Abstract
To further our understanding of the effects of nicotine on the molecular responses of macrophages during virus or virus-like infections, poly(I:C)-stimulated macrophage-like RAW264.2 cells or mouse primary peritoneal macrophages were challenged with nicotine; and their molecular responses were evaluated using a qRT-PCR array, antibody array, ELISA, Western blotting, and Ca2+ imaging. Of 51 genes expressed in the Toll-like receptor (TLR) and RIG-I-like receptor (RLR) pathways, mRNA expression of 15 genes in RAW264.7 cells was attenuated by nicotine, of which mRNA expression of IL-6, TNF-α, and IL-1β was confirmed to be attenuated in peritoneal macrophages. Concurrently, nicotine treatment attenuated the release of IL-6 and TNF-α from poly(I:C)-stimulated macrophages. However, when poly(I:C)-stimulated macrophages were challenged with nicotine plus α-bungarotoxin (α-BTX), secretion of IL-6 and TNF-α was found to be in a level seen with poly(I:C) stimulation only, indicating that α7-nAChR, a highly Ca2+ permeable ion channel sensitive to blockade by α-BTX, is involved in this process. Furthermore, results from an antibody array indicated that nicotine treatment attenuated the phosphorylation of 82 sites, including Thr286 on CaMKIIα, from poly(I:C)-stimulated RAW264.7 cells, of which 28 are expressed in the downstream cascade of Ca2+ signaling. Coincidentally, poly(I:C)-stimulated macrophages showed attenuated expression of phosphorylated CaMKIIα when pretreated with nicotine. In addition, nicotine attenuated intracellular Ca2+ signal from poly(I:C)-stimulated RAW264.7 cells. Collectively, these results indicate that poly(I:C)-induced molecular responses of macrophages could be significantly attenuated by nicotine.
Introduction
Recreational abuse of drugs is a public health concern with far-reaching clinical implications. Molecular and epidemiologic evidence suggests that viral infection severity is affected by nicotine consumption (Kark et al., 1982; Friedman, 1996; Sopori, 2002; Arcavi and Benowitz, 2004; Razani-Boroujerdi et al., 2004; Lifson and Lando, 2012 ). Nicotine is a ligand of nicotinic acetylcholine receptors (nAChR). Although numerous nAChR subtypes can be construed in theory because of the availability of 16 nAChR gene products, only a few, such as α7*, α4*, α6*, and α3* (* = known or presumed additional subunits), having physiologic relevance have been described. Functional consequences of nAChR in nonneuronal tissues such as immune and endothelial cells are emerging (Razani-Boroujerdi et al., 2007; Pena et al., 2011). Immune cells such as monocytes (Yoshikawa et al., 2006), macrophages (Matsunaga et al., 2001; Wang et al., 2003; Wang et al., 2004; de Jonge et al., 2005), and T lymphocytes (Razani-Boroujerdi et al., 2007) express nAChR subunit(s) (Sato et al., 1999; Hao et al., 2011). Binding of nAChR agonists such as nicotine, acetylcholine, GTS-21, and DMPP to α7- or α7-like nAChR attenuates or suppresses inflammation (Borovikova et al., 2000; Wang et al., 2003; Blanchet et al., 2006; Razani-Boroujerdi et al., 2007; Mikulski et al., 2010; Kox et al., 2011). Hence, cholinergic signaling via nAChR in immune cells is the subject of attempts to develop treatments for inflammatory diseases/disorders and gives rise to the concept of a “cholinergic anti-inflammatory pathway” (Pavlov et al., 2003).
Toll-like receptor (TLR) pathways, especially TLR4, the receptor for Gram-negative bacterial lipopolysaccharide (LPS) (Wittebole et al., 2010), α7-nAChR, and multiple signaling pathways (Sugano et al., 1998; Wang et al., 2004; de Jonge et al., 2005; Arredondo et al., 2006; Hamano et al., 2006; Park et al., 2008; Cui and Li, 2010) are involved in the anti-inflammatory effect of nicotine. Stimulation of TLRs activates transcription factor nuclear factor-κB (NF-κB) and stress-activated protein kinases (Slack et al., 2000; Beutler, 2004) involved in the expression of mRNAs of inflammatory cytokines. In a sepsis model, nicotine inhibited TLR4-induced inflammation and improved survival by interacting with α7-nAChR (Wang et al., 2003, 2004; Cui and Li, 2010). Treatment with cholinergic agonist(s) activates the JAK2-STAT3 pathway and suppresses NF-κB activity (de Jonge et al., 2005; Yoshikawa et al., 2006). Nicotine also suppresses expression of TLR4 antigens on monocytes and production of tumor necrosis factor (TNF)-α peptides by human peripheral blood mononuclear cells (PBMCs) in the presence of LPS (Hamano et al., 2006). These actions are blocked by a nonselective and a selective α7-nAChR antagonist, mecamylamine and α-bungarotoxin, respectively. Moreover, a NF-κB and a p38 MAPK inhibitor mimicked the actions of nicotine in the presence of LPS (Hamano et al., 2006). Both GTS-21, an α7-nAChR partial agonist, and nicotine inhibit the release of proinflammatory cytokines by PBMCs, monocytes, and whole blood independent of the TLR stimulated. The effects of GTS-21 and nicotine are not blocked by nAChR antagonists, whereas the JAK2 inhibitor AG490 abolished the effects. GTS-21 downregulated monocyte cell-surface expression of TLR2 and TLR4 antigens. Quantitative PCR analysis demonstrated that the anti-inflammatory effect of GTS-21 is mediated at the transcriptional level and involves JAK2-STAT3 activation (Kox et al., 2009). The absence of blocking by nAChR antagonists in human leukocytes suggests different pharmacological properties of α7-nAChR in leukocytes than in other cell types (Kox et al., 2009). These data imply a role for nicotine or nicotinic agonists in modulating cytokine production and TLR expression in sepsis and inflammation.
Endosomal TLR3 is an important mediator of virus-induced inflammation. Downstream signaling is dependent on the TRIF pathway. Interaction of TLR3 with double-stranded viral RNA triggers inflammatory responses protecting the host from infection. Cytoplasmic RIG-I-like receptors (RLRs), which share downstream cascades with TLR3, also sense virus RNA and affect virus-induced inflammation (Kumar et al., 2006).
To understand how nicotine alters the responses of a host to viruses, macrophages stimulated with poly(I:C) (mimicking virus infection) were treated with nicotine. We first used a qRT-PCR array to measure mRNA expression of 51 key genes to determine how genes in the TLR pathways could be modulated by nicotine. We then used an array containing more than 1,000 protein-phosphorylation-targeted antibodies to examine protein activity. Western blotting was conducted to measure phosphorylation of CaMKIIα, a key Ca2+/CAM (calmodulin)-dependent kinase. We also measured intracellular Ca2+ flux. Nicotine attenuated mRNA and protein expression of interleukin (IL)-6, TNF-α, and other cytokines and altered the phosphorylation of many kinases/phosphoproteins, including CaMKIIα with poly(I:C) stimulation only, and intracellular Ca2+ flux. In sum, nicotine attenuates poly(I:C)-induced responses in macrophages.
Materials and Methods
Cell Culture, Primary Cell Preparation, and Treatments.
Mouse RAW264.7 macrophages were purchased from the American Type Culture Collection (Manassas, VA) and cultured in high-glucose DMEM supplemented with 10% fetal bovine serum (FBS; GIBCO Invitrogen, Grand Island, NY) at 37°C in a humidified atmosphere of 95% air and 5% CO2. When the cells reached about 80% confluence, they were treated with 5 μM nicotine (Sigma, St. Louis, MO) for 10 minutes or with PBS buffer and then stimulated with either poly(I:C) at a concentration of 20 μg/ml or control buffer for the indicated period of time. Thus, RAW264.7 macrophages received these treatments: PBS alone, poly(I:C) alone, nicotine alone, or poly(I:C) + nicotine. In some experiments, RAW264.7 macrophages were also incubated with α-bungarotoxin [α-BTX; a α7-nicotinic acetylcholine receptor (nAChR)-specific antagonist] at 1 μg/ml for 30 minutes before nicotine administration. Hence, the additional treatment options, depending on the nature of experiments, were α-BTX alone, α-BTX + nicotine, and α-BTX + nicotine + poly(I:C).
Primary peritoneal macrophages, which were harvested by intraperitoneal lavage with sterile PBS from C57BL/6 mice previously injected for 3 days with 4% thioglycolate solution, were used in some experiments. For some experiments, these macrophages were cultured and treated in the same way as the RAW264.7 cells.
All experiments were carried out in accordance with the Institutional Animal Care and Use Committee at the University of Virginia and were consistent with federal guidelines.
RNA Isolation and Quantitative RT-PCR Array.
Total RNA was isolated from treated mouse RAW264.7 or primary peritoneal macrophages using Trizol reagent (Invitrogen, Carlsbad, CA). To eliminate any residual DNA, each sample was treated with RNase-free DNase I at 37°C for 30 minutes, followed by inactivation at 65°C for 10 minutes. The quantitative RT-PCR array was conducted as described previously (Cao et al., 2011; Wei et al., 2011; Cui et al., 2012). Briefly, 2 μg of total RNA was reverse transcribed using Superscript II RT, and the mixture was incubated at 25°C for 10 minutes, 42°C for 1.5 hours, and 70°C for 15 minutes. The RT product was amplified in a volume of 10 µl containing 5 µl 2× Power SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) and combined sense and antisense primers (3 µl; final concentration 250 nM) in a 384-well plate using the 7900HT fast real-time PCR System (Applied Biosystems) with the following thermal cycling conditions: 1 cycle at 50°C for 2 minutes, initial denaturation at 95°C for 10 minutes, 40 cycles of denaturation at 95°C for 15 seconds, and annealing/extension at 60°C for 1 minute. The mRNA expression of genes studied was normalized to the mRNA expression of β-actin and then analyzed using a comparative Ct method (Winer et al., 1999). The relative gene expression was compared among different treatment groups by the analysis of variance (ANOVA) test via MATLAB (The Mathworks, Natick, MA). Table 1 provides detailed information on primer sequences for genes of interest, which were designed using Primer Express Software 3.0 (Applied Biosystems, Carlsbad, CA).
TABLE 1.
Primers included in qRT-PCR array for measurement of relative mRNA expression in treated or untreated RAW264.7 cells
| Gene Symbol | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| β-ACTIN * | TGCCGCATCCTCTTCCTC | CGCCTTCACCGTTCCAGT |
| CD14 | AGCCTATCTGGGCTGCTCAA | CACCAGAAGCAACAGCAACAA |
| ECSIT | GGGCCGGAAGACCTCCTT | GGTGTTGGGCAGTTACCATGT |
| EIF2AK2 | AAACTGCCGGAACATCCTCTAG | GGGAAACACCATTACTTGTCATAGAC |
| ELK1 | TGACCCACCCAAACAAACCT | GGTAACTGGCAGGAATTAGAGAAGA |
| FADD | ACTGCCATGGACCCATTCC | CGCGGCACAAGAACTTGAG |
| GAPDH * | CAGCAGTAGCAGCAGACTATTAAACTAAA | GCTGGCACTGCACAAGAAGA |
| IFNA4 | ACTGGTCAGCCTGTTCTCTAGGA | GGACTGTCAAGGCCCTCTTG |
| IFNAR1 | TGGGCAGTGTGACCTTTTCA | GTTGTAGTATGTTGACATTCAGGCACTT |
| IFNAR2 | CAGACTACATCGTGCCTGCAA | GGTCTGTAAAGCCAACGATCTCA |
| IFNB1 | CCATCATGAACAACAGGTGGAT | GAGAGGGCTGTGGTGGAGAA |
| IFNγ | TTGCCAAGTTTGAGGTCAACAA | TGGTGGACCACTCGGATGA |
| IKKα | GGCACAGTAACCCCTCCAGTAT | CCACACATGTCAGAGGATGTTCA |
| IKKβ | AGCTGTCCTTACCCTGCTGAGT | CAGGTCTCGATGGATGATTCTG |
| IKKε | GGTGTCACCGCAACCTATGG | TCATTACAAAGCTCCTGCATGTG |
| IKKγ | GAGCCAGAGCCGTTTGGA | CTGCTCGAATCCTTCCCTCTAA |
| IL-12A | CATCCAGCAGCTCCTCTCAGT | GCAAGGGTGGCCAAAAAGA |
| IL-12B | TGGTGCAAAGAAACATGGACTT | CACATGTCACTGCCCGAGAGT |
| IL-1β # | AGGACATGAGCACCTTCTTTTCC | ACGTCACACACCAGCAGGTTAT |
| IL-6 # | TCGGAGGCTTAATTACACATGTTC | CATACAATCAGAATTGCCATTGC |
| IPS-1 | ACCTGTCCTGCCTCACAGCTA | TCCCGGTTCCCGATCTG |
| IRAK1 | AATGGCTCCTTGGAGGATCA | AGTCGTTGAGGCCAGGAAAGT |
| IRAK4 | TGTCCTGCCTGGATGGTACA | GCTGTCCCCTGAGCAACCT |
| IRF-3 | TTCGTGGCAGATCTGATTGC | ACATTTCCCCCATGCAGAAC |
| IRF-7 | CCCCAGCCGGTGATCTTT | TGGAGAAGCGTCTCTGTGTAGTG |
| LY96 | GTTCTGAACCCTGCATAAGACTGA | TCTATGGAGTTGACACTGATGAATAGG |
| MDA-5 | TGGGATGGACGCAGATGTT | CAGTGAGTGTGGGTTTGACATAGC |
| MEKK1 | TCCAGTAACATACACAGGCCAAAG | GCGTCCCCTAGTTTGCTTGTAC |
| MyD88 | GCCAGCGAGCTAATTGAGAAA | TCCTTGCTCTGTAGATAATCGTCAGA |
| NFKB1 | TGGCAGCTCTTCTCAAAGCA | CCAAGAGTCGTCCAGGTCATAGA |
| NFKBIA | CTGCACACCCCAGCATCTC | CAGACACGTGTGGCCATTGT |
| NIK | CCCCATCCTTTCCCATTCTAC | TTTGTCCAGGACGGCAGAGT |
| PGLYRP1 | CCCATCTGGAATCCCATGTC | CACCCCACATTCCAGAAGATTT |
| PPARA | CAAGGCCTCAGGGTACCACTAC | GCCGAATAGTTCGCCGAAA |
| RELA | GCCCATGGAGTTCCAGTACTTG | GTCCTTTTGCGCTTCTCTTCA |
| RIG-I | CGGCACCCAGAAATATGAACA | CTCCTCTTTGTCTGCCATCTGA |
| RIP-1 | CCCCACCGTGCAGATCA | AGGACCACGGTGCCAAAG |
| RIPK-1 | GAACTATTCGCTGGTGATGGAGTA | CACGATTATCCTTCCTTTCAATGA |
| TAB1 | ACCACACCACCGAGAACGA | CGCCCATCTGCTTGATCTTC |
| TAB2 | TCGGCATTTCTGAGCCAAA | CAGGCGACCTTCTTGAACTTTAG |
| TAK1 | AGTGGCTTACCTGCACAGCAT | CAGCAAGTTTGGAGGCTTGAG |
| TBK1 | GGTGGGCGGGATGAATC | TGATGTTGCCTGGCTTGATATC |
| TIRAP | GGCCTGCACTATGGCTTCAT | TGCCTGAACCAGTCAGCTATCTT |
| TLR3 | TTAAAAGACCCTCTGTGCAGAAGA | CGCAAACAGAGTGCATGGTT |
| TLR4 | ACTCTGATCATGGCACTGTTCTTC | TCCATGCATTGGTAGGTAATATTAGG |
| TNF-α # | GGTCCCCAAAGGGATGAGAA | TGAGGGTCTGGGCCATAGAA |
| TNFAR1 | GGTAATTCTGGGAAGCCGTAAA | TGCCCCTGCGGATGAA |
| TNFRSF1A | TCACAAACCCCCAGGACTCA | GATAGAAGGCAAAGACCTAGCAAGA |
| TOLLIP | TGTGGACTCGTTCTACCTTGAGATC | GGTCCAAGCTATGCGGTCAT |
| TRAF6 | TCTGCAAAGCCTGCATCATC | GATTTTCCAGCAGTATTTCATTGTCA |
| TRAM | TGCCCCTGCGGATGAA | CCCATCAATCCAACCCTTTTATT |
| TRIF | CCACCTTCTGTGAGGAATTTCAG | TCGATGGCATCTTGGAGACA |
Genes serving as internal controls.
Genes assayed for relative mRNA expression in treated or untreated mouse primary peritoneal macrophages.
Enzyme-Linked Immunosorbent Assay.
The IL-6 and TNF-α concentrations in the culture supernatant liquids of treated RAW264.2 and primary peritoneal macrophages were measured with enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s protocols.
Calcium Imaging.
The RAW 264.7 mouse macrophages cultured on glass-bottom 35-mm dishes were loaded with 2.5 µM of the fluorescent indicator fluo-4 AM (Invitrogen) in DMEM buffer containing Pluronic F-127 (0.05%; Invitrogen) for 1 hour at 37°C. After labeling, cells were washed 3 times with Tyrode’s solution and then de-esterified for 30 minutes in DMEM at 23°C. The cells were examined using an inverted epifluorescence microscope IX81 (Olympus, Center Valley, PA), and images were captured by a Hamamatsu 12-bit digital CCD camera (C9300-221). Images were collected for 10 minutes at 1-second intervals under UPlanFL N PH 10×/0.30 objective lenses using IPLab software (BD Biosciences). To evaluate intracellular calcium [Ca2+]i dynamics, in a given field of view, individual cells were outlined manually, and the average fluorescence intensity of the selected cells (usually 6–8 cells per experiment) was determined using the ImageJ processing program developed at the National Institutes of Health (http://rsb.info.nih.gov/ij/). All fluorescence measurements are expressed as a ratio of fluorescence intensity (F) to the basal fluorescence intensity (F0). Data collected from three independent experiments were analyzed by ANOVA test via MATLAB.
Western Blot Analysis.
Treated or control macrophages were washed with PBS and digested with 500 μl of RIPA buffer (50 mM Tris-HCl pH 7.5, 0.5% deoxycholic acid, 0.1% SDS, 150 mM NaCl, 1% NP-40). After incubation on ice for 30 minutes, the samples were centrifuged at 12,000 rpm for 15 minutes, and the supernatant liquids were collected. For each sample, 10 μg of total protein was electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel and then transferred electrophoretically to polyvinylidenedifluoride (PVDF) membranes (Millipore, Bedford, MA). After blocking with 1% BSA in TBST buffer at room temperature for 1 hour, the filters were incubated with primary antibody (1:1000) overnight at 4°C. Immunoreactivity was detected using the SuperSignal West Pico Chemiluminescent Substract kit (Pierce, Rockford, IL), and the preparations were exposed to X-ray film. After the films were developed, they were scanned on a Microtek ScanMaker i800 with ScanWizard 5.5 at a resolution of 600 dpi for quantitative analysis with ImageQuant 5.1 (Molecular Dynamics, Sunnyvale, CA). The amounts of phosphorylated CaMKIIα (p-CaMKIIα) were normalized to α-tubulin, and the significance of the difference between the treated and control samples was determined by Student’s t test via MATLAB.
Hybridization of Protein Antibody Array and Data Analysis.
The protein antibody array, Signaling Explorer Antibody Array, was purchased from Full Moon Biosystems, Inc. (Sunnyvale, CA) and processed according to the protocol suggested by the manufacturer. Briefly, 25 μl of purified RAW264.7 cell lysate (at a concentration of 7 μg/μl) was incubated with 3 μl of biotin/DMF (10 μg/μl) for 30 minutes at room temperature. The array was treated with blocking solution for 45 minutes and then incubated with the biotin-labeled protein in 6 ml of coupling buffer for 2 hours on an orbital shaker at 35 rpm. The array was washed thoroughly and then incubated for 20 minutes with Cy3-streptavidin diluted in detection buffer. After a second washing, the hybridized array was air-dried and captured using the ScanArray Gx scanner, and the intensity of each probe was quantified with the ScanArray Express microarray analysis system (PerkinElmer, Waltham, CA).
Three independent cell cultures for each experimental group were prepared under the same conditions and used for the antibody array experiments. The expression of each protein contained in the array was normalized to β-actin (included in the array as an internal control), and then to the saline control group.
Statistical and Bioinformatics Analysis.
Depending on the specific experiment, statistical significance was determined by Student’s t test or ANOVA in MATLAB. All results are expressed as the mean ± S.E.M. for at least three independent experiments. The genes significantly regulated by each treatment were examined by ingenuity pathway analysis (IPA; https://analysis.ingenuity.com), where downregulated or upregulated genes are indicated in green or red, respectively, as commonly seen in microarray-related reports.
Results
Nicotine Reduces mRNA Expression for 15 Key Genes from a Panel of 51 Genes in Innate Immune Pathways in Poly(I:C)-stimulated RAW264.7 Macrophages.
To investigate the effects of nicotine on gene expression at the mRNA level during virus infection or virus-like treatment, mouse RAW264.7 macrophages were exposed to 5 μM nicotine for 10 minutes prior to stimulation with poly(I:C) 20 μg/ml. Results from a custom-designed qRT-PCR array, which contained 51 key genes involved in the TLR3, TLR4, and RLRs signaling pathways (see Table 1 for a detailed list), indicated that nicotine significantly reduced mRNA expression for 15 of them (Table 2).
TABLE 2.
mRNA expression significantly suppressed by nicotine treatment in poly(I:C)-induced RAW264.7 cells (n ≥ 3/group)
| Gene Symbol | Name | Poly(I:C)/ Control (mean ± S.E.M.)* | Nic+Poly(I:C)/ Control (mean ± S.E.M.)* | Ratio# (R) | P Value |
|---|---|---|---|---|---|
| CD14 | CD14 molecule | 0.79 ± 0.04 | 0.54 ± 0.02 | 0.68 | 0.006 |
| EIF2AK2 | Eukaryotic translation initiation factor 2-alpha kinase 2 | 3.26 ± 0.14 | 2.48 ± 0.21 | 0.76 | 0.007 |
| FADD | Fas (TNFRSF6)-associated via death domain | 0.49 ± 0.03 | 0.27 ± 0.09 | 0.55 | 0.021 |
| IKKε | Inhibitor of kappaB kinase epsilon | 2.52 ± 0.05 | 1.74 ± 0.95 | 0.69 | <0.001 |
| IL-1β | Interleukin 1 beta | 6.02 ± 0.34 | 3.25 ± 0.85 | 0.54 | 0.006 |
| IL-6 | Interleukin 6 | 57.83 ± 2.76 | 27.54 ± 7.98 | 0.48 | <0.001 |
| IRAK-4 | Interleukin-1 receptor-associated kinase 4 | 0.57 ± 0.03 | 0.36 ± 0.05 | 0.63 | 0.009 |
| IRF-7 | Interferon regulatory factor 7 | 146.62 ± 11.62 | 91.1 ± 12.97 | 0.62 | 0.002 |
| LY96 | Lymphocyte antigen 96 | 0.54 ± 0.02 | 0.37 ± 0.01 | 0.69 | 0.002 |
| MDA-5 | Melanoma differentiation associated protein-5 | 9.79 ± 0.53 | 6.83 ± 0.04 | 0.70 | <0.001 |
| PPARA | Peroxisome proliferator activated receptor alpha | 3.01 ± 0.20 | 1.13 ± 0.23 | 0.38 | <0.001 |
| RIG-I | Retinoic acid-inducible gene-I | 21.48 ± 0.31 | 15.33 ± 3.30 | 0.71 | 0.028 |
| TIRAP | Toll-interleukin 1 receptor (TIR) domain containing adaptor protein | 0.71 ± 0.03 | 0.5 ± 0.09 | 0.70 | 0.029 |
| TLR3 | Toll-like receptor 3 | 3.08 ± 0.16 | 1.89 ± 0.61 | 0.61 | 0.030 |
| TNF-α | Tumor necrosis factor alpha | 1.67 ± 0.12 | 1.1 ± 0.22 | 0.66 | 0.026 |
Expression of each gene was normalized to β-actin and then divided by PBS-control group.
Ratio = [Nic + poly(I:C)]/poly(I:C).
To predict the biologic relations among genes whose expressions were reduced by nicotine treatment, we performed functional group analysis using the gene ontology enrichment program implemented in IPA bioinformatics software. These genes were classified into three pathways, i.e., TLR3, TLR4, and RLR (Fig. 1). For the TLR3 pathway, mRNA expression of nine genes was significantly reduced by nicotine (P < 0.05; Fig. 1A). These were eukaryotic translation initiation factor 2-alpha kinase 2 (EIF2AK2; R = 0.76; P = 0.007), Fas-associated via death domain (FADD; R = 0.55; P = 0.021), inhibitor of kappaB kinase epsilon (IKKε; R = 0.69; P < 0.001), IL-1β (R = 0.54; P = 0.006), IL-6 (R = 0.48; P < 0.001), interferon regulatory factor 7 (IRF-7; R = 0.62; P = 0.002), peroxisome proliferator activated receptor alpha (PPARA; R = 0.38; P < 0.001), TLR3 (R = 0.61; P = 0.03), and TNF-α (R = 0.66; P = 0.026).
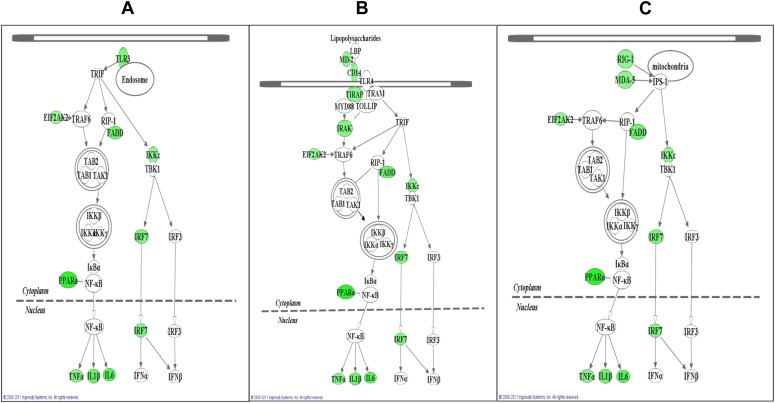
Fig. 1.
Genes whose mRNA expression is suppressed by nicotine in RAW264.7 cells under poly(I:C) stimulation compared with those under poly(I:C) stimulation only are mapped to three gene networks, as predicted by ingenuity pathway analysis (IPA). Cells were stimulated with poly(I:C) at 20 μg/ml with and without prior treatment with 5 μM nicotine and then subjected to quantitative RT-PCR analysis (qRT-PCR) using an array. A total of 51 genes was assayed, including two control genes (β-actin and GAPDH) (Table 1). These genes could be classified into three pathways following IPA analysis: (A) TLR3, which is a major receptor for poly(I:C), depends on the TRIF pathway, and leads to activation of IRFs and NF-κB; (B) TLR4, which depends on both the TRIF and MyD88 pathways and mediates bacteria-induced inflammation; and (C) RLR, whose receptor mRNA expression can be induced by TLR3 and that shares similar downstream signaling with TLR3 and plays a key role in virus-induced innate immune responses. mRNA expression of 15 genes was found to be significantly suppressed in RAW264.7 cells when nicotine-treated cells were further challenged with poly(I:C) (Table 1) compared with the poly(I:C)-only treated cells (P < 0.05). These significantly downregulated genes are highlighted in green in the three pathways described above. For a detailed summary of expression change of each gene, please see Table 2.
In the TLR4 pathway (Fig. 1B), mRNA expression of 12 genes was significantly reduced by nicotine. These were CD14 molecule (CD14; R = 0.68; P = 0.006), EIF2AK2 (R = 0.76; P = 0.007), FADD (R = 0.55; P = 0.021), IKKε (R = 0.69; P < 0.001), IL-1β (R = 0.54; P = 0.006), IL-6 (R = 0.48; P < 0.001), interleukin-1 receptor-associated kinase 4 (IRAK4; R = 0.63; P = 0.009), IRF-7 (R = 0.62; P = 0.002), lymphocyte antigen 96 (LY96; R = 0.69; P = 0.002), PPARA (R = 0.38; P < 0.001), Toll-interleukin 1 receptor [TIR] domain-containing adaptor protein (TIRAP; R = 0.70; P = 0.029), and TNF-α (R = 0.66; P = 0.026).
Because 1) retinoic acid-inducible gene-I (RIG-I) and melanoma differentiation-associated protein-5 (MDA-5) play key roles in sensing double-stranded RNA (dsRNA), as does TLR3, and 2) they are involved in TLR3 signaling downstream and can be induced by TLR3, we measured the mRNA expression of these two sensor genes and their adaptor, IPS-1. As shown in Table 2, mRNA expression of RIG-I and MDA-5 was significantly reduced in poly(I:C)-stimulated macrophages by treatment with nicotine (P < 0.05). By combining the mRNA expression results from the three genes and the genes involved in TLR pathways, we found that all the genes in the RLR pathway were suppressed by nicotine, as were the genes in TLR3 and TLR4.
Nicotine Treatment Reduces Release of IL-6 and TNF-α Proteins from Poly(I:C)-stimulated RAW264.7 Macrophages.
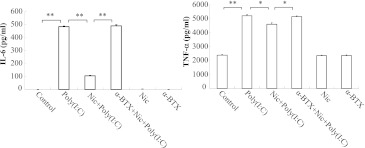
Because both IL-6 and TNF-α are products of TLR3 signaling and are representative proinflammatory cytokines, we used them as indicators of pathway activation and the inflammation state of the cell. As shown in Fig. 2, the protein concentrations of poly(I:C)-induced IL-6 and TNF-α were significantly (P < 0.05) lower in the presence of nicotine, with a maximum inhibition of about 80% and 20%, respectively, as measured by ELISA.
Fig. 2.
Protein expression of proinflammatory cytokines in RAW264.7 cells. Cells were treated with α-BTX 1 μg/ml for 30 minutes and then 5 μM nicotine for 10 minutes followed by stimulation with poly(I:C) 20 μg/ml for 12 hours. The protein concentrations of IL-6 and TNF-α were measured by ELISA. Data are shown as the mean ± S.E.M. from three independent experiments performed under identical conditions (*P < 0.05; **P < 0.01).
α-Bungarotoxin Treatment Indicates that the Inhibitory Effect of Nicotine on Release of Inflammatory Cytokines from Poly(I:C)-induced RAW264.7 Cells Involves α7-nAChR.
Earlier studies (Wang et al., 2003) showed that nicotine inhibits the expression of proinflammatory cytokines in TLR4-activated macrophages by specifically interacting with α7-nAChR. Given the similar considerations, in this study, we used α-BTX, an α7-nAChR-specific antagonist, to determine whether α7-nAChR was involved in the release of proinflammatory cytokines from poly(I:C)-induced RAW264.7 cells. When poly(I:C)-stimulated cells were treated with nicotine plus α-BTX, the inhibitory effect of nicotine on poly(I:C)-induced release of inflammatory cytokines was blocked, as determined by ELISA (Fig. 2). This indicated that nicotine inhibits TNF-α and IL-6 release through a process that involves α7-nAChR.
Nicotine Reduces Proinflammatory Cytokine Expression from Poly(I:C)-stimulated Mouse Primary Peritoneal Macrophages at Both the mRNA and Protein Levels.
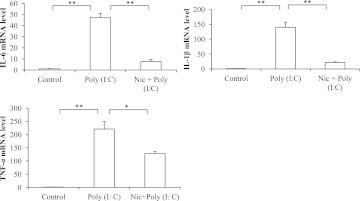
To reproduce and confirm the earlier findings from RAW264.7 cells in an independent system that also would reflect the inflammatory indices ex vivo, we examined the expression of a few key proinflammatory cytokines at both the mRNA and protein levels in mouse primary peritoneal macrophages. To this end, peritoneal macrophages received treatment identical to that given to RAW264.7 cells [i.e., 5 μM nicotine exposure following stimulation with poly(I:C) at 20 μg/ml]. Expression of IL-6, TNF-α, and IL-1β mRNA was significantly suppressed by nicotine, with a maximum reduction of 42%, 84%, and 84%, respectively (Fig. 3). These results were very similar to the ones shown for TNF-α, IL-1β, and IL-6 mRNA expression from similarly treated RAW264.7 cells.
Fig. 3.
Nicotine’s effect on proinflammatory cytokine mRNA expression in poly(I:C)-stimulated mouse primary peritoneal macrophages. Macrophages were first treated with 5 μM nicotine for 10 minutes and then treated with poly(I:C) 20 μg/ml for 4 hours. The expression of IL-1β, IL-6, and TNF-α mRNA was measured by qRT-PCR. Data are shown as the mean ± S.E.M. from three independent experiments performed under identical conditions (*P < 0.05; **P < 0.01).
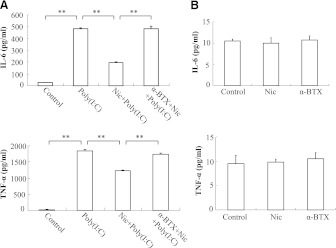
Next, we used an ELISA to measure the expression of TNF-α and IL-6 at the protein level and found that nicotine suppressed poly(I:C)-induced TNF-α and IL-6 protein accumulation in the primary culture medium with a maximum inhibition of 35% and 60%, respectively (Fig. 4). We further found that the anti-cytokine release effect of nicotine was blocked by α-BTX, whereas treatment with nicotine or α-BTX alone had no effect on proinflammatory cytokine expression. These results again indicated that nicotine inhibited the release of the proinflammatory cytokines TNF-α and IL-6 from poly(I:C)-treated primary mouse peritoneal macrophages. This was consistent with our earlier findings in RAW264.7 cells.
Fig. 4.
Quantitation of proinflammatory cytokine proteins in mouse primary peritoneal macrophages. (A) macrophages were treated with α-BTX at 1 μg/ml for 30 minutes and then 5 μM nicotine for 10 minutes, followed by stimulation with poly(I:C) at 20 μg/ml for 12 hours. The production of IL-6 and TNF-α was measured by ELISA. (B) macrophages were treated with PBS (control), 5 μM nicotine, or α-BTX at 1 μg/ml for 12 hours; and the amounts of IL-6 and TNF-α protein were measured by ELISA. Data are shown as the mean ± S.E.M. from three independent experiments performed under identical conditions (**P < 0.01).
Nicotine Reduces Poly(I:C)-induced Protein Phosphorylation in RAW264.2 Cells.
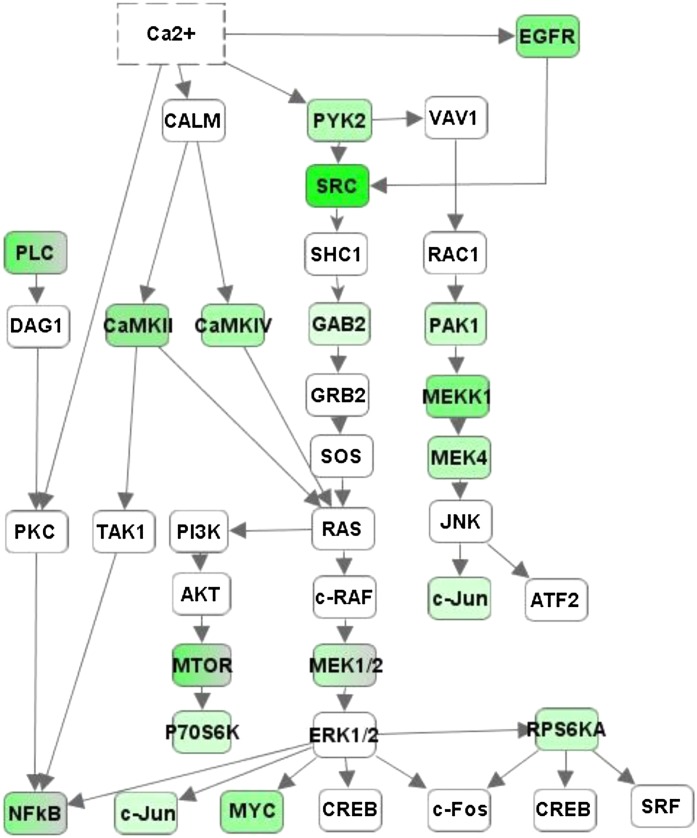
To reveal the modulatory effect of nicotine on signaling pathway activity during poly(I:C) stimulation, we used an antibody array that contained 1318 phosphoprotein-targeted antibodies. We found that 82 sites were significantly inhibited by nicotine in poly(I:C)-stimulated RAW264.7 cells (P < 0.05). Then we performed pathway analysis for the 82 proteins and found that 28 of them are involved in the downstream region of the calcium signaling pathway. As shown in Fig. 5, these proteins can be classified into four branches as follows: 1) calcium/calmodulin-activated kinase signaling, such as calcium/calmodulin-dependent protein kinase IV (CaMKIV); 2) protein tyrosine kinase 2 beta signaling, such as PYK2; 3) epidermal growth factor receptor signaling, such as EGFR; and 4) PKC signaling, such as phospholipase C, gamma 1 (PLCG1) and phospholipase C, gamma 2 (PLCG2). It is clear that some of the branches share downstream molecules, such as the v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (SRC); GRB2-associated binding protein 2 (GAB2); mitogen-activated protein kinase kinase 1 (MEKK1); mitogen-activated protein kinase kinase 2 (MEKK2); p21 protein (Cdc42/Rac)-activated kinase 1 (PAK1); mitogen-activated protein kinase kinase kinase 1 (MEKKK1); mitogen-activated protein kinase kinase 4 (MEKK4); mechanistic target of rapamycin (serine/threonine kinase; mTOR); ribosomal protein S6 kinase, 90 kDa, polypeptide 1 (P90RSK); ribosomal protein S6 kinase, 70 kDa, polypeptide 1 (P70S6KB1); and ribosomal protein S6 kinase, 70 kDa, polypeptide 2 (P70S6KB2). These pathways lead to activation of multiple transcription factors, including NF-κB, which is responsible for the transcription of many inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 (Yoshikawa et al., 2006). The phosphorylation of two subunits of NF-κB was also significantly suppressed by nicotine in poly(I:C)-stimulated RAW264.7 cells, including v-relreticuloendotheliosis viral oncogene homolog A and nuclear factor of kappa light polypeptide gene enhancer in B cells 1 (NF-κB1). A detailed description of the phospho-sites for each gene and their quantitative parameters, such as the mean ± S.E.M. [(nicotine + poly(I:C))/poly(I:C)] ratio (R), P value, and FDR are provided in Table 3.
Fig. 5.
Nicotine’s effect on multiple signal transducers in the downstream pathway of calcium. Using IPA software, a calcium downstream pathway containing four signaling branches was generated: CAMK, PYK2, EGFR, and PLC. Significantly downregulated genes are shown in green. For a detailed summary of changes in each phospho-site, please see Table 3.
TABLE 3.
Effect of nicotine treatment on poly(I:C)-triggered phosphorylation of multiple calcium-inducible genes (n = 3/group) in RAW264.7 cells
| Name | Gene Name | Phosphorylation Site | Poly (I:C)/Control (Mean ± S.E.M.)* | Nic+Poly (I:C)/Control (Mean ± S.E.M.) * | Ratio # (R) | P Value | FDR |
|---|---|---|---|---|---|---|---|
| MEK4 | Mitogen-activated protein kinase kinase 4 | Ser257 | 1.84 ± 0.13 | 0.94 ± 0.43 | 0.51 | <0.001 | 0.003 |
| Myc | v-Myc myelocytomatosis viral oncogene homolog | Ser373 | 2.2 ± 0.03 | 0.83 ± 0.28 | 0.38 | <0.001 | <0.001 |
| P70S6KB1 | Ribosomal protein S6 kinase, 70kDa, polypeptide 1 | Ser411 | 1.65 ± 0.92 | 1.28 ± 0.07 | 0.78 | <0.001 | 0.019 |
| P70S6KB1 | Ribosomal protein S6 kinase, 70kDa, polypeptide 1 | Thr389 | 1.74 ± 0.24 | 0.91 ± 0.68 | 0.52 | <0.001 | 0.010 |
| PAK1 | p21 Protein (Cdc42/Rac)-activated kinase 1 | Ser204 | 1.75 ± 0.17 | 1.16 ± 0.64 | 0.66 | <0.001 | <0.001 |
| PLCG2 | Phospholipase C, gamma 2 | Tyr1217 | 3.09 ± 0.79 | 1.25 ± 0.07 | 0.40 | <0.001 | 0.001 |
| mTOR | Mechanistic target of rapamycin (serine/threonine kinase) | Ser2481 | 2.71 ± 0.82 | 0.72 ± 0.15 | 0.26 | <0.001 | 0.037 |
| P70S6KB1 | Ribosomal protein S6 kinase, 70kDa, polypeptide 1 | Ser371 | 1.92 ± 0.31 | 0.44 ± 0.09 | 0.23 | 0.001 | 0.031 |
| PLCG1 | Phospholipase C, gamma 1 | Tyr771 | 2.08 ± 0.28 | 0.91 ± 0.29 | 0.44 | 0.001 | 0.029 |
| PLCG1 | Phospholipase C, gamma 1 | Tyr1253 | 2.24 ± 0.23 | 0.54 ± 0.15 | 0.24 | 0.002 | 0.047 |
| PLCG2 | Phospholipase C, gamma 2 | Tyr753 | 2.3 ± 0.35 | 0.65 ± 0.12 | 0.28 | 0.002 | 0.041 |
| MEK2 | Mitogen-activated protein kinase kinase 2 | Thr222 | 2.11 ± 0.17 | 1.18 ± 0.18 | 0.56 | 0.005 | 0.073 |
| P90RSK | Ribosomal protein S6 kinase, 90kDa, polypeptide 1 | Thr573 | 0.6 ± 0.08 | 0.38 ± 0.09 | 0.63 | 0.005 | 0.072 |
| PYK2 | Protein tyrosine kinase 2 beta | Tyr402 | 2.95 ± 0.62 | 1.41 ± 0.17 | 0.48 | 0.005 | 0.073 |
| P70S6kB2 | Ribosomal protein S6 kinase, 70kDa, polypeptide 2 | Ser423 | 1.81 ± 0.39 | 1.32 ± 0.78 | 0.73 | 0.007 | 0.095 |
| NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | Ser337 | 1.28 ± 0.06 | 1.05 ± 0.07 | 0.82 | 0.009 | 0.109 |
| JNK | Mitogen-activated protein kinase 8 | Tyr185 | 1.45 ± 0.19 | 1.97 ± 0.22 | 1.35 | 0.011 | 0.12 |
| RELA | v-Rel reticuloendotheliosis viral oncogene homolog A | Ser529 | 1.09 ± 0.33 | 0.33 ± 0.13 | 0.31 | 0.024 | 0.212 |
| Src | v-Src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog | Ser75 | 2.09 ± 0.55 | 0.36 ± 0.18 | 0.17 | 0.024 | 0.212 |
| EGFR | Epidermal growth factor receptor | Tyr1092 | 4.3 ± 1.7 | 1.72 ± 0.27 | 0.4 | 0.033 | 0.261 |
| MEK1 | Mitogen-activated protein kinase kinase 1 | Ser298 | 1.65 ± 0.65 | −0.41 ± 0.67 | −0.25 | 0.038 | 0.291 |
| CaMKIIα | Ca2+/calmodulin-dependent protein kinases II | Thr286 | 1.20 ± 0.002 | 0.74 ± 0.07 | 0.62 | 0.038 | 0.291 |
| mTOR | Mechanistic target of rapamycin (serine/threonine kinase) | Ser2448 | 2.69 ± 0.87 | 1.89 ± 0.13 | 0.7 | 0.039 | 0.293 |
| MEKK1 | Mitogen-activated protein kinase kinase kinase 1 | Thr1381 | 2.87 ± 2.04 | 0.55 ± 0.10 | 0.19 | 0.042 | 0.304 |
| CaMK4 | Calcium/calmodulin-dependent protein kinase IV | Thr196/200 | 1.95 ± 0.17 | 0.84 ± 0.31 | 0.43 | 0.045 | 0.323 |
| GAB2 | GRB2-associated binding protein 2 | Tyr643 | 1.22 ± 0.14 | 1.2 ± 0.40 | 0.98 | 0.046 | 0.323 |
| c-Jun | Jun proto-oncogene | Thr91 | 1.79 ± 0.42 | 1.47 ± 0.65 | 0.82 | 0.047 | 0.323 |
| EGFR | Epidermal growth factor receptor | Tyr1016 | 1.59 ± 0.29 | 1.26 ± 0.45 | 0.79 | 0.047 | 0.323 |
Amount of protein of each phosphorylation site was normalized to β-actin and then divided by PBS-control group.
Ratio = [Nic + poly(I:C)]/poly(I:C); FDR = false discovery rate.
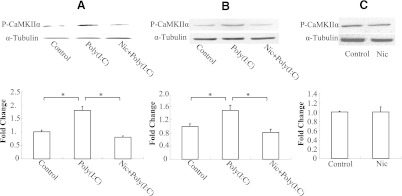
Nicotine Suppresses Poly(I:C)-stimulated Phosphorylation of CaMKIIα in Both RAW264.7 and Primary Peritoneal Macrophages.
It was shown earlier that RAW264.7 cells stimulated with poly(I:C) rapidly increase their CaMKIIα activity (Liu et al., 2008), which is accompanied by a significant increase in CaMKIIα phosphorylation (Thr286). Thus, we used CaMKIIα as a reporter gene in the TLR3 signaling pathway to study the effect of nicotine on Ca2+ signaling and verify the antibody array result. As expected, stimulation with poly(I:C) led to an increase in phosphorylation of CaMKIIα (Fig. 6), whereas nicotine treatment significantly reduced poly(I:C)-induced phosphorylation of CaMKII-α both in RAW264.7 cells (Fig. 6A) and in peritoneal macrophages (Fig. 6B) (43% and 57% reduction, respectively). The phosphorylation of CaMKIIα was also measured in peritoneal macrophages treated with nicotine alone, but no significant difference was detected compared with the control group (Fig. 6C).
Fig. 6.
Phosphorylation of CaMKIIα in RAW264.7 cells and mouse peritoneal macrophages. RAW264.7 cells (A) and mouse peritoneal macrophages (B) were treated with 5 μM nicotine for 10 minutes followed by stimulation with poly(I:C) at 20 μg/ml for 30 minutes. (C) mouse peritoneal macrophages were incubated with 5 μM nicotine or PBS (control) for 30 minutes. Phospho-CaMKIIα and α-tubulin were detected by Western blotting and quantitated using ImageQuant5.1. Data are shown as the mean ± S.E.M. from three independent experiments performed under identical conditions (*P < 0.05).
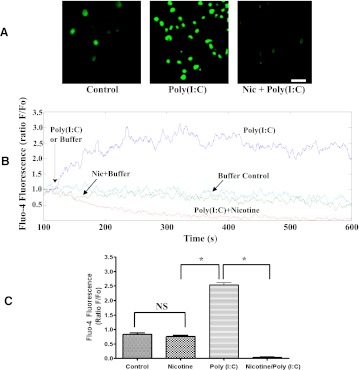
Nicotine Reduces Poly(I:C)-induced Intracellular Calcium Increase in RAW264.7 Cells.
Consistent with the results of an earlier study (Liu et al., 2008) we showed that RAW264.7 cells stimulated with poly(I:C) increase mobilization of their Ca2+ from internal stores (Fig. 7). To further understand how nicotine suppresses poly(I:C)-induced inflammation, we determined whether nicotine treatment could modify poly(I:C)-induced [Ca2+]i changes in RAW 264.7 cells. To this end, RAW264.7 cells were loaded with calcium fluorescent dye fluo-4, incubated with nicotine or vehicle control for 5–10 minutes, and then challenged with poly(I:C) 20 μg/ml [poly(I:C)+nicotine and poly(I:C), respectively] or with PBS buffer (nicotine and control, respectively) (Fig. 7A) and subjected to real-time calcium imaging. In control buffer-treated cells, poly(I:C) produced a rapid rise in intracellular Ca2+ release (Fig. 7, B and C). The increase was almost immediate and reached a maximum by 3.7 minutes after agonist addition (Fig. 7, B and C). In contrast, prior treatment of 5–10 minutes with nicotine prevented any poly(I:C)-induced calcium increase. Concurrently, application of 5 µM nicotine alone had no detectable effect on [Ca2+]i in RAW264.7 cells, which is in agreement with the findings of Mikulski et al. (2010) showing that nicotine treatment did not result in a calcium change in rat alveolar macrophages.
Fig. 7.
Nicotine treatment suppresses poly(I:C)-induced intracellular Ca2+ increase in mouse RAW 264.7 macrophages. After nicotine treatment, RAW 264.7 macrophages were stimulated with TLR3 agonist poly(I:C) (20 μg/ml) or PBS. (A) typical fluorescence images of control and poly(I:C)-stimulated RAW264.7 cells captured at 4 minutes of recording in the presence or absence of nicotine. Scale bar represents 50 µm. (B) representative time courses of intracellular Ca2+ changes (fluo-4 fluorescence expressed as a ratio of individual cell fluorescence intensity [F] to its basal fluorescence intensity [F0]) in response to stimulation with poly(I:C), poly(I:C) + nicotine, or nicotine alone from 8 cells per experiment. In the nicotine-only treatment group, nicotine (5 μg/ml) was added at the same time point with the poly(I:C) + nicotine group, and the fluorescence curve was recorded simultaneously to compare with the control group. (C) quantitative analysis of the inhibitory effect of nicotine on poly(I:C)-induced intracellular Ca2+ increase is captured at 4 minutes of stimulation. The fluorescence of nicotine-alone treatment group was also recorded at the same time point to compare with control group. Data are shown as mean ± S.E.M. from three independent experiments (*P < 0.001).
Discussion
Nicotine, a major addictive component of tobacco smoke, exerts an anti-inflammatory effect in immune cells and promotes viral infection (Razani-Boroujerdi et al., 2004). To evaluate the effect of nicotine on virus-triggered inflammatory pathways, we used mouse macrophage-like RAW264.7 cells and primary peritoneal macrophages as models for poly(I:C)-induced inflammation. Both cell types were treated with nicotine and then challenged with poly(I:C), a synthetic dsRNA ligand for TLR3 commonly used to mimic viral dsRNA exposure, to induce innate immune responses (Ranjith-Kumar et al., 2007). Of the 51 genes examined at the mRNA level in RAW264.7 cells, expression of 15, including those of IL-6 and TNF-α, was significantly reduced by nicotine. Some of these results, at both the mRNA (IL-6, TNF-α, and IL-1β) and protein (IL-6 and TNF-α) levels, were confirmed in primary mouse peritoneal macrophages used to evaluate inflammation ex vivo. Moreover, in both models, the inhibitory effect of nicotine on proinflammatory cytokine release was blocked by α-BTX. Furthermore, nicotine treatment reduced phosphorylation of 82 kinases in poly(I:C)-treated RAW264.2 cells, 27 of which are presumably involved in the Ca2+ signaling pathway. Further verification by Western blotting showed nicotine treatment led to reduced levels of phospho-CaMKIIα. In addition, nicotine treatment diminished intracellular [Ca2+]i flux in poly(I:C)-treated cells. These results suggest involvement of calcium or Ca2+ signaling or both in innate TLR3-triggered immune responses.
To study the effect of nicotine on viral-mediated innate immune signaling, we conducted two investigations: first, screening for mRNA expression of 51 genes and, second, measuring protein phosphorylation using an antibody array containing 1,318 targets. Nicotine attenuated the molecular responses seen with poly(I:C) stimulation that typify TLR3-mediated virus-triggered inflammation. Moreover, the ELISA results complemented the RT-PCR array findings and vice versa. These results also indicate that transcriptional activation or repression of IL-6 and TNF-α (among others) following poly(I:C) stimulation or poly(I:C) + nicotine treatment accompanied an increase or decrease, respectively, in proinflammatory cytokines: an indication that gene activation or repression is involved in their actions, although other mechanisms (Tsoyi et al., 2011) could also be active. Similarly, antibody array results complement Western blotting findings and vice versa: CamKIIα phosphorylation was reduced in nicotine + poly(I:C)-treated cells in both assays.
Although cigarette smoking exaggerates symptomatic responses to virus infection (Kark and Lebiush, 1981; Kark et al., 1982; Cohen et al., 1993; Arcavi and Benowitz, 2004) and nicotine promotes influenza A virus infection (Razani-Boroujerdi et al., 2004) while suppressing inflammatory responses, the molecular bases of these observations are not well understood. So far, the investigations of the anti-inflammatory effect of nicotine focused on bacteria- or LPS-induced inflammation, mostly mediated by TLR4. Although both TLR3 and TLR4 belong to the TLR family, the information gleaned from TLR4 studies cannot fully explain the regulatory effect of nicotine on TLR3, as their signaling cascades/pathways are different. First, TLR4 triggers both MyD88- and TRIF-dependent pathways, whereas TLR3 activates only the TRIF-dependent pathway. Second, they use different adaptors to trigger the pathway: TRAM is specific for the TLR4-triggered TRIF-dependent pathway (Oshiumi et al., 2003). Third, they have different subcellular locations: TLR4 on cell surfaces and TLR3 in endolysosomal compartments (Le Goffic et al., 2007). In TLR4-activated macrophages, nicotine apparently suppresses NF-κB activation and proinflammatory cytokine expression through α7-nAChR (Wang et al., 2003; Wang et al., 2004; Cui and Li, 2010). GTS-21, a selective α7-nAChR agonist, can attenuate TLRs-triggered cytokine expression (Rosas-Ballina et al., 2009). Our results also indicate that nicotine reduces the release of proinflammatory cytokines from TLR3-activated macrophages, and this response potentially involves (α7- or α7-like) nAChR, which is sensitive to activation by nicotine and blockade by α-BTX but refractory to promoting intracellular Ca2+. These results also indicate that nicotine attenuates TLR3 signaling following viral infection.
Previous investigations of the anti-inflammatory effect of nicotine were limited to study of signaling molecules/transcription factors, such as NF-κB, IκB, Jak2, and STAT3 (de Jonge et al., 2005; Blanchet et al., 2006; Yoshikawa et al., 2006; Cheng et al., 2007). Protein phosphorylation plays an important role in signal transduction from pathogen to inflammatory products. These phosphoproteins, mostly kinases, construct a range of signaling cascades downstream of TLRs and RLRs and are deeply involved in modulating the anti-inflammatory effect of nicotine. For example, phosphorylation of NF-κB is significantly suppressed by nicotine in macrophages (Wang et al., 2004). The Jak2-STAT3 pathway can be activated by nicotine in TLR4-induced macrophages and could interfere with NF-κB activation (de Jonge et al., 2005). Also, MAPKs are involved in the α7-nAChR-mediated anti-inflammatory effect against TLRs in human monocytes (Rosas-Ballina et al., 2009). However, these studies focused on one or at most two signaling molecules/pathways. In contrast, in the current study, we used a large-scale array to screen the protein phosphorylation profile of poly(I:C)-induced or poly(I:C) + nicotine-treated macrophages. An IPA analysis showed that 28 phospho-sites had reduced phosphorylation in nicotine-treated cells and could be involved in downstream cascades of Ca2+ signaling. It can be inferred that calcium signaling is part of the regulatory effect of nicotine during poly(I:C)-induced inflammation or nicotine-treated anti-inflammation.
Calcium (Ca2+) is a secondary messenger that impacts various biologic processes (Berridge et al., 2000). Activation of α7-nAChR increases the intracellular calcium concentration in both neurons and some nonneuron cells (Vijayaraghavan et al., 1992; Bertrand et al., 1993; Sopori et al., 1998; Shoop et al., 2001; Blanchet et al., 2006). However, the α7-nAChR expressed on the surface of RAW264.7 cells or peritoneal macrophages, apparently activated by nicotine and sensitive to inhibition by α-BTX, is not able to raise [Ca2+]i, because nicotine-treated cells yield [Ca2+]i signal that is almost the same as in the negative-control cells. This observation of the lack of [Ca2+]i signal presumably in the presence of (α7) nAChR and its agonist was reported earlier (Blumenthal et al., 1997; Rakhilin et al., 1999; Sharma and Vijayaraghavan, 2001; Mikulski et al., 2010).
nAChR-mediated cytoplasmic calcium signals could be attributable to 1) direct calcium flux through the nAChR; 2) indirect calcium influx through voltage-dependent calcium channels, which are activated by nAChR-mediated depolarization; and 3) calcium-induced calcium release (triggered by the first two sources) from the endoplasmic reticulum (ER) through ryanodine receptors and inositol (1,4,5)-triphosphate receptors (IP3Rs) (Shen and Yakel, 2009). In addition, nAChR activity can be regulated by cytoplasmic calcium concentrations, suggesting a complex reciprocal relationship (Shen and Yakel, 2009). In addition, in alveolar macrophages (Mikulski et al., 2010), nicotine treatment prior to application of ATP (agonist for purinergic receptors) reduces the intracellular [Ca2+]i signal, although nicotine alone does not affect [Ca2+]i. However, α-BTX abrogates nicotine’s effect on [Ca2+]i in ATP-induced macrophages. It is possible that this kind of α7-nAChR requires additional components or signaling events to be able to raise [Ca2+]i. Such rare instances in which nAChR requires additional components or activation of nAChR connects to other signaling pathway have been reported (Khakh et al., 2000, 2005; Razani-Boroujerdi et al., 2007; Mikulski et al., 2010). For example, T cells express α7-nAChR subunits that require a functional T-cell receptor (TCR) and leukocyte-specific protein tyrosine kinase for a nicotine-induced Ca2+ response (Razani-Boroujerdi et al., 2007). Very recently, it was shown that concurrent depolarizing treatments along with a slightly chronically elevated cytosolic Ca2+ is required for action of nicotine through a mechanism involving α-bungarotoxin-sensitive (presumably α7) nAChR and secondarily T-type voltage-gated calcium channels in Parkinson disease-vulnerable rat midbrain dopamine neurons (Toulorge et al., 2011). Another possible explanation for the inability of RAW264.7 macrophages to sustain high calcium concentrations in the presence of nicotine could be rapid and persistent desensitization of the α7-nAChR. Desensitized nAChRs, although possessing high affinity for ligand binding, tend to be in a closed conformation, which may lead to reduced cytoplasmic [Ca2+] concentrations. Other possible reasons for these observations could be species- and tissue-specific differences and plasticity in receptor expression under unnatural or pathologic conditions (Prasse et al., 2009).
Our earlier explanations for the effect of nicotine in poly(I:C)-stimulated or nonstimulated macrophages solely assumed the action of nicotine at or around the cell surface bound α7-nAChRs. However, it is well known that nicotine can permeate membranes (as a result of its favorable pKa or log P value) readily (Lester et al., 2009). Hence nicotine can enter the cytoplasm and ER and other organelles and concentrate many folds higher in organelles than the cytoplasm or extracellular concentration (Lester et al., 2009). Hence additional mechanisms that may govern the observed effect of nicotine on poly(I:C)-stimulated or nonstimulated macrophages may involve regulatory effect of nicotine on gene expression (activation or deactivation) due to its presence in cytoplasm and/or organelles. Thus it is entirely plausible that nicotine trapped in the ER and other organelles could be involved in downregulating the release of Ca2+ from intracellular stores from poly(I:C)-stimulated macrophages.
An emerging hypothesis (SePhaChARNS: selective pharmacological chaperone of acetylcholine receptor number and stoichiometry) that is gaining ground (and mostly shown to be valid so far for neuronal α4β2-nAChR), substantiates that physiologically relevant manipulations of nAChRs take place in the ER, not at the cell surface membrane (Kuryatov et al., 2005; Srinivasan et al., 2011, 2012), and a sustained interaction between nicotine and nascent nAChRs exerts control over nAChR trafficking (ER export and retention/retrieval) and stoichiometry. An overall increase of plasma membrane nAChRs results from the stabilization of assembled receptors by nicotine and this trapping cause massive desensitization of nAChR in the exocytic pathway without affecting pharmacological chaperoning within the ER and it does not affect the function of surface expressed nAChRs (Lester et al., 2009). Such a phenomenon of pharmacological chaperoning or upregulation of α7- or α7-like nAChR in macrophages is plausible but needs further experimental validation. Some of these upregulated receptors possibly could primarily be activated by acute nicotine, whereas others could primarily be desensitized as is shown for α4β2-nAChR expressed in nigrostriatal dopamine pathway (Xiao et al., 2009). Hence the lack of detectable Ca2+ flux in nicotine-treated macrophages, in part, could be attributed to the presence of acutely activated and desensitized nAChRs.
Poly(I:C)-induced calcium release from internal stores in macrophages (de Bouteiller et al., 2005; Liu et al., 2008) promotes phosphorylation of CaMKIIα, which interacts with TAK-1 and activates NF-κB-induced expression of proinflammatory cytokines (Liu et al., 2008). Whereas nicotine-treated cells cannot mobilize [Ca2+]i, poly(I:C)-stimulated cells show massive mobilization of [Ca2+]i. But nicotine treatment prior to stimulation with poly(I:C) significantly reduces the [Ca2+]i signal. Thus, nicotine is acting as a negative regulator of [Ca2+]i mobilization in poly(I:C)-stimulated cells. However, it remains to be determined how TLRs induce calcium release and whether nicotine alters [Ca2+] through ion channels on the cell surface or blockage of intracellular calcium stores (although we have hypothesized earlier that nicotine available in the ER and/or other organelles may influence the release of intracellular Ca2+). Further studies are required to identify the upstream signaling events involved in nicotine’s modulation of intracellular calcium in poly(I:C)-stimulated macrophages. On a hypothetical note, the observed effect of nicotine could also in part be due to its direct or indirect effect on TLR3 expression.
It is proposed that nicotine or cholinergic agonists could be advantageous compared with treatments using proinflammatory cytokine antibodies in preventing macrophages from releasing proinflammatory cytokines (Tracey and Abraham, 1999; Tracey, 2002). However, at the same time, intentional (nicotine treatment) or unintentional (nicotine inhalation through cigarettes or any other form of smoking) use of nicotine could be dangerous to patients suffering from virus infection. Similarly, nicotine could have negative consequences because of its reported significant immunosuppressive effect in both animal models and clinical studies (Geng et al., 1996; Mills et al., 1997; Denda et al., 1998; Guslandi, 1999; Matri et al., 2000). The findings from our study contribute to a better understanding of the action of nicotine during viral infection, which is vital for the safe clinical use of cholinergic agonists.
Acknowledgments
The authors thank Dr. David L. Bronson for excellent editing of this manuscript.
Abbreviations
- AMPK
AMP-activated protein kinase
- ANOVA
analysis of variance
- α-BTX
α-bungarotoxin
- [Ca2+]i
intracellular calcium
- CaMKIV
calcium/calmodulin-dependent protein kinase IV
- EGFR
epidermal growth factor receptor signaling
- EIF2AK2
eukaryotic translation initiation factor 2-alpha kinase 2
- ELISA
enzyme-linked immunosorbent assay
- ER
endoplasmic reticulum
- FADD
Fas-associated via death domain
- GAB2
GRB2-associated binding protein 2
- IKKε
inhibitor of kappaB kinase epsilon
- IL-6
interleukin-6
- IPA
ingenuity pathway analysis
- IRAK4
interleukin-1 receptor-associated kinase 4
- IRF-7
interferon regulatory factor 7
- LPS
lipopolysaccharide
- MEK1
mitogen-activated protein kinase kinase 1
- MEK2
mitogen-activated protein kinase kinase 2
- MEK4
mitogen-activated protein kinase kinase 4
- MEKK1
mitogen-activated protein kinase kinase kinase
- nAChR
nicotinic acetylcholine receptor
- PAK1
p21 protein (Cdc42/Rac)-activated kinase 1
- PKY2
protein tyrosine kinase 2 beta
- PLCG1
phospholipase C, gamma 1
- PLCG2
phospholipase C, gamma 2
- PPARA
peroxisome proliferator activated receptor alpha
- RLR
RIG-I-like receptor
- SRC
v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog
- TIRAP
Toll-interleukin 1 receptor (TIR) domain-containing adaptor protein
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
- VDCCs
voltage-dependent calcium channels
Authorship Contributions
Participated in research design: Cui, Saucerman, Li.
Conducted experiments: Cui, Zhao, Wei, Polanowska-Grabowska.
Performed data analysis: Cui, Wang, Polanowska-Grabowska, Saucerman.
Wrote or contributed to the writing of the manuscript: Cui, Dash, Chang, Saucerman, Gu, Li.
Footnotes
The project was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grants R01DA013783, R01DA016149, R01DA026356]; and the National Natural Science Foundation of China [Grant 81273223]. W.-Y.C. was partially supported by the China Scholarship Council.
References
- Arcavi L, Benowitz NL. (2004) Cigarette smoking and infection. Arch Intern Med 164:2206–2216 [DOI] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. (2006) Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J 20:2093–2101 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21 [DOI] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiéry A, Bertrand S, Changeux JP. (1993) Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc Natl Acad Sci USA 90:6971–6975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. (2004) Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430:257–263 [DOI] [PubMed] [Google Scholar]
- Blanchet MR, Israël-Assayag E, Daleau P, Beaulieu MJ, Cormier Y. (2006) Dimethyphenylpiperazinium, a nicotinic receptor agonist, downregulates inflammation in monocytes/macrophages through PI3K and PLC chronic activation. Am J Physiol Lung Cell Mol Physiol 291:L757–L763 [DOI] [PubMed] [Google Scholar]
- Blumenthal EM, Conroy WG, Romano SJ, Kassner PD,, Berg DK. (1997) Detection of functional nicotinic receptors blocked by alpha-bungarotoxin on PC12 cells and dependence of their expression on post-translational events. J Neurosci 17:6094–6104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405:458–462 [DOI] [PubMed] [Google Scholar]
- Cao J, Dwyer JB, Mangold JE, Wang J, Wei J, Leslie FM, Li MD. (2011) Modulation of cell adhesion systems by prenatal nicotine exposure in limbic brain regions of adolescent female rats. Int J Neuropsychopharmacol 14:157–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PY, Lee YM, Law KK, Lin CW, Yen MH. (2007) The involvement of AMP-activated protein kinases in the anti-inflammatory effect of nicotine in vivo and in vitro. Biochem Pharmacol 74:1758–1765 [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, Russell MA, Jarvis MJ, Smith AP. (1993) Smoking, alcohol consumption, and susceptibility to the common cold. Am J Public Health 83:1277–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui WY, Li MD. (2010) Nicotinic modulation of innate immune pathways via α7 nicotinic acetylcholine receptor. J Neuroimmune Pharmacol 5:479–488 [DOI] [PubMed] [Google Scholar]
- Cui WY, Wang J, Wei J, Cao J, Chang SL, Gu J, Li MD. (2012) Modulation of innate immune-related pathways in nicotine-treated SH-SY5Y cells. Amino Acids 43:1157–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bouteiller O, Merck E, Hasan UA, Hubac S, Benguigui B, Trinchieri G, Bates EE, Caux C. (2005) Recognition of double-stranded RNA by human toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J Biol Chem 280:38133–38145 [DOI] [PubMed] [Google Scholar]
- de Jonge WJ, van der Zanden EP, The FO, et al. (2005) Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6:844–851 [DOI] [PubMed] [Google Scholar]
- Denda A, Endoh T, Tang Q, Tsujiuchi T, Nakae D, Konishi Y. (1998) Prevention by inhibitors of arachidonic acid cascade of liver carcinogenesis, cirrhosis and oxidative DNA damage caused by a choline-deficient, L-amino acid-defined diet in rats. Mutat Res 402:279–288 [DOI] [PubMed] [Google Scholar]
- Friedman H. (1996) Drugs of abuse as possible co-factors in AIDS progression: summary of panel discussion. Adv Exp Med Biol 402:225–228 [DOI] [PubMed] [Google Scholar]
- Geng Y, Savage SM, Razani-Boroujerdi S, Sopori ML. (1996) Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J Immunol 156:2384–2390 [PubMed] [Google Scholar]
- Guslandi M. (1999) Long-term effects of a single course of nicotine treatment in acute ulcerative colitis: remission maintenance in a 12-month follow-up study. Int J Colorectal Dis 14:261–262 [DOI] [PubMed] [Google Scholar]
- Hamano R, Takahashi HK, Iwagaki H, Yoshino T, Nishibori M, Tanaka N. (2006) Stimulation of alpha7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes. Shock 26:358–364 [DOI] [PubMed] [Google Scholar]
- Hao J, Simard AR, Turner GH, Wu J, Whiteaker P, Lukas RJ, Shi FD. (2011) Attenuation of CNS inflammatory responses by nicotine involves α7 and non-α7 nicotinic receptors. Exp Neurol 227:110–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kark JD, Lebiush M. (1981) Smoking and epidemic influenza-like illness in female military recruits: a brief survey. Am J Public Health 71:530–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kark JD, Lebiush M, Rannon L. (1982) Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N Engl J Med 307:1042–1046 [DOI] [PubMed] [Google Scholar]
- Khakh BS, Fisher JA, Nashmi R, Bowser DN,, Lester HA. (2005) An angstrom scale interaction between plasma membrane ATP-gated P2X2 and alpha4beta2 nicotinic channels measured with fluorescence resonance energy transfer and total internal reflection fluorescence microscopy. J Neurosci 25:691–6920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Zhou X, Sydes J, Galligan JJ, Lester HA. (2000) State-dependent cross-inhibition between transmitter-gated cation channels. Nature 406:405–410 [DOI] [PubMed] [Google Scholar]
- Kox M, Pompe JC, Gordinou de Gouberville MC, van der Hoeven JG, Hoedemaekers CW, Pickkers P. (2011) Effects of the α7 nicotinic acetylcholine receptor agonist GTS-21 on the innate immune response in humans. Shock 36:5–11 [DOI] [PubMed] [Google Scholar]
- Kox M, van Velzen JF, Pompe JC, Hoedemaekers CW, van der Hoeven JG, Pickkers P. (2009) GTS-21 inhibits pro-inflammatory cytokine release independent of the Toll-like receptor stimulated via a transcriptional mechanism involving JAK2 activation. Biochem Pharmacol 78:863–872 [DOI] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Kato H, et al. (2006) Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med 203:1795–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. (2005) Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol Pharmacol 68:1839–1851 [DOI] [PubMed] [Google Scholar]
- Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. (2007) Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol 178:3368–3372 [DOI] [PubMed] [Google Scholar]
- Lester HA, Xiao C, Srinivasan R, Son CD, Miwa J, Pantoja R, Banghart MR, Dougherty DA, Goate AM, Wang JC. (2009) Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. AAPS J 11:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson AR, Lando HA. (2012) Smoking and HIV: Prevalence, Health Risks, and Cessation Strategies. Curr HIV/AIDS Rep 9:223–220 [DOI] [PubMed] [Google Scholar]
- Liu X, Yao M, Li N, Wang C, Zheng Y, Cao X. (2008) CaMKII promotes TLR-triggered proinflammatory cytokine and type I interferon production by directly binding and activating TAK1 and IRF3 in macrophages. Blood 112:4961–4970 [DOI] [PubMed] [Google Scholar]
- Matri S, Karoui S, Filali A. (2000) [Tobacco and inflammatory bowel disease]. Tunis Med 78:693–698 [PubMed] [Google Scholar]
- Matsunaga K, Klein TW, Friedman H, Yamamoto Y. (2001) Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol 167:6518–6524 [DOI] [PubMed] [Google Scholar]
- Mikulski Z, Hartmann P, Jositsch G, et al. (2010) Nicotinic receptors on rat alveolar macrophages dampen ATP-induced increase in cytosolic calcium concentration. Respir Res 11:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CM, Hill SA, Marks R. (1997) Transdermal nicotine suppresses cutaneous inflammation. Arch Dermatol 133:823–825 [PubMed] [Google Scholar]
- Oshiumi H, Sasai M, Shida K, Fujita T, Matsumoto M, Seya T. (2003) TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-beta. J Biol Chem 278:49751–49762 [DOI] [PubMed] [Google Scholar]
- Park SY, Baik YH, Cho JH, Kim S, Lee KS, Han JS. (2008) Inhibition of lipopolysaccharide-induced nitric oxide synthesis by nicotine through S6K1-p42/44 MAPK pathway and STAT3 (Ser 727) phosphorylation in Raw 264.7 cells. Cytokine 44:126–134 [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. (2003) The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med 9:125–134 [PMC free article] [PubMed] [Google Scholar]
- Peña VB, Bonini IC, Antollini SS, Kobayashi T, Barrantes FJ. (2011) alpha 7-type acetylcholine receptor localization and its modulation by nicotine and cholesterol in vascular endothelial cells. J Cell Biochem 112:3276–3288 [DOI] [PubMed] [Google Scholar]
- Prasse A, Stahl M, Schulz G, et al. (2009) Essential role of osteopontin in smoking-related interstitial lung diseases. Am J Pathol 174:1683–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhilin S, Drisdel RC, Sagher D, McGehee DS, Vallejo Y, Green WN. (1999) alpha-Bungarotoxin receptors contain alpha7 subunits in two different disulfide-bonded conformations. J Cell Biol 146:203–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjith-Kumar CT, Miller W, Xiong J, et al. (2007) Biochemical and functional analyses of the human Toll-like receptor 3 ectodomain. J Biol Chem 282:7668–7678 [DOI] [PubMed] [Google Scholar]
- Razani-Boroujerdi S, Boyd RT, Dávila-García MI, Nandi JS, Mishra NC, Singh SP, Pena-Philippides JC, Langley R, Sopori ML. (2007) T cells express alpha7-nicotinic acetylcholine receptor subunits that require a functional TCR and leukocyte-specific protein tyrosine kinase for nicotine-induced Ca2+ response. J Immunol 179:2889–2898 [DOI] [PubMed] [Google Scholar]
- Razani-Boroujerdi S, Singh SP, Knall C, Hahn FF, Peña-Philippides JC, Kalra R, Langley RJ, Sopori ML. (2004) Chronic nicotine inhibits inflammation and promotes influenza infection. Cell Immunol 230:1–9 [DOI] [PubMed] [Google Scholar]
- Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdés-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ. (2009) The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med 15:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato KZ, Fujii T, Watanabe Y, Yamada S, Ando T, Kazuko F, Kawashima K. (1999) Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci Lett 266:17–20 [DOI] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. (2001) Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci USA 98:4148–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen JX, Yakel JL. (2009) Nicotinic acetylcholine receptor-mediated calcium signaling in the nervous system. Acta Pharmacol Sin 30:673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoop RD, Chang KT, Ellisman MH,, Berg DK. (2001) Synaptically driven calcium transients via nicotinic receptors on somatic spines. J Neurosci 21:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JL, Schooley K, Bonnert TP, Mitcham JL, Qwarnstrom EE, Sims JE, Dower SK. (2000) Identification of two major sites in the type I interleukin-1 receptor cytoplasmic region responsible for coupling to pro-inflammatory signaling pathways. J Biol Chem 275:4670–4678 [DOI] [PubMed] [Google Scholar]
- Sopori M. (2002) Effects of cigarette smoke on the immune system. Nat Rev Immunol 2:372–377 [DOI] [PubMed] [Google Scholar]
- Sopori ML, Kozak W, Savage SM, Geng Y, Kluger MJ. (1998) Nicotine-induced modulation of T Cell function. Implications for inflammation and infection. Adv Exp Med Biol 437:279–289 [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, Miwa J, Lester HA. (2011) Nicotine up-regulates alpha4beta2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol 137:59–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Richards CI, Xiao C, Rhee D, Pantoja R, Dougherty DA, Miwa JM, Lester HA. (2012) Pharmacological chaperoning of nicotinic acetylcholine receptors reduces the endoplasmic reticulum stress response. Mol Pharmacol 81:759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano N, Shimada K, Ito K, Murai S. (1998) Nicotine inhibits the production of inflammatory mediators in U937 cells through modulation of nuclear factor-kappaB activation. Biochem Biophys Res Commun 252:25–28 [DOI] [PubMed] [Google Scholar]
- Toulorge D, Guerreiro S, Hild A, Maskos U, Hirsch EC, Michel PP. (2011) Neuroprotection of midbrain dopamine neurons by nicotine is gated by cytoplasmic Ca2+. FASEB J 25:2563–2573 [DOI] [PubMed] [Google Scholar]
- Tracey KJ. (2002) The inflammatory reflex. Nature 420:853–859 [DOI] [PubMed] [Google Scholar]
- Tracey KJ, Abraham E. (1999) From mouse to man: or what have we learned about cytokine-based anti-inflammatory therapies? Shock 11:224–225 [PubMed] [Google Scholar]
- Tsoyi K, Jang HJ, Kim JW, et al. (2011) Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxid Redox Signal 14:2057–2070 [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Pugh PC, Zhang ZW, Rathouz MM, Berg DK. (1992) Nicotinic receptors that bind alpha-bungarotoxin on neurons raise intracellular free Ca2+. Neuron 8:353–362 [DOI] [PubMed] [Google Scholar]
- Wang H, Liao H, Ochani M, et al. (2004) Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med 10:1216–1221 [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, et al. (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421:384–388 [DOI] [PubMed] [Google Scholar]
- Wei J, Wang J, Dwyer JB, Mangold J, Cao J, Leslie FM, Li MD. (2011) Gestational nicotine treatment modulates cell death/survival-related pathways in the brains of adolescent female rats. Int J Neuropsychopharmacol 14:91–106 [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CK, Shackel I, Williams PM. (1999) Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 270:41–49 [DOI] [PubMed] [Google Scholar]
- Wittebole X, Castanares-Zapatero D, Laterre PF. (2010) Toll-like receptor 4 modulation as a strategy to treat sepsis. Mediators Inflamm 2010:568396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Nashmi R, McKinney S, Cai H, McIntosh JM,, Lester HA. (2009) Chronic nicotine selectively enhances alpha4beta2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway . J Neurosci 29:12428–12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H, Kurokawa M, Ozaki N, Nara K, Atou K, Takada E, Kamochi H, Suzuki N. (2006) Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin Exp Immunol 146:116–123 [DOI] [PMC free article] [PubMed] [Google Scholar]