Chao E, Zuercher WJ, Willson TM, Collins JL, Redinbo MR. (2007) Crystal structure of the PXR-T1317 complex provides a scaffold to examine the potential for receptor antagonism. Bioorg Med Chem 15:2156–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. (2012) FAT SIGNALS—lipases and lipolysis in lipid metabolism and signaling. Cell Metab 15:279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cheng X, Aleksunes LM, Klaassen CD. (2012) Transcription factor-mediated regulation of carboxylesterase enzymes in livers of mice. Drug Metab Dispos 40:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Bie J, Wang J, Marqueen SA, Ghosh S. (2012) Identification of a novel intracellular cholesteryl ester hydrolase (carboxylesterase 3) in human macrophages: compensatory increase in its expression after carboxylesterase 1 silencing. Am J Physiol Cell Physiol 15:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Song J, Chow WN, St Clair RW, Rudel LL, Ghosh S. (2007) Macrophage-specific transgenic expression of cholesteryl ester hydrolase significantly reduces atherosclerosis and lesion necrosis in Ldlr mice. J Clin Invest 117:2983–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.