Abstract
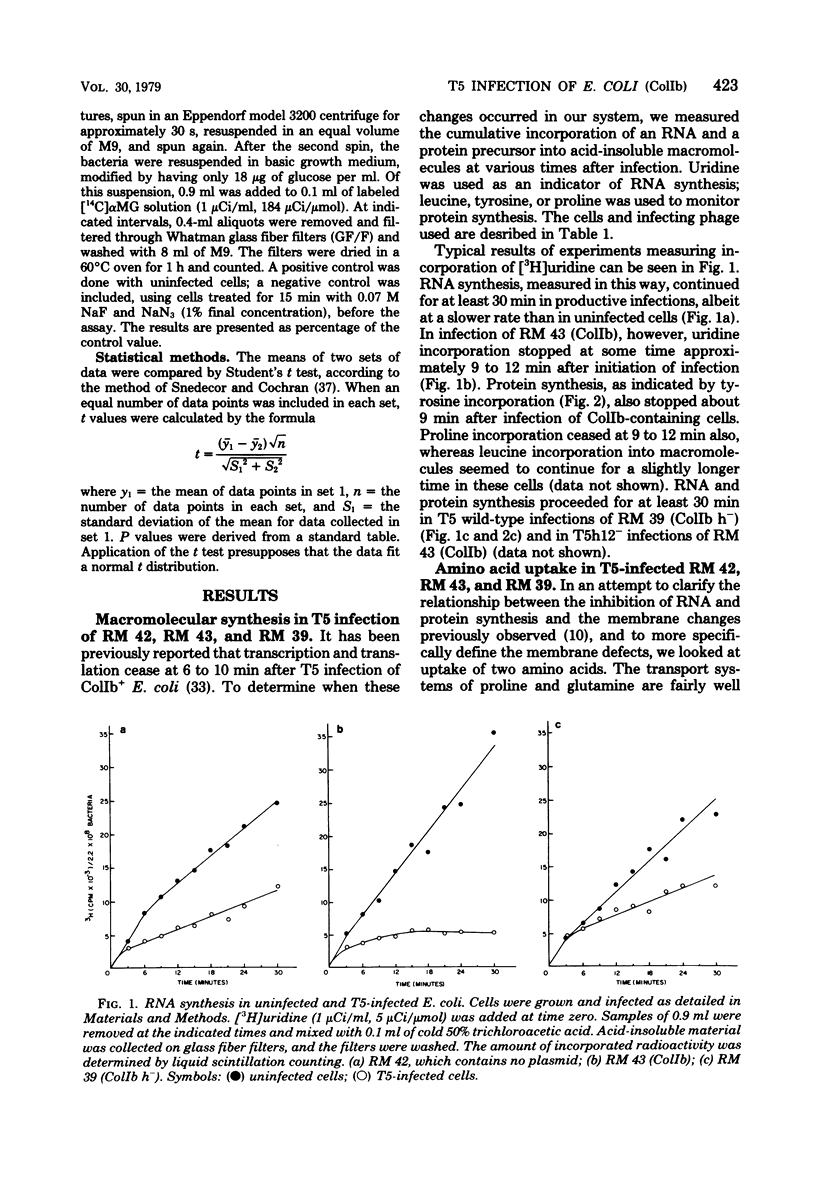
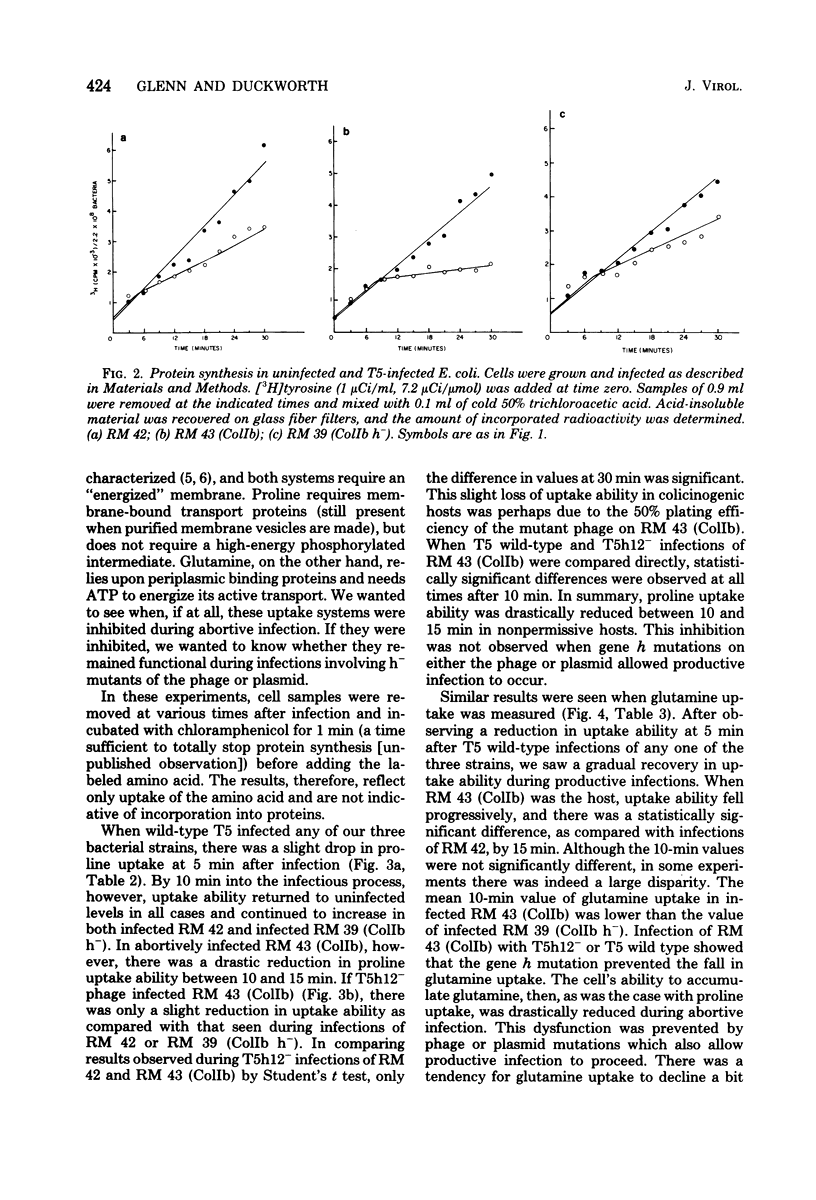
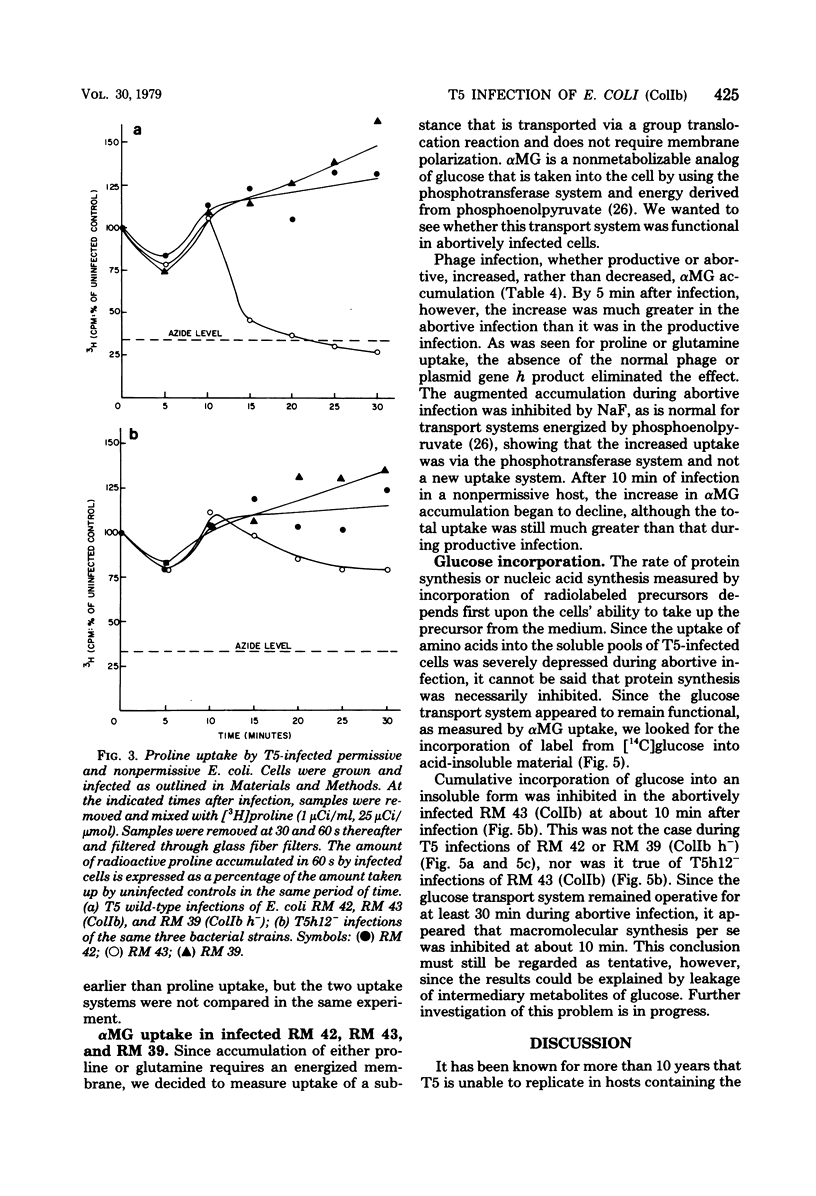
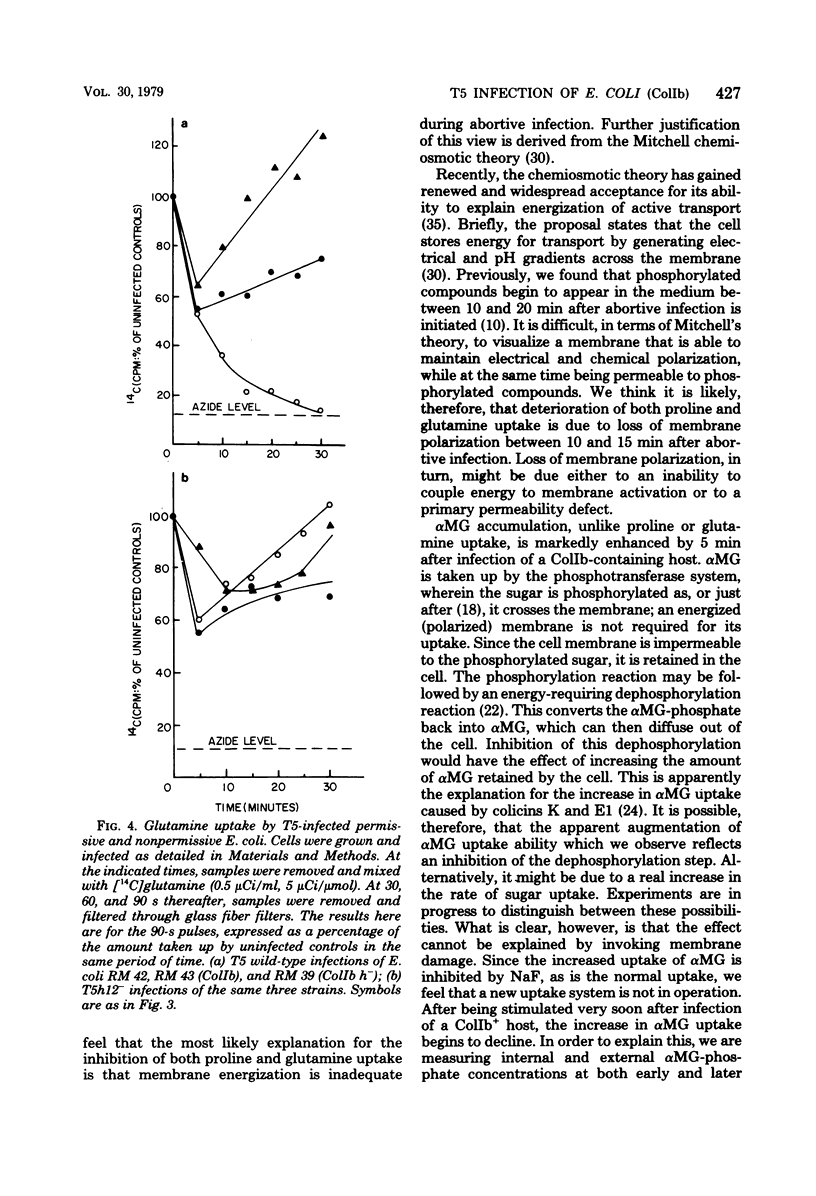
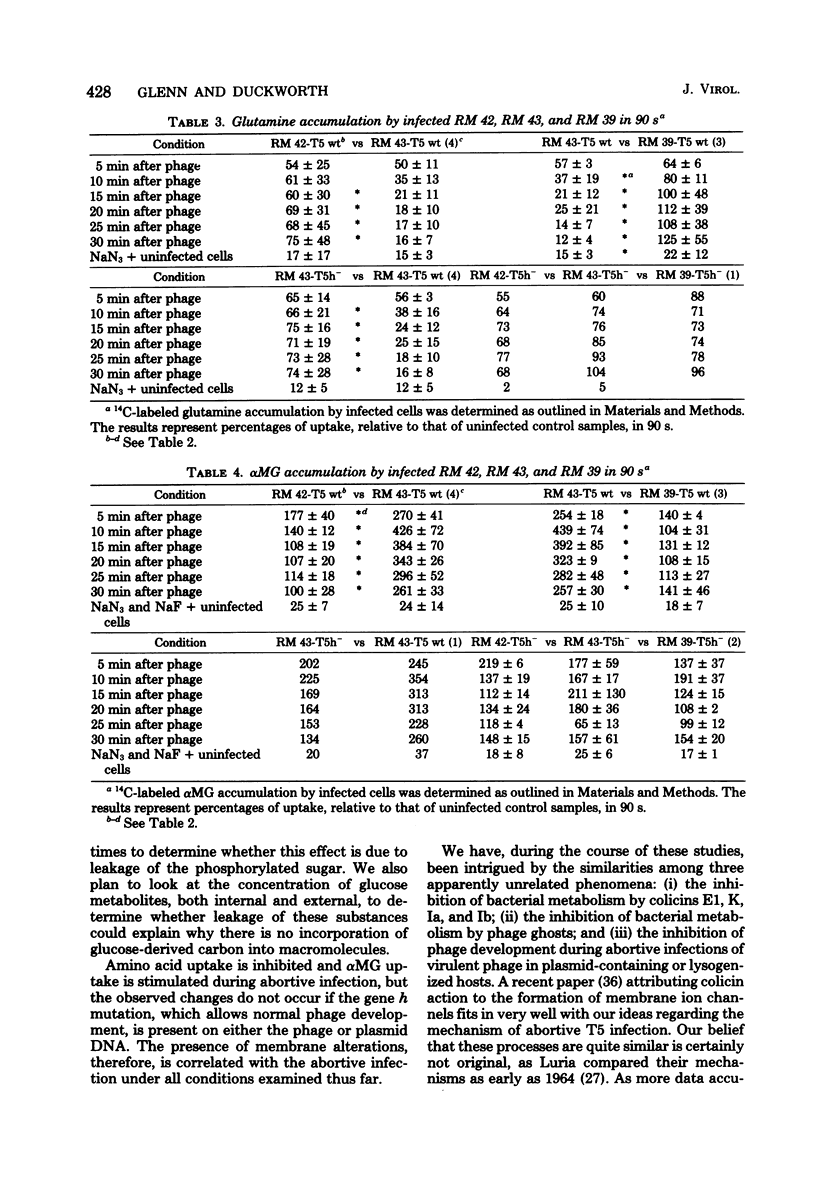
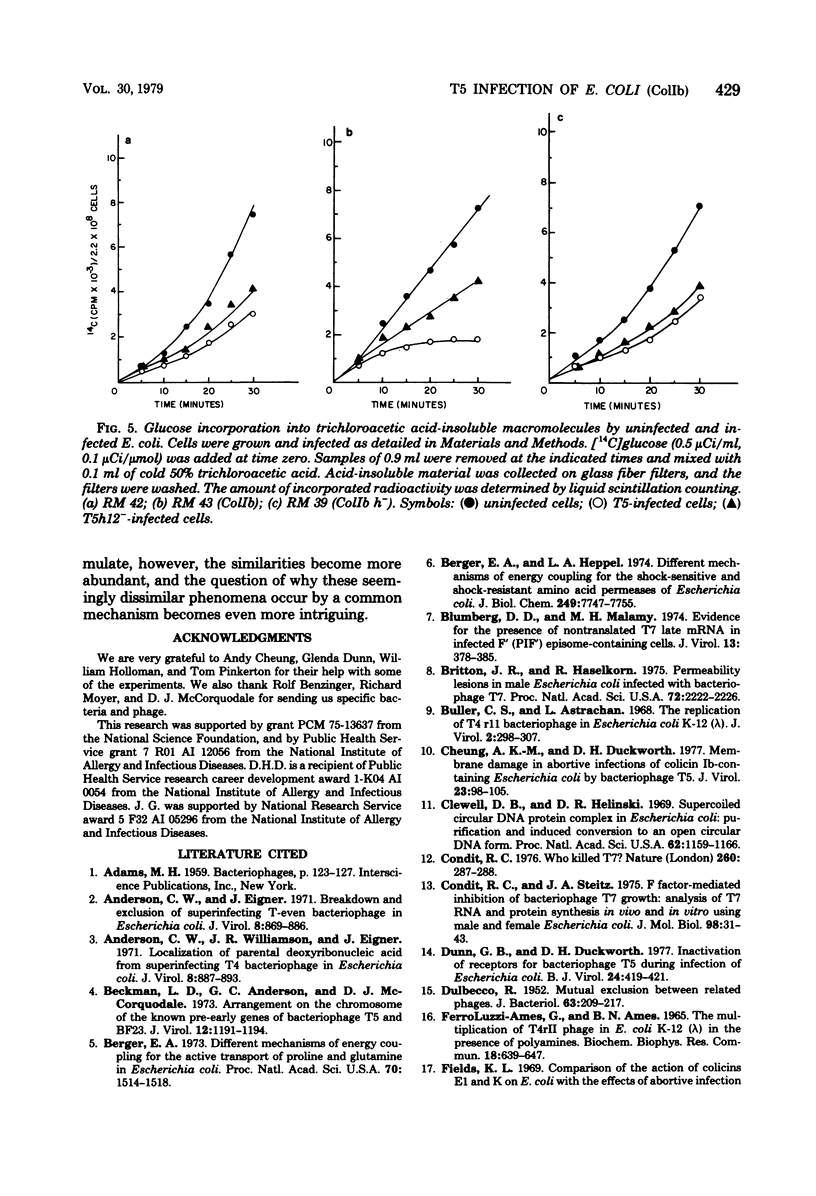
T5 bacteriophage cannot replicate in Escherichia coli containing the colicinogenic factor ColIb. We show that active transport of proline and glutamine begins to decline at about 10 min after infection, the same time at which macromolecular synthesis stops during abortive infection. Uptake of alpha-methylglucoside is stimulated, however, and this change is evident even by 5 min after infection. These changes in membrane function do not occur during infections that are productive because of mutations on the plasmid or phage. The results suggest that the abortive infection is caused by membrane depolarization.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Eigner J. Breakdown and exclusion of superinfecting T-even bacteriophage in Escherichia coli. J Virol. 1971 Dec;8(6):869–886. doi: 10.1128/jvi.8.6.869-886.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Williamson J. R., Eigner J. Localization of parental deoxyribonucleic acid from superinfecting T4 bacteriophage in Escherichia coli. J Virol. 1971 Dec;8(6):887–893. doi: 10.1128/jvi.8.6.887-893.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman L. D., Anderson G. C., McCorquodale D. J. Arrangement on the chromosome of the known pre-early genes of bacteriophages T5 and BF23. J Virol. 1973 Nov;12(5):1191–1194. doi: 10.1128/jvi.12.5.1191-1194.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Blumberg D. D., Malamy M. H. Evidence for the presence of nontranslated T7 late mRNA in infected F'(PIF+) episome-containing cells. J Virol. 1974 Feb;13(2):378–385. doi: 10.1128/jvi.13.2.378-385.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J. R., Haselkorn R. Permeability lesions in male Escherichia coli infected with bacteriophage T7. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2222–2226. doi: 10.1073/pnas.72.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller C. S., Astrachan L. Replication of T4rII bacteriophage in Escherichia coli K-12 (lambda). J Virol. 1968 Apr;2(4):298–307. doi: 10.1128/jvi.2.4.298-307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. K., Duckworth D. H. Membrane damage in abortive infections of colicin Ib-containing Escherichia coli by bacteriophage T5. J Virol. 1977 Jul;23(1):98–105. doi: 10.1128/jvi.23.1.98-105.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Steitz J. A. F factor-mediated inhibition of bacteriophage T7 growth: analysis of T7 RNA and protein synthesis in vivo and in vitro using male and female Escherichia coli. J Mol Biol. 1975 Oct 15;98(1):31–43. doi: 10.1016/s0022-2836(75)80099-4. [DOI] [PubMed] [Google Scholar]
- DULBECCO R. Mutual exclusion between related phages. J Bacteriol. 1952 Feb;63(2):209–217. doi: 10.1128/jb.63.2.209-217.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G. B., Duckworth D. H. Inactivation of receptors for bacteriophage T5 during infection of Escherichia coli B. J Virol. 1977 Oct;24(1):419–421. doi: 10.1128/jvi.24.1.419-421.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A. Physiological effects of rII mutations in bacteriophage T4. Virology. 1961 Jun;14:151–163. doi: 10.1016/0042-6822(61)90190-8. [DOI] [PubMed] [Google Scholar]
- Gachelin G. Studies on the alpha-methylglucoside permease of Escherichia coli. A two-step mechanism for the accumulation of alpha-methylglucoside 6-phosphate. Eur J Biochem. 1970 Oct;16(2):342–357. doi: 10.1111/j.1432-1033.1970.tb01088.x. [DOI] [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. Preparation, purification, and properties of E. coli virus T2. J Gen Physiol. 1952 May;36(1):17–28. doi: 10.1085/jgp.36.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFEE P., ENGLESBERG E., LAMY F. THE GLUCOSE PERMEASE SYSTEM IN BACTERIA. Biochim Biophys Acta. 1964 Mar 30;79:337–350. [PubMed] [Google Scholar]
- Herman R. C., Moyer R. W. In vivo repair of bacteriophage t5 DNA: an assay for viral growth control. Virology. 1975 Aug;66(2):393–407. doi: 10.1016/0042-6822(75)90212-3. [DOI] [PubMed] [Google Scholar]
- Hull R., Moody E. E. Isolation and genetic characterizaion of Escherichia coli K-12 mutations affecting bacteriophage T5 restriction by the ColIb plasmid. J Bacteriol. 1976 Jul;127(1):229–236. doi: 10.1128/jb.127.1.229-236.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M. Effects of colicins K and E1 on the glucose phosphotransferase system. Biochim Biophys Acta. 1976 Aug 13;440(2):403–411. doi: 10.1016/0005-2728(76)90074-8. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf K., Bujard H. Structure and function of the genome of coliphage T5. Transcription in vitro of the "nicked" and "nick-free" T5+ DNA. Eur J Biochem. 1975 May 6;53(2):371–385. doi: 10.1111/j.1432-1033.1975.tb04077.x. [DOI] [PubMed] [Google Scholar]
- LURIA S. E. ON THE MECHANISMS OF ACTION OF COLICINS. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:73. [PubMed] [Google Scholar]
- McCorquodale D. J. The T-odd bacteriophages. CRC Crit Rev Microbiol. 1975 Dec;4(2):101–159. doi: 10.3109/10408417509111574. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mizobuchi K., Anderson G. C., McCorquodale D. J. Abortive infection by bacteriophage BF23 due to the colicin Ib factor. I. Genetic studies of nonrestricted and amber mutants of bacteriophage BF23. Genetics. 1971 Jul;68(3):323–340. doi: 10.1093/genetics/68.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Fu A. S., Szabo C. Regulation of bacteriophage T5 development by ColI factors. J Virol. 1972 May;9(5):804–812. doi: 10.1128/jvi.9.5.804-812.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Ozeki H. Early abortive lysis by phage BF23 in Escherichia coli K-12 carrying the colicin Ib factor. J Virol. 1968 Nov;2(11):1249–1254. doi: 10.1128/jvi.2.11.1249-1254.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The electrochemical proton gradient in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):848–854. doi: 10.1021/bi00624a006. [DOI] [PubMed] [Google Scholar]
- Schein S. J., Kagan B. L., Finkelstein A. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978 Nov 9;276(5684):159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- Strobel M., Nomura M. Restriction of the growth of bacteriophage BF23 by a colicine I (Col I-P9) factor. Virology. 1966 Apr;28(4):763–765. doi: 10.1016/0042-6822(66)90263-7. [DOI] [PubMed] [Google Scholar]
- Szabo C., Dharmgrongartama B., Moyer R. W. The regulation of transcription in bacteriophage T5-infected Escherichia coli. Biochemistry. 1975 Mar 11;14(5):989–997. doi: 10.1021/bi00676a018. [DOI] [PubMed] [Google Scholar]
- Szabo C., Moyer R. W. Purification and properties of a bacteriophage T5-modified form of Escherichia coli RNA polymerase. J Virol. 1975 Apr;15(4):1042–1046. doi: 10.1128/jvi.15.4.1042-1046.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UETAKE H., LURIA S. E., BURROUS J. W. Conversion of somatic antigens in Salmonella by phage infection leading to lysis or lysogeny. Virology. 1958 Feb;5(1):68–91. doi: 10.1016/0042-6822(58)90006-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]



