Abstract
Aim
Protection achieved by ischemic preconditioning is dependent on A2B adenosine receptors (A2BAR) in rabbit and mouse hearts and, predictably, an A2BAR agonist protects them. But it is controversial whether cardiomyocytes themselves actually express A2BAR. The present study tested whether A2BAR could be demonstrated on rat cardiomyocytes.
Methods and Results
Isolated rat hearts experienced 30 min of ischemia and 120 min of reperfusion. The highly selective, cell-permeant A2BAR agonist BAY60-6583 (500 nM) infused at reperfusion reduced infarct size from 40.4±2.0 % of the risk zone in control hearts to 19.9±2.8 % indicating that A2BAR are protective in rat heart as well. Furthermore, BAY60-6583 reduced calcium-induced mitochondrial permeability transition in isolated rat cardiomyocytes. A2BAR protein could be demonstrated in isolated cardiomyocytes by western blotting. In addition, message for A2BAR was found in individual cardiomyocytes using quantitative RT-PCR. Surprisingly, immunofluorescence microscopy did not show A2BAR on the cardiomyocyte's sarcolemma but rather at intracellular sites. Co-staining with MitoTracker Red in isolated cardiomyocytes revealed A2BAR are localized to mitochondria. Western blot analysis of a mitochondrial fraction from either rat heart biopsies or isolated cardiomyocytes revealed a strong A2BAR band.
Conclusions
Thus the present study demonstrates that activation of A2BAR is strongly cardioprotective in rat heart and suppresses transition pores in isolated cardiomyocytes, and A2BAR are expressed in individual cardiomyocytes. However, surprisingly, A2BAR are present in or near mitochondria rather than on the sarcolemma as are other adenosine receptors. Because A2BAR signalling is thought to result in inhibition of mitochondrial transition pores, this convenient location may be important.
Keywords: adenosine A2B receptors, cardioprotection, mitochondria
Introduction
Ischemic preconditioning (IPC) triggers powerful endogenous signalling that ultimately makes the heart resistant to infarction from ischemia. A key step in preconditioning's signal transduction pathway is activation of adenosine A2B receptors (A2BAR) early in reperfusion [8,10,25,43]. Ischemic postconditioning, another endogenous cardioprotective phenomenon, also depends on activation of A2BAR [33]. Analogously the highly selective A2B agonist BAY60-6583 given at reperfusion reduces infarct size in rabbit hearts following prolonged myocardial ischemia [21].
A2BAR are G protein-coupled receptors (GPCR) that communicate through both Gαs and Gαq, which in turn lead to increased cytosolic cAMP, inositol trisphosphate and Ca2+ [23]. There is agreement that coronary endothelial cells contain A2BAR [14,39], but there is discrepant information about the expression of A2B on cardiomyocytes, the cells that IPC must ultimately protect. To address this question, Yang et al. [47] replaced exon 1 of the A2BAR gene in transgenic mice with a reporter construct containing β-Gal. They did not detect β-Gal expression in heart muscle indicating that expression of the receptors was either absent or too low to detect. On the other hand, Morrison and colleagues saw a positive inotropic response to the potent, albeit nonselective, A2BAR agonist 5’- (N-ethylcarboxamido) adenosine (NECA) in A2aAR knockout mice suggesting that some A2BAR must reside on or within cardiomyocytes [30]. Furthermore, Liang and Haltiwanger [22] studied contractility and cAMP production of cultured chick embryonic ventricular cells and again found responses to A2BAR agonists. Finally, a recent report by Xin at al. [45] contributed to the controversy; they could find no functional evidence for sarcolemmal A2BAR in isolated rabbit cardiomyocytes.
In the present study we address the question whether A2BAR are expressed in rat ventricular cardiomyocytes. First we tested whether a selective A2BAR agonist is as protective in intact rat hearts and isolated cardiomyocytes as it is in rabbits. Then, using antibodies to A2BAR we performed immunofluorescence, FACS analysis, and western blotting to try to localize the receptors.
Methods
The present study was conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The experimental protocols were approved by the local authorities of Mecklenburg-Vorpommern (Germany) and the University of North Carolina (USA).
Isolated rat hearts and infarct size measurement
Adult Wistar rats of either sex were used. Heart isolation and determination of infarct size were performed as described previously in detail [26]. Control hearts (n=6) were exposed to 30 min of regional ischemia by occluding a snared coronary artery and then 120 min of reperfusion. The second group (n=10) was treated with 500 nM BAY60-6583 for 65 min starting 5 min before reperfusion. The third group (n=6) received 500 nM BAY60-6583 in the presence of the highly selective A2BAR antagonist MRS1754 (20 nM). At the end of the experiment risk zone was determined with green fluorescent microspheres injected into the aorta perfusate after religation of the snared coronary artery and infarct size was determined with triphenyltetrazolium chloride staining of the sliced left ventricle. Infarct size is presented as a percentage of the risk zone. BAY60-6583 and MRS1754 were dissolved in dimethyl sulfoxide (DMSO) before being further diluted in perfusion buffer, resulting in DMSO concentrations lower than 0.01 %. The ability of BAY60-6583 to enter cardiomyocytes has been demonstrated (supplemental Fig. S1).
Isolated rat cardiomyocytes and assessment of mitochondrial membrane potential ΔΨm
Hearts were excised and retrogradely perfused with Krebs-Henseleit-buffer containing 25 μM calcium and collagenase type II at 37°C as previously described [12]. Viable ventricular myocytes were separated by centrifugation in a 4% bovine serum albumin gradient, seeded on laminin coated four-well plates and cultured in medium M199 containing 0.2% BSA, 5 mM creatine, 2 mM L-carnithine, 5 mM taurine and penicillin and streptomycin (each 100 U/ml). Four hours after isolation cells were washed once and loaded for 20 min with 100 nM tetramethylrhodamine ethyl ester (TMRE) (Molecular Probes Inc., Eugene, OR, USA) which causes cells to fluoresce in proportion to their mitochondrial membrane potential (ΔΨm). A reduction in TMRE fluorescence served as an indicator of the loss of ΔΨm, which would occur with mitochondrial permeability transition pore (mPTP) formation. If required, BAY 60-6583 (100 nM) and MRS1754 (100 nM) were added 5 min before TMRE loading to mimic a preconditioning protocol. Afterwards cells were washed with TMRE-free medium and then incubated with the selective calcium ionophore calcimycin (1 μM), which is known to induce mPTP formation similar to that seen at reperfusion following myocardial ischemia [32]. Fluorescence intensity was measured at 582 nm (FL-2 channel) using a Becton Dickinson (Heidelberg, Germany) FACSCalibur and CellQuest software after 35 min of calcimycin exposure and expressed as the mean fluorescence for each measurement. Four groups of TMRE-loaded myocytes were studied: treatment with calcimycin alone or with the additional agents BAY 60-6583, MRS1754, or BAY 60-6583 plus MRS1754 (each n=9).
Isolated mitochondria from intact rat hearts and isolated cardiomyocytes
Cardiac mitochondria were isolated using standard centrifugation techniques. Briefly, hearts were minced in ice-cold solution A (250 mM sucrose, 10 mM Tris-HCl, 0.1 mM EGTA and 0.5% BSA, pH 7.4). Tissue pieces were then homogenized using a Potter S (Braun Biotech International, Melsungen, Germany) at 1000 rpm and subjected to stepwise centrifugation. The homogenate was centrifuged at 500 g and the supernatant at 9,000 g. The resulting pellet (crude mitochondrial fraction) was resuspended in ice-cold solution B (250 mM sucrose, 10 mM Tris-HCl, 0.1 mM EGTA, pH 7.4). To further purify mitochondria the resulting suspension was alternately spun at 9,000g and washed 5 times.
Mitochondria were harvested from isolated cardiomyocytes at the end of the cell isolation procedure by directly suspending the cells in solution A. The rest of the preparation was identical to that described above.
Isolation and purification of cardiac sarcolemma
To obtain purified sarcolemma two different protocols were used. First, hearts were homogenized as described above and the homogenate centrifuged at 9,000 g to remove intracellular organelles including mitochondria. The supernatant was centrifuged at 100,000 g and the pellet was resuspended in Tris-HCl (5 mM, pH 7.4) and further used for Western blot analysis. The second protocol included an additional pellet purification step with a sucrose gradient (38 % sucrose in 5 mM HEPES, pH 7.4). The pellet was centrifuged at 280,000 g and the interphase containing purified sarcolemma was washed in Tris-sucrose (10 mM and 250 mM, pH 7.4) prior to Western blot analysis.
A2BAR expression by quantitative RT-PCR
Isolated rat cardiomyocytes were plated in a glass chamber and observed at 30 X using an Olympus BX-51 microscope. Under micromanipulator control cardiomyocytes (5-10 cells) were harvested by drawing them into micropipettes pulled from 1.5 mm diameter borosilicate glass (Drummond Scientific Company, Broomall, PA). Care was taken to exclude all other cell types. After collection, cells were immediately transferred to a 500 μL microcentrifuge tube containing protease K and RNAase inhibitors by breaking the pipette tip against the bottom of the tube and expelling the contents of the pipette. Total mRNA was isolated using Trizol (Invitrogen) reagent. RNA was converted to cDNA using reverse transcriptase. GAPDH and A2BAR mRNA sequences were identified using Rat Genome Resources of NCBI (http://www.ncbi.nlm.nih.gov). Primer pairs for GAPDH (forward: CAGGTTGTCTCCTGCGACTT, reverse: ATGTAGGCCATGAGGTCCAC) and A2BAR (forward: CCAAGGACAAGCCCAAATG, reverse: CCGTCTGGCAGAGAACGTAT) were designed using Primer3 program. Gene expressions were assessed by quantitative RT-PCR using QuantiTect SYBR Green PCR Kit (Qiagen) and ABI 7900HT Fast Real-Time PCR System. Relative quantities of message were calculated from cycle threshold (Ct) measurements. Ct values were standardized to GAPDH for each sample and then subjected to standard calculations. Dissociation curves were also initially evaluated for each qPCR sample to qualitatively confirm assay integrity. The amplified product was analyzed on an agarose gel to confirm its expected molecular size. Additionally, two target bands were selected from two samples and purified with Wizard SV gel and PCR clean-up system (Promega, WI). The PCR products (10 ng) and the primers (10 pmol) were used for RNA sequencing at the University of North Carolina Genome Analysis Facility. The raw data were processed and analyzed with Sequencher (Version 4.8.) and ChromasPro.
Immunofluorescence
Either cryosections of the heart, isolated cardiomyocytes, or mitochondria were studied. Cryosections were prepared after rinsing the heart of blood with ice cold saline (0.9%) delivered retrogradely through the aorta. After cutting the heart from base to apex into parallel slices which were snap-frozen in liquid nitrogen, 5 μm-thick cryosections were cut from the snap-frozen slices (Leica CM 1900, Leica Microsystems, Nussloch, Germany). Heart sections were fixed on SuperFrost Plus microscope slides (R. Langenbrinck, Teningen, Germany) in acetone and permeabilized in 0.5% Triton X-100 in PBS. After a blocking step in 5% FCS in PBS for one hour, staining was carried out overnight at 4°C using either the primary A2BAR antibody or affinity-purified IgG in a corresponding concentration. A secondary antibody conjugated to Alexa Fluor 488 was used. Staining of the nuclei was performed by using propidium iodide (1 μg/ml, Sigma, St. Louis, MO). Adult ventricular cardiomyocytes were isolated as described above. Cells were plated on laminin-coated coverslips and incubated in modified CCT-medium 199 as previously described [34]. After 4 h of incubation cardiomyocytes were stained with a mitochondria-specific dye (50 nM MitoTracker Red CMXRos, Invitrogen, Karlsruhe, Germany) and fixed in 2% paraformaldehyde. Finally, the protocol described above for cryosections was used. MitoTracker-loaded cells were first exposed to the A2BAR primary antibody. Native cells were incubated with either a combination of primary antibodies against A2BAR and α-actinin or against A2BAR and Na+/K+-ATPase and then exposed to secondary antibodies conjugated to Alexa Fluor 488 and Alexa Fluor 568 (Invitrogen, Karlsruhe, Germany). Staining of the nuclei was performed by 4'-6-diamidino-2-phenylindole (DAPI) incubation (0.2 μg/ml, Roth, Karlsruhe, Germany). Fluorescent micrographs were taken with a confocal laser scanning microscope (Leica SP5, Wetzlar, Germany). Representative pictures from at least four independent heart or cell preparations are shown. Additionally human embryonic kidney (HEK) 293 cells overexpressing human A2BAR were treated with the A2B antibody and then a fluorochrome-linked secondary antibody. These experiments are more fully described in the online supplement.
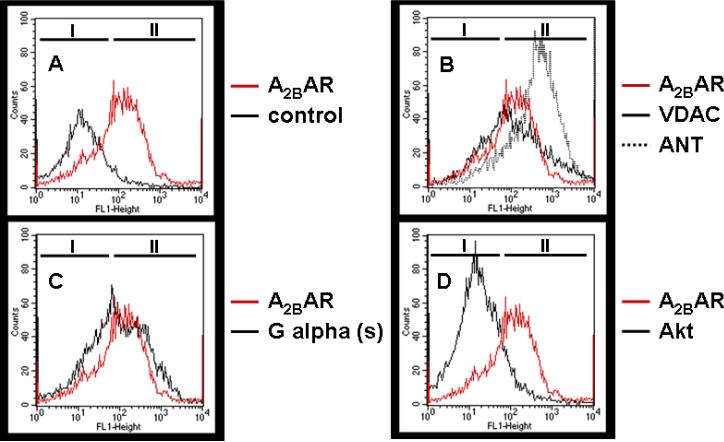
Mitochondria were isolated as described above, blocked in Solution A containing BSA, and incubated with primary antibodies (0.1 μg/200 μg mitochondria) against A2BAR, Gαs, and markers of mitochondria [voltage-dependent anion channel (VDAC), an outer membrane mitochondrial protein, and adenine nucleotide transporter (ANT), an inner membrane mitochondrial protein] and cytosol (Akt). In control experiments the primary antibody was omitted. After washing in Solution A probes were incubated with secondary antibody conjugated to Alexa Fluor 488, washed again, and analysed using the FL-1 flow cytometer channel (Becton Dickinson FACSCalibur, Heidelberg, Germany) and CellQuest software. Data were gated to exclude debris, and fluorescence of 20,000 particles was computed. Representative FL-1 histograms from three independent mitochondria isolations are presented.
Western blot analysis
Transmural biopsies of the left ventricle were snap-frozen in liquid nitrogen and tissue lysate was prepared as described previously [12]. Accordingly, cell lysates from isolated cardiomyocytes were prepared after trypsinization and sonication. Mitochondrial and sarcolemmal samples were isolated as described above. In 50 μg samples of extracts proteins were separated by standard SDS-PAGE methods and immunoreactive bands were detected by enhanced chemiluminescence with LumiGLO (Cell Signaling Technology, Beverly, MA). Representative western blots from at least three independent preparations are presented. In addition, in preliminary experiments western blotting in HEK293 cells overexpressing human A2BAR and in A2BAR knockout mice were done to document the selectivity of the anti-A2B antibody and are more fully described in the online supplement.
Preparation of mitoplasts with digitonin digestion
Digitonin (Sigma, Saint Louis, MO) was prepared as described elsewhere [41] with slight modifications. Briefly, digitonin was recrystalized with hot ethanol and dried at 100°C to produce a fine powder. A 2% stock solution was than made by adding an appropriate volume of solution B. Aliquots of mitochondrial suspensions (200 μg/100 μl solution B) were placed on ice and 10 μl digitonin were added every minute up to 10 times, resulting in final concentrations of 0, 0.75, 1.5, 2.25, 3.0 and 3.75 mg digitonin/mg mitochondrial protein. The suspensions were incubated on ice for 30 min with oxygen access and gentle stirring and were diluted with solution B. The diluted mitochondrial solutions were again fractionated by centrifugation as described above and prepared for western blotting with antibodies to A2BAR, ANT, and VDAC. Experiments were repeated twice and representative western blots are presented.
Antibodies
The following antibodies and concentrations were used: rabbit polyclonal anti-human A2BAR (1:50 for immunofluorescence microscopy and 1:500 for western blotting), goat polyclonal anti-human calnexin (1:500), goat polyclonal anti-human ANT (1:500), goat polyclonal VDAC-1 (1:500), rabbit polyclonal anti-rat Gαs (1:500), and donkey anti-goat horseradish peroxidase linked IgG (1:5000). All were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal anti-mouse Akt (1:1000), normal rabbit IgG, horse anti-mouse horseradish peroxidase linked IgG, and goat anti-rabbit horseradish peroxidase linked IgG (1:5000) were from Cell Signaling Technology (Beverly, MA), while Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 568-conjugated goat anti-mouse IgG (1:200 each) were from Invitrogen (Karlsruhe, Germany). Monoclonal mouse anti-rabbit Na+/K+-ATPase (1:250 for immunofluorescence and 1:2000 for western blotting) was from Millipore (Temicula, CA) and monoclonal mouse anti α-actinin (1:2000) from Sigma (St. Louis, MO).
Statistics
Data are presented as mean ± SEM. Differences in infarct sizes, risk zones and TMRE fluorescence amongst groups were compared by one-way ANOVA with Fisher LSD post hoc testing. P<0.05 was considered to be significant.
Results
Infarct size measurements after A2BAR activation
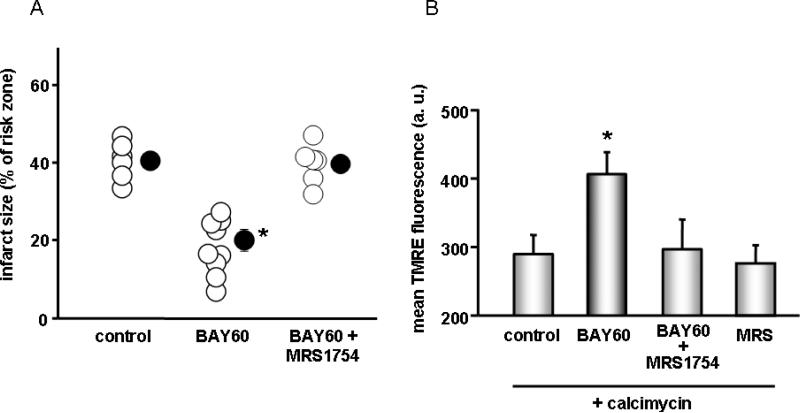
As shown in Fig. 1A treating rat hearts at reperfusion with the highly selective A2BAR agonist BAY60-6583 protected against ischemia/reperfusion injury. Protection was blocked by co-administration of the selective A2BAR antagonist MRS1754, thus, A2B agonists are also protective in the rat heart.
Figure 1.
A. Infarct size in isolated rat hearts expressed as a percentage of the risk zone. Open circles denote individual experiments, while filled-in circles are group means±SEM. *p<0.001 B. Assessment of mitochondrial membrane potential ΔΨm in isolated cardiomyocytes. Whereas control cells were treated with only calcimycin, other cells were also exposed to BAY 60-6583 alone or a combination of BAY 60-6583 and MRS1754. *p<0.05
Assessment of mitochondrial membrane potential ΔΨm in isolated rat cardiomyocytes
Isolated cardiomyocytes were stressed with the calcium ionophore calcimycin which causes mPTP formation related to calcium overload. Fig. 1B illustrates that treatment with BAY60-6583 preserved ΔΨm compared to calcimycin-treated control cells and protection was blocked by MRS1754. These results reveal an A2BAR-mediated response in individual cardiomyocytes.
A2BAR mRNA expression in individual rat cardiomyocytes
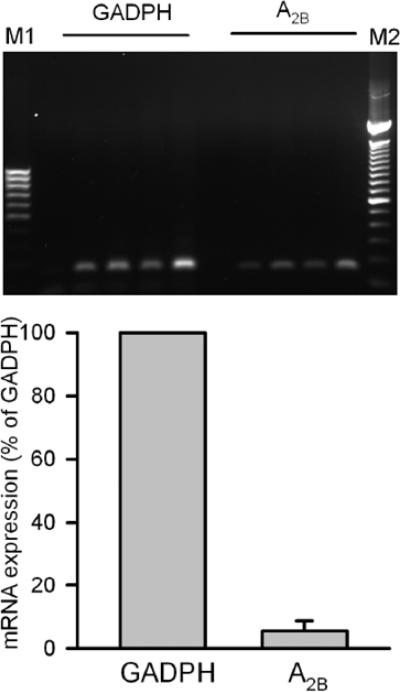
Single cardiac myocytes were selected under a microscope and placed in the PCR tube to insure that no other cell types were included. Quantitative RT-PCR analysis demonstrated A2BAR mRNA expression in rat cardiomyocytes (Fig. 2). A2BAR mRNA was expressed as a percentage of the housekeeping gene GAPDH, and easily detected in all of the tubes tested. The agarose gel revealed that the amplified product was the predicted size based on our primers. Finally, sequences from two of our samples were analysed. The sequencing results showed 100 % homology with the A2B sequence in the GeneBank [gi|8392863|ref|NM_017161.1 Rattus norvegicus adenosine A2B receptor (Adora2b) mRNA].
Figure 2.
A2BAR-mRNA expression in isolated rat cardiomyocytes. Quantitative RT-PCR showed A2BAR mRNA copy count was low but measurable as compared with GAPDH. The gel demonstrates that the amplified transcripts were of the size these primers were expected to produce.
Immunofluorescence
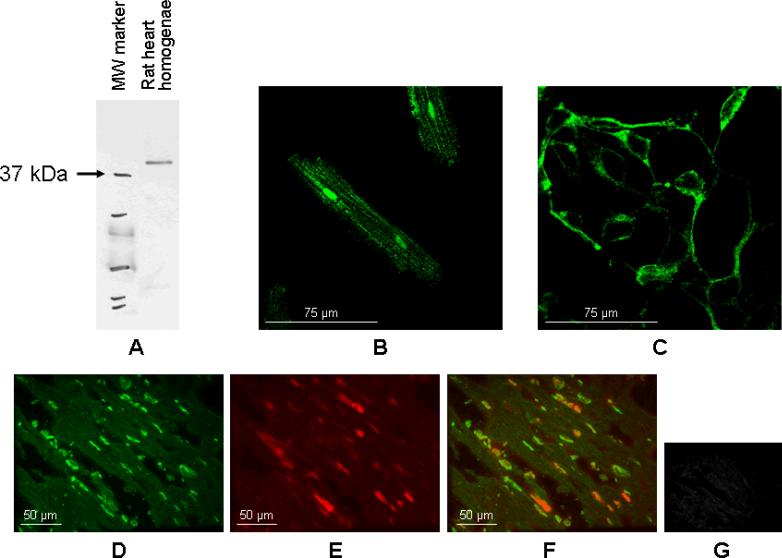
Intracellular distribution of A2BAR in rat heart and isolated rat cardiomyocytes was analyzed by immunofluorescence microscopy. A western blot of heart homogenate with the antibody used for immunohistochemistry reveals a single strong band making nonspecific staining unlikely (Fig. 3A). In response to the same antibody, HEK293 cells overexpressing human A2BAR showed a similar single band which was not present in normal HEK293 cells (supplemental Fig. S2). Furthermore, heart homogenate of A2BAR knockout mice no longer showed the clear single band seen in wildtype animal hearts (supplemental Fig. S2), suggesting that the antibody used indeed immunodetects A2BAR. Fig. 3B shows punctate staining of the myocyte's cytosol and its nuclei but no staining of the sarcolemma. In comparison, an immunofluorescent micrograph of HEK293 cells overexpressing A2BAR recorded at the same magnification showed receptor localization at the plasma membrane (Fig. 3C and supplemental Fig. S3). To make sure the absence of membrane staining was not an artefact of the collagenase digestion used to disperse the myocytes, we examined a cryosection of a rat ventricle. Figure 3D shows anti-A2BAR staining in green, and Fig. 3E shows propidium iodide staining the nuclei red. Fig. 3F is the merged image. In addition to the cytosolic and nuclear staining, string-like structures between the cells stained for A2BAR and were presumed to be capillaries. Again there was no staining of cell membranes. Incubation of cardiomyocytes with purified IgG showed no staining (Fig. 3G). Cryosections from wild-type mouse hearts showed a similar pattern which was fully absent in A2BAR knock-out animals (supplemental Fig. S4), further indicating specificity of the signal.
Figure 3.
A. Western blot of heart homogenate with anti-A2B antibody revealing a single strong band. In Fig. 3B-3G cardiomyocytes, HEK293 cells overexpressing A2BAR, and cryosections of rat heart slices were stained with A2BAR antibody coupled to a fluorochrome (green). B. Isolated rat cardiomyocytes with punctate intracellular and nuclear staining, but no staining of sarcolemma. C. Plasma membrane staining in HEK293 cells overexpressing A2BAR at identical magnification as in Fig. 3B. D. Slices of rat left ventricle showing diffuse intracellular and nuclear A2BAR staining. E. Propidium iodide staining of the nuclei. F. Merged image of Figs. 3D and 3E. G. Control with cardiomyocytes incubated with IgG.
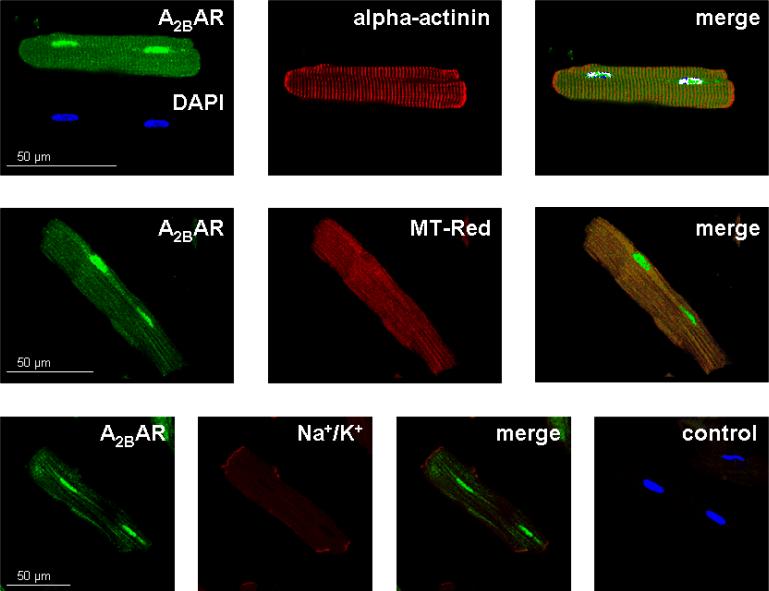
Staining of cardiomyocytes with anti-A2BAR antibody (green), DAPI (blue) to label nuclei, and anti-α-actinin antibody (red) to localize Z bands demonstrates integrity of isolated myocytes and shows clear localization of staining within the nuclei, but there is no association with the α-actinin-labelled Z bands (Fig. 4, upper panels). To test the hypothesis that A2BAR might be located on mitochondria, co-staining with anti-A2B antibody and MitoTracker Red was performed. As shown in the merged picture in Fig. 4, middle panel, there was good co-localization of A2BAR and MitoTracker suggesting the receptors are indeed on or very near the mitochondria. The lower panels of Fig. 4 showed only weak co-staining of A2BARs and the sarcolemmal Na+/K+-ATPase, additionally demonstrating the intracellular A2BAR localization. As expected, no immunoreactivity was detected in control experiments with the corresponding IgG, and only the nuclei stained with DAPI were visible (Fig. 4, lower panel, right).
Figure 4.
Isolated rat cardiomyocytes were stained with A2BAR antibody coupled to a fluorochrome (green). Additionally, nuclei, Z bands, mitochondria and Na+/K+-ATPase were detected using DAPI, α-actinin antibody, MitoTracker Red and Na+/K+-ATPase antibody, respectively. A2BAR green fluorescence was observed in nuclei but not in plasma membranes or Z bands (upper panel). Intracellular staining for A2BAR localized to mitochondria (middle panel). Intracellular A2BAR staining did not co-localize with sarcolemmal Na+/K+-ATPase (lower panel). DAPI-stained nuclei are visible in control cardiomyocytes incubated with IgG instead of specific primary antibody (lower panel, right image).
Mitochondrial localization of A2BARs was further demonstrated by FACS analysis of isolated mitochondria treated with antibodies recognizing A2BAR and marker proteins of different compartments. As shown in Fig. 5A, A2BAR antibody binding to isolated mitochondria was recognized by a shift of fluorescence intensity (population II) when compared to controls (no primary antibody, population I). To further demonstrate that the analyzed particles are most likely mitochondria, we treated them with antibodies against mitochondrial marker proteins (VDAC, ANT). As seen in Fig. 5B, there is staining of A2BAR, VDAC and ANT, and there is a rightward shift towards population II cells. The same was true for Gαs (Fig. 5C), but not for the cytosolic marker Akt (Fig. 5D).
Figure 5.
Isolated mitochondria were stained with different primary antibodies and corresponding secondary antibody coupled to green fluorescent 488 dye and subjected to FACS analysis. A. Anti-A2B antibody-stained mitochondria showed a marked rightward shift. Mitochondria stained with mitochondrial marker proteins VDAC and ANT (B) and Gαs (C) similarly showed rightward shifts, suggesting mitochondrial A2BAR and G protein co-localization. D. The purity of the mitochondrial preparation was demonstrated by using Akt as a cytosolic marker which had no effect.
Western blot analysis
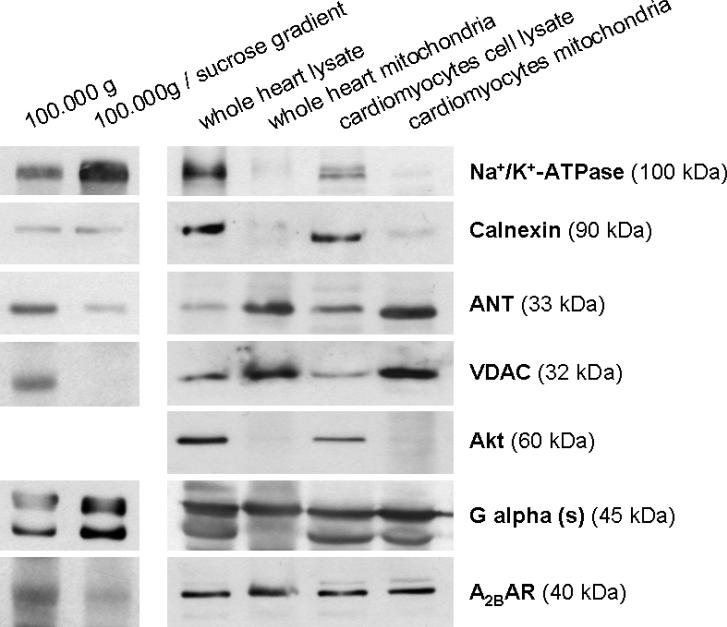
Fig. 6 shows that A2BAR immunoreactivity was detected in western blots of mitochondrial preparations from left ventricular biopsies as well as isolated rat cardiomyocytes. In all preparations the A2BAR band appeared at approximately 40 kDa, its expected size. In addition to the A2BAR band in the mitochondrial fractions, the mitochondrial membrane proteins ANT and VDAC were also evident and enriched compared to whole heart and cell lysates. Gαs is known to couple to A2BAR and was detected in all fractions. In contrast, neither mitochondrial fraction (whole heart or cardiomyocyte) demonstrated immunoreactivity to the plasma membrane marker Na+/K+-ATPase, the sarcoplasmic reticulum marker (calnexin), or the cytosolic marker (Akt). Mitochondria purification using an additional percoll gradient showed no decrease in A2BAR content (supplemental Fig. S5) Furthermore, two different sarcolemmal preparations were analysed, a 100,000 g pellet in which mitochondrial contamination should be reduced by an upstream centrifugation step, and a 100,000 g pellet further purified by a sucrose gradient. The high Na+/K+-ATPase immunoreactivity of this preparation purified by sucrose gradient demonstrates sarcolemmal enrichment (Fig. 6). While the normal 100,000 g pellet still contained mitochondrial (ANT, VDAC) and SR (calnexin) contamination, this was much less the case when the sucrose gradient step was added. Hence, the sucrose gradient was able to reduce mitochondrial contamination, while SR was still present. A clear reduction of A2BAR in the sucrose purified fraction supports our hypothesis of mitochondrial A2BAR localization. Not surprisingly, strong signals for Gαs were present in both preparations related to the abundant presence of GPCRs in the sarcolemma.
Figure 6.
Western blot of different rat heart preparations revealed mitochondrial localization of A2BAR protein. Purity of mitochondrial samples from hearts and isolated cardiomyocytes was demonstrated by co-staining of different marker proteins. The heart mitochondria samples were free of plasma membrane (Na+/K+-ATPase), sarcoplasmic reticulum (calnexin), and cytosol (Akt). Absence of A2BAR in sarcolemma was demonstrated by two different membrane preparations in which Na+/K+-ATPase was enriched. 100,000 g pellets still contained SR and mitochondria and, therefore, stained positive for A2BAR, while further purification with a sucrose gradient greatly reduced mitochondrial contamination together with A2BAR staining.
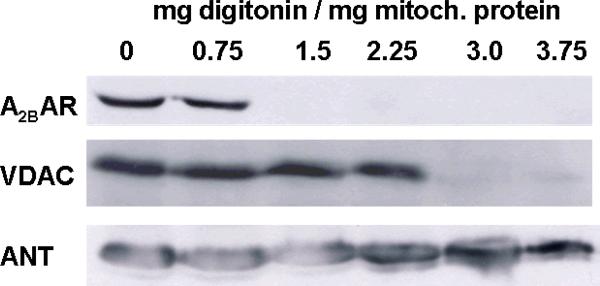
Mitochondria were incubated with increasing concentrations of digitonin to remove the outer mitochondrial membrane (Fig. 7). Higher digitonin concentrations dissolve the outer membrane of mitochondria as is confirmed by the loss of VDAC, a protein only present in the outer membrane. Conversely, the inner mitochondrial membrane marker ANT persists at all concentrations of digitonin. Increasing concentrations of digitonin also caused loss of A2BAR suggesting that they are also predominantly located in or near the outer mitochondrial membrane.
Figure 7.
Western blot analysis of mitochondrial preparations treated with increasing digitonin concentrations. The signal for the outer membrane marker voltage-dependent anion channel (VDAC) is lost at higher concentrations, while the inner membrane marker protein adenine nucleotide transporter (ANT) is still present even at the highest digitonin concentration. Since A2BAR and VDAC bands were similarly affected by digitonin, a location for the former in the outer mitochondrial membrane is assumed.
Discussion
Considerable evidence indicates activation of A2BAR is cardioprotective. A2BAR selective agonists limit infarct size in hearts subjected to ischemia/reperfusion [10,21,46]. In the present study BAY60-6583 also protected the isolated rat heart when infused at the time of reperfusion. Protection from postconditioning initiated with either ischemia or a PKC activator could be blocked by co-administration of MRS1754 [21,33] which is a highly selective inhibitor of A2BAR [19]. While these data imply that functional receptors must reside in the heart, there is debate as to whether cardiomyocytes actually express them in their plasma membranes. We were able to detect A2BAR mRNA transcripts using quantitative RT-PCR in individually selected cardiomyocytes uncontaminated with other cell types. Furthermore, western blot analysis of both heart biopsy and isolated cardiomyocyte lysate showed a strong A2BAR band.
Since the A2BAR is a GPCR, we assumed that immunofluorescence would reveal plasma membrane staining. To our surprise, the A2BAR antibody did not bind to the cardiomyocyte sarcolemma as expected, but rather caused structures at intracellular sites to fluoresce. Co-staining with propidium iodide or DAPI revealed high expression in nuclei. There was also a chain-like pattern of fluorescent staining of intracellular structures with the A2B antibody which resembled the pattern seen with mitochondrial stains in myocytes. The merged image of A2BAR staining with staining generated by MitoTracker Red, a mitochondria-specific dye, indicated co-localization of A2BAR and mitochondria. To further assess the likelihood that A2BAR were in the sarcolemma, we exposed isolated cardiomyocytes simultaneously to antibodies to A2BAR and Na+/K+-ATPase and found almost no co-localization of these two proteins. In ventricular muscle cells surface receptors are often located on the T-tubules which overlay the Z-lines deep in the cell. However, co-localization of A2BAR with Z-lines stained with anti-actin antibody was also not observed.
In contrast to the findings in cardiomyocytes, HEK293 cells overexpressing A2BAR indeed showed typical membrane staining by fluorochrome-linked A2BAR antibodies. While our observations are consistent with recent reports of A2BAR localization [27,31], we have no explanation for the differences between these cell models of A2BAR overexpression and the pattern seen in primary adult cardiomyocytes.
Although it has been generally assumed that GPCRs are only trafficked to the plasma membrane, there is evidence that GPCR can also localize and function in intracellular compartments, such as the nucleus for the β-adrenergic receptor [7]. Also, mitochondrial Ca2+ transport has been linked to the presence of P2Y-like receptors on mitochondria [2] and Gαi subunits have been found in mitochondria of HEK293T cells [24]. Furthermore, mitochondria are known to contain non-GPCR for estrogens, glucocorticoids, androgens, and thyroid hormones, as well as the retinoid receptor α and the peroxisome proliferator-activated receptor β [36].
Western blot analysis of mitochondrial preparations from hearts as well as isolated cardiomyocytes showed A2BAR immunoreactivity (Fig. 6). The mitochondrial fractions appeared to be nearly free of contamination with sarcolemma and sarcoplasmic reticulum as revealed by a lack of reactivity to anti-Na+/K+-ATPase and anti-calnexin antibodies, respectively. To further demonstrate an intracellular A2BAR localization we tried to isolate sarcolemma free of mitochondria and SR, which we hypothesized would not contain A2BAR. First, a simple preparation including a centrifugation step at lower speed followed by a high speed centrifugation step to pellet the sarcolemma was not sufficient because we still saw contamination by mitochondria and SR proteins and, therefore, also A2BAR. Further treatment with a sucrose gradient markedly reduced mitochondrial contamination. Since the A2BAR signal also decreased without affecting SR content, A2BAR localization in or near mitochondria is likely. Our digitonin studies additionally suggest A2BAR may reside in the outer mitochondrial membrane. Nevertheless our studies do not unequivocally prove that A2BAR reside within mitochondria; they may in fact only be loosely attached on the outside. Moriyama and Sitkovsky [29] recently showed that A2BAR are poorly trafficked to the membrane in some cells leaving many receptors in the endoplasmic reticulum of the Golgi. However, the location of the receptors in our study is not consistent with a peri-nuclear Golgi locus.
The molecular weight of our A2BAR band was near 40 kDa which closely approximates the expected 38.18 kDa size. Previous studies have reported a tissue- and species-dependent protein size between 31 and 50 kDa. For example, hexahistidine/FLAG-tagged human A2BAR recombinantly expressed in HEK293 cells have molecular weights of 34.8 kDa and after deglycosylation 31.3 kDa [23]. Puffinbarger et al. [37] demonstrated human A2BAR with differing sizes, 35 kDa in small intestine, and 50-52 kDa in thymus, colon and placenta. Jackson et al. [18] presented evidence for a 52 kDa A2BAR in rat aorta and preglomerular microvasculature. It is difficult to explain the observed differences, but it is suspected that the higher molecular weights were the result of antibodies cross-reacting with some non-receptor epitope. In the present study the fact that the A2B band disappeared in tissue from A2B knockout mice argues against non-specific binding of our antibody to another protein or receptor subtype.
Functional relevance of cardiac A2BAR could be demonstrated by a BAY60-6583-mediated decrease in infarct size after ischemia/reperfusion, and this protection was blocked with the highly-selective A2BAR antagonist MRS1754. To test if cardiomyocytes, the cells that must be protected at reperfusion, respond to A2BAR stimulation, we used a cell model of intracellular calcium stress which mirrors the detrimental calcium increase occurring at reperfusion [32]. Cardiomyocytes were stained with TMRE, and it is well accepted that a loss in TMRE fluorescence is correlated with a loss of mitochondrial membrane potential (ΔΨm), which in turn presumably indicates mPTP opening. When BAY60-6583 was added before the calcium ionophore, cells were less prone to calcium-induced depolarization of ΔΨm. This effect was inhibited by MRS1754. Confirmation of our results comes from a recent study in which NECA, a potent A2AR agonist, suppressed mitochondrial transition pore formation in isolated rat cardiomyocytes and, again, this protection could be blocked with MRS1754 [44].
We do not know how A2BAR might be directed to mitochondria. Like 99% of the mitochondrial proteins A2BAR are not part of the mitochondrial genome. Rather they are encoded in the nuclear DNA. After being synthesized in the cytosol they must be transported to the mitochondria. Most mitochondrial proteins carry importing sequences which are recognized by cytosol-exposed receptors present in the mitochondrial outer membrane called “translocase of the outer membrane” (TOM). Depending on the imported protein's target they are transported by TOM and possibly by TIM (translocase of the inner membrane) complexes [28]. Mitochondrial targeting moieties are quite variable and can be present anywhere in the poly-peptide structure. However, as analyzed by either TargetP or MITOPROT programs the A2BAR amino acid sequence does not contain any known importation sequence [11]. This does not exclude the possibility that mitochondria will incorporate them, however, as some imported mitochondrial proteins do not contain any of these sequences. This was recently found to be the case for connexin 43 which is present in myocardial mitochondria [4,6,38].
Although still controversial, the survival kinases ERK, Akt and GSK-3β are central to the protection conferred by pre- and postconditioning in the rat heart [13,15,42] and they can localize in mitochondria as well. As reviewed by Poderoso et al. [35] activation of protein kinase A (PKA), MEK1/2 and ERK1/2 leads to signal complex formation at the mitochondrion surface. GSK-3β which is cardioprotective when inhibited by phosphorylation is present in mitochondria [17], and Akt reportedly accumulates in mitochondria following PI3-kinase activation [3]. In our study we used Akt as a cytosolic marker and did not see immunoreactivity in our mitochondrial preparations. But our cells were in a basal, unstimulated state.
A2BAR couple through stimulatory G proteins (Gαs) which in turn are known to produce cAMP via adenylyl cyclase. Indeed several G proteins were identified in mitochondria of HEK293T cells [24]. And we found Gαs in our mitochondrial preparation. Sardanelli et al. [40] showed PKA in mitochondria from rat hearts, while Acin-Perez et al. [1] demonstrated cAMP production within mitochondria and the subsequent activation of mitochondrial PKA. Furthermore, nitric oxide synthase and its product nitric oxide could also be demonstrated within mitochondria [20]. Although the presence of A2BAR in or near mitochondria is likely, the demonstration of a functional relevance of these receptor complexes surely requires further study.
There is growing evidence that protection afforded by pre- and postconditioning is related to kinase- and signalling molecule-dependent inhibition of mitochondrial transition pores upon reperfusion [5,9,16] and that A2BAR control them [33]. It therefore seems quite conceivable that A2BAR localized on mitochondria could be involved in this signalling cascade.
Supplementary Material
Acknowledgments
We thank Thomas Krahn and Barbara Albrecht (Bayer HealthCare Wuppertal, Germany) for providing BAY60-6583 and performing the concentration measurements (see supplemental material). We thank Dr J Linden, University of Virginia, Charlottesville, VA, for the HEK293-A2BAR cells, and Dr H Eltzschig, University of Colorado, Denver, CO for the A2BAR knock-out mice (see supplemental material).
Funding
The study was in part supported by grants from the National Institutes of Health Heart, Lung and Blood Institute HL-20648 (T.K., J.M.D.) and the Deutsche Forschungsgemeinschaft (T.K.).
Footnotes
Conflict of interest
None.
References
- 1.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. DOI: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belous AE, Jones CM, Wakata A, Knox CD, Nicoud IB, Pierce J, Chari RS. Mitochondrial calcium transport is regulated by P2Y1- and P2Y2-like mitochondrial receptors. J.Cell Biochem. 2006;99:1165–1174. doi: 10.1002/jcb.20985. DOI: 10.1002/jcb.20985. [DOI] [PubMed] [Google Scholar]
- 3.Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. DOI: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di Lisa F, Heusch G, Schulz R. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res. 2005;67:234–244. doi: 10.1016/j.cardiores.2005.04.014. DOI: 10.1016/j.cardiores.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–85. doi: 10.1007/s00395-010-0124-1. DOI: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boengler K, Stahlhofen S, van de Sand A, Gres P, Ruiz-Meana M, Garcia-Dorado D, Heusch G, Schulz R. Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res Cardiol. 2009;104:141–7. doi: 10.1007/s00395-009-0007-5. DOI: 10.1007/s00395-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 7.Boivin B, Lavoie C, Vaniotis G, Baragli A, Villeneuve LR, Ethier N, Trieu P, Allen BG, Hébert TE. Functional β-adrenergic receptor signalling on nuclear membranes in adult rat and mouse ventricular cardiomyocytes. Cardiovasc Res. 2006;71:69–78. doi: 10.1016/j.cardiores.2006.03.015. DOI: 10.1016/j.cardiores.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MV, Yang X-M, Liu GS, Heusch G, Downey JM. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial KATP channels. Circ Res. 2001;89:273–8. doi: 10.1161/hh1501.094266. DOI: 10.1161/hh1501.094266. [DOI] [PubMed] [Google Scholar]
- 9.Di Lisa F, Canton M, Menabò R, Dodoni G, Bernardi P. Mitochondria and reperfusion injury. The role of permeability transition. Basic Res Cardiol. 2003;98:235–241. doi: 10.1007/s00395-003-0415-x. DOI: 10.1007/s00395-003-0415-x. [DOI] [PubMed] [Google Scholar]
- 10.Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. DOI: 10.1161/circulationaha.106.669697. [DOI] [PubMed] [Google Scholar]
- 11.Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. DOI: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- 12.Förster K, Paul I, Solenkova N, Staudt A, Cohen MV, Downey JM, Felix SB, Krieg T. NECA at reperfusion limits infarction and inhibits formation of the mitochondrial permeability transition pore by activating p70S6 kinase. Basic Res Cardiol. 2006;101:319–326. doi: 10.1007/s00395-006-0593-4. DOI: 10.1007/s00395-006-0593-4. [DOI] [PubMed] [Google Scholar]
- 13.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. DOI: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Haynes J, Jr, Obiako B, Thompson WJ, Downey JM. Adenosine-induced vasodilation: receptor characterization in pulmonary circulation. Am J Physiol Heart Circ Physiol. 1995;268:H1862–H1868. doi: 10.1152/ajpheart.1995.268.5.H1862. [DOI] [PubMed] [Google Scholar]
- 15.Heusch G, Boengler K, Schulz R. Cardioprotection. Nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–9. doi: 10.1161/CIRCULATIONAHA.108.805242. DOI: 10.1161/circulationaha.108.805242. [DOI] [PubMed] [Google Scholar]
- 16.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the holy grail of cardioprotection. Basic Res Cardiol. 2010;105:151–4. doi: 10.1007/s00395-009-0080-9. DOI: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 17.Hoshi M, Sato M, Kondo S, Takashima A, Noguchi K, Takahashi M, Ishiguro K, Imahori K. Different localization of tau protein kinase I/glycogen synthase kinase-3β from glycogen synthase kinase-3α in cerebellum mitochondria. J Biochem. 1995;118:683–685. doi: 10.1093/oxfordjournals.jbchem.a124965. [DOI] [PubMed] [Google Scholar]
- 18.Jackson EK, Zhu C, Tofovic SP. Expression of adenosine receptors in the preglomerular microcirculation. Am J Physiol Renal Physiol. 2002;283:F41–F51. doi: 10.1152/ajprenal.00232.2001. DOI: 10.1152/ajprenal.00232.2001. [DOI] [PubMed] [Google Scholar]
- 19.Ji X, Kim YC, Ahern DG, Linden J, Jacobson KA. [3H]MRS 1754, a selective antagonist radioligand for A2B adenosine receptors. Biochem Pharmacol. 2001;61:657–663. doi: 10.1016/s0006-2952(01)00531-7. DOI: 10.1016/s0006-2952(01)00531-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanai AJ, Pearce LL, Clemens PR, Birder LA, Van Bibber MM, Choi S-Y, de Groat WC, Peterson J. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci U S A. 2001;98:14126–14131. doi: 10.1073/pnas.241380298. DOI: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuno A, Critz SD, Cui L, Solodushko V, Yang X-M, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007;43:262–271. doi: 10.1016/j.yjmcc.2007.05.016. DOI: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang BT, Haltiwanger B. Adenosine A2a and A2b receptors in cultured fetal chick heart cells. High- and low-affinity coupling to stimulation of myocyte contractility and cAMP accumulation. Circ Res. 1995;76:242–251. doi: 10.1161/01.res.76.2.242. [DOI] [PubMed] [Google Scholar]
- 23.Linden J, Thai T, Figler H, Jin X, Robeva AS. Characterization of human A2B adenosine receptors: radioligand binding, western blotting, and coupling to Gq in human embryonic kidney 293 cells and HMC-1 mast cells. Mol Pharmacol. 1999;56:705–713. [PubMed] [Google Scholar]
- 24.Lyssand JS, Bajjalieh SM. The heterotrimeric G protein subunit Gαi is present on mitochondria. FEBS Lett. 2007;581:5765–5768. doi: 10.1016/j.febslet.2007.11.044. DOI: 10.1016/j.febslet.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 25.Maas JE, Wan TC, Figler RA, Gross GJ, Auchampach JA. Evidence that the acute phase of ischemic preconditioning does not require signaling by the A2B adenosine receptor. J Mol Cell Cardiol. 2010;49:886–893. doi: 10.1016/j.yjmcc.2010.08.015. DOI: 10.1016/j.yjmcc.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maas O, Donat U, Frenzel M, Rütz T, Kroemer HK, Felix SB, Krieg T. Vardenafil protects isolated rat hearts at reperfusion dependent on GC and PKG. Br J Pharmacol. 2008;154:25–31. doi: 10.1038/bjp.2008.71. DOI: 10.1038/bjp.2008.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matharu AL, Mundell SJ, Benovic JL, Kelly E. Rapid agonist-induced desensitization and internalization of the A2B adenosine receptor is mediated by a serine residue close to the COOH terminus. J Biol Chem. 2001;276:30199–30207. doi: 10.1074/jbc.M010650200. DOI: 10.1074/jbc.M010650200. [DOI] [PubMed] [Google Scholar]
- 28.Mokranjac D, Neupert W. Thirty years of protein translocation into mitochondria: unexpectedly complex and still puzzling. Biochim Biophys Acta. 2009;1793:33–41. doi: 10.1016/j.bbamcr.2008.06.021. DOI 10.1016/j.bbamcr.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Moriyama K, Sitkovsky MV. Adenosie A2A receptor is involved in cell surface expression of A2B receptor. J Biol Chem. 2010;285:39271–88. doi: 10.1074/jbc.M109.098293. DOI: 10.1074/jbc.M109.098293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison RR, Talukder MA, Ledent C, Mustafa SJ. Cardiac effects of adenosine in A2A receptor knockout hearts: uncovering A2B receptors. Am J Physiol Heart Circ Physiol. 2002;282:H437–H444. doi: 10.1152/ajpheart.00723.2001. DOI: 10.1152/ajpheart.00723.2001. [DOI] [PubMed] [Google Scholar]
- 31.Mundell SJ, Matharu AL, Kelly E, Benovic JL. Arrestin isoforms dictate differential kinetics of A2B adenosine receptor trafficking. Biochemistry. 2000;39:12828–12836. doi: 10.1021/bi0010928. DOI: 10.1021/bi0010928. [DOI] [PubMed] [Google Scholar]
- 32.Penzo D, Petronilli V, Angelin A, Cusan C, Colonna R, Scorrano L, Pagano F, Prato M, Di Lisa F, Bernardi P. Arachidonic acid released by phospholipase A2 activation triggers Ca2+-dependent apoptosis through the mitochondrial pathway. J Biol Chem. 2004;279:25219–25225. doi: 10.1074/jbc.M310381200. DOI: 10.1074/jbc.M310381200. [DOI] [PubMed] [Google Scholar]
- 33.Philipp S, Yang X-M, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–314. doi: 10.1016/j.cardiores.2006.02.014. DOI: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Piper HM, Probst I, Schwartz P, Hutter FJ, Spieckermann PG. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982;14:397–412. doi: 10.1016/0022-2828(82)90171-7. DOI: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- 35.Poderoso C, Maloberti P, Duarte A, Neuman I, Paz C, Maciel FC, Podesta EJ. Hormonal activation of a kinase cascade localized at the mitochondria is required for StAR protein activity. Mol Cell Endocrinol. 2009;300:37–42. doi: 10.1016/j.mce.2008.10.009. DOI: 10.1016/j.mce.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Psarra AM, Sekeris CE. Nuclear receptors and other nuclear transcription factors in mitochondria: regulatory molecules in a new environment. Biochim Biophys Acta. 2008;1783:1–11. doi: 10.1016/j.bbamcr.2007.10.021. DOI: 10.1016/j.bbamcr.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 37.Puffinbarger NK, Hansen KR, Resta R, Laurent AB, Knudsen TB, Madara JL, Thompson LF. Production and characterization of multiple antigenic peptide antibodies to the adenosine A2b receptor. Mol Pharmacol. 1995;47:1126–1132. [PubMed] [Google Scholar]
- 38.Rottlaender D, Boengler K, Wolny M, Michels G, Endres-Becker J, Motloch LJ, Schwaiger A, Buechert A, Schulz R, Heusch G, Hoppe UC. Connexin 43 acts as a cytoprotective mediator of signal transduction by stimulating mitochondrial KATP channels in mouse cardiomyocytes. J Clin Invest. 2010;120:1441–53. doi: 10.1172/JCI40927. DOI:10.1172/jci40927. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Rubino A, Ralevic V, Burnstock G. Contribution of P1-(A2b subtype) and P2-purinoceptors to the control of vascular tone in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1995;115:648–652. doi: 10.1111/j.1476-5381.1995.tb14981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sardanelli AM, Signorile A, Nuzzi R, Rasmo DD, Technikova-Dobrova Z, Drahota Z, Occhiello A, Pica A, Papa S. Occurrence of A-kinase anchor protein and associated cAMP-dependent protein kinase in the inner compartment of mammalian mitochondria. FEBS Lett. 2006;580:5690–5696. doi: 10.1016/j.febslet.2006.09.020. DOI: 10.1016/j.febstel.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Schnaitman C, Erwin VG, Greenawalt JW. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967;32:719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs. No causal role for RISK activation. Circ Res. 2009;104:15–8. doi: 10.1161/CIRCRESAHA.108.186429. DOI: 10.1161/circresaha.108.186429. [DOI] [PubMed] [Google Scholar]
- 43.Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol Heart Circ Physiol. 2006;290:H441–H449. doi: 10.1152/ajpheart.00589.2005. DOI: 10.1152/ajpheart.00589.2005. [DOI] [PubMed] [Google Scholar]
- 44.Xi J, McIntosh R, Shen X, Lee S, Chanoit G, Criswell H, Zvara DA, Xu Z. Adenosine A2A and A2B receptors work in concert to induce a strong protection against reperfusion injury in rat hearts. J Mol Cell Cardiol. 2009;47:684–690. doi: 10.1016/j.yjmcc.2009.08.009. DOI: 10.1016/j.yjmcc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin W, Cohen MV, Rich TC, Downey JM. Which preconditioning-associated G protein-coupled receptors are expressed on the sarcolemma? FASEB J. 2009;23:793. [Google Scholar]
- 46.Xu Z, Mueller RA, Park S-S, Boysen PG, Cohen MV, Downey JM. Cardioprotection with adenosine A2 receptor activation at reperfusion. J Cardiovasc Pharmacol. 2005;46:794–802. doi: 10.1097/01.fjc.0000188161.57018.29. [DOI] [PubMed] [Google Scholar]
- 47.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. DOI: 10.1172/jci27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.