Abstract
The encapsulation and packaging reliability in fully integrated, fully wireless 100 channel Utah Slant Electrode Array (USEA)/integrated neural interface-recording version 5 (INI-R5) has been evaluated by monitoring the extended long term in-vitro functional stability and recording longevity. The INI encapsulated with 6-μm Parylene-C was immersed in phosphate buffer saline (PBS) at room temperature for a period of over 12 months. The USEA/INI-R5, while being soaked was powered and configured wirelessly through 2.765 MHz inductive link and the transmitted frequency shift keying (FSK) modulated radio-frequency (RF) (900 MHz Industrial, scientific, medical-ISM band) signal was also recorded wirelessly as a function of soak time. In order to test the long term recording ability, in-vitro wireless recording was performed in agarose for few channels. The full functionality and the ability of the electrodes to record artificial neural signals even after 12 months of PBS soak provides a measure of encapsulation reliability, the functional and recording stability in fully integrated wireless neural interface and potential usefulness for future chronic implants.
1. Introduction
Implantable biomedical devices such as microelectrode arrays for neural recording/stimulation are being developed as viable treatments for sensory and movement disorders [1–3]. One example of this technology is Utah Electrode Array (UEA)/Utah Slant Electrode Array (USEA) microelectrodes that are designed to record/stimulate many neurons simultaneously [2]. One of the most significant challenges is the percutaneous connector, which is likely to cause infections during chronic use [4]. To eliminate the use of wired connections, significant research efforts have been applied to the development of fully integrated wireless neural interfaces that can wirelessly transmit the power and the neuronal data [5–8]. For chronic use, these implantable integrated wireless neural interface devices must function in-vivo for years. The failure of chronic neural implants can occur due to both physiological reasons as well as failure of the encapsulation, leading short circuits, delamination of films or corrosion of contacts and interconnects, rendering the active and passive electronic components in the device non-functional or destroying them permanently. Packaging and encapsulation that can protect the electronic circuitry of neural interface from the harsh in-vivo environment, is a major challenge. Based on chemical resistance against the biological media and the diffusion of the media into the encapsulation material, various materials for example, Urethanes, polyimide, Teflon, Parylenes, silicon nitride (Si3N4), amorphous silicon carbide (a-SiC), diamond-like carbon (DLC) and historically known silicones [9–14] have been investigated. The evaluation of the material stability focuses on the verifications based on measurements of electrical leakage, resistivity, impedance, transport rates for H2O and ionic species, dissolution rates from infrared (IR) spectra [11, 13, 14]. With the requirement of reliable hermiticity, selective removal of the electrode tips and the biocompatibility issues makes the selection of encapsulating material much more complicated. The encapsulation layer should not only prevent the direct contact of the electrical components to the biological environment but also be biocompatible. Both aspects, protection of the living tissue against INI device and the protection of the INI chip against the biological environment demonstrate the importance of an encapsulation which is stable over the intended lifespan of the implant of several years. Clearly, the long term stability and the functionality of the INI device must be ensured before a chronic implantation.
Parylene-C has been recognized a viable material for implant encapsulation with biocompatibility, inertness, non-toxicity, capable of being deposited in conformal nanoscale layers and easily patternable to expose the electrode tips for sensing. Food and Drug Administration (FDA) approval of Parylene-C is well documented and currently comply with class VI polymer requirements. The CVD Parylene films possess low dielectric constant (~3), good high frequency properties, and high resistivity (8.8×1016 Ωm) which makes them a good candidate dielectric barrier material for neural interface devices. Our previous investigations involving encapsulation and electrical insulation properties of Parylene-C have shown that the impedance of interdigitated electrodes (IDEs) are stable in 0.15 mM phosphate buffered saline PBS for more than one year [11]. However, in a fully integrated wireless device the variation in the geometry (topography), surface properties, and several additional processing steps (for example, flip chip integration, tip deinsulation) that are not involved in the IDE’s test structures can invalidate the prediction of failure. All these factors can alter the diffusion rates of mobile ions present in PBS, and the hydrolytic effects in a fully integrated wireless device in comparison to the IDEs and does not necessarily assure an acceptable packaging and encapsulation for a chronic implant.
In this context, we demonstrate the extended in-vitro long-term functional stability and the encapsulation reliability of fully integrated, fully wireless 100 channel neural interface based on USEA/integrated neural interface-recording version 5 (INI-R5) which is designed to record from peripheral nerves. The neural recordings using fully-integrated, fully-wireless, 100 channel USEA/INI-R5 from sciatic nerve in an anesthetized cat and the real-time decodes based upon these recordings have been recently reported [15, 16]. We recently, also reported the functionality and stability of the INI device encapsulated with Parylene-C for over 150 days [8]. The extended in-vitro stability demonstrated in this report, together with the recent acute animal experiments demonstration constitute major milestone, the encapsulation reliability, functional stability, and the recording longevity in a fully integrated wireless neural interface.
2. Experimental
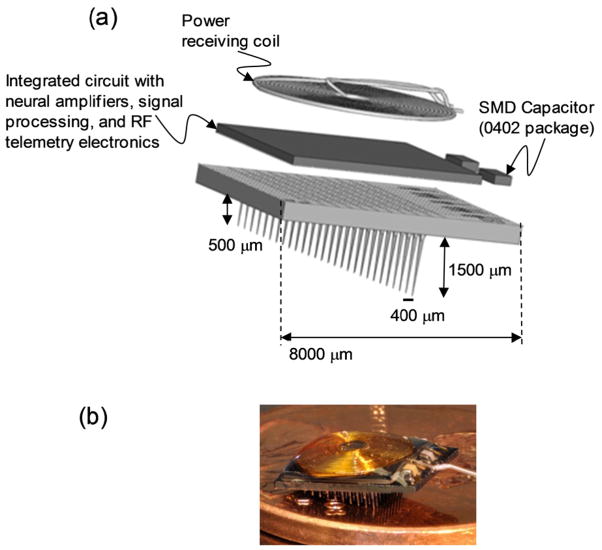
The integrated device used for long term functional study consist of a 100 channel wireless neural recording application-specific integrated circuit (ASIC) designated as INI-R5, and was flip chip bonded to 10×10 USEA using Au/Sn reflow soldering. The details of the system integration are described elsewhere [6]. An inductive wireless link provides power, clock and command signals to the chip. Silquest A-174® silane (GE silicones) was vaporized onto the integrated device as an adhesion promoter for Parylene-C. Finally, the biocompatible polymer Parylene-C with a thickness of 6-μm was chemically vapor deposited using a Paratech 3000 labtop deposition system (Paratech Coating, Inc.). The electrode tips for sensing were de-insulated by exposing the tips (30–50 μm) with an aluminum foil mask followed by a DC pulsed oxygen plasma (Oxford Plasmalab 80 Plus PECVD system, Oxford Instruments). Fig. 1 shows the schematic with approximate dimensions and the photograph of a fully integrated USEA/INI-R5 wireless neural interface.
Fig. 1.
(a) Schematic with approximate dimensions, and (b) the photograph of the Utah Slant Electrode Array/integrated neural interface-recording version 5(USEA/INI-R5) device.
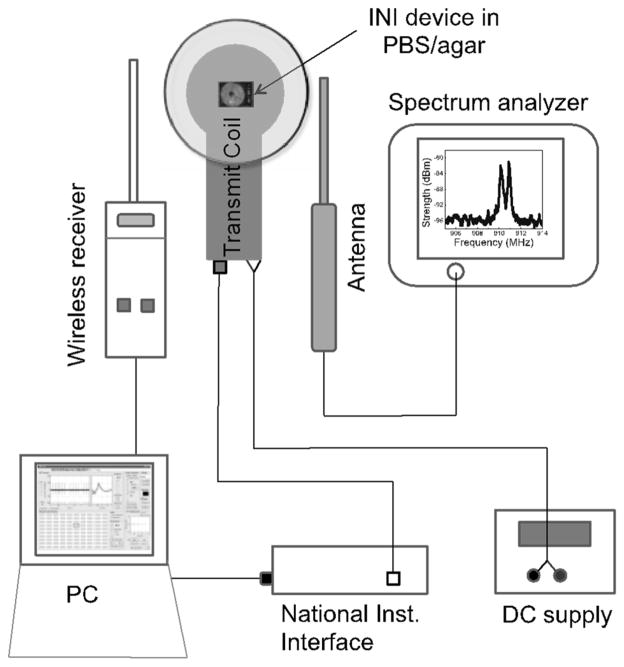
A custom made transmit coil that uses a class E power amplifier to create a 2.765 MHz waveform of up to 80 Vrms from a 10 V supply was used to deliver the power. The resulting AC magnetic field was inductively coupled to the receiving coil present in the integrated neural interface (INI) and was supplied to the chip in the INI. The transmitted radio-frequency (RF) signal was detected using two different antenna-receiver systems. A monopole antenna connected to the spectrum analyzer (Agilent E4402B) was used for displaying the RF spectra. The second antenna receiver system was a custom developed wireless receiver board that displays the frequency and signal strength, and decodes the real time information in MATLAB on a PC through the universal serial bus (USB) interface. PBS (1% NaCl) in a covered glass Petri dish with 11 mm depth was used for long term soak testing (PBS depth was maintained at constant level all the time). The long term soak test in PBS was performed at room temperature. For generating the artificial neural signals into the agarose, a Grass SD-9 pulse stimulator was used. The experimental set-up for long-term PBS soak, and agar testing is shown in Fig. 2.
Fig. 2.
Experimental set-up for long term phosphate buffer saline (PBS) soak/agarose testing of fully integrated wireless neural interface.
3. Results and discussion
3.1 Long term RF functionality in PBS
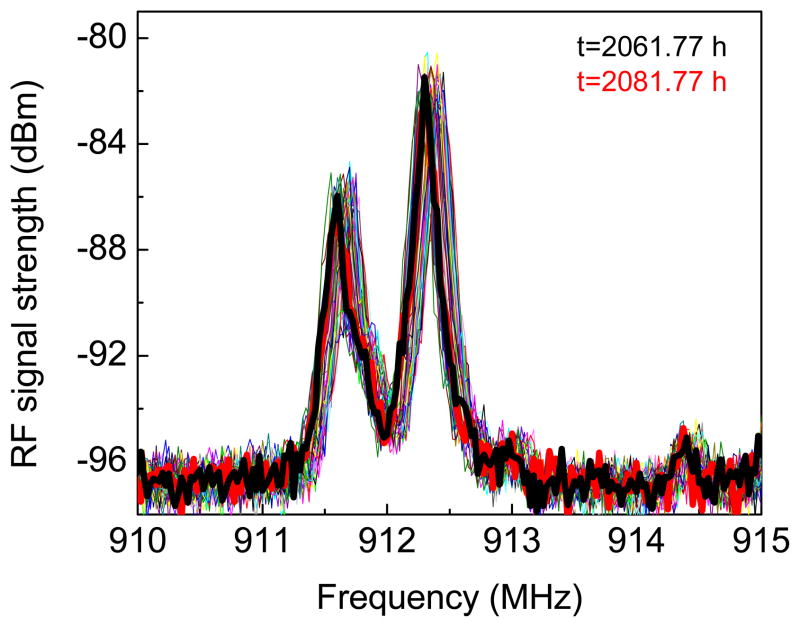
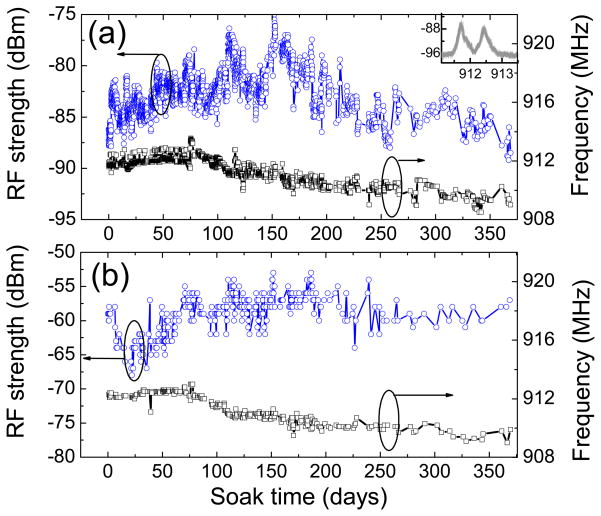
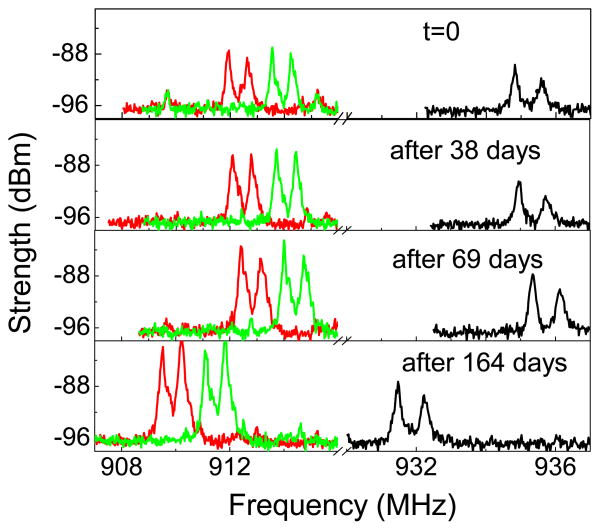
The USEA/INI-R5 device, while in PBS, was powered and configured wirelessly through 2.765 MHz inductive link, and the transmitted frequency shift keying (FSK) modulated RF signal (900–928 MHz Industrial, Scientific, Medical-ISM band) was recorded wirelessly as a function of soak time. The chip in the INI device contains an 8-bit register that can take a value between ‘0’ and ‘255’ and sets the command transmitter frequency in the range of 902-928 MHz (ISM band) [5]. In order to investigate the long term RF functional stability of the INI device in PBS, the FSK modulated RF signal for telemetry frequency ‘0’ that corresponds to 911.9 MHz, as measured using the monopole antenna connected to the spectrum analyzer was monitored as a function of soak time. Fig. 3 shows the RF spectra transmitted wirelessly from the fully integrated USEA/INI-R5 neural interface immersed in PBS for different time periods. The peak RF signal strength and the respective frequency as a function of soak time extracted from such spectra are shown in Fig. 4(a). The FSK modulated RF signal analyzed on custom developed wireless receiver board is shown in Fig. 4(b). It can be observed that the values of signal strength and the frequency measured by wireless receiver board are different than the values measured on spectrum analyzer. The discrepancies between the signal strengths measured by receiver board and the spectrum analyzer can be expected because of two different antenna-receiver systems. Additionally, it appears that there is an oscillating noise pattern in the RF signal strength. This oscillating noise pattern in the signal strength could possibly due to the variations in the temperature as the data was recorded at room temperature. However, the important information to be noted here is that the INI device is fully functional i.e. powers up as well as responds to the telemetry frequency commands while being immersed in PBS. It can be seen from Fig. 4(a) and 4(b) that the RF functionality of the fully integrated INI device is maintained in PBS even after 368 days. However, it also appears that there is a decrease in the frequency of the RF signal over soak days. In order to reveal this, FSK modulated wireless RF spectra from the INI device in PBS upon power up, and command telemetry frequency ‘0’ and ‘4’ are depicted in Fig. 5 for different soak days. It can be seen clearly that after 164 days in PBS, there is a decrease in the RF frequency for both the power-up and the command signals. The variation in the RF signal strength and the transmission frequency over 12 months lies within 15% and 0.4% of the initial values, respectively.
Fig. 3.
RF signature from a fully integrated USEA/INI-R5 device in PBS solution at different soak times demonstrating in-vitro functional stability.
Fig. 4.
Transmitted wireless RF signal monitored as a function of soak time in PBS (antenna was at 8 cm from the device). (a) Peak RF signal strengths and the respective frequencies as extracted from the spectra measured using the spectrum analyzer (inset shows few consecutive RF spectra at 20 min intervals). (b) RF signal strengths and the respective frequencies as monitored on the custom-developed wireless receiver board.
Fig. 5.
FSK modulated RF spectra transmitted wirelessly from INI in PBS at t=0, and after t=38 days, t=69 days, and t=164 days upon power up (black curve), and command telemetry frequency ‘0’ (red curve) and telemetry frequency ‘4’ (green curve).
Further, the responsiveness of the INI device to the commands for changing the telemetry frequency checked at t=0, and after t=150 days, t=211 days, t=276 days, and t=368 days corresponding to the telemetry frequency ‘0’ and ‘4’ at t=0 using the wireless receiver board are compared in Table 1. Interestingly, the INI device continued to receive power and commands, and transmitted the RF signature even after 368 days of soaking in PBS at RT. This long term full functionality in the wireless RF signal clearly demonstrates that the electronic circuitry has remained unaffected in the PBS solution even after more than 12 months of soaking. Therefore, the Parylene-C coating is providing an effective protection of the INI from ingress of the moisture and other ionic species that are present in the PBS.
Table 1.
FSK modulated wireless RF signal characteristics for telemetry frequency ‘0’ and ‘4’ measured through the PBS using custom made wireless receiver, when antenna was placed at 8 cm from the device.
| Soak time (days) | Telemetry frequency | RF signal frequency (MHz) | RF signal strength (dBm) |
|---|---|---|---|
| t=0 | ‘0’ | 911.2 | −57 |
| ‘4’ | 912.5 | −59 | |
| t=150 | ‘0’ | 910.6 | −57 |
| ‘4’ | 912.5 | −57 | |
| t=211 | ‘0’ | 910.1 | −55 |
| ‘4’ | 911.9 | −60 | |
| t=276 | ‘0’ | 910.1 | −59 |
| ‘4’ | 911.6 | −60 | |
| t=368 | ‘0’ | 909.9 | −57 |
| ‘4’ | 911.5 | −62 |
3.2. In-vitro recording longevity in agar
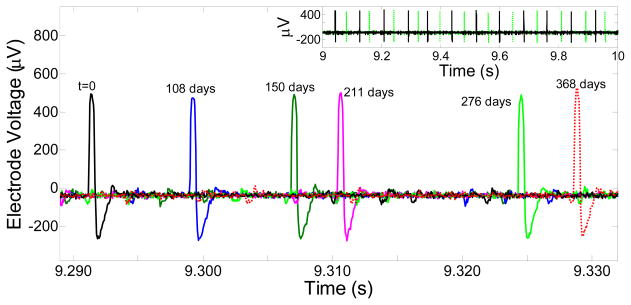
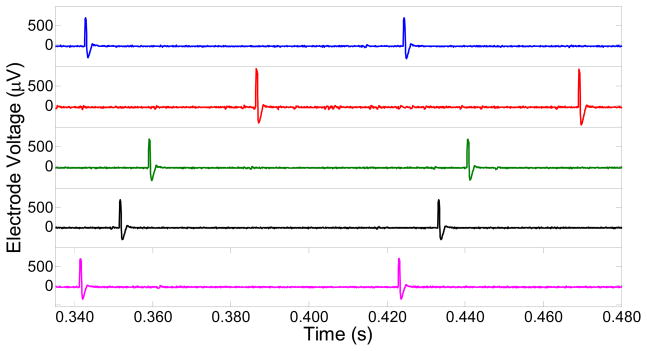
In order to test the long term recording ability of the electrodes in the INI device that was soak tested, in-vitro wireless recording was performed in agarose gel in a petri dish for few channels at time t=0, 108, 150, 211, 276, and 368 days of PBS soaking. The agarose impedance measured between the testing probe and the reference Pt electrode separated at 8 cm distance was 5 kΩ at 1 kHz frequency (10 nA current signal). The impedance of the agarose gel for the given distance was kept similar every time while performing the in-vitro testing after different soak periods of time. The integrated neural interface was taken out from the PBS solution, and was inserted into the agarose gel in the petri dish. The artificial neural signals were introduced into the agarose gel from a SD-9 Grass stimulator using the Pt electrodes. The input signals from the SD-9 consisted of a biphasic cyclic voltage pulse (2 Vpp) with a frequency of 12 pulses/s (0.4 ms duration). During artificial neural signal recordings, the Pt electrodes for injecting the signal into agarose were also separated by 8 cm for maintaining the similar impedance and consistence voltage drop (for μm lengths, the voltage will be dropped to a few hundred mV). Fig. 6 shows the real time wirelessly recorded artificial action potentials from one channel at t=0 and after soaking the INI device in PBS for t=108, t=150, t=211, t=276, t=276, and t=368 days. The inset compares the recording of the artificial action potentials (for soak time t=0 days and t=276 days) at different time scale. The peak-to-peak voltage (Vpp) of the artificial action potential waveform that was wirelessly recorded from the INI at t=0, soak time t=108 days, soak time t=150 days, soak time t=211 days, soak time t=276 days, and soak time t=368 days is 745±32 μV, 723±13 μV, 691±97 μV, 783±50 μV, 796±25 μV, and 754 ±12 μV respectively (these values are average of Vpp that were recorded for over 60 s in real time). The respective noise levels were 99±15 μV, 101±26 μV, 98±2 μV, 92±8 μV, 111±4 μV, and 90±14 μV. The variance in the recorded signal/noise amplitude lies within 14% and indicates that the long term soaking did not have much influence on the tip deinsulated areas of the electrodes, and the ability to record the artificial neural signals. These in-vitro recordings show that for a given channel, the ability to track the artificial action potential is maintained even after 368 days of PBS soak. Similar long term recording ability was observed for few other channels/electrodes, and is shown in Fig. 7 for five different electrodes that were picked randomly, and seemed to work during the in-vitro recording in agar after 368 days of PBS soak. Furthermore, some of the integrated and encapsulated INI devices implanted in animals in acute experiments could survive the impact of the pneumatic inserter as well as immersion in the real biological environment for several hours. The devices successfully recorded and wirelessly transmitted digitized electrical activity in live anesthetized animals. Although, a statistical description based on large number of devices would provide further insight into the reliability of the packaging, the in-vitro stability together with the acute animal experiments demonstration constitute major milestone, the encapsulation reliability, functional stability, and the recording longevity in a fully integrated wireless neural interface.
Fig. 6.
In-vitro wirelessly recorded artificial action potentials that were injected from Grass SD-9 stimulator into the agarose dish. Recording is shown from one channel at time t = 0, and after soaking the INI device in PBS for 108, 150, 211, 276, and 368 days to demonstrate the recording longevity of particular channel in UTAH/INI-R5 device after PBS soaking. Inset shows the recording of the artificial action potentials at t = 0, and after soaking in PBS for 276 days at different time scales.
Fig. 7.
In-vitro wireless recording ability for five different electrodes in the INI after 368 days of PBS soak. The artificial action potentials were injected from Grass SD-9 stimulator into the agarose dish.
4. Summary
In summary, the encapsulation and packaging reliability of Parylene-C in fully integrated wireless neural interfaces is evaluated by monitoring the long term in-vitro functional stability and recording longevity in USEA/INI-R5 system. The device was fully functional (wireless RF power/command and data transmission) even after 368 days soaking without significant change in the transmitted RF signal strength and the transmission frequency. Additionally, the in-vitro recording of the artificial neural signals performed in agarose shows the ability of the channels in the INI device to track artificial action potentials with signal/noise amplitude within 14% variation for more than 368 days of PBS soaking. These in-vitro results provide a measure of the encapsulation reliability, functional stability, and the recording longevity in a fully integrated wireless neural interface. The evaluation of the neural interface in PBS over 12 months demonstrates its stability in a real physiological environment, and the usefulness of wireless neural interface for future chronic implants.
Acknowledgments
This work was supported in part by NIH/NINDS Contract 1R01NS064318-01A1 and by DARPA under contract N66001-06-C-8005 through the John’s Hopkins Applied Physics Laboratory award No. 908164. The authors gratefully acknowledge Fraunhofer IZM for developing and carrying out the flip chip bonding.
Biographies
Asha Sharma received her Ph.D. from Indian Institute of Technology Kanpur, India in 2006. She was a Postdoctoral Fellow at Georgia Institute of Technology, Atlanta, until 2008. Following that, she worked at the University of Utah, Salt Lake City, from 2009–2011 as a Postdoctoral Researcher in the Microsystems Laboratory, focused her research on fully integrated wireless neural interfaces. She is currently a Research Scientist-II at Georgia Institute of Technology, Atlanta, in the School of ECE. Her primary research interests are in the application of organic electronics and photonics to the biology and medicine for the next generation of biocompatible, low cost, and clean-environment healthcare technologies.
Loren Rieth received his B.S. degree in Materials Science from The Johns-Hopkins University, Baltimore, MD, in 1994. He received his Ph.D. in Materials Science and Engineering from the University of Florida, Gainesville, FL, in 2001. From 2001 to 2003, he was a Postdoctoral Research Associate at the University of Utah, Salt Lake City, UT, and continued on at the University of Utah as a Research Assistant Professor in Materials Science (2003–2005), and Electrical and Computer Engineering (2004–present). His research is focused on deposition and characterization of thin film materials for sensors (chemical, physical, and biological), MEMS, BioMEMS, and energy production.
Prashant Tathireddy received his bachelor’s degree in chemical technology from the Osmania University, Hyderabad, India in 1997. In 2000 he joined the department of chemical engineering at the University of Utah, Salt Lake City, UT. There he received his PhD degree in 2005. Currently, he is a Research Assistant Professor in the department of electrical and computer engineering, University of Utah. His research interests are microfluidics, microsensors and also include development and fabrication of implantable microdevices.
Reid R. Harrison received the B.S. degree in electrical engineering from the University of Florida, Gainesville, in 1994 and the Ph.D. degree from the California Institute of Technology, Pasadena, in 2000. He joined the University of Utah, Salt Lake City, in 2000, where was an Associate Professor of Electrical and Computer Engineering and an Adjunct Associate Professor of Bioengineering through 2010. In 2003, he founded Intan Technologies, LLC and joined them full time in 2010. His research interests include lowpower analog and mixed-signal complementary metal–oxide semiconductor circuit design, integrated electronics for neural interfaces, and other biomedical devices and hardware for biologically inspired computational systems.
Hermann Oppermann received the Dipl.Ing. degree in materials science and technologies from the Technical University of Clausthal, Germany, in 1986, and the Dr.Ing. degree in material physics from the Technical University of Berlin, Germany, in 1992. He joined the Microperipheric Center, Technical University of Berlin, working in the area of AuSn solder development, flip-chip and chip-scale packages. Since 1998, he has been with the Fraunhofer Institut für Zuverlässigkeit und Mikrointegration, Berlin. His research interests include interconnect metallurgy and the packaging of MEMs, power- and high-temperature electronics, optoelectronics, and solid-state lighting.
Matthias Klein received the MS degree in physics from the University of Kiel, Germany, in 1997. He joined the Center of Microperipheric Technologies at the Technical University of Berlin in 1997, where heworked as a Research Scientist. Since 2002 he is working at the Fraunhofer IZM, Berlin. His main research is the development of chip interconnection and packaging techniques. He is especially engaged in thermo-compression and thermo-sonic flip chip bonding as well as mechanical bumping technologies.
Michael Töpper received his MS degree in chemistry from University of Karlsruhe and PhD in material science fromTechnical University of Berlin, Germany. Since 1994 he is with the Packaging Research Team at the Technical University Berlin and Fraunhofer IZM. In 1999 he became head of a research group at Fraunhofer IZM. Dr. Topper has authored and co-authored more than 130 papers and book chapters related to electronic packaging. In 2000 he was co-founder of an International Consortium for Advanced Packaging (SECAP). In 2003 he was honoured with the Fraunhofer IZM science award for his research work on BCB applications. He is member of IEEE-CPMT, IMAPS and MRS and currently the chair of the IEEE Technical CommitteeWafer Level Packaging.
Erik Jung received the diploma degree in physics and physical chemistry from the University of Kaiserslautern, Germany, in 1994. He then joined the Fraunhofer IZM, Berlin, Germany, working in the area of chip interconnect technology. Since 1997, he has been heading the group Flip Chip and CSP Assembly at the Fraunhofer IZM as a R&D technical manager. Since late 2001, he has been responsible for the IZM’s MEMS Packaging Research Program, one of six research foci of the institute.
Richard A. Normann is a Distinguished Professor of Bioengineering and Ophthalmology at the University of Utah, Salt Lake City, where he conducts research on sensory encoding and information processing by neural ensembles in the vertebrate central and peripheral nervous systems. He is also developing high-electrode-count microelectrode arrays that can be used for basic applied research in neuroprosthetics.
Gregory A. Clark received the B.A. degree in psychology from Brown University, Providence, RI, and the Ph.D. degree in biology from the University of California, Irvine. He is presently Associate Professor and Chair of the Neural Interface Track in the Department of Bioengineering at the University of Utah, Salt Lake City. His research focuses in the use of neuroprostheses to restore sensory and motor function after spinal cord injury or limb loss, primarily using the peripheral nervous system of animal model systems.
Florian Solzbacher received his M.Sc. in electrical engineering from the Technical University Berlin in 1997 and his Ph.D. from the Technical University Ilmenau in 2003. He is Director of the Microsystems Laboratory at the University of Utah and a faculty member in the Departments of Electrical and Computer Engineering, Materials Science and Bioengineering; and he is responsible for the Utah branch office of the Fraunhofer IZM, Germany. Dr. Solzbacher is co-founder of First Sensor Technology GmbH, an established supplier to the automotive and process control industry in the USA, Europe and Asia. He is Chairman of the German Association for Sensor Technology AMA. He is author of over 70 scientific and engineering publications and book chapters on MEMS devices, technologies and markets for Harsh Environments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donoghue JP. Connecting cortex to machines: recent advances in brain interfaces. Nature Neuroscience. 2002;5:1085–1088. doi: 10.1038/nn947. [DOI] [PubMed] [Google Scholar]
- 2.Normann RA. Technology Insight: future neuroprosthetic therapies for disorders of the nervous system. Nature Clinical Practice Neurology. 2007;3:444–452. doi: 10.1038/ncpneuro0556. [DOI] [PubMed] [Google Scholar]
- 3.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 4.Scott SH. Neuroscience - Converting thoughts into action. Nature. 2006;442:141–142. doi: 10.1038/442141a. [DOI] [PubMed] [Google Scholar]
- 5.Harrison RR, Kier RJ, Chestek CA, Gilja V, Nuyujukian P, Ryu S, Greger B, Solzbacher F, Shenoy KV. Wireless neural recording with single low-power integrated circuit. IEEE Trans Neural Syst Rehabil Eng. 2009;17:322–329. doi: 10.1109/TNSRE.2009.2023298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison RR, Kier RJ, Kim S, Rieth L, Warren DJ, Ledbetter NM, Clark GA, Solzbacher F, Chestek CA, Gilja V, Nuyujukian P, Ryu SI, Shenoy KV. BioCAS 2008. IEEE; Piscataway, NJ, USA: 2008. A wireless neural interface for chronic recording; pp. 125–128. [Google Scholar]
- 7.Chestek CA, Gilja V, Nuyujukian P, Kier RJ, Solzbacher F, Ryu SI, Harrison RR, Shenoy KV. HermesC: low-power wireless neural recording system for freely moving primates. IEEE Trans Neural Syst Rehabil Eng. 2009;17:330–338. doi: 10.1109/TNSRE.2009.2023293. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A, Rieth L, Tathireddy P, Harrison R, Solzbacher F. Long term in-vitro stability of fully integrated wireless neural interfaces based on Utah Slant Electrode Array. Appl Phys Lett. 2010 doi: 10.1063/1.3318251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lago N, Ceballos D, Rodriguez FJ, Stieglitz T, Navarro X. Long term assessment of axonal regeneration through polyimide regenerative electrodes to interface the peripheral nerve. Biomaterials. 2005;26:2021–2031. doi: 10.1016/j.biomaterials.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Hsu JM, Tathireddy P, Rieth L, Norman AR. Characterization of a-SiCx : H thin films as an encapsulation material for integrated silicon based neural interface devices. Thin Solid Films. 2007;516:34–41. doi: 10.1016/j.tsf.2007.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jui-Mei H, Rieth L, Normann RA, Tathireddy P, Solzbacher F. Encapsulation of an integrated neural interface device with parylene C. IEEE Transactions on Biomedical Engineering. 2009;56:23–29. doi: 10.1109/TBME.2008.2002155. [DOI] [PubMed] [Google Scholar]
- 12.Roy RK, Lee KR. Biomedical applications of diamond-like carbon coatings: A review. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2007;83B:72–84. doi: 10.1002/jbm.b.30768. [DOI] [PubMed] [Google Scholar]
- 13.Cogan SF, Edell DJ, Guzelian AA, Liu YP, Edell R. Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. Journal of Biomedical Materials Research - Part A. 2003;67:856–867. doi: 10.1002/jbm.a.10152. [DOI] [PubMed] [Google Scholar]
- 14.Wu JL, Pike RT, Wong CP, Kim NP, Tanielian MH. Evaluation and characterization of reliable non-hermetic conformal coatings for microelectromechanical system (MEMS) device encapsulation. IEEE Transactions on Advanced Packaging. 2000;23:721–728. [Google Scholar]
- 15.Warren DJ, Ledbetter NM, Kier RJ, Sharma A, Rieth LW, Solzbacher F, Harrison RR, Clark GA. Neuroscience. 2009 [Google Scholar]
- 16.Warren DJ, Ledbetter NM, Kier RJ, Sharma A, Rieth LW, Normann RA, Solzbacher F, Harrison RR, Clark GA. Wireless Recordings from Nerve and Brain Obtained with Fully Integrated 100-channel Utah (Slanted) Electrode Arrays. Neural Interfaces Conference; 2010. [Google Scholar]