Years of developing cancer therapies targeted to specific molecular drivers have yielded some crucial advances, yet only 5% of such therapies tested in the clinic prove efficacious, indicating a need for marked improvement in predictive preclinical research1. Given that cancers are complex diseases with diverse molecular etiologies, the most predictive preclinical models require accurate engineering for the induction of cancer in situ.
Recently, several studies in such genetically engineered mice (GEM) have shown promise for obtaining insight into clinical challenges to diagnosis and treatment and for guiding clinical research. One such breakthrough occurred in evaluating therapeutics for pancreatic ductal adenocarcinoma2, a deadly cancer with no effective treatment, where mice with inducible genetic aberrations common to the human disease specifically in the pancreatic ductal epithelium developed pancreatic cancers that are histopathologically similar to those in humans3.
In particular, the study by Olive et al.2 used a GEM pancreatic cancer model to uncover a potential major impediment to the treatment of these cancers—their relative impermeability to drug perfusion. These mice were engineered to incorporate targeted point mutations in the genetic loci of the endogenous oncoprotein Kras and the tumor suppressor p53, which are found in 95% and 75% of human ductal pancreatic tumors, respectively. When mice with in situ cancers in the pancreas were treated with gemcitabine (Gemzar, Eli Lilly), the standard of care for ductal pancreatic cancer, responses were minimal, just as observed in the clinic. When tumors were examined over time for vascular delivery of the drug, tissue perfusion within the tumor was minimal. In fact, the vasculature in these cancers was both sparse and poorly functional, probably explaining the poor perfusion of gemcitabine in the tumor and the lack of therapeutic effect.
To test whether altering the stroma to increase perfusion would also increase the drug efficacy, tumor-bearing mice (identified by ultrasound or imaging) were treated with a Smoothened inhibitor (IPI-926, Infinity Pharmaceuticals) ten days before treatment with gemcitabine. Smoothened is a mediator of the Hedgehog signaling pathway, which forms a paracrine signaling axis in pancreatic cancers4-6. The Sonic Hedgehog ligand is overexpressed and released from neoplastic epithelial cells and activates downstream signaling in nearby stromal cells, promoting desmoplasia, a dense, fibrous tumor stroma7.
Remarkably, the inhibition of Smoothened depleted the stroma from established tumors and facilitated the delivery of multiple drug agents, resulting in a more than twofold increase in survival of mice when it was administered in combination with gemcitabine, compared with mice that did not receive the Smoothened inhibitor2 (Fig. 1).
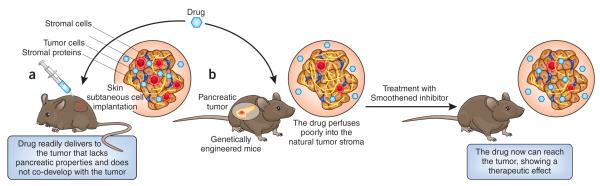
Figure 1.
A de novo tumor model mimics the growth patterns of the human tumor and allows testing of alternative antitumor therapies to use in clinical trials. (a) Tumor pancreatic cancer cells that are implanted under the skin in immunosuppressed mice develop into tumors with less dense stroma—that lacks pancreatic properties and does not co-develop with the evolving tumor—and numerous blood vessels. This allows the administered drug to readily perfuse into the tumor and have a therapeutic effect. (b) GEM with mutations in the pancreatic ductal epithelial develop in situ tumors that develop de novo with the tumor microenvironment. These tumors show more stroma and fewer blood vessels, which results in poor perfusion of the drug into the natural tumor stroma. Tumor-bearing GEM treated with Smoothened inhibitor, an inhibitor of paracrine signals in pancreatic cancers, have increased amounts of drug in the tumors, which have less stroma, and show a strong therapeutic response to the antitumor drug.
The results of the study by Olive et al.2 are striking for several reasons. First, if the model is, in fact, representative of the human disease, the observation that pancreatic cancers are resistant to drug uptake may explain why virtually every therapy tested for this disease has failed8 and represents, therefore, a breakthrough in advancing therapeutic effectiveness. Second, the use of the GEM model facilitated testing for therapeutic agents that promote effective drug delivery, resulting in the development of a protocol for combined therapy that targets both the microenvironment, with the Smoothened inhibitor, and the tumor cells, with gemcitabine. Finally, this study2 showed the importance of using relevant models for preclinical assessment.
It was crucial in the study2 to use a mouse model in which cancer developed de novo, evolving within the natural microenvironment and mimicking the pancreatic tumor development in humans. Tumors derived from human pancreatic cancer cells implanted under the skin of immunocompromised mice—the standard in preclinical evaluation—were well perfused and responsive to gemcitabine2. It is unlikely that de novo cancer models will be required for effective preclinical therapeutic assessment of all cancers. Nevertheless, given that we understand so little about stromal interactions during tumor progression, therapeutic studies in de novo cancer models may be required for all cancers to assess the full range of treatment possibilities.
Despite the intriguing and promising possibilities for human disease management suggested by the GEM pancreatic cancer studies, a question arises about whether outcomes in the cancer mouse model predict a real human disease mechanism. Answering this crucial question requires research in humans. The mouse studies, however, have provided a hypothesis to test in the clinic.
More important, these basic findings in mouse models are en route to be tested in drug efficacy studies in humans by several investigators, underscoring that relevant preclinical findings can be translated into clinical research design. For instance, trials influenced or guided by the preclinical mouse studies currently include a phase 1b-2 trial of gemcitabine with IPI-926 versus vehicle in metastatic pancreatic ductal adenocarcinoma and four trials of GDC-0449 (an inhibitor of Smoothened; Vismodegib, Genentech-Roche) in metastatic pancreatic ductal adenocarcinoma: a phase 1 toxicity study, carried out at the Mayo Clinic, in combination with gemcitabine and erlotinib (Tarceva, Genentech-Roche), a pilot study by the National Cancer Institute on the effects of GDC-0449 plus gemcitabine on circulating tumors cells, a phase 2 study of gemcitabine plus GDC0449 versus vehicle at the University of Chicago and a phase 2 trial of gemcitabine in combination with GDC0449 and Abraxane (Abraxis Pharmaceuticals) at the Johns Hopkins University Hospital (http://clinicaltrials.gov/).
In addition, a phase 1 study of GDC0449 in presurgical patients at the Cancer Research UK Cambridge Research Institute will acquire before-after-treatment biopsies from locally confined tumors to directly evaluate the changes in stroma and vasculature that were predicted by the mouse studies by Olive et al.2. Furthermore, recent promising phase 2 results with the combination of abraxane and gemcitabine in individuals with pancreatic ductal adenocarcinoma (D.D. Von Hoff (Translational Genomics Research Institute), personal communication) raise the question as to whether abraxane might also act similarly by targeting the stroma. Considering that the study by Olive et al.2 is over a year old, this is a remarkably rapid turnaround for the translation of a preclinical finding into clinical trials.
The mouse studies have shown that drug-induced stromal modification can be used to improve overall drug delivery. It is a major advance to incorporate such basic preclinical findings so quickly into the design and interpretation of human studies. Results from the above clinical trials and their successors, however, will be required to know how well studies in a biologically and genetically relevant mouse cancer model can predict clinical outcomes.
In addition to predicting efficacy, the mouse studies proposed a mechanism that can now be evaluated directly in the earliest clinical trials. This upends the traditional clinical trial pathway in which molecular mechanisms are usually sought, that is, only after large-scale efficacy trials have been successful.
In the best scenario, these early clinical trials will find both signs of efficacy and data in support of a molecular mechanism. At worst, the samples generated in human trials will allow a better understanding of why the drugs failed.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Reichert JM, Wenger JB. Drug Discov. Today. 2008;13:30–37. doi: 10.1016/j.drudis.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Olive KP, et al. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hruban RH, et al. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 4.Tian H, et al. Proc. Natl. Acad. Sci. USA. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan-Stevaux O, et al. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yauch RL, et al. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 7.Bailey JM, et al. Clin. Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong HQ, Carr K, Abbruzzese JL. Drugs. 2006;66:1059–1072. doi: 10.2165/00003495-200666080-00003. [DOI] [PubMed] [Google Scholar]