Abstract
Inhibition of the bile salt export pump (BSEP) can cause intracellular accumulation of bile acids and is a risk factor for drug-induced liver injury in humans. Antiretroviral protease inhibitors lopinavir (LPV) and ritonavir (RTV) are reported BSEP inhibitors. However, the consequences of LPV and RTV, alone and combined (LPV/r), on hepatocyte viability, bile acid transport, and endogenous bile acid disposition in rat hepatocytes have not been examined. The effect of LPV, RTV, and LPV/r on cellular viability and the disposition of [3H]taurocholic acid (TCA) and [14C]chenodeoxycholic acid (CDCA) was determined in sandwich-cultured rat hepatocytes (SCRH) and suspended rat hepatocytes. Lactate dehydrogenase and ATP assays revealed a concentration-dependent effect of LPV and RTV on cellular viability. LPV (5 µM), alone and combined with 5 µM RTV, significantly decreased [3H]TCA accumulation in cells + bile of SCRHs compared with control. LPV/r significantly increased [3H]TCA cellular accumulation (7.7 ± 0.1 pmol/mg of protein) compared with vehicle and 5 µM LPV alone (5.1 ± 0.7 and 5.0 ± 0.5 pmol/mg of protein). The [3H]TCA biliary clearance was reduced significantly by LPV and RTV and further reduced by LPV/r. LPV and RTV did not affect the initial uptake rates of [3H]TCA or [14C]CDCA in suspended rat hepatocytes. LPV (50 µM), RTV (5 µM), and LPV/r (5 and 50 µM/5 µM) significantly decreased the accumulation of total measured endogenous bile acids (TCA, glycocholic acid, taurochenodeoxycholic acid, glycochenodeoxycholic acid, and α/β-tauromuricholic acid) in SCRH. Quantification of endogenous bile acids in SCRH may reveal important adaptive responses associated with exposure to known BSEP inhibitors.
Introduction
Antiretroviral protease inhibitors (PIs) continue to be a mainstay in the treatment of human immunodeficiency (HIV) infection. Despite their success, PIs have been associated with drug-induced liver injury (DILI), which is one of the most common adverse events leading to the discontinuation of PI-inclusive antiretroviral therapy (Sulkowski et al., 2000; Bongiovanni et al., 2005). Liver injury occurred in 1% to 9.5% of PI-treated patients in randomized clinical trials conducted before US Food and Drug Administration approval (Sulkowski, 2004). Retrospective and prospective cohort studies report an overall incidence rate between 5% and 23% for hepatotoxicity associated with PI-inclusive drug therapy. However, the PI dose and the definition of hepatotoxicity varied across the studies (Sulkowski, 2003). In particular, ritonavir (RTV)-containing regimens reportedly increased the risk of hepatotoxicity by 8.6-fold (Sulkowski et al., 2000). RTV is now administered at subtherapeutic (and subtoxic) doses to enhance systemic concentrations of coadministered PIs. One commonly prescribed PI combination is lopinavir (LPV) and ritonavir (LPV/r). Reportedly, patients on highly active antiretroviral therapy containing LPV/r who experienced liver failure exhibited higher LPV/r plasma concentrations compared with patients with normal functioning livers (package insert, Abbott Laboratories, Abbott Park, IL).
One proposed mechanism for DILI is that drugs or their metabolites impair the function of transport proteins responsible for the efflux of bile acids from the hepatocyte (McRae et al., 2006; Marion et al., 2007; Wolf et al., 2010). Bile acids can cause cellular necrosis and apoptosis as a result of mitochondrial damage and disruption of cell membranes due to the detergent-like effects of these molecules (Pauli-Magnus et al., 2005). Interference with the efflux of bile acids from hepatocytes could cause intracellular accumulation of bile acids, leading to toxicity.
The major transport protein responsible for biliary excretion of bile acids from the hepatocyte is the bile salt export pump (BSEP). Recent studies have shown that many drugs implicated in DILI inhibit BSEP (Morgan et al., 2010). PIs, including LPV and RTV, also can inhibit bile acid transport via BSEP (McRae et al., 2006; Dawson et al., 2012), supporting the idea that intracellular accumulation of bile acids may be a mechanism for DILI observed in patients treated with this combination (Morgan et al., 2010; Dawson et al., 2012). If this is correct, we reasoned that the combination of LPV and RTV used in the clinic may have an additive or even synergistic effect on BSEP inhibition, resulting in an increased risk of DILI.
To our knowledge, the effect of PI combinations on hepatocyte viability and bile acid uptake or efflux has not been studied previously. Therefore, we examined the effects of LPV, alone and combined with RTV, on hepatocyte viability, bile acid transport, and endogenous bile acid disposition in rat hepatocytes. We hypothesized that each PI would cause hepatocellular accumulation of bile acids and toxicity and that coadministration of RTV and LPV would have at least an additive effect on bile acid accumulation and toxicity.
Materials and Methods
Chemicals.
[3H]Taurocholic acid (TCA, 5 Ci/mmol; purity > 97%) was purchased from PerkinElmer Inc. (Waltham, MA). [14C]Chenodeoxycholic acid (CDCA; 50 mCi/mmol; purity > 97%) and [14C]inulin (2.8 mCi/g, purity > 97%) were purchased from American Radiolabeled Chemicals, Inc. (St. Louis, MO). RTV was obtained initially from the National Institutes of Health AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (Germantown, MD). In addition, RTV, LPV, and d4 TCA were purchased from Toronto Research Chemicals (Toronto, ON, Canada). The d8 TCA was purchased from Martrex, Inc. (Minnetonka, MN). All other deuterated bile acids were purchased from CDN Isotopes, Inc. (Pointe-Claire, QC, Canada). The bile acids α- and β-tauromuricholic acid (α/β-TMCA) were purchased from Steraloids, Inc. (Newport, RI). TCA, lactate dehydrogenase (LDH), ATP, Triton X-100, Hanks’ balanced salt solution (HBSS) premix, HBSS modified (with no calcium chloride, magnesium sulfate, phenol red, and sodium bicarbonate) premix, dexamethasone, and collagenase (type IV) were purchased from Sigma-Aldrich (St. Louis, MO). Dimethyl sulfoxide (DMSO) was obtained from Fisher Scientific (Fairlawn, NJ). GIBCO-brand fetal bovine serum, recombinant human insulin, and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Invitrogen (Carlsbad, CA). Insulin, transferrin, and selenium (ITS) Universal Culture Supplement Premix and Matrigel Basement Membrane Matrix were obtained from BD Biosciences (Palo Alto, CA). The CellTiter-Glo Luminescent Cell Viability Assay was purchased from Promega (Madison, WI). LDH Cytotoxicity Detection Kit was purchased from Roche Applied Sciences (Indianapolis, IN). All other chemicals and reagents were of analytical grade and were readily available from commercial sources.
Hepatocyte Isolation and Culture in a Sandwich Configuration.
Hepatocytes were isolated from male Wistar rats (270–300 g) obtained from Charles River Laboratories, Inc. (Raleigh, NC) using a two-step collagenase perfusion method, previously described (LeCluyse et al., 1996). Animals had free access to water and food before surgery and were allowed to acclimate for at least 5 days. All animal procedures complied with the guidelines of the Institutional Animal Care and Use Committee (University of North Carolina at Chapel Hill, Chapel Hill, NC).
Hepatocytes were seeded at 1.75 × 106 cells per well on six-well, or 0.35 × 106 cells per well on 24-well, BioCoat collagen plates in DMEM containing 5% fetal bovine serum, 10 µM insulin, 1 µM dexamethasone, 2 mM l-glutamine, 1% MEM nonessential amino acids, 100 units of penicillin G sodium, and 100 µg of streptomycin sulfate. Cells were incubated for 2 h at 37°C in a humidified incubator (95% O2, 5% CO2) and allowed to attach to the collagen substratum, after which time the medium was aspirated to remove unattached cells and replaced with fresh medium. Approximately 24 h later, cells were overlaid with BD Matrigel at a concentration of 0.25 mg/ml in ice-cold feeding medium (DMEM with 1% ITS, 0.1 µM dexamethasone, 2 mM l-glutamine, 1% MEM nonessential amino acids, 100 units of penicillin G sodium, and 100 µg/ml of streptomycin sulfate). The culture medium was changed daily thereafter. Rat hepatocytes were cultured for at least 3 days to allow for the formation of bile canalicular networks.
Cytotoxicity and Cell Viability Assays.
After 24-h exposure to PIs, intracellular ATP levels were measured using the CellTiter-Glo Luminescent Cell Viability Assay. All reagents were allowed to equilibrate to room temperature before use. The CellTiter-Glo Reagent was prepared by adding lyophilized CellTiter-Glo substrate to CellTiter-Glo buffer and mixing by vortex. Hepatocytes cultured in 24-well plates were allowed to equilibrate for at least 30 min to reach room temperature before the assay was performed. Medium was aspirated from each well twice and replaced with equal volumes of fresh feeding medium and CellTiter-Glo reagent. Plates were placed on an orbital shaker for 2 min to induce cell lysis and then incubated at room temperature for 10 min to allow the luminescent signal to stabilize.
Leakage of LDH into sandwich-cultured rat hepatocyte (SCRH) medium was determined using the LDH Cytotoxicity Detection Kit. Briefly, on day 3, SCRHs in 24-well plates were exposed to PIs for 24 h, after which cell-free supernatant was collected and aliquots were placed in individual wells of a 96-well plate. The substrate mixture was added to the culture supernatant and incubated for 30 min. During this time, LDH released from hepatocytes into the supernatant reduced the tetrazolium salt 2-(4-iodophenyl)-3-(4- nitrophenyl)-5-phenyl-2H-tetrazolium chloride (INT) to formazan by a coupled enzymatic reaction. After incubation, formazen formation was measured directly in the 96-well opaque-walled microplate by an enzyme-linked immunosorbent assay absorbance plate reader at 492 nm. To compare assays directly, LDH data were converted to viability and expressed as a percentage of control by subtracting the degree of toxicity (%) from 100%. Maximum cell death was represented by the values measured after complete cell lysis by 0.5% Triton X-100.
Bile Acid ([3H]TCA and [14C]CDCA) Accumulation Studies in Sandwich-Cultured Rat Hepatocytes.
The model bile acid, TCA, and the unconjugated organic acid, CDCA, were used for transport studies. Day 4 SCRHs seeded in 24-well plates were washed three times (20 s per wash) and coincubated for 10 min with Ca2+-containing (standard; cells + bile) or Ca2+-free (cells) HBSS buffer containing EGTA to maintain or disrupt tight junctions, respectively. Next, hepatocytes were coincubated for 10 min with TCA (1 µM cold TCA plus 0.07 µM [3H]TCA) or CDCA (1 µM cold CDCA plus 4 µM [14C]CDCA) in the presence or absence of individual or combined PIs in standard HBSS at 37°C. Cells were then aspirated twice, and uptake was terminated by rinsing wells with 2 ml of ice-cold standard HBSS. After rinsing, cells were lysed with 0.1% Triton X-100 in phosphate-buffered saline and placed on an orbital shaker for 20 min. Aliquots of sample (500 µl) and dosing solution (100 µl) were collected for quantification of radioactivity by liquid scintillation counting. Another 500-µl aliquot of sample was reserved for protein quantification using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). To correct for nonspecific binding to the collagen substratum, [3H]TCA and [14C]CDCA accumulation in BioCoat plates without cells was subtracted from raw values.
[3H]TCA and [14C]CDCA Initial Uptake in Suspended Rat Hepatocytes.
The initial uptake of TCA (1 µM cold TCA plus [3H]TCA, 60 nCi/ml) and CDCA (0.5 µM cold CDCA plus 0.5 µM [14C]CDCA; 25 nCi/ml) in suspended rat hepatocytes was measured in the presence of vehicle (DMSO), LPV (10 µM), or RTV (5 µM), alone and combined, using methods previously described (Leslie et al., 2007). Uptake studies were performed in Na+-containing buffer to measure total uptake (Na+-dependent and Na+-independent) and Na+-free, choline-containing buffer (Na+-independent uptake only). Na+-dependent uptake was calculated by subtracting the Na+-independent uptake from the total uptake. Briefly, cells were washed twice in ice-cold buffer containing sodium chloride or choline chloride (137 mM NaCl or choline chloride, 0.8 mM MgSO4, 10 mM HEPES, 1.2 mM CaSO4, 0.86 mM K2HPO4, 0.14 mM KH2PO4, and 5 mM glucose, pH 7.4). Cells were resuspended at 1.0 × 106 cells/ml in the same buffer, kept on ice, and used immediately in experiments. Hepatocyte suspensions (4 ml; n = 3 livers, in triplicate) were preincubated in bottom-inverted Erlenmeyer flasks at 37°C for 5 min; 0.1% DMSO or PIs were added 30 s before the addition of [3H]TCA (1 μM of unlabeled TCA plus [3H]TCA, 60 nCi/ml). At 15, 30, and 45 s, 200-µl samples of the cell suspension were collected and placed in a 0.4-ml polyethylene tube containing a top layer of silicone oil:mineral oil [82:18 (v/v), 100 µl] and a bottom layer of 3M KOH (50 µl) and immediately centrifuged. Radioactivity in the cell pellet and in the supernatant was measured by liquid scintillation counting. Adherent fluid volume was determined by incubating cells with [14C] inulin (60 nCi/ml) as reported by Baur and colleagues (1975). Uptake was normalized to protein concentrations for individual hepatocyte suspensions as determined by the BCA protein assay reagent kit. Cellular viability of the suspended hepatocytes (>90%) was determined by trypan blue exclusion at the beginning and end of each experiment.
Accumulation of Endogenous Bile Acids in Cells + Bile, Cells, and Culture Medium of Sandwich-Cultured Rat Hepatocytes.
After 24-h exposure to vehicle or PIs, 1-ml aliquots of medium were collected from day 4 SCRH in six-well format and stored at −80°C until analysis. The remaining culture medium was aspirated from all wells, and triplicate wells were rinsed with 1.5 ml/well of warmed HBSS containing calcium (cells + bile) or HBSS without calcium (cells alone). After rinses, the wells were aspirated twice, and another 1.5 ml of HBSS, with or without calcium, was added to the wells, and cells were incubated at 37°C for 4 min. After incubation, the HBSS buffer was aspirated from all wells. Plates were sealed and stored at −80°C until analysis.
Liquid Chromatography Coupled with Tandem Mass Spectrometry Analysis.
Culture medium and cell lysate samples were prepared for liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) analysis as described previously (Marion et al., 2011). Briefly, six endogenous conjugated bile acid species [TCA, glycocholic acid (GCA), taurochenodeoxycholic acid (TCDCA), glycochenodeoxycholic acid (GCDCA), and α/β-TMCA] were detected simultaneously; 10 µl of sample or calibration standards were injected onto a Shimadzu binary high-performance liquid chromatography system (Columbia, MD). Chromatographic conditions used were as follows: 60% 0.5 mM ammonium acetate:40% MeOH (solvent A) and 20% 0.5 mM ammonium acetate:80% MeOH (solvent B) at a flow rate of 50 µl/min. The initial mobile phase was 70% solvent A:30% solvent B. The gradient was increased rapidly to 100% of solvent B for 2 to 15 min and then returned to initial conditions (solvent A) for 1 min. The autosampler was maintained at 4°C and rinsed with 1500 µl of 50:50 (v/v) 50% methanol:50% water after aspiration. Methanol (100%) was added at 10 µl per minute as a postcolumn solvent. Tandem MS used to quantify analytes was performed using a Thermo Electron TSQ Quantum Discovery MAX (Thermo Fisher Scientific, Inc., Charlotte, NC) with an Ion Max ESI (Thermo Fischer Scientific, Inc.) source in negative ion electrospray ionization mode using selected reaction monitoring. The concentration ranges of the standard curves for rat cell lysate and medium of each bile acid were 0.5–100 pmol per well and 0.5–50 pmol/100 µl of medium, respectively. For a detailed list of the transitions monitored at unit resolution, see Marion et al. (2011).
When rat lysate and medium samples were analyzed initially, LC-MS/MS raw data were collected on α- and β-TMCA, but they were not processed. Both α- and β-TMCA have the same MS precursor and product negative ions as TCA; thus, their MS data were collected in the same analytical run as TCA. Once standards for α- and β-TMCA became available, they were used to confirm the identity of the LC-MS/MS response in the TCA channel thought to be α/β-TMCA. Because of the chromatographic separation used here, TCA was well resolved from α- and β-TMCA; however, α- and β-TMCA, which are stereoisomers, were measured collectively (designated α/β-TMCA). Using recently generated standard curves for β-TMCA from rat lysate (10–2000 pmol/well) and medium (1.0–500 pmol/100 µl), the original raw data collected for α/β-TMCA, along with the data for the other bile acids, were processed. The new α/β-TMCA standard curves were not generated with a stable isotope equivalent but were corrected for endogenous α/β-TMCA background. Similarly, the raw data for the glycine conjugates of α- and β-muricholic acid were collected but not processed in the original analytical run. Unfortunately, standards for these glycine conjugates currently are not commercially available.
Data Analysis.
Cells + bile and cellular concentrations of bile acids were calculated based on estimates of hepatocyte intracellular volume (6.83 µl/well) and the number of cells per well (Lee and Brouwer, 2010). Medium concentrations were calculated based on a volume of 1.5 ml per well. For bile acid accumulation studies, the in vitro biliary excretion index (BEI; %), defined as the percentage of accumulated substrate residing within the bile canaliculi, was calculated using B-CLEAR technology (Qualyst Transporter Solutions, Durham, NC) according to the following equation:
(Liu et al., 1999b). The in vitro biliary clearance (Clbile) was calculated based on the following equation:
where AUCmedium represents the product of the incubation time (10 min) and the initial concentration in the incubation medium. Statistical analyses (one-way analysis of variance and Bonferroni’s multiple comparison post-test) were performed using GraphPadPrism 3.0 (GraphPad Software, Inc., San Diego, CA). In all cases, P < 0.05 was considered statistically significant.
Results
Assessment of Cellular Viability in Sandwich-Cultured Rat Hepatocytes.
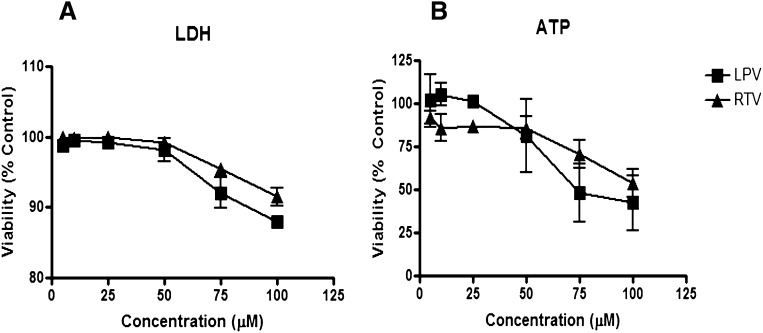
Prior studies have shown that it takes 3 days for rat hepatocytes to regain polarity in sandwich culture (Dunn et al., 1991; Liu et al., 1998; Liu et al., 1999a). Because polarity is necessary to assess the vectorial transport of bile acids, we examined the effects of 24-h RTV and LPV treatment, alone and combined, on cellular viability and bile acid disposition between culture day 3 and day 4. LDH release and cellular ATP content were measured after individual and combination treatment with LPV and RTV. Alone, LPV and RTV demonstrated concentration-dependent effects on cellular viability; the observed differences between the two treatments were not significant (Fig. 1). Toxicity was not detected, or was minimal, at concentrations <50 µM for each PI. Since toxicity may affect metabolic and transport processes involved in bile acid disposition in the SCRH model, PI concentrations ≤50 µM were used in subsequent studies. Cellular viability after exposure to the combination of LPV (5–50 µM) and RTV (5 µM) was comparable to LPV alone (Table 1), and the trend toward increased toxicity at 50 µM of LPV was not statistically significant.
Fig. 1.
Effect of 24-h exposure to lopinavir or ritonavir on hepatocyte viability in sandwich-cultured rat hepatocytes. Day 3 SCRH were treated with LPV (squares; 5–100 µM) or RTV (triangles; 5–100 µM) for 24 h. After incubation, lactate dehydrogenase release (A) and cellular ATP levels (B) were measured. Data are presented as mean ± SEM (n = 3).
TABLE 1.
Effect of lopinavir exposure for 24 h, in the presence or absence of ritonavir, on sandwich-cultured rat hepatocyte viability
Day 3 sandwich-cultured rat hepatocytes were treated for 24 h with LPV in the absence or presence of 5 µM ritonavir (LPV/r). Data represent mean ± SEM (n = 3 livers in triplicate).
|
LPV |
LPV/r |
|||
|---|---|---|---|---|
|
Viability (% Control) |
||||
| LPV Dosing Concentration (µM) | LDH Assay | ATP Assay | LDH Assay | ATP Assay |
| 5 | 99 ± 1 | 102 ± 15 | 99 ± 1 | 81 ± 7 |
| 10 | 100 ± 1 | 105 ± 7 | 99 ± 1 | 80 ± 7 |
| 25 | 99 ± 1 | 101 ± 1 | 98 + 1 | 79 ± 4 |
| 50 | 98 ± 2 | 81 ± 22 | 88 ± 8 | 68 ± 25 |
[3H]TCA and [14C]CDCA Accumulation in Sandwich-Cultured Rat Hepatocytes.
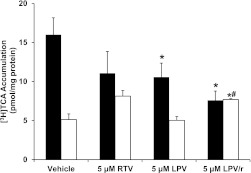
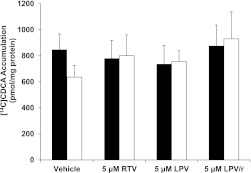
Accumulation of [3H]TCA (1.07 µM) or [14C]CDCA (5 µM) from the culture medium into cells + bile versus cells alone was measured after 10-min coincubation with vehicle (0.1% DMSO), RTV (5 µM), LPV (5–50 µM), or combined LPV and 5 µM RTV (LPV/r). As shown in Fig. 2, the mean accumulation of [3H]TCA in cells + bile was reduced by both LPV and RTV when administered alone, and the reduction was significant for LPV. A significant reduction in [3H]TCA accumulation in cells + bile relative to vehicle treatment also was observed for the combination treatment LPV/r (from 16.0 ± 2.2 to 7.6 ± 1.2 pmol/mg protein). It appeared that the coadministration of LPV with RTV resulted in additional reduction in the concentration of [3H]TCA in cells + bile compared with LPV treatment alone, but this decrease was not significant. The hepatocyte (cell) concentration of [3H]TCA was not significantly increased by RTV or LPV alone (Fig. 2). However, when RTV was combined with LPV, the hepatocyte concentration of [3H]TCA (7.7 ± 0.1 pmol/mg protein) was significantly increased relative to the cellular concentrations observed with either vehicle or 5 µM LPV alone (5.1 ± 0.7 and 5.0 ± 0.5 pmol/mg protein, respectively). When the same experiment was repeated with [14C]CDCA, the treatments did not significantly alter the accumulation of [14C]CDCA species in cells + bile or cells alone (Fig. 3).
Fig. 2.
Effect of lopinavir and ritonavir, alone and combined, on [3H]TCA accumulation in sandwich-cultured rat hepatocytes. [3H]TCA accumulation in cells + bile (black bars) and cells (white bars), in day 4 SCRH were determined after a 10-min coincubation with RTV (5 µM) and LPV (5 µM), alone or combined (LPV/r) (mean ± SEM; n = 3 livers in triplicate; analysis of variance followed by a Bonferroni post test, *, versus cells + bile vehicle control; #, versus 5 µM LPV alone; P < 0.05).
Fig. 3.
Effect of LPV and ritonavir RTV, alone and combined, on the accumulation of [14C]CDCA species in sandwich-cultured rat hepatocytes. The [14C]CDCA accumulation of [14C]CDCA species in cells + bile (black bars) and cells (white bars), in day 4 SCRH was determined after a 10-min coincubation with RTV (5 µM) and LPV (5 µM), alone or combined (LPV/r) (mean ± SEM; n = 3 livers in triplicate).
Biliary Excretion of [3H]TCA and [14C]CDCA in Sandwich-Cultured Rat Hepatocytes.
The calculated BEI (%) for [3H]TCA was reduced by both LPV and RTV alone, and further reduced by the combination treatment (Table 2). The calculated biliary clearance values (Clbile) followed a similar pattern, but the reductions caused by RTV and LPV were statistically significant relative to vehicle treatment. Moreover, the reduction in Clbile observed with the combination of LPV and RTV was significantly greater than that observed with LPV alone, suggesting an additive effect on impaired biliary clearance. Concentrations exceeding 10 µM LPV virtually ablated the BEI of [3H]TCA, regardless of coadministration with RTV (data not shown).
TABLE 2.
Effect of lopinavir and ritonavir on the biliary excretion index (BEI) and in vitro biliary clearance of [3H]taurocholic acid (TCA) and [14C]chenodeoxycholic acid (CDCA) in sandwich-cultured rat hepatocytes
Data from Figures 2 and 3 were used to calculate the biliary excretion index and in vitro biliary clearance (Clbile), as described in the methods, in the absence or presence (LPV/r) of 5 µM RTV. Data represent mean ± SEM (n = 3 livers in triplicate; analysis of variance followed by a Bonferroni post-test). Initial concentrations of TCA and CDCA in incubation medium were 1.07 µM and 5 µM, respectively.
|
BEI |
Clbile |
|||
|---|---|---|---|---|
| [3H]TCA | [14C]CDCA | [3H]TCA | [14C]CDCA | |
| % | ml/min/kg | |||
| Vehicle | 68 ± 3 | 27 ± 2 | 8.7 ± 1.3 | 37.2 ± 8.1 |
| 5 µM RTV | 21 ± 15 | BLQ | 2.5 ± 2.1a | BLQ |
| 5 µM LPV | 49 ± 11 | BLQ | 4.4 ± 1.7a | BLQ |
| 5 µM LPV/r | 9 ± 5 | BLQ | 0.61 ± 0.35b | BLQ |
BLQ, below limit of quantitation.
Versus vehicle control, P < 0.05.
Versus 5 µM LPV alone, P < 0.05.
[14C]CDCA cellular concentrations in vehicle-treated hepatocytes were 120-fold greater compared with [3H]TCA, and the BEI of [14C]CDCA species was 2- to 3-fold lower than for [3H]TCA. Thus, a decrease in biliary excretion might not affect the cellular accumulation of [14C]CDCA to the same extent as that of [3H]TCA. LPV, alone or combined with RTV, reduced the BEI and Clbile of [14C]CDCA species to values below the limit of quantitation (Table 2).
[3H]TCA and [14C]CDCA Initial Uptake in Suspended Rat Hepatocytes.
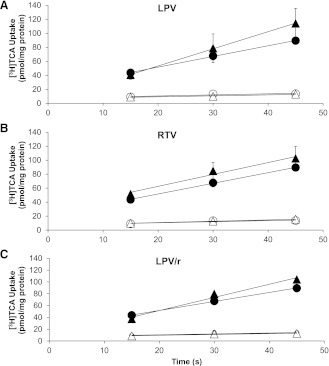
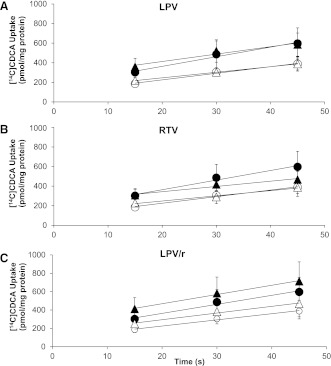
To determine whether inhibition of bile acid uptake contributed to the reduction in Clbile caused by the PIs, [3H]TCA and [14C]CDCA influx into hepatocytes was measured during the linear uptake time interval (15–45 s) in suspended rat hepatocytes (Iga and Klaassen, 1982; Marion et al., 2007). Initial uptake rates of [3H]TCA in Na+-containing and Na+-free buffer were 1.53 ± 0.11 and 0.15 ± 0.07 pmol/s per milligram of protein, respectively (n = 3; Fig. 4). LPV (10 µM), RTV (5 µM), and LPV/r had no effect on the initial uptake rates of [3H]TCA in Na+-containing or Na+-free buffer compared with vehicle control. Similarly, LPV, RTV, and LPV/r had no effect on the initial uptake rates of [14C]CDCA in Na+-containing and Na+-free buffer of vehicle control hepatocytes (9.92 ± 3.02 and 6.73 ± 2.19 pmol/s per milligram of protein, respectively; n = 3; Fig. 5).
Fig. 4.
Effect of LPV and RTV, alone and combined, on the Na+-dependent and Na+-independent uptake of [3H]TCA into freshly isolated suspended rat hepatocytes. [3H]TCA accumulation in freshly isolated rat hepatocytes was determined after preincubation with LPV (10 µM) (A) or RTV (5 µM) (B), alone and in combination (C), in the absence or presence of sodium. Closed and open circles represent vehicle-treated cells in Na+-containing or Na+-free buffer, respectively. Closed and open triangles represent treated cells in Na+-containing or Na+-free buffer, respectively. Uptake into cells is reported as picomoles per milligram (pmol/mg) of protein (mean ± SEM; n = 3 livers in triplicate).
Fig. 5.
Effect of LPV and RTV, alone and combined, on the Na+-dependent and Na+-independent uptake of [14C]CDCA into freshly isolated suspended rat hepatocytes. Accumulation of [14C]CDCA species in freshly isolated rat hepatocytes was determined after preincubation with LPV (10 µM) (A) or RTV (5 µM) (B), alone and in combination (C), in the absence or presence of sodium. Closed and open circles represent vehicle-treated cells in Na+-containing or Na+-free buffer, respectively. Closed and open triangles represent treated cells in Na+-containing or Na+-free buffer, respectively. Uptake into cells is reported as picomoles per milligram of protein (pmol/mg) (mean ± SEM; n = 3 livers in triplicate).
Accumulation of Endogenous Bile Acids in Cells + Bile, Cells, and Medium of Sandwich-Cultured Rat Hepatocytes.
TCA, GCA, TCDCA, GCDCA, and α/β-TMCA were measured in cells + bile, cells, and medium of SCRH. Taurine-conjugated bile acids accounted for most (approximately 99%) of the bile acid species detected in vehicle-treated SCRH, consistent with data from in vitro rat studies published previously (Barth et al., 2006). Concentrations (micromoles per liter) of each bile acid species in cells + bile, cells, and medium of vehicle-treated SCRH are listed in Table 3. The α- and β-TMCA species constituted most of the total measured bile acid pool and appeared predominantly in the cells of SCRH. The BEI value of endogenous TCA (49%) was in the same range as the BEI calculated after the addition of 1 µM [3H]TCA (68%; Table 2). It is not possible to calculate the biliary clearance of endogenously synthesized bile acids based on the current study design.
TABLE 3.
Bile acid concentrations (μM) in cells + bile, cells, and medium, and biliary excretion index values for each bile acid species in day 4 sandwich-cultured rat hepatocytes
Data represent mean ± SEM (n = 3 livers in triplicate). Calculations assume a hepatocyte volume of 6.83 µl/well. The biliary excretion index was calculated as described in the methods.
| Species | Cells + Bile | Cells | Medium | BEI |
|---|---|---|---|---|
| % | ||||
| TCA | 5.14 ± 1.71 | 2.61 ± 1.78 | 0.651 ± 0.127 | 49 |
| GCA | 0.20 ± 0.06 | 0.13 ± 0.08 | 0.07 ± 0.03 | 35 |
| TCDCA | 1.07 ± 0.20 | 0.63 ± 0.20 | 0.017 ± 0.003 | 41 |
| GCDCA | 0.12 ± 0.08 | 0.07 ± 0.04 | 0.004 ± 0.003 | 42 |
| α/β-TMCA | 168 ± 65 | 133 ± 72 | 1.59 ± 0.37 | 20 |
| Total | 174 ± 67 | 137 ± 74 | 2.34 ± 0.412 |
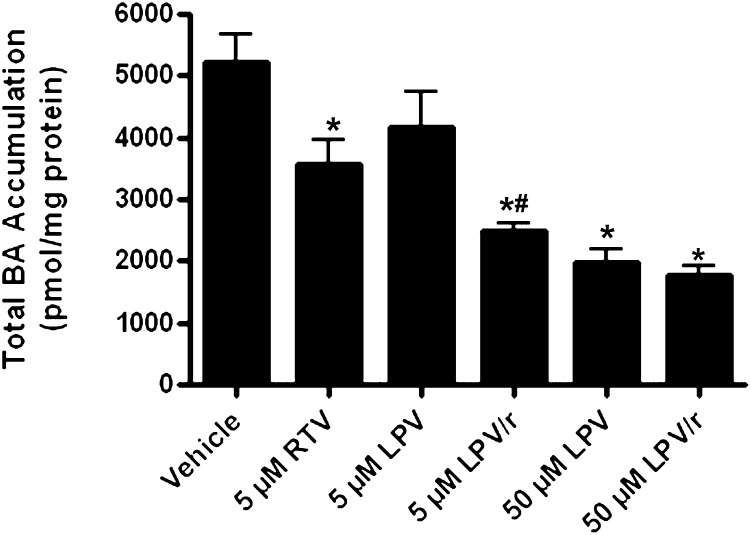
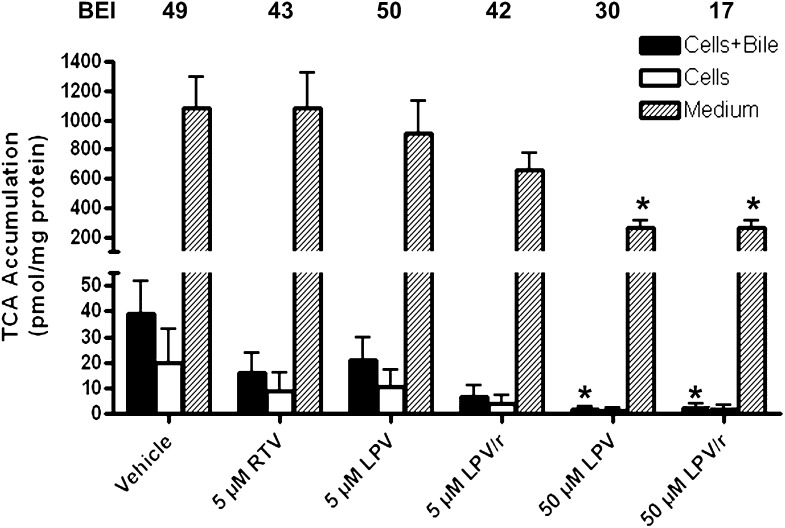
Accumulation of total endogenous bile acids (sum of TCA, GCA, TCDCA, GCDCA, and α/β-TMCA) in medium, cells, and bile of SCRH also was determined after 24-h incubation with vehicle, LPV (5 or 50 µM), and RTV (5 µM), alone or combined. Surprisingly, all treatments, except 5 µM LPV, significantly decreased total bile acid accumulation compared with vehicle control. This marked reduction in total measured bile acids is consistent with the observation that 24-h LPV exposure yielded minimal apparent toxicity to SCRH at these concentrations (Fig. 1; Table 1). The addition of 5 µM RTV to low-dose LPV (5 µM) significantly decreased total bile acid accumulation relative to both vehicle and 5 µM LPV alone (Fig. 6). The addition of 5 µM RTV to 50 µM LPV did not further decrease endogenous bile acid accumulation relative to 50 µM LPV alone (Figs. 6–9).
Fig. 6.
Accumulation of total measured bile acids (sum of TCA, GCA, TCDCA, GCDCA, and α/β-TMCA) in sandwich-cultured rat hepatocytes (cells, bile, and medium). Bile acids were measured after 24-h treatment with vehicle (0.1% DMSO), RTV (5 µM), and LPV (5 or 50 µM), alone or combined (mean ± SEM; n = 4 livers in triplicate; analysis of variance followed by a Bonferroni post-test; *, versus vehicle control; #, versus 5 µM LPV, P < 0.05).
Fig. 9.
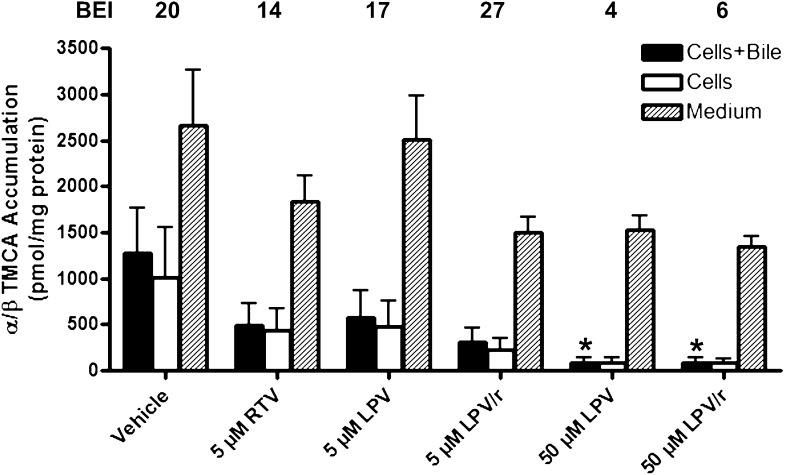
Accumulation of α/β-TMCA in cells + bile (solid bars), cells (open bars), and medium (hatched bars) and BEI (%) values in sandwich-cultured rat hepatocytes. Bile acids were measured after 24-h treatment with vehicle (0.1% DMSO), RTV (5 µM), and LPV (5 or 50 µM), alone or combined (mean ± SEM; n = 4 livers in triplicate; analysis of variance followed by a Bonferroni post test; *, versus vehicle control; #, versus 5 µM LPV, P < 0.05).
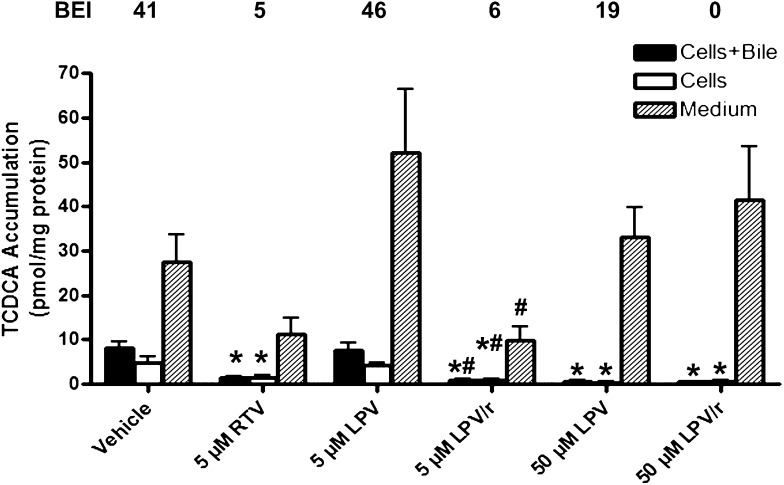
The addition of 5 µM RTV to low-dose LPV (5 µM) significantly decreased TCDCA accumulation in cells + bile and cells alone relative to both vehicle and 5 µM LPV alone (Fig. 8). In contrast, TCA and α/β-TMCA accumulation in cells + bile and cells alone was not significantly influenced by LPV (5 µM), either alone or combined with RTV (Figs. 7 and 9, respectively). LPV (50 µM) significantly reduced TCA accumulation in medium and cells + bile (Fig. 7), TCDCA accumulation in cells + bile and cells alone (Fig. 8), and α/β-TMCA accumulation in cells + bile (Fig. 9), relative to vehicle. Interestingly, TCDCA accumulation in cells + bile and cells alone was significantly decreased by 5 µM RTV (Fig. 8). Notably, the BEI of TCDCA was markedly decreased by RTV, alone or in combination with LPV (values at the top of Fig. 8).
Fig. 8.
Accumulation of TCDCA in cells + bile (solid bars), cells (open bars), and medium (hatched bars) and BEI (%) values in sandwich-cultured rat hepatocytes. Bile acids were measured after 24-h treatment with vehicle (0.1% DMSO), RTV (5 µM), and LPV (5 or 50 µM), alone or combined (mean ± SEM; n = 4 livers in triplicate; analysis of variance followed by a Bonferroni post test; *, versus vehicle control; #, versus 5 µM LPV, P < 0.05).
Fig. 7.
Accumulation of TCA in cells + bile (solid bars), cells (open bars), and medium (hatched bars) and BEI (%) values in sandwich-cultured rat hepatocytes. Bile acids were measured after 24-h treatment with vehicle (0.1% DMSO), RTV (5 µM), and LPV (5 or 50 µM), alone or combined (mean ± SEM; n = 4 livers in triplicate; analysis of variance followed by a Bonferroni post test; *, versus vehicle control, P < 0.05).
The GCA accumulation in cells + bile was significantly decreased from control by 5 µM LPV combined with 5 µM RTV (1.53 ± 0.42 versus 0.14 ± 0.14 pmol per milligram of protein) and nearly abolished by exposure to high-dose LPV, in the absence and presence of RTV. GCDCA was essentially undetectable in cells + bile and cells of SCRH treated with 5 µM LPV combined with RTV or with high-dose LPV (50 µM), alone or combined with 5 µM RTV. Medium GCA and GCDCA were not statistically different after PI exposure relative to vehicle control values.
Discussion
Inhibition of BSEP-mediated biliary excretion of bile acids is a proposed mechanism of DILI. LPV and RTV inhibit BSEP in vitro and are associated with hepatotoxicity. HIV treatment regimens frequently combine RTV with other PIs to improve oral bioavailability, but they are associated with increased liver toxicity. The present work characterizes the interactions between hepatocytes, PIs, and bile acids. We hypothesized that the addition of RTV to LPV would result in increased toxicity and intracellular accumulation of bile acids in SCRH.
SCRH regain in vivo–like morphologic properties, including the development of tight junctions, polarized transport, and functional canalicular networks; metabolic capacity (e.g., bile acid synthesis and secretion) and functional regulatory machinery are well documented in SCRH (Swift et al., 2010). Periodic contractions of the networks, as described for isolated hepatocyte couplets or hepatocyte groups (Oshio and Phillips, 1981; Phillips et al., 1982), return canalicular contents to the medium (LeCluyse et al., 1994). Mathematical modeling of SCRH data consistently requires a rate constant for substrate flux from the canalicular compartment (Liu et al., 1999b; Hoffmaster et al., 2005; Lee et al., 2010). Thus, when cultured under appropriate conditions, SCRH do not exhibit biliary stasis, intracellular bile acids are within the range of previously reported values (Marion et al., 2012), and SCRH exhibit toxicity when BSEP is inhibited (Kemp and Brouwer, 2004; Marion et al., 2007; Ogimura et al., 2011). The content of reduced glutathione in SCRH is normal and is decreased markedly by exposure to toxicants that deplete glutathione in vivo (Kiang et al., 2011). Therefore, we selected the SCRH model for the present studies. Indisputably, species differences exist between rodents and humans regarding drug-mediated toxicity and drug-transporter interactions. For example, potent inhibition of the bile salt analog cholyl-glycylamido-fluorescein by RTV in rat, but not in human, hepatocytes has been reported (Ye et al., 2010). However, rodents remain the major preclinical in vivo screen to assess the potential hepatotoxicity of new drug candidates.
Contrary to our hypothesis, LPV/r did not significantly increase toxicity relative to LPV alone (Table 1). Nonetheless, short-term (10-min) exposure of SCRH to LPV/r further increased TCA cellular accumulation compared with LPV alone (Fig. 2). It is important to note that our transport inhibition studies were conducted after 10 min of PI exposure, whereas toxicity was assessed after 24 h of PI exposure. The lack of toxicity observed at 24 h may indicate that normal-functioning hepatocytes respond to cellular injury via hepatoprotective mechanisms. Alternatively, feedback mechanisms may downregulate bile acid synthesis or upregulate bile acid efflux, causing only transient increases in intracellular bile acids.
As expected from previous reports (McRae et al., 2006), RTV inhibited [3H]TCA Clbile and BEI. LPV also inhibited the Clbile of [3H]TCA; coincubation with RTV resulted in further inhibition. It should be noted that the additional reduction in [3H]TCA Clbile and BEI resulting from the addition of RTV to LPV is consistent with additive effects of each drug and not a synergistic interaction. Doubling the LPV concentration (to 10 µM) ablated Clbile and BEI for [3H]TCA. Similar effects were observed when LPV (5 µM) was coadministered with RTV (5 µM).
The effects of LPV and RTV on the BEI and Clbile of [14C]CDCA species were similar to those observed with [3H]TCA; values were reduced below the limit of quantitation. In contrast to the result with [3H]TCA, we were unable to detect any effect of LPV alone or LPV/r on the cellular content of [14C]CDCA species because cellular accumulation was already extensive for these bile acid species. Thus, cellular concentrations of [14C]CDCA species are less sensitive to a modest decrease in canalicular efflux.
Because the marked effects of the PIs on biliary excretion of [3H]TCA and [14C]CDCA species were not associated with similar increases in hepatocyte content of bile acids, we speculated that the PIs differentially inhibited basolateral uptake of bile acids. Modulating the Na+ content of the buffer provides an accurate estimate of the contribution of the Na+-dependent transporter, Ntcp, and the sodium-independent organic anion transporting polypeptides (Oatps) to total uptake. Basolateral uptake of TCA is governed primarily by Ntcp and to a lesser extent by Oatps (Pauli-Magnus et al., 2005). Conversely, CDCA uptake is driven predominantly by Oatps, whereas Ntcp contributes to a lesser degree (Marion et al., 2011). Consistent with previous work, about 90% of the initial uptake rate of TCA into hepatocytes preincubated with vehicle (0.1% DMSO) was Ntcp-mediated; about 10% was driven by sodium-independent transporter-mediated processes (presumably Oatps). Conversely, about 69% of transporter-mediated [14C]CDCA uptake occurred in Na+-free buffer, consistent with published reports that Oatp transporters are primarily responsible for initial CDCA uptake (Kemp et al., 2005; Marion et al., 2011). LPV and RTV, alone and combined, did not affect the initial uptake of [3H]TCA or [14C]CDCA under Na+-containing and Na+-free conditions. Based on these findings, we concluded that disruption of canalicular efflux is the primary mechanism responsible for the PI-mediated decrease in Clbile of [3H]TCA and [14C]CDCA species.
This article reports, for the first time, the effects of PIs on the disposition of bile acids synthesized by SCRH. Although the bile acid pool comprises numerous bile acid species, this study focused on quantification of taurine- and glycine-conjugated cholic acid and chenodeoxycholic acid because of their potential cytotoxicity (Danielsson, 1973b; Danielsson, 1973a; Ellis et al., 1998). Additionally, the aforementioned bile acids are common to both human and rodent bile. The rodent-specific α/β-TMCA species represent most of the rat bile acid pool. Secondary bile acids (produced via intestinal metabolism) are not synthesized in the SCRH system and thus were not quantified (Thomas et al., 2008). BEI values for endogenous TCA were comparable to those estimated after the addition of [3H]TCA. However, very different results were obtained when we investigated the effects of the PIs on intracellular concentrations of endogenously synthesized TCA. Contrary to our results with exogenous [3H]TCA administration and 10-min PI exposure, LPV treatment (50 µm; 24 h) significantly decreased the accumulation of endogenous TCA and α/β-TMCA in cells + bile (Figs. 7 and 9). The addition of RTV to 50 µM LPV had little additive effect. However, the addition of RTV to low-dose LPV (5 µM) significantly reduced the accumulation of endogenously synthesized total bile acids and TCDCA in SCRH relative to LPV alone (Figs. 6 and 8). The extent of the combined effect of LPV and RTV on Bsep inhibition differs considerably between 10-min and 24-h exposure. In addition, RTV-mediated inhibition of CYP3A4 metabolism may increase cellular LPV or RTV concentrations, which may alter bile acid synthesis. The precise mechanism(s) responsible for PI-mediated decreases in endogenous bile acids are the subject of ongoing studies. These data also suggest that LPV and RTV alter the synthesis and biliary excretion of individual bile acids differentially.
The remarkable decrease in total measured bile acid content may be due to reduced bile acid synthesis. Consistent with this conclusion, 24-h RTV (15–100 µM) exposure reportedly perturbed bile acid synthesis in a concentration-dependent manner by decreasing the activity of cholesterol 7α-hydroxylase, the rate-limiting enzyme in bile acid catabolism (Zhou et al., 2006). Based on these findings, the observed decrease in total measured bile acids after PI exposure in SCRH probably involves regulatory feedback mechanisms that promptly reduce synthesis of bile acids as a protective mechanism. An important conclusion drawn from our studies is that quantification of hepatocellular concentrations of endogenous bile acids may be required when establishing a relationship between drug-mediated inhibition of hepatic transporters and hepatotoxicity.
An important question is how the effects of LPV and RTV on bile acid excretion from hepatocytes relate to the hepatotoxicity observed clinically. At steady state, LPV and RTV are 98% to 99% bound to plasma proteins (albumin and AAG). The average unbound fraction of LPV in plasma was 0.73% and ranged from 0.14% to 1.68% (Fayet et al., 2008). Total and unbound LPV plasma concentrations in HIV-infected patients ranged from 677 to 23,767 ng/ml (∼1–38 µM) and 4.2 to 209.2 ng/ml (0.007–0.33 µM), respectively. PI concentrations selected for these studies exceeded reported unbound plasma concentrations because the clinically relevant unbound intracellular PI concentrations are unknown, but may exceed systemic concentrations as a result of transporter-mediated accumulation in hepatocytes and inhibition of intracellular metabolism and excretion.
In summary, 10-min LPV and RTV exposure reduced biliary excretion and, consequently, intracellular accumulation of TCA in SCRH. However, after 24-h exposure to LPV and RTV, we were unable to demonstrate even additive toxicity. We observed a marked reduction in hepatocyte accumulation of endogenous bile acids (sum total of TCA, GCA, TCDCA, GCDCA, and α/β-TMCA), primarily attributed to decreased α/β-TMCA. These observations do not necessarily refute a role for bile acid transport inhibition in DILI observed in patients treated with PIs. This is because most patients treated with PIs do not develop hepatotoxicity. We speculate that initial PI-mediated increases in cellular bile acid concentrations initiate a cascade of events that enables hepatocytes to remain healthy in most patients. This adaptive response includes mechanisms that decrease hepatocyte content of bile acids, most likely involving reduced synthesis. Such responses may not occur in all patients treated with these drugs. If such deficiencies have a genetic basis, their identification could lead to a personalized medicine approach to avoid DILI in PI-containing regimens.
Abbreviations
- α/β-TMCA
α/β-tauromuricholic acid
- BA
bile acid
- BEI
biliary excretion index
- BLQ
below limit of quantitation
- BSEP
bile salt export pump
- CDCA
chenodeoxycholic acid
- GCA
glycocholic acid
- GCDCA
glycochenodeoxycholic acid
- LDH
lactate dehydrogenase
- LPV
lopinavir
- RTV
ritonavir
- SCRH
sandwich-cultured rat hepatocytes
- TCA
taurocholic acid
- TCDCA
taurochenodeoxycholic acid
Authorship Contributions:
Participated in research design: Griffin, Brouwer.
Conducted experiments: Griffin, Perry, St. Claire.
Performed data analysis: Griffin, Perry, St. Claire, Watkins, Brouwer.
Wrote or contributed to the writing of the manuscript: Griffin, Watkins, Brouwer.
Footnotes
This work was supported by the National Institutes of Health [Grant R01-GM41935].
References
- Barth A, Braun J, Müller D. (2006) Bile acid transport and metabolism in rat liver slices. Exp Toxicol Pathol 57:313–319 [DOI] [PubMed] [Google Scholar]
- Baur H, Kasperek S, Pfaff E. (1975) Criteria of viability of isolated liver cells. Hoppe Seylers Z Physiol Chem 356:827–838 [DOI] [PubMed] [Google Scholar]
- Bongiovanni M, Cicconi P, Landonio S, Meraviglia P, Testa L, Di Biagio A, Chiesa E, Tordato F, Bini T, Monforte A. (2005) Predictive factors of lopinavir/ritonavir discontinuation for drug-related toxicity: results from a cohort of 416 multi-experienced HIV-infected individuals. Int J Antimicrob Agents 26:88–91 [DOI] [PubMed] [Google Scholar]
- Danielsson H. (1973a) Effect of biliary obstruction on formation and metabolism of bile acids in rat. Steroids 22:567–579 [DOI] [PubMed] [Google Scholar]
- Danielsson H. (1973b) Influence of dietary bile acids on formation of bile acids in rat. Steroids 22:667–676 [DOI] [PubMed] [Google Scholar]
- Dawson S, Stahl S, Paul N, Barber J, Kenna JG. (2012) In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab Dispos 40:130–138 [DOI] [PubMed] [Google Scholar]
- Dunn JC, Tompkins RG, Yarmush ML. (1991) Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog 7:237–245 [DOI] [PubMed] [Google Scholar]
- Ellis E, Goodwin B, Abrahamsson A, Liddle C, Mode A, Rudling M, Bjorkhem I, Einarsson C. (1998) Bile acid synthesis in primary cultures of rat and human hepatocytes. Hepatology 27:615–620 [DOI] [PubMed] [Google Scholar]
- Fayet A, Béguin A, de Tejada BM, et al. (2008) Determination of unbound antiretroviral drug concentrations by a modified ultrafiltration method reveals high variability in the free fraction. Ther Drug Monit 30:511–522 [DOI] [PubMed] [Google Scholar]
- Hoffmaster KA, Zamek-Gliszczynski MJ, Pollack GM, Brouwer KLR. (2005) Multiple transport systems mediate the hepatic uptake and biliary excretion of the metabolically stable opioid peptide [D-penicillamine2,5]enkephalin. Drug Metab Dispos 33:287–293 [DOI] [PubMed] [Google Scholar]
- Iga T, Klaassen CD. (1982) Uptake of bile acids by isolated rat hepatocytes. Biochem Pharmacol 31:211–216 [DOI] [PubMed] [Google Scholar]
- Kaletra (lopinavir/ritonavir) US Prescribing Information. Abbott Laboratories, North Chicago, IL. Available at http://www.kaletra.com/ (last accessed: March 2, 2011).
- Kemp DC, Brouwer KLR. (2004) Viability assessment in sandwich-cultured rat hepatocytes after xenobiotic exposure. Toxicol In Vitro 18:869–877 [DOI] [PubMed] [Google Scholar]
- Kemp DC, Zamek-Gliszczynski MJ, Brouwer KLR. (2005) Xenobiotics inhibit hepatic uptake and biliary excretion of taurocholate in rat hepatocytes. Toxicol Sci 83:207–214 [DOI] [PubMed] [Google Scholar]
- Kiang TK, Teng XW, Surendradoss J, Karagiozov S, Abbott FS, Chang TK. (2011) Glutathione depletion by valproic acid in sandwich-cultured rat hepatocytes: role of biotransformation and temporal relationship with onset of toxicity. Toxicol Appl Pharmacol 252:318–324 [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Audus KL, Hochman JH. (1994) Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol 266:C1764–C1774 [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Bullock PL, Parkinson A, Hochman JH. (1996) Cultured rat hepatocytes. Pharm Biotechnol 8:121–159 [DOI] [PubMed] [Google Scholar]
- Lee JK, Brouwer KR. (2010) Determination of intracellular volume of rat and human sandwich-cultured hepatocytes (Abstract ID 1595). The Toxicologist. Toxicol Sci 114 (Suppl):339 [Google Scholar]
- Lee JK, Marion TL, Abe K, Lim C, Pollock GM, Brouwer KLR. (2010) Hepatobiliary disposition of troglitazone and metabolites in rat and human sandwich-cultured hepatocytes: use of Monte Carlo simulations to assess the impact of changes in biliary excretion on troglitazone sulfate accumulation. J Pharmacol Exp Ther 332:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie EM, Watkins PB, Kim RB, Brouwer KL. (2007) Differential inhibition of rat and human Na+-dependent taurocholate cotransporting polypeptide (NTCP/SLC10A1) by bosentan: a mechanism for species differences in hepatotoxicity. J Pharmacol Exp Ther 321:1170–1178 [DOI] [PubMed] [Google Scholar]
- Liu X, Brouwer KLR, Gan LS, Brouwer KR, Stieger B, Meier PJ, Audus KL, LeCluyse EL. (1998) Partial maintenance of taurocholate uptake by adult rat hepatocytes cultured in a collagen sandwich configuration. Pharm Res 15:1533–1539 [DOI] [PubMed] [Google Scholar]
- Liu X, LeCluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, Meier PJ, Brouwer KLR. (1999a) Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol 277:G12–G21 [DOI] [PubMed] [Google Scholar]
- Liu X, LeCluyse EL, Brouwer KR, Lightfoot RM, Lee JI, Brouwer KLR. (1999b) Use of Ca2+ modulation to evaluate biliary excretion in sandwich-cultured rat hepatocytes. J Pharmacol Exp Ther 289:1592–1599 [PubMed] [Google Scholar]
- Marion TL, Leslie EM, Brouwer KLR. (2007) Use of sandwich-cultured hepatocytes to evaluate impaired bile acid transport as a mechanism of drug-induced hepatotoxicity. Mol Pharm 4:911–918 [DOI] [PubMed] [Google Scholar]
- Marion TL, Perry CH, St Claire RL, Brouwer KLR. (2012) Endogenous bile acid disposition in rat and human sandwich-cultured hepatocytes. Toxicol Appl Pharmacol 261:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion TL, Perry CH, St Claire RL, Yue W, Brouwer KLR. (2011) Differential disposition of chenodeoxycholic acid versus taurocholic acid in response to acute troglitazone exposure in rat hepatocytes. Toxicol Sci 120:371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae MP, Lowe CM, Tian X, Bourdet DL, Ho RH, Leake BF, Kim RB, Brouwer KLR, Kashuba AD. (2006) Ritonavir, saquinavir, and efavirenz, but not nevirapine, inhibit bile acid transport in human and rat hepatocytes. J Pharmacol Exp Ther 318:1068–1075 [DOI] [PubMed] [Google Scholar]
- Morgan RE, Trauner M, van Staden CJ, Lee PH, Ramachandran B, Eschenberg M, Afshari CA, Qualls CW, Jr, Lightfoot-Dunn R, Hamadeh HK. (2010) Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci 118:485–500 [DOI] [PubMed] [Google Scholar]
- Ogimura E, Sekine S, Horie T. (2011) Bile salt export pump inhibitors are associated with bile acid-dependent drug-induced toxicity in sandwich-cultured hepatocytes. Biochem Biophys Res Commun 416:313–317 [DOI] [PubMed] [Google Scholar]
- Oshio C, Phillips MJ. (1981) Contractility of bile canaliculi: implications for liver function. Science 212:1041–1042 [DOI] [PubMed] [Google Scholar]
- Pauli-Magnus C, Stieger B, Meier Y, Kullak-Ublick GA, Meier PJ. (2005) Enterohepatic transport of bile salts and genetics of cholestasis. J Hepatol 43:342–357 [DOI] [PubMed] [Google Scholar]
- Phillips MJ, Oshio C, Miyairi M, Katz H, Smith CR. (1982) A study of bile canalicular contractions in isolated hepatocytes. Hepatology 2:763–768 [DOI] [PubMed] [Google Scholar]
- Sulkowski MS. (2003) Hepatotoxicity associated with antiretroviral therapy containing HIV-1 protease inhibitors. Semin Liver Dis 23:183–194 [DOI] [PubMed] [Google Scholar]
- Sulkowski MS. (2004) Drug-induced liver injury associated with antiretroviral therapy that includes HIV-1 protease inhibitors. Clin Infect Dis 38 (Suppl 2):S90–S97 [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. (2000) Elevated liver enzymes following initiation of antiretroviral therapy. JAMA 283:2526–2527 [PubMed] [Google Scholar]
- Swift B, Pfeifer ND, Brouwer KLR. (2010) Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab Rev 42:446–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. (2008) Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 7:678–693 [DOI] [PubMed] [Google Scholar]
- Wolf KK, Vora S, Webster LO, Generaux GT, Polli JW, Brouwer KLR. (2010) Use of cassette dosing in sandwich-cultured rat and human hepatocytes to identify drugs that inhibit bile acid transport. Toxicol In Vitro 24:297–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZW, Van Pelt J, Camus S, Snoeys J, Augustijns P, Annaert P. (2010) Species-specific interaction of HIV protease inhibitors with accumulation of cholyl-glycylamido-fluorescein (CGamF) in sandwich-cultured hepatocytes. J Pharm Sci 99:2886–2898 [DOI] [PubMed] [Google Scholar]
- Zhou H, Gurley EC, Jarujaron S, Ding H, Fang Y, Xu Z, Pandak WM, Jr, Hylemon PB. (2006) HIV protease inhibitors activate the unfolded protein response and disrupt lipid metabolism in primary hepatocytes. Am J Physiol Gastrointest Liver Physiol 291:G1071–G1080 [DOI] [PubMed] [Google Scholar]