Abstract
Tolbutamide and gliclazide block the KATP channel Kir6.2/Sur1, causing membrane depolarization and stimulating insulin secretion in pancreatic beta cells. We examined the ability of the EPAC-selective cAMP analog 8-pCPT-2′-O-Me-cAMP-AM to potentiate the action of these drugs and the mechanism that might account for it. Insulin secretion stimulated by both 200 μM tolbutamide and 20 μM gliclazide, concentrations that had equivalent effects on membrane potential, was inhibited by thapsigargin (1 μM) or the L-type Ca2+ channel blocker nicardipine (2 μM) and was potentiated by 8-pCPT-2′-O-Me-cAMP-AM at concentrations ≥2 μM in INS-1 cells. Ca2+ transients stimulated by either tolbutamide or gliclazide were inhibited by thapsigargin or nicardipine and were significantly potentiated by 8-pCPT-2′-O-Me-cAMP-AM at 5 μM but not 1 μM. Both tolbutamide and gliclazide stimulated phospholipase C activity; however, only gliclazide did so independently of its activity at KATP channels, and this activity was partially inhibited by pertussis toxin. 8-pCPT-2′-O-Me-cAMP-AM alone (5 μM) did not stimulate insulin secretion, but did increase intracellular Ca2+ concentration significantly, and this activity was inhibited by 25 μM 2-aminoethoxydiphenylborate (2-APB) or the removal of extracellular Ca2+. 8-pCPT-2′-O-Me-cAMP-AM potentiation of insulin secretion stimulated by tolbutamide was markedly inhibited by 2-APB (25 μM) and enhanced by the PKC inhibitor bisindolylmaleimide I (1 μM). Our data demonstrate that the actions of both tolbutamide and gliclazide are strongly potentiated by 8-pCPT-2′-O-Me-cAMP-AM, that gliclazide can stimulate phospholipase C activity via a partially pertussis toxin-sensitive mechanism, and that 8-pCPT-2′-O-Me-cAMP-AM potentiation of tolbutamide action may involve activation of a 2-APB-sensitive Ca2+ influx.
Introduction
Sulfonylurea drugs, such as tolbutamide and gliclazide, have been used for decades to stimulate insulin secretion and decrease blood glucose levels in type 2 patients with diabetes with insufficient endogenous insulin secretion (Groop, 1992). These drugs bypass the metabolic steps required for glucose-stimulated insulin secretion and mimic the effect of an increase in ATP/ADP ratio (Rorsman and Trube, 1985; Dunne and Petersen, 1986) on the KATP channel composed of the Kir6.2 and SUR1 subunits (Babenko et al., 1998). Binding of sulfonylureas to the KATP channel favors channel closing (Schmid-Antomarchi et al., 1987) and subsequent membrane depolarization because open KATP channels permit the efflux of K+ ions and the maintenance of the membrane potential close to the equilibrium potential for K+ (Cook and Hales, 1984). Depolarization of the membrane potential of pancreatic beta cells leads to the activation of several varieties of voltage-gated Ca2+ channels (Horvath et al., 1998), including the L-type channels Cav1.2 and Cav1.3 (Seino et al., 1992). The subsequent rise in intracellular Ca2+ triggers the exocytosis of insulin (Wollheim et al., 1975) via dense core vesicles that are shuttled to (Van Obberghen et al., 1975), and eventually fuse with, the plasma membrane (Barg et al., 2001; Shibasaki et al., 2007).
Recently, exchange protein directly activated by cAMP 2 (EPAC2) was identified as a novel cellular target for some members of the sulfonylurea drug class (Zhang et al., 2009). EPAC2 is a guanine nucleotide exchange factor (GEF) for the low molecular weight GTP binding protein Rap1 (Kawasaki et al., 1998). In the cAMP-bound form, EPAC2 accelerates the exchange of GDP for GTP at the nucleotide binding site of Rap1 (Kawasaki et al., 1998). Along with the activation of protein kinase A (Ding and Gromada, 1997), activation of EPAC2 contributes to the potentiation of glucose-stimulated insulin secretion by incretin hormones that stimulate adenylyl cyclase activity, such as GLP-1 (Leech et al., 2010a). The GTP-bound form of Rap1 is implicated in the activation of phospholipase C-ε (Dzhura et al., 2010) in pancreatic beta cells, in augmenting the number of insulin granules in close proximity to sites of exocytosis on the plasma membrane (Shibasaki et al., 2007), and in the priming of insulin granules for exocytosis (Eliasson et al., 2003).
EPAC2 is thought to exist in a signaling complex with several other peripheral membrane proteins. EPAC2 interacts directly with SUR1 (Shibasaki et al., 2004) and with the CAZ protein piccolo (Fenster et al., 2000), which forms a dimer with the related protein RIM2 (Wang et al., 2000), in a Ca2+-dependent manner (Fujimoto et al., 2002). In addition, the voltage-gated Ca2+ channel Cav1.2 interacts with the C2 domains of RIM2 and piccolo via the intracellular II-III loop (Shibasaki et al., 2004). This signaling complex of scaffolding proteins (RIM2, piccolo), cAMP effector (EPAC2), and ion channel subunits (SUR1, Cav1.2) is of special significance because membrane depolarization-dependent calcium influx via Cav1.2 channels has been implicated in triggering Ca2+-induced Ca2+ release from the ER in pancreatic beta cells (Liu et al., 2006), a process that is amplified by cAMP, at least in part, through EPAC2 (Kang et al., 2003; Liu et al., 2006). The role of intrinsic EPAC2 stimulation by sulfonylureas on insulin secretion or the underlying Ca2+ dynamics in beta cells is currently unknown.
Tolbutamide and gliclazide, which both stimulate insulin secretion from pancreatic beta cells by block of KATP channels, differ in their ability to bind and activate EPAC2. Zhang et al. (2009), reported that tolbutamide at concentrations ≥30 μM significantly activated Rap1 in an EPAC2-dependent manner, whereas gliclazide at concentrations up to 30 nM did not. We used electrophysiological analysis to identify concentrations of tolbutamide and gliclazide that had equivalent effects on KATP channel activity both in terms of block of current and induction of membrane depolarization. We then used these concentrations of tolbutamide and gliclazide to compare stimulation of insulin secretion, stimulation of increases in intracellular Ca2+ concentration, and potentiation of these activities by the EPAC-selective cAMP analog (ESCA) 8-pCPT-2′-O-Me-cAMP-AM in INS-1 cells. Because activation of phospholipase C is a consequence of EPAC2 activation in pancreatic beta cells, we also assayed phospholipase C activation by tolbutamide and gliclazide in the absence and presence of diazoxide to prevent membrane depolarization and Ca2+ influx via voltage-gated Ca2+ channels. To gain insight into the mechanism whereby EPAC activation potentiates sulfonylurea action, we assayed 8-pCPT-2′-O-Me-cAMP-AM modulation of phospholipase C activity and intracellular Ca2+ concentration in INS-1 cells.
Materials and Methods
Chemicals.
U73122 and U73343 were from Tocris (Minneapolis, MN). 8-pCPT-2′-O-Me-cAMP and 8-pCPT-2′-O-Me-cAMP-AM were from Biolog (Bremen, Germany). Rp-cAMP was from Santa Cruz Biotechnology (Santa Cruz, CA). Ryanodine was from Calbiochem (San Diego, CA). All other chemical reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Cell Culture.
INS-1 cells were grown in RPMI medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 11 mg/ml sodium pyruvate, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM/β-mercaptoethanol at 37°C, 5% CO2.
Electrophysiological Assay.
Electrophysiological measurements in INS-1 cells were recorded at room temperature using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) and filtered at 1 kHz (six-pole Bessel filter, −3 dB). Electrodes were pulled from borosilicate glass (VWR, West Chester, PA) and fire-polished to resistances of 2–5 MΩ. For current-clamp experiments and voltage-clamp experiments to measure KATP channel current, the intracellular solution contained (in mM) 90 K2SO4, 10 NaCl, 1 MgCl2, 1.1 EGTA, 0.1 CaCl2, 5 HEPES, 0.3 ATP, 0.2 GTP. The extracellular solution used for measuring KATP currents contained (in mM) 138 NaCl, 5.6 KCl, 11.1 glucose, 10 HEPES, 1.2 MgCl2, 2.6 CaCl2, and the same solution with the addition of 2.5 mM glucose was used in current-clamp experiments. The pH of solutions was adjusted to 7.4 with NaOH, and the osmolality was adjusted to 290–300 mOsm. Whole cell KATP currents were elicited by 1.3-s steps of ±20 mV from a holding potential of −70 mV. Data were acquired at a sampling frequency of 1 kHz. The membrane potential of INS-1 cells was measured using gap-free recording at a sampling frequency of 1 kHz in I = 0 current-clamp mode. The KATP channel opener, diazoxide (300 µM), was transiently applied to maximally open KATP channels, before application of tolbutamide or gliclazide. Tolbutamide and gliclazide solutions were prepared from stocks dissolved in 0.1 M NaOH made fresh daily. Diazoxide solutions were prepared from stocks dissolved in DMSO. For recordings of voltage-gated Ca2+ channel currents, the bath solution contained (in mM) 150 Tris, 10 BaCl2, 4 MgCl2. The intracellular solution contained (in mM) 130 N-methyl-d-glucamine, 10 EGTA, 60 HEPES, 2 ATP, and 1 MgCl2. The pH of both solutions was adjusted to 7.3 with methanesulfonic acid, and the osmolality was corrected to 290–300 mOsM. Current-voltage relationship data were collected by applying 100-ms test depolarizations from −50 to +60 mV in 10-mV increments from a holding potential of −70 mV. Data were acquired at a sampling frequency of 10 kHz and filtered at 1 kHz.
Insulin Secretion Assay.
INS-1 cells were plated in 24-well tissue culture plate at 50–70% confluency and incubated overnight in RPMI medium supplemented as described above at 37°C, 5% CO2. Immediately before the assay, cells were washed twice in phosphate-buffered saline [PBS: 137 mM NaCl, 10 mM phosphate, 2.7 mM KCl (pH 7.4)], and preincubated with a modified Kreb-Ringer buffer [KRBH: 134 mM NaCl, 3.5 mM KCl, 1.2 mM KH2PO4, 0.5 mM MgSO4, 1.5 mM CaCl2, 5 mM NaHCO3, 10 mM HEPES (pH 7.4)] supplemented with 0.05% fatty acid free BSA (KRBH buffer) alone or containing indicated concentrations of inhibitors for 30 min at 37°C, 5% CO2. The buffer was decanted and replaced with fresh KRBH buffer alone (basal condition) or KRBH containing the indicated concentrations of sulfonylurea with or without inhibitors for 1 hour at 37°C, 5% CO2. Secreted insulin was assayed using an ELISA for rat insulin (High-Range EIA kit, ALPCO Diagnostics, Salem, NH). Cells were lysed in 20 mM Na2HPO4, 150 mM NaCl, 0.1% Triton X-100, 800 nM aprotinin, 50 μM leupeptin, 1 μg/ml pepstatin, 1 mM benzamidine, 1 mM 4-(2-aminoethyl)benzenesulfonylfluoride, 10 μg/ml calpain inhibitor I, and 10 μg/ml calpain inhibitor II (pH 7.4), and cellular protein in each well was determined using the BCA assay (Thermo Scientific, Rockford, IL).
Intracellular Ca2+ Assays.
INS-1 cells were plated at 100% confluency in black-walled 96 well plates (Corning Life Sciences, Lowell, MA) in RPMI supplemented as described above, and incubated overnight at 37°C, 5% CO2. Cells were washed twice with PBS and incubated with 5 μM Fura-2/acetoxymethyl ester (Fura-2 AM; Molecular Probes, Eugene, OR) diluted in KRBH for 1 hour at 37°C, 5% CO2. The KRBH containing Fura-2 AM was then removed, and the cells were washed twice with KRBH and equilibrated for 30 min at 37°C, 5% CO2 in the KRBH alone, or with indicated concentrations of inhibitors or 8-pCPT-2′-O-Me-cAMP-AM. When pertussis toxin was used, 25 ng/well was added to cells in 96-well plates approximately 18 hours before assay. Cells were stimulated by injection of the indicated concentration of sulfonylurea or 8-pCPT-2′-O-Me-cAMP-AM (or buffer control), and changes in intracellular Ca2+ concentrations were measured by recording the ratio of fluorescence intensities at 508/20 nm resulting from excitation of Fura-2 at 340/11 nm or 380/20 nm (center/band pass) using a Synergy 4 multi-mode microplate reader (BioTek, Winooski, VT). For experiments injecting sulfonylureas, ratios were acquired every 0.7 s for 15 s before injection and 2 minutes after injection of stimuli. For experiments injecting 8-pCPT-2′-O-Me-cAMP-AM, ratios were acquired every 5 s for 2 minutes before injection and at least 8 minutes after injection. Data were corrected for any injection artifact by subtracting the change in fluorescence ratio measured in cells injected with KRBH alone.
IP1 Assays.
INS-1 cells were plated at approximately 65,000 cells/well in white 96-well tissue culture plates (PerkinElmer, Waltham, MA) in the presence of 2.5 mM glucose overnight at 37°C and 5% CO2. A prestimulation buffer was added to the cells for 1 hour before stimulation [10 mM HEPES, 1 mM CaCl2, 0.5 mM MgCl2, 4.2 mM KCl, 146 mM NaCl (pH 7.4)]. The prestimulation buffer was decanted, and treatments were made in a stimulation buffer containing LiCl to block inositol phosphate degradation [10 mM HEPES, 1 mM CaCl2, 0.5 mM MgCl2, 4.2 mM KCl, 146 mM NaCl, 5 mM LiCl (pH 7.4)]. Cells were incubated with treatments for 1 hour at 37°C, 5% CO2. When pertussis toxin was used, 25 ng/well was added to cells in 96-well plates approximately 18 hours before assay. IP1 levels were measured using the IP-One Tb Homogeneous Time-Resolved Fluorescence Resonance Energy Transfer (HTRF-FRET) Kit from Cisbio (Bedford, MA) according to the manufacturer’s instructions. The 620 nm/650 nm fluorescence ratio (excitation wavelength of 330 nM) was measured using a Synergy 4 fluorescent plate reader (BioTek). The concentration of IP1 in each sample was determined by comparison of the 620 nm/650 nm ratio to those for a standard curve of IP1 concentrations.
Data Analysis.
Data were analyzed using SigmaPlot 11.0. Data are shown as means ± S.E. Statistical significance was determined using one-way analysis of variance and the Tukey's post hoc test unless otherwise indicated. P < 0.05 was considered significant.
Results
Electrophysiological Characterization of Tolbutamide and Gliclazide Activity in INS-1 Cells.
Both tolbutamide and gliclazide bind to and block the KATP channel in pancreatic beta cells. To compare the effects of these two drugs and determine whether tolbutamide might also activate EPAC2 in INS-1 cells, we determined the potency of KATP channel block and the dose response curve for membrane depolarization in INS-1 cells (Fig. 1). In the whole cell voltage-clamp mode, an alternating voltage-step protocol (stepping to −50 mV or −90 mV from a holding potential of −70 mV) elicited inward (at −90 mV) and outward (at −50 mV) K+ currents through KATP channels (Fig. 1A). These currents were blocked by increasing concentrations of either sulfonylurea drug, as indicated by the decrease in current amplitude in both directions. Plots of the percent of current block at each of several different concentrations of either tolbutamide or gliclazide yielded dose response curves that were fit as described in Materials and Methods. As expected, gliclazide was more potent in blocking of KATP channel current than was tolbutamide (gliclazide IC50 = 143 ± 23 nM; tolbutamide IC50 = 2.6 ± 0.7 μM; Fig. 1B).
Fig. 1.
Tolbutamide and gliclazide induce similar KATP current block and membrane depolarization in INS-1 cells. (A) representative trace of the dose-dependent tolbutamide block of KATP current measured using whole cell voltage clamp. Whole cell current was elicited by a ±20-mV step from a holding potential of −70 mV. (B) percentage of the whole cell KATP current blocked by tolbutamide (n = 4–11) or gliclazide (n = 3–8). IC50 values were determined by fitting the data using the equation Relative Current = 1/[1 + 10^(logIC50 − [drug])]. (C) representative trace of membrane potential depolarization elicited by tolbutamide (500 µM) or gliclazide (200 µM) and reversed by diazoxide (300 µM). (D) dose-dependent effect of tolbutamide (n = 8—18) or gliclazide (n = 7–27) on membrane depolarization. EC50 values were determined by fitting the data to the equation Relative Depolarization = 1 − {1/[1 + (EC50/[drug])]}.
Because the block of KATP channels leads to membrane depolarization in pancreatic beta cells, we also measured the dose dependence for the membrane depolarization induced by tolbutamide or gliclazide. In current-clamp recordings using the zero-current injection mode, the resting membrane potential of INS-1 cells was found to be −75.1 ± 0.75 mV. Application of high concentrations of either tolbutamide (500 μM) or gliclazide (200 μM) caused similar, strong depolarization of the membrane potential that often led to firing of action potentials at voltages greater than −50 mV (Fig. 1C). Membrane potential repolarization occurred rapidly upon washout of tolbutamide. Washout of gliclazide was slower, but could be accelerated by application of 300 μM diazoxide, a KATP channel “opener” (Fig. 1C). To compare the potency of tolbutamide and gliclazide in stimulating membrane potential depolarization, increasing concentrations of either drug were applied to INS-1 cells under current clamp. Tolbutamide and gliclazide both led to progressively greater depolarization of the membrane potential (Fig. 1D). Similar to block of KATP channel current, gliclazide induced membrane depolarization more potently than did tolbutamide. The EC50 for membrane depolarization by tolbutamide was determined to be 21.5 ± 10 μM with a maximum depolarization of 27.1 ± 4.2 mV. The EC50 for membrane depolarization by gliclazide was determined to be 4.2 ± 0.9 μM with a maximum depolarization of 32.6 ± 3.8 mV. Because the concentrations of 20 μM gliclazide and 200 μM tolbutamide were maximally effective in their ability to induce membrane depolarization and were within error of each other, we used these concentrations for subsequent experiments to compare the ability of these sulfonylurea drugs to stimulate activities in INS-1 cells.
Stimulation of Insulin Secretion and Ca2+ Transients by Tolbutamide and Gliclazide.
The concentrations of 20 μM gliclazide and 200 μM tolbutamide were used to induce insulin secretion in INS-1 cells using the static incubation method (Fig. 2). As expected from the electrophysiological characterization of the drugs, these concentrations of the sulfonylureas stimulated insulin secretion that was significantly different from basal secretion. Secretion stimulated by both 20 μM gliclazide and 200 μM tolbutamide was completely inhibited by 2 μM nicardipine, an L-type-selective Ca2+ channel blocker (Fig. 2A). Nicardipine was used at 2 μM because we had shown previously that it completely inhibits L-type channels but does not substantially inhibit P/Q-type Ca2+ channels at this concentration (Lin et al., 2011). To assess the role of internal stores of Ca2+ in sulfonylurea-stimulated insulin secretion, the ability of 1 μM thapsigargin, an inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+- ATPase (SERCA), to inhibit insulin secretion stimulated by either gliclazide or tolbutamide was determined. We found that thapsigargin completely inhibited secretion stimulated by 20 μM gliclazide and significantly, but incompletely, blocked insulin secretion stimulated by 200 μM tolbutamide (Fig. 2B). Thus, our results suggest that both influx of Ca2+ via L-type Ca2+ channels and release of internal stores of Ca2+ are required for maximal insulin secretion in response to gliclazide and tolbutamide stimulation.
Fig. 2.
Modulation of tolbutamide- and gliclazide-stimulated insulin secretion and Ca2+ transients by nicardipine and thapsigargin. (A) insulin secretion stimulated by either 20 μM gliclazide (white bars) or 200 μM tolbutamide (black bars) is completely inhibited by 2 μM nicardipine. (B) insulin secretion stimulated by 20 μM gliclazide (white bars) is completely inhibited, and secretion stimulated by 200 μM tolbutamide (black bars) is partially inhibited by 1 μM thapsigargin. ***P < 0.001, **P < 0.01, *P < 0.05 compared with basal; †††P < 0.001, ††P < 0.01, †P < 0.05 compared with nicardipine or thapsigargin. (C–F) increases in intracellular Ca2+ concentration are shown as increases in the ratio of intensity of fluorescence at 510 nm excited at 340 nm or 380 nm (340 nm/380 nm ratio). Black circles represent the response to 200 μM tolbutamide (C and E) or 20 μM gliclazide (D and F). Data shown were corrected by subtracting the increase in 340 nm/380 nm ratio observed upon addition of KRBH buffer alone. White circles in (C and E) and (D and F) represent the portion of the response to tolbutamide or gliclazide, respectively, inhibited by the indicated agent. Nicardipine (2 μM) completely inhibited the Ca2+ transient stimulated by either tolbutamide (C) or gliclazide (E). Preincubation with 2 μM thapsigargin (1 μM final concentration) selectively inhibited the rapid peak of the response stimulated by either tolbutamide (D) or gliclazide (F). Data shown are the mean ± S.E. of at least three representative experiments performed in quadruplicate.
Because sulfonylurea-stimulated insulin secretion was inhibited by nicardipine and thapsigargin, we examined the changes in intracellular Ca2+ concentration induced by tolbutamide or gliclazide. INS-1 cells in 96-well plates were loaded with the Ca2+ indicator Fura-2-AM, and changes in the ratio of fluorescence intensity at 510 nm after excitation at 340 nm and 380 nm (340 nm/380 nm ratio) were measured upon injection of tolbutamide (final concentration of 200 μM) or gliclazide (final concentration 20 μM). The changes in 340 nm/380 nm ratio upon sulfonylurea injection were corrected by subtracting the changes in 340 nm/380 nm ratio measured in replicate wells of INS-1 cells upon injection of buffer only. The net change in fura2 fluorescence ratio, reflecting the net change in intracellular Ca2+ concentration stimulated by tolbutamide or gliclazide, is shown in Fig. 2. Both drugs induced a biphasic rise in intracellular Ca2+ concentration, with a rapid peak that decayed to an elevated plateau that persisted until the end of the 2-minute measurement (black circles: tolbutamide, Fig. 2, C and E; gliclazide, Fig. 2, D and F). Pretreatment of INS-1 cells with 2 μM nicardipine for 30 minutes before injection of sulfonylureas completely inhibited this response. The portion of the response to each sulfonylurea that was sensitive to nicardipine [sulfonylurea response − (sulfonylurea + nicardipine response)] is shown as white circles in Fig. 2C (tolbutamide) and Fig. 2D (gliclazide). Pretreatment of INS-1 cells with 1 μM thapsigargin for 30 minutes before injection of sulfonylureas selectively inhibited the rapid peak in intracellular Ca2+ concentration, with minimal effect on the sustained plateau phase stimulated by either tolbutamide or gliclazide. The portion of the response to each sulfonylurea that was sensitive to thapsigargin pretreatment is shown as white circles in Fig. 2E (tolbutamide) and Fig. 2F (gliclazide). Thus, Ca2+ transients stimulated by either tolbutamide or gliclazide are completely blocked by nicardipine, whereas thapsigargin selectively inhibits the early phase of the transient. The close correlation between block of insulin secretion and block of the early phase of the Ca2+ transient by nicardipine and thapsigargin suggests that this rapid peak in intracellular Ca2+ concentration is particularly important in sulfonylurea-stimulated insulin secretion in INS-1 cells.
Potentiation of Sulfonylurea-Stimulated Insulin Secretion by 8-pCPT-2′-O-Me-cAMP-AM.
Because tolbutamide is reported to directly activate EPAC2 (Zhang et al., 2009), we next asked if the insulin secretion stimulated by 200 μM tolbutamide or 20 μM gliclazide was differentially potentiated by the EPAC-selective cAMP analog 8-pCPT-2′-O-Me-cAMP-AM. As shown in Fig. 3A, insulin secretion was stimulated with 200 μM tolbutamide in the absence or presence of 0.1, 0.5, 1.0, 2.0, or 5.0 μM 8-pCPT-2′-O-Me-cAMP-AM. Tolbutamide-stimulated insulin secretion was significantly potentiated by 8-pCPT-2′-O-Me-cAMP-AM at concentrations ≥2.0 μM. The potentiation of secretion over that stimulated by tolbutamide alone was 1.47-fold and 1.92-fold at 2 μM and 5 μM 8-pCPT-2′-O-Me-cAMP-AM, respectively. When the same concentrations of 8-pCPT-2′-O-Me-cAMP-AM were used in combination with 20 μM gliclazide to stimulate insulin secretion (Fig. 3A), we found that significant potentiation of the response occurred at concentrations ≥2.0 μM as well. The potentiation of secretion over that stimulated by gliclazide alone was 1.47-fold and 2.1-fold at 2 μM and 5 μM 8-pCPT-2′-O-Me-cAMP-AM, respectively. Moreover, the insulin secretion stimulated by 200 μM tolbutamide (199.3 ± 13 ng insulin/mg protein; n = 14) is not significantly different from that stimulated by 20 μM gliclazide (237 ± 13 ng insulin/mg protein; n = 12). 8-pCPT-2′-O-Me-cAMP-AM at concentrations up to 5 μM had no effect on insulin secretion in the absence of sulfonylurea (basal = 121 ± 8 ng insulin/mg protein, 5 μM 8-pCPT-2′-O-Me-cAMP-AM only = 156 ± 6 ng insulin/mg protein; n = 9 in three separate experiments). Thus the concentration dependence and extent of potentiation of both 200 μM tolbutamide and 20 μM gliclazide-stimulated insulin secretion by 8-pCPT-2′-O-Me-cAMP-AM are essentially identical.
Fig. 3.
Potentiation of tolbutamide- or gliclazide-stimulated insulin secretion by the EPAC-selective cAMP analog (ESCA) 8-pCPT-2′-O-Me-cAMP-AM. (A) 200 μM tolbutamide and 20 μM gliclazide both stimulated insulin secretion that was significantly potentiated by 8-pCPT-2′-O-Me-cAMP-AM (ESCA) at concentrations ≥2 μM. ††P < 0.01, †P < 0.05 compared with sulfonylurea and sulfonylurea + all concentrations of 8-pCPT-2′-O-Me-cAMP-AM; ***P < 0.001, *P < 0.05 compared with sulfonylurea alone (n = 9–14 in 3–5 independent experiments). (B) potentiation of tolbutamide- and gliclazide-stimulated insulin secretion by 8-pCPT-2′-O-Me-cAMP-AM is not blocked by the selective PKA inhibitor Rp-cAMPs (100 μM). ***P < 0.001 compared with sulfonylurea alone; sulfonylurea and ESCA +/− Rp-cAMPs were not significantly different (P = 0.089 for Tolb, P = 0.91 for Glz) (n = 9 in 3 independent experiments). (C) potentiation of tolbutamide- and gliclazide-stimulated insulin secretion by 5 μM 8-pCPT-2′-O-Me-cAMP-AM is completely blocked by the L-type Ca2+ channel blocker nicardipine (2 μM). ***P < 0.001, **P < 0.01, *P < 0.05 compared with basal (n = 9 in 3 independent experiments).
To ensure that the potentiation of insulin secretion by 8-pCPT-2′-O-Me-cAMP-AM that we observed with both tolbutamide and gliclazide was not mediated by cross-activation of protein kinase A (PKA), we tested the ability of the PKA-specific inhibitor Rp-adenosine-3′,5′-cyclic monophosphorothioate (Rp-cAMPs) to inhibit the potentiation of sulfonylurea-stimulated insulin secretion by 8-pCPT-2′-O-Me-cAMP-AM. As shown in Fig. 3B, 100 μM Rp-cAMPs did not significantly inhibit the potentiation of either tolbutamide- or gliclazide-stimulated insulin secretion by 5 μM 8-pCPT-2′-O-Me-cAMP-AM. However, 2 μM nicardipine completely blocked insulin secretion stimulated by tolbutamide or gliclazide in the presence of 5 μM 8-pCPT-2′-O-Me-cAMP-AM (Fig. 3C). Thus, 5 μM 8-pCPT-2′-O-Me-cAMP-AM potentiates insulin secretion stimulated by tolbutamide or gliclazide in a manner that does not involve PKA, but does require activation of L-type Ca2+ channels.
Potentiation of Sulfonylurea-Stimulated Intracellular Ca2+ Transients by 8-pCPT-2′-O-Me-cAMP-AM.
Because 5 μM, but not 1 μM, 8-pCPT-2′-O-Me-cAMP-AM potentiated insulin secretion stimulated by either tolbutamide or gliclazide, we tested the ability of these concentrations of 8-pCPT-2′-O-Me-cAMP-AM to potentiate the Ca2+ transient stimulated by tolbutamide or gliclazide. Fura2-loaded INS-1 cells were pretreated with 0, 1, or 5 μM 8-pCPT-2′-O-Me-cAMP-AM for 30 minutes prior to injection of either buffer only, 200 μM tolbutamide, or 20 μM gliclazide. The net change in fura2 fluorescence ratio (sulfonylurea response − buffer only response) is shown for a representative experiment with tolbutamide (Fig. 4A) or gliclazide (Fig. 4B). Pretreatment with 5 μM 8-pCPT-2′-O-Me-cAMP-AM markedly and selectively increased the early peak of the Ca2+ transient stimulated by either sulfonylurea over pretreatment with buffer alone or 1 μM 8-pCPT-2′-O-Me-cAMP-AM. Area under the curve (AUC) analysis for the entire postinjection time course of three separate experiments revealed that pretreatment with 5 μM, but not 1 μM, 8-pCPT-2′-O-Me-cAMP-AM significantly increased the Ca2+ integral induced by tolbutamide or gliclazide compared with control cells (Fig. 4, C and D, respectively). Thus, just as observed for potentiation of sulfonylurea-stimulated insulin secretion, 5 μM, but not 1 μM, 8-pCPT-2′-O-Me-cAMP-AM significantly increased the magnitude of the Ca2+ transient in response to sulfonylurea stimulation, and the concentration threshold for potentiation of the tolbutamide response did not differ from that for gliclazide.
Fig. 4.
Potentiation of Ca2+ transients by the EPAC-selective cAMP analog (ESCA) 8-pCPT-2′-O-Me-cAMP-AM. (A and B) increase in fura2 340 nm/380 nm ratio in response to application of 200 μM tolbutamide (A) or 20 μM gliclazide (B) to INS-1 cells preincubated with KRBH buffer only (black circles), 1 μM (white circles), or 5 μM (black triangles) 8-pCPT-2′-O-Me-cAMP-AM (ESCA). Data shown are mean ± S.E. from a representative experiment done in quadruplicate and were corrected by subtracting the change in 340 nm/380 nm ratio stimulated by injection of KRBH buffer only on to cells given the same pretreatment. C and D, area under the curve analysis of the Ca2+ transients stimulated by tolbutamide or gliclazide alone or in the presence of 1 μM or 5 μM 8-pCPT-2′-O-Me-cAMP-AM. The Ca2+ integrals for the entire poststimulation period with preincubations of 1 μM or 5 μM 8-pCPT-2′-O-Me-cAMP-AM for three separate experiments were normalized to the Ca2+ integral for tolbutamide (C) or gliclazide (D) in cells preincubated with KRBH buffer alone. The Ca2+ integral for 5 μM 8-pCPT-2′-O-Me-cAMP-AM + tolbutamide or gliclazide was significantly different from that for either sulfonylurea alone. ***P < 0.001 (n = 12 in 3 separate experiments).
Activation of Phospholipase C by Tolbutamide or Gliclazide in INS-1 Cells.
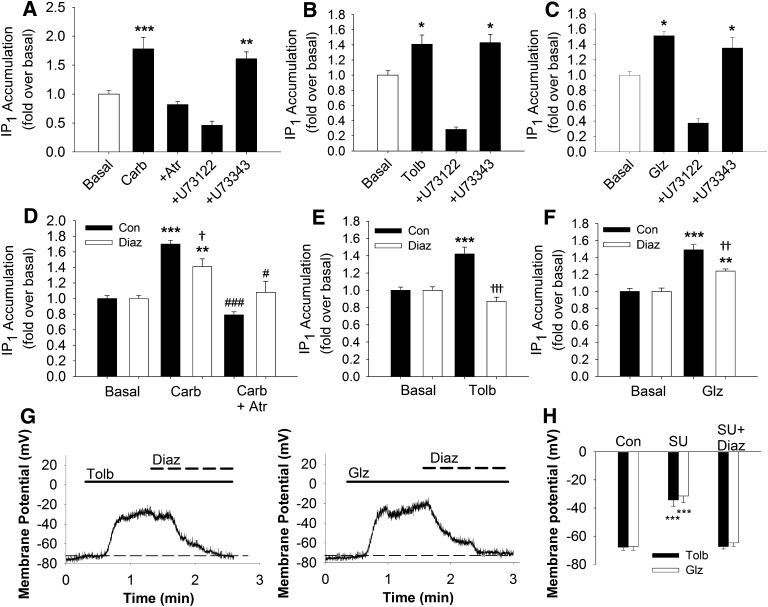
Because tolbutamide, but not gliclazide, is reported to directly activate EPAC2, we compared the ability of gliclazide and tolbutamide to stimulate phospholipase C activity in these cells. The concentrations of inositol-1-phosphate (IP1) in the presence of LiCl [to inhibit inositol-1-phosphate phosphatase (Hallcher and Sherman, 1980)] after stimulation with the muscarinic receptor agonist carbachol (500 μM) was measured in INS-1 cells using homogeneous time-resolved FRET (HTRF). Muscarinic acetylcholine receptor agonists, such as carbachol, stimulate phospholipase C activity in pancreatic beta cells (Yada et al., 1995) and INS-1 cells (Jacobo et al., 2009). The phospholipase C inhibitor U73122 and its inactive analog U73343 were used to define specific phospholipase C activity stimulated by 500 μM carbachol, 200 μM tolbutamide, or 20 μM gliclazide in the HTRF assay for IP1. As shown in Fig. 5A, 500 μM carbachol stimulated IP1 accumulation in INS-1 cells that was completely blocked by either 100 μM atropine, a muscarinic receptor antagonist, or 10 μM U73122, but not 10 μM U73343. Stimulation of INS-1 cells with 200 μM tolbutamide or 20 μM gliclazide also resulted in a significant increase in IP1 accumulation over basal levels that was completely inhibited by 10 μM U73122, but not 10 μM U73343 (Fig. 5, B and C). Although U73122 and U73343 gave the expected results in our experiments measuring IP1 accumulation, we did not use these compounds in experiments measuring changes in intracellular Ca2+ concentration because we found that 10 μM U73122 irreversibly blocks ∼80% and 10 μM U73343 reversibly blocks ∼50% of the voltage-gated Ca2+ channel activity in INS-1 cells (Supplemental Fig. 1).
Fig. 5.
Stimulation of phospholipase C activity by carbachol and the sulfonylureas tolbutamide and gliclazide. (A) 500 μM carbachol stimulated significant accumulation of IP1 over basal levels in INS-1 cells that was completely blocked by either 100 μM atropine or 10 μM U73122, but not 10 μM U73343. B and C, 200 μM tolbutamide (B) or 20 μM gliclazide (C) stimulated significant IP1 accumulation over basal levels in INS-1 cells that was completely blocked by 10 μM U73122, but not 10 μM U73343. D and F, IP1 accumulation stimulated by 500 μM carbachol (D), 200 μM tolbutamide (E), or 20 μM gliclazide (F) in the presence (white bars) or absence (black bars) of 300 μM diazoxide. ***P < 0.001, **P < 0.01, *P < 0.05 compared with basal; †††P < 0.001, ††P < 0.01, †P < 0.05 compared with indicated stimulus in the absence of diazoxide; ###P < 0.001, #P < 0.05 compared with carbachol alone. (G) representative whole cell current-clamp traces of tolbutamide (200 μM)- or gliclazide (20 μM)-induced membrane depolarization and reversal by diazoxide. Solid lines represent the time during which the indicated sulfonylurea was applied to the cell. Bold dashed lines represent the time during which 300 μM diazoxide was applied to the cell. The thin dashed line represents membrane potential of the cell before perfusion of sulfonylureas. (H) summarized data comparing the resting membrane potential (Con), the membrane potential in the presence of the indicated sulfonylurea (SU), and membrane potential in the presence of sulfonylurea and 300 μM diazoxide (SU+Diaz). Tolbutamide: Con = −67.9 ± 2 mV; SU = −34.3 ± 5 mV; DU + Diaz = −67.3 ± 2 mV (n = 5–6). Gliclazide: Con = −67.4 ± 3 mV; SU = −31.5 ± 5 mV; DU + Diaz = −64.8 ± 2 mV (n = 5–6). ***P < 0.001 compared with control or SU + Diaz.
Because membrane depolarization is reported to stimulate phospholipase C activity in INS-1 cells (Thore et al., 2004), we further asked if carbachol, tolbutamide, or gliclazide could stimulate IP1 accumulation in the presence of the KATP channel opener diazoxide. As expected, 500 μM carbachol stimulated a significant increase in IP1 accumulation over basal levels that was completely blocked by 100 μM atropine in the presence or absence of 300 μM diazoxide (Fig. 5D). However, diazoxide incompletely, but significantly, reduced the IP1 accumulation stimulated by carbachol, suggesting that membrane depolarization contributes to carbachol activation of phospholipase C activity. In contrast, diazoxide completely inhibited the ability of 200 μM tolbutamide to stimulate IP1 accumulation above basal levels (Fig. 5E). Similar to what was observed with carbachol, 20 μM gliclazide significantly stimulated IP1 accumulation over basal levels in the presence or absence of diazoxide, although this increase was significantly greater in the absence of diazoxide (Fig. 5F). To ensure that membrane potential depolarization was not contributing to the diazoxide-resistant IP1 accumulation observed with gliclazide, we measured the membrane potential in INS-1 cells stimulated with either 200 μM tolbutamide or 20 μM gliclazide and subsequently treated with 300 μM diazoxide in the continued presence of sulfonylurea drug. Figure 5G shows representative traces of whole cell current-clamp recordings of INS-1 cells depolarized with 200 μM tolbutamide (Fig. 5G, left) or 20 μM gliclazide (Fig. 5G, right). After the membrane depolarization induced by each drug had reached its maximum, 300 μM diazoxide was coapplied with the sulfonylureas. With both tolbutamide and gliclazide, the resting membrane potential was rapidly re-established when 300 μM diazoxide was present. Figure 5H summarizes the results of five or six separate experiments with 200 μM tolbutamide or 20 μM gliclazide and 300 μM diazoxide and shows that the membrane potential in the presence of diazoxide and tolbutamide or gliclazide is not different from the resting membrane potential before application of either sulfonylurea drug. Taken together, our results show that gliclazide, but not tolbutamide, is capable of stimulating phospholipase C activity independently of its KATP channel-blocking activity.
Evidence That Gliclazide Stimulates a Gαi/o Protein-Coupled Receptor.
One possibility that might explain the KATP channel-independent activity of gliclazide is that it activates a G protein-coupled receptor that can stimulate phospholipase C activity. Therefore, we asked whether pertussis toxin, an inhibitor of Gαi/o function, could interfere with the gliclazide stimulation of phospholipase C activity in the presence of diazoxide. Figure 6A shows that pretreatment of INS-1 cells in 96-well plates with 25 ng/well of pertussis toxin for 18 hours at 37°C, 5% CO2 significantly, but incompletely, inhibited the ability of 20 μM gliclazide to stimulate IP1 accumulation in the presence of 300 μM diazoxide. Increasing the amount of pertussis toxin used in the pretreatment to 50 ng/well did not further increase the extent of inhibition (data not shown). In contrast, pertussis toxin pretreatment had no effect on basal IP1 levels in the presence of 300 μM diazoxide (Fig. 6A). If gliclazide is, in fact, able to stimulate phospholipase C activity in the absence of membrane depolarization via activation of a Gαi/o-coupled receptor, then gliclazide should also stimulate a Ca2+ transient in the presence of diazoxide. Figure 6B is a representative experiment showing 20 μM gliclazide stimulation of Ca2+ transients in fura2-loaded INS-1 cells in the absence or presence of 300 μM diazoxide. A rise in intracellular Ca2+ concentration in the presence of diazoxide was clearly detectable. The magnitude of the response (area under the curve) to gliclazide in the presence of diazoxide was significantly greater than that stimulated by 200 μM tolbutamide under the same conditions (Fig. 6B, inset).
Fig. 6.
Evidence for KATP channel-independent activities of gliclazide. (A) stimulation of phospholipase C activity in the presence of diazoxide by gliclazide in INS-1 cells is partially inhibited by pertussis toxin (PTx). In the absence of PTx (white bars), 20 μM gliclazide (Glz) significantly stimulates IP1 accumulation in the presence of 300 μM diazoxide (Diaz). In cells pretreated with PTx (black bars), basal IP1 accumulation in the presence of diazoxide is not different from non-pertussis toxin-treated cells, but gliclazide-stimulated IP1 accumulation is significantly reduced compared with control cells. Data shown are mean ± S.E. from four separate experiments done in triplicate. (***P < 0.001 compared with the corresponding control; †P < 0.05 compared with Glz + Diaz without PTx pretreatment). (B) representative experiment illustrating the transient rise in intracellular Ca2+ concentration in INS-1 cells stimulated by 20 μM Glz in the absence (black circles) or presence (white circles) of 300 μM Diaz. (Inset) area under the curve analysis of Ca2+ transients measured in INS-1 cells stimulated with either tolbutamide (Tolb) or gliclazide in the presence of Diaz. **P < 0.01 (n = 6–8 in 3–4 independent experiments). (C and D) increase in fura2 340 nm/380 nm ratio in response to application of 200 μM tolbutamide (C) or 20 μM gliclazide (D) to INS-1 cells preincubated with KRBH only (black circles). White circles in (C) and (D) represent the portion of the response to Tolb or Glz, respectively, inhibited by preincubation with 20 μM ryanodine (Ryn). Preincubation with Ryn completely inhibited the early peak of the transient stimulated by Tolb (C) but not Glz (D). Data shown are mean ± S.E. of representative experiments performed in quadruplicate. (E) removal of extracellular Ca2+ reduces, but does not eliminate, the Ca2+ transient stimulated by 20 μM Glz or 20 μM Glz + 20 μM Ryn. In experiments labeled 0 Ca2+, KRBH with no added Ca2+ was used. Data shown are mean ± S.E. for 6–15 independent experiments performed in quadruplicate. Area under the curve analysis for 6 (Glz alone, Glz + 0 Ca2+, Glz + Ryn + 0 Ca2+) or 15 (Glz + Ryn) independent experiments revealed no significant difference between the Ca2+ transients stimulated by Glz or Glz + Ryn in either the absence or presence of 2 mM Ca2+. However, reducing extracellular Ca2+ significantly reduced the Ca2+ transients stimulated by Glz alone or Glz + Ryn (P < 0.05 and P < 0.01, respectively; not shown). (F) ryanodine-resistant Ca2+ transient stimulated by gliclazide is not inhibited by PTx. Increase in fura2 340 nm/380 nm ratio in response to application of 20 μM gliclazide to INS-1 cells preincubated with KRBH only (black circles), 20 μM Ryn (white circles), or 20 μM Ryn after an 18 hours of pretreatment with PTx (gray diamonds). Data from cells pretreated with PTx only were omitted for clarity. Data shown are mean ± S.E. and are representative of six independent experiments performed in quadruplicate. Area under the curve analysis of six independent experiments revealed no significant difference between Glz alone, Glz + PTx, Glz + Ryn, or Glz + Ryn + PTx (not shown).
We next examined the ability of ryanodine to block Ca2+ transients stimulated by tolbutamide or gliclazide. We reasoned that Ca2+ transients stimulated by Ca2+-induced Ca2+ release would be sensitive to ryanodine, but Ca2+ release stimulated by activation of phospholipase C via IP3 receptors would not. We found that pretreatment of INS-1 cells with 20 μM ryanodine for 30 minutes before injection of sulfonylureas completely inhibited the early phase of the rise in intracellular Ca2+ in response to 200 μM tolbutamide (Fig. 6C; ryanodine-sensitive: white circles). However, pretreatment of INS-1 cells with 20 μM ryanodine did not strongly inhibit the Ca2+ transient stimulated by 20 μM gliclazide (Fig. 6D; ryanodine-sensitive: white circles). We next tested the requirement for physiologic concentrations of extracellular Ca2+ in the ryanodine-resistant transients stimulated by gliclazide. Figure 6E shows that in KRBH with zero added Ca2+, 20 μM gliclazide was still able to stimulate a robust increase in intracellular Ca2 concentration in the presence or absence of 20 μM ryanodine. To examine a possible role of Gαi/o-coupled receptors in this gliclazide-stimulated, ryanodine-insensitive Ca2+ transient, we pretreated INS-1 cells in 96-well plates with 25 ng/well of pertussis toxin (PTx) for 18 hours at 37°C, 5% CO2 and measured the Ca2+ transients stimulated by 20 μM gliclazide in the presence of 20 μM ryanodine. A representative of six independent experiments is shown in Fig. 6F. Area under the curve analysis of these six experiments, normalized to the gliclazide response, revealed that PTx pretreatment did not significantly reduce the ability of gliclazide to stimulate a rise in intracellular Ca2+ concentration in the presence of ryanodine. Together, the data in Fig. 6 suggest that gliclazide stimulates phospholipase C activity independently of KATP channel block and that this activity is, in part, mediated by a Gαi/o-dependent mechanism. This unique activity of gliclazide is correlated with stimulation of a more robust Ca2+ transient in the presence of diazoxide compared with tolbutamide. Moreover, the unique ability of gliclazide to stimulate a Ca2+ transient independently of activation of ryanodine receptors does not involve a Gαi/o-dependent mechanism and does not require influx of Ca2+ across the plasma membrane.
8-pCPT-2′-O-Me-cAMP Does Not Enhance Membrane Depolarization Stimulated by 200 μM Tolbutamide or 20 μM Gliclazide or Voltage-Gated Ca2+ Channel Activity in INS-1 Cells.
To further elicit the mechanism of 8-pCPT-2′-O-Me-cAMP potentiation of insulin secretion stimulated by tolbutamide or gliclazide in INS-1 cells, we used whole cell current-clamp measurements of membrane potential depolarization induced by these sulfonylureas in the presence or absence of 8-pCPT-2′-O-Me-cAMP. In these experiments 5 μM 8-pCPT-2′-O-Me-cAMP, not the acetoxymethyl ester, was included in the pipette solution and allowed to dialyze into the cells for 10 min before either 200 μM tolbutamide or 20 μM gliclazide was applied via bath perfusion. We found that addition of 5 μM 8-pCPT-2′-O-Me-cAMP to the pipette solution did not significantly alter the extent of membrane potential depolarization induced by either sulfonylurea (Fig. 7A). The effect of 5 μM 8-pCPT-2′-O-Me-cAMP dialysis into the cells on whole cell voltage-gated Ca2+ channel currents was also assessed. Addition of 5 μM 8-pCPT-2′-O-Me-cAMP to the pipette solution did not change the Ba2+ current density or the voltages at which whole cell voltage-dependent Ba2+ currents activated, from a holding potential of −70 mV, at either 3 min or >10 min after break-in compared with control cells (Fig. 7, B and C). However, an approximately −15 mV shift in the reversal potential of voltage-dependent Ba2+ current was observed after 10 minutes with 5 μM 8-pCPT-2′-O-Me-cAMP in the pipette (Fig. 7C). This shift in reversal potential was likely due to an increase in membrane permeability to the organic cation N-methyl-d-glucamine (NMDG) present in the intracellular solution, because it was abolished by equalizing the NMDG concentration in the intracellular and extracellular solutions (Fig. 7C). In contrast, no shift was observed in controls cells in which 0.1% DMSO (vehicle for 8-pCPT-2′-O-Me-cAMP) was included in the intracellular solution. The kinetics of current activation and inactivation were also unaffected by 5 μM 8-pCPT-2′-O-Me-cAMP as illustrated in Fig. 7D, which shows Ba2+ current measured at 0 mV from a holding potential of −70 mV, in a single cell, at break-in and 10 minutes after break-in. Thus, at a concentration that significantly potentiated insulin secretion, 8-pCPT-2′-O-Me-cAMP had no detectable effect on membrane potential depolarization induced by either 200 μM tolbutamide or 20 μM gliclazide or on the amplitude and voltage dependence of voltage-gated Ca2+ channel activity in INS-1 cells. However, 5 μM 8-pCPT-2′-O-Me-cAMP, applied via the patch pipette, did enhance membrane permeability to the organic cation NMDG.
Fig. 7.
Effect of intracellular application of the EPAC-selective cAMP analog (ESCA) 8-pCPT-2′-O-Me-cAMP on sulfonylurea-stimulated membrane depolarization and voltage-dependent Ca2+ channel activity in INS-1 cells. (A) membrane potential changes measured using whole cell current clamp during application of tolbutamide or gliclazide in the absence (black bars) or presence (gray bars) of 5 μM 8-pCPT-2′-O-Me-cAMP (ESCA) (n = 6–10). 8-pCPT-2′-O-Me-cAMP was included in the intracellular solution of the patch pipette, and the cell was held for at least 10 min to allow for equilibration of 8-pCPT-2′-O-Me-cAMP into the cell. After 10 minutes, tolbutamide or gliclazide was applied and changes in membrane potential were recorded. (B) whole cell IBa density (pA/pF) measured at 0 mV from a holding potential of −70 mV using whole cell voltage clamp. 8-pCPT-2′-O-Me-cAMP (5 μM) was included in the intracellular solution of the patch pipette. Once consistent, currents were recorded 3 minutes after break-in and then again 10 minutes after break-in. (n = 11). (C) current-voltage relationship curves of IBa measured using whole cell voltage clamp. Currents were elicited by 100-ms depolarizations from −50 to +60 mV in 10-mV increments, from a holding potential of −70 mV, in the absence (control, gray triangles) or presence of 5 μM 8-pCPT-2′-O-Me-cAMP in the patch pipette 3 minutes (black circles) or 10 minutes (gray circles) after break-in (n = 15). The shift in reversal potential observed >10 min after break-in with 8-pCPT-2′-O-Me-cAMP in the patch pipette is abolished when the concentration of N-methyl-d-glucamine (NMDG) is equalized on both sides of the membrane by replacing 130 mM of Tris in the extracellular solution with 130 mM NMDG (black triangles) (n = 6). The shift in reversal potential is not observed at >10 minutes after break-in when 0.1% DMSO only (vehicle; gray triangles) is added to the intracellular solution. (D) representative whole cell voltage clamp trace of IBa measured at 0 mV, from a holding potential of −70 mV, with 5 μM of 8-pCPT-2′-O-Me-cAMP at 3 min after break-in (control) or 10 min after break-in in the same INS-1 cell.
Activation of Phospholipase C Activity by 8-pCPT-2′-O-Me-cAMP-AM.
Because activation of EPAC2 leads to activation of PLC-ε via Rap1 (Dzhura et al., 2010), we examined the ability of the concentrations of 8-pCPT-2′-O-Me-cAMP-AM that gave significant potentiation of insulin secretion by tolbutamide or gliclazide to stimulate an increase in cellular phospholipase C activity. As expected, 500 μM carbachol stimulated an increase in IP1 accumulation in INS-1 cells that was completely blocked by 100 μM atropine (Fig. 8A). Concentrations of 8-pCPT-2′-O-Me-cAMP-AM from 1-20 μM did not stimulate IP1 accumulation above the basal level. However, 50 μM 8-pCPT-2′-O-Me-cAMP-AM applied to INS-1 cells significantly increased IP1 accumulation to an extent that was not different from that stimulated by 500 μM carbachol (Fig. 8A). Because 5 μM 8-pCPT-2′-O-Me-cAMP-AM was sufficient to strongly potentiate both insulin secretion and Ca2+ transients stimulated by tolbutamide or gliclazide, we looked for other evidence that 5 μM 8-pCPT-2′-O-Me-cAMP-AM could, in fact, stimulate phospholipase C activity. If the HTRF assay for IP1 is not sensitive enough to detect very low concentrations of IP1 generated by 5 μM 8-pCPT-2′-O-Me-cAMP-AM, we reasoned that a small rise in intracellular Ca2+ stimulated by low levels of phospholipase C activation might be detectable. Therefore, we applied increasing concentrations of 8-pCPT-2′-O-Me-cAMP-AM to fura2-loaded INS-1 cells and measured the change in 340 nm/380 nm ratio over 8-10 minutes. This longer time scale was used because 8-pCPT-2′-O-Me-cAMP-AM must be de-esterified intracellularly before it becomes active. We found that 8-pCPT-2′-O-Me-cAMP-AM dose dependently increased intracellular Ca2+ concentrations and that a significant increase over basal was observed at concentrations as low as 2 μM and 5 μM (Fig. 8B). The rise in intracellular Ca2+ stimulated by 5 μM 8-pCPT-2′-O-Me-cAMP-AM was significantly blocked by preincubation of cells with 25 μM 2-aminoethoxydiphenylborate (2-APB) (Fig. 8C). Because 2-APB is able to block both IP3 receptors and TRP channels (Bootman et al., 2002), we examined the ability of 5 μM 8-pCPT-2′-O-Me-cAMP-AM to stimulate Ca2+ transients in the absence of extracellular Ca2+. We found that 5 μM 8-pCPT-2′-O-Me-cAMP-AM stimulation of Ca2+ transients in INS-1 cells was abolished by removal of Ca2+ from the extracellular solution (Fig. 8C). Thus, even though we were unable to detect an increase in IP1 levels stimulated by 5 μM 8-pCPT-2′-O-Me-cAMP-AM, it does stimulate a small, but detectable, increase in intracellular Ca2+ that could mediate the potentiation of sulfonylurea action.
Fig. 8.
The EPAC-selective cAMP analog (ESCA) 8-pCPT-2′-O-Me-cAMP stimulates phospholipase C activity and a slow, persistent rise in intracellular Ca2+ in INS-1 cells. (A) 8-pCPT-2′-O-Me-cAMP-AM significantly stimulates IP1 accumulation at 50 μM, but not at 1,5,10, or 20 μM in INS-1 cells (black bars). A positive control for phospholipase C activation, carbachol (500 μM), stimulates IP1 accumulation that is completely blocked by 100 μM atropine. ***P < 0.001 compared with basal (one-way analysis of variance with Tukey’s post hoc test). (B) representative experiments showing fura2 340 nm/380 nm ratio over a 10-minute interval following application of 2,5,25, or 50 µM 8-pCPT-2′-O-Me-cAMP-AM (n = 2–7 in 7 independent experiments). Concentrations as low as 2 µM 8-pCPT-2′-O-Me-cAMP-AM displayed a sustained rise in intracellular Ca2+. At 7 minutes post injection, the Δ340 nm/380 nm ratio was significantly different from KRBH injection for all concentrations of 8-pCPT-2′-O-Me-cAMP-AM (2 μM, P < 0.05; 5 μM, P < 0.05; 25 μM, P < 0.001; 50 μM, P < 0.001). (C) area under the curve analysis of Ca2+ transients stimulated by 5 µM 8-pCPT-2′-O-Me-cAMP-AM alone in the presence of 25 µM 2-APB or in the absence of extracellular Ca2+. Data from 3–4 separate experiments performed in quadruplicate are shown as mean ± S.E. ***P < 0.001 compared with 8-pCPT-2′-O-Me-cAMP-AM stimulation alone. (D) 25 μM 2-APB markedly reduces potentiation of tolbutamide-stimulated insulin secretion by 5 μM 8-pCPT-2′-O-Me-cAMP-AM. Data were normalized to secretion stimulated by 200 μM tolbutamide alone. Data shown are mean ± S.E. of three independent experiments. ***P < 0.001, **P < 0.01, compared with tolbutamide + 2-APB; ###P < 0.001, compared with tolbutamide alone; †††P < 0.001 compared with tolbutamide + ESCA + 2-APB.
Because 2-APB inhibits the 8-pCPT-2′-O-Me-cAMP-AM-stimulated increase in intracellular Ca2+ concentration, we tested the ability of 2-APB to inhibit 8-pCPT-2′-O-Me-cAMP-AM potentiation of tolbutamide-stimulated insulin secretion. We chose tolbutamide over gliclazide for these experiments to avoid the KATP channel-independent activity of gliclazide. As shown in Fig. 8D, 5 μM 8-pCPT-2′-O-Me-cAMP-AM increased insulin secretion stimulated by 200 μM tolbutamide by approximately twofold, whereas addition of 25 μM 2-APB alone did not significantly affect tolbutamide-stimulated insulin secretion . However, when INS-1 cells were treated with 25 μM 2-APB + 5 μM 8-pCPT-2′-O-Me-cAMP-AM, tolbutamide-stimulated insulin secretion was not different from that measured in the absence of either compound. Thus, 25 μM 2-APB significantly blocked both the increase in intracellular Ca2+ concentration and the potentiation of tolbutamide-stimulated insulin secretion stimulated by 5 μM 8-pCPT-2′-O-Me-cAMP-AM in INS-1 cells.
Modulation of 8-pCPT-2′-O-Me-cAMP-AM Potentiation of Tolbutamide Action by Bisindolylmaleimide I.
Protein kinase C (PKC) is a potential downstream effector of the small increase in intracellular Ca2+ stimulated by low concentrations of 8-pCPT-2′-O-Me-cAMP-AM. PKC is activated by Ca2+ and is reported to phosphorylate RYR2 and enhance Ca2+-induced Ca2+ release in cardiac muscle downstream of EPAC activation (Oestreich et al., 2009). We therefore examined the ability of the broad-spectrum PKC inhibitor bisindolylmaleimide I (BIS) to inhibit potentiation of tolbutamide action by 8-pCPT-2′-O-Me-cAMP-AM (Fig. 9). As shown in Fig. 9A, preincubation with 1 μM BIS significantly increased the amplitude of the Ca2+ transient stimulated by 200 μM tolbutamide to a similar extent as 5 μM 8-pCPT-2′-O-Me-cAMP-AM. The combination of BIS and 8-pCPT-2′-O-Me-cAMP-AM also significantly increased the amplitude of the Ca2+ transient stimulated by tolbutamide, but there was no further increase over that observed with BIS or 8-pCPT-2′-O-Me-cAMP-AM alone. We next examined the ability of BIS to potentiate insulin secretion stimulated by tolbutamide. Consistent with the modulation of Ca2+ transients, 1 μM BIS also potentiated the insulin secretion stimulated by 200 μM tolbutamide (Fig. 9C). In contrast to the experiments measuring Ca2+ transients, the combination of 1 μM BIS + 5 μM 8-pCPT-2′-O-Me-cAMP-AM potentiated insulin secretion stimulated by tolbutamide to a greater extent than 1 μM BIS alone (Fig. 9D). Because inhibition of PKC potentiates both tolbutamide-stimulated Ca2+ transients and insulin secretion, it is unlikely that activation of PKC by EPAC2/Rap1 stimulation of phospholipase C activity plays a role in the potentiation of sulfonylurea-stimulated Ca2+ transients or insulin secretion.
Fig. 9.
Tolbutamide action is enhanced by the PKC inhibitor bisindolylmaleimide I. (A and B) increase in fura2 340 nm/380 nm ratio in response to application of 200 μM tolbutamide (A) to INS-1 cells preincubated with KRBH buffer only (white diamonds), 5 μM 8-pCPT-2′-O-Me-cAMP-AM (gray squares), 1 μM bisindolylmaleimide (BIS) (black triangles) or both 5 μM 8-pCPT-2′-O-Me-cAMP-AM (ESCA) and 1 μM BIS (white circles). Data shown are mean ± S.E. from a representative experiment done in quadruplicate (A). Area under the curve analysis of Ca2+ transients (B) that were normalized to the Ca2+ integrals for tolbutamide in the absence of any treatment. The Ca2+ integrals for 200 μM tolbutamide plus 5 μM 8-pCPT-2′-O-Me-cAMP-AM or 1 μM bisindolylmaleimide or both 5 μM 8-pCPT-2′-O-Me-cAMP-AM and 1 μM bisindolylmaleimide were each significantly greater than tolbutamide alone. Data shown are mean ± S.E. for three separate experiments (n = 9). ***P < 0.001, **P < 0.01. (C) potentiation of tolbutamide-stimulated insulin secretion by 1 μM BIS. Insulin secretion stimulated by 200 μM tolbutamide in the presence of 1 μM BIS was significantly greater than that stimulated by 200 μM tolbutamide alone. Insulin secretion in the presence of 1 μM BIS alone was not different from basal. Data shown are mean ± S.E. for three separate experiments (n = 9). ***P < 0.001, **P < 0.01 compared with basal; †††P < 0.001, ††P < 0.01 compared with BIS alone; ##P < 0.01 compared with tolbutamide alone. (D) insulin secretion stimulated by 200 μM tolbutamide + 1 μM BIS is further increased by 5 μM 8-pCPT-2′-O-Me-cAMP-AM. Data shown are mean ± S.E. for three independent experiments. ***P < 0.001, compared with tolbutamide alone; ††P < 0.01 compared with tolbutamide + BIS.
Discussion
Potentiation of Tolbutamide- or Gliclazide-stimulated Insulin Secretion and Ca2+ Transients by the EPAC-selective cAMP Analog 8-pCPT-2′-O-Me-cAMP Is Not Different in INS-1 Cells.
Previous studies reported that the sulfonylurea tolbutamide is able to directly activate EPAC2 while the structurally distinct sulfonylurea gliclazide is not (Zhang et al., 2009). We tested this conclusion in the rat pancreatic β-cell line INS-1 using a pharmacological approach. If tolbutamide activates EPAC2 in INS-1 cells, we would expect that tolbutamide might stimulate insulin secretion to a greater extent than gliclazide at concentrations of the drugs that are equivalent in terms of their activity at the KATP channels. In fact, we found that at such concentrations, tolbutamide and gliclazide stimulated insulin secretion to the same extent (Fig. 3A). In addition, if tolbutamide was able to activate EPAC2 directly in INS-1 cells, we would expect that gliclazide-stimulated secretion would be more sensitive to potentiation by 8-pCPT-2′-O-Me-cAMP-AM. However, we found that the concentration at which 8-pCPT-2′-O-Me-cAMP-AM potentiation of insulin secretion reached significance was identical for both sulfonylureas (i.e., 2 μM; Fig. 3A). In addition, the Ca2+ transient stimulated by either sulfonylurea was significantly potentiated by 8-pCPT-2′-O-Me-cAMP-AM at 5 μM, but not at 1 μM (Fig. 4). These findings are inconsistent with a direct activation of EPAC2 by 200 μM tolbutamide, but not 20 μM gliclazide, in INS-1 cells.
8-pCPT-2′-O-Me-cAMP Potentiation of Sulfonylurea-Stimulated Insulin Secretion and Ca2+ Transients Does Not Require Activation of PKA or Enhancement of Membrane Depolarization.
The mechanism by which 8-pCPT-2′-O-Me-cAMP-AM potentiates insulin secretion by sulfonylureas was also examined. The concentrations of 8-pCPT-2′-O-Me-cAMP-AM used in this study are very likely to be selective for activation of EPAC over PKA because even 1 mM 8-pCPT-2′-O-Me-cAMP activated <25% of PKA activity at physiologic concentrations of the enzyme (Christensen et al., 2003). However, sulfonylurea stimulation of pancreatic β-cells has been reported to stimulate adenylyl cyclase activity (Grill and Cerasi, 1978). Therefore, endogenously produced cAMP, and activation of PKA, could potentially contribute to the activities of tolbutamide and gliclazide observed in this study. To test this possibility, we asked if the PKA-selective inhibitor Rp-cAMPs could interfere with 8-pCPT-2′-O-Me-cAMP-AM potentiation of sulfonylurea-stimulated insulin secretion; however, a role for PKA activity was excluded because 100 μM Rp-cAMPs had no effect (Fig. 3). This result contrasts with a previous report that potentiation of glucose-stimulated insulin secretion by 8-pCPT-2′-O-Me-cAMP-AM was markedly attenuated by inhibitors of PKA in human islets (Chepurny et al., 2010). This discrepancy may result from differences between glucose stimulation of β-cells, which generates many glucose metabolites, and sulfonylurea stimulation, which more specifically regulates membrane potential.
A previous study (Leech et al., 2010b) found that 8-pCPT-2′-O-Me-cAMP-AM at concentrations of 10 μM and 50 μM increased the sensitivity of KATP channels in excised membrane patches to relatively low concentrations of tolbutamide. However, as channel block by tolbutamide approached saturation, this effect of 8-pCPT-2′-O-Me-cAMP-AM became insignificant. Our analysis of the electrophysiological effects of 5 μM 8-pCPT-2′-O-Me-cAMP-AM on INS-1 cells did not reveal any potentiation of sulfonylurea-induced membrane depolarization or any enhancement of voltage-gated Ca2+ channel activity (Fig. 7). This is not surprising given that the concentrations of sulfonylureas were saturating both in terms of their effect on membrane potential and in block of whole cell KATP channel currents (Fig. 1). Moreover, the concentration of 8-pCPT-2′-O-Me-cAMP-AM used in the electrophysiological experiments reported here were lower than those used by Leech et al. (2010b). Thus, the ability of 8-pCPT-2′-O-Me-cAMP-AM to potentiate insulin secretion and Ca2+ transients in our experiments was independent of any enhancement of electrical activity stimulated by the sulfonylureas.
Potentiation of Sulfonylurea-Stimulated Insulin Secretion by 8-pCPT-2′-O-Me-cAMP-AM Involves Ca2+ Influx via L-type Voltage-Gated Ca2+ Channels and Enhanced Release of Ca2+ from Internal Stores.
Insulin secretion and Ca2+ transients (Fig. 2) stimulated by tolbutamide and gliclazide were both blocked by the L-type Ca2+ channel blocker nicardipine. Moreover, insulin secretion stimulated by both sulfonylureas was substantially blocked by unloading intracellular stores of Ca2+ with thapsigargin (Fig. 2). Thapsigargin selectively inhibited the early peak of the Ca2+ transient stimulated by either tolbutamide or gliclazide (Fig. 2). Interestingly, the major effect of 8-pCPT-2′-O-Me-cAMP-AM on sulfonylurea-stimulated Ca2+ transients was to markedly increase the amplitude of this early peak (Fig. 4). These data suggest that Ca2+ influx via L-type Ca2+ channels upon sulfonylurea stimulation of INS-1 cells causes a rapid release of Ca2+ from internal stores that is greatly amplified by 8-pCPT-2′-O-Me-cAMP-AM via EPAC2. This enhancement of Ca2+ release is likely the driving factor behind the enhancement of insulin secretion because the concentration threshold of 8-pCPT-2′-O-Me-cAMP-AM for both activities is essentially the same.
Gliclazide, But Not Tolbutamide, Stimulates a Depolarization-independent Activation of Phospholipase C Activity in INS-1 Cells.
If tolbutamide were able to directly activate EPAC2 and Rap1 in INS-1 cells, it may have a unique ability to activate phospholipase C-ε and mobilize Ca2+ from internal stores via an RYR2-independent, phospholipase C/IP3 receptor-dependent mechanism. We found, however, that both tolbutamide and gliclazide markedly stimulated phospholipase C activity as assessed by accumulation of IP1. It was previously reported that glucose stimulates phospholipase C activity that depends upon Ca2+ influx via L-type Ca2+ channels and protein kinase C activation in INS-1 cells (Thore et al., 2004). However, our experiments measuring gliclazide- and tolbutamide-stimulated phospholipase C activity in the presence of diazoxide uncovered a unique ability of gliclazide to activate phospholipase C independently of its KATP channel blocking activity (Fig. 5). The mechanism accounting for this activity remains unknown. However, the unique activities of gliclazide that we have identified in this study, including its similarity to carbachol in stimulating IP1 accumulation in the presence or absence of diazoxide, the partial sensitivity of this activity to pertussis toxin, the ability to stimulate a diazoxide-insensitive Ca2+ transient, and the ability to stimulate a ryanodine-insensitive Ca2+ transient in the presence or absence of physiologic concentrations of extracellular Ca2+ (Fig. 6), suggest that gliclazide may activate a GPCR that stimulates phospholipase C (Fig. 10A). Attractive possibilities include cannabinoid or purinergic receptors. CB1 and CB2 receptors are present and coupled to activation of phospholipase C and Ca2+ mobilization in the rat pancreatic β-cell line Rinm5F (De Petrocellis et al., 2007) and mouse pancreatic β-cells (Li et al., 2010). The CB1 receptor inverse agonists rimonabant and ibipinabant are reported to act as KATP channel openers (Lynch et al., 2012) at low micromolar concentrations, suggesting some similarity in the structural requirements for KATP channel and CB1 receptor modulation. Additionally, various subtypes of P2Y receptors are expressed in pancreatic beta cells, some of which activate PLC in response to binding of ATP and other nucleotides (Burnstock and Novak, 2012). Alternatively, our data do not exclude the possibility that gliclazide has a direct effect on PLC activity that is synergistic with Gi and is therefore partially inhibited by pertussis toxin. Whatever the mechanism, our results provide a potential explanation for some unique therapeutic advantages of gliclazide. For example, gliclazide carries a reduced risk for secondary beta cell failure in patients with type 2 diabetes compared with the sulfonylurea glibenclamide (Satoh et al., 2005). In addition, two large clinical studies have reported a reduced risk of death by cardiovascular disease in type 2 diabetics taking gliclazide compared with similar patients taking other sulfonylureas including glibenclamide and tolbutamide (Jorgensen et al., 2010; Schramm et al., 2011). Interestingly, gliclazide is reported to inhibit several activities of oxidized low-density lipoprotein in human endothelial cells (Li and Renier, 2009) and to reduce adhesion of monocytes to endothelial cells (Renier et al., 2003) —early steps in atherosclerosis. Determining whether these beneficial effects of gliclazide can be attributed to the non-KATP channel-dependent activity reported here will clearly require further investigation.
Fig. 10.
Model for the gliclazide KATP channel-independent activity of gliclazide and the EPAC-mediated potentiation of sulfonylurea action. (A) model for the KATP channel-independent activity of gliclazide. Both tolbutamide and gliclazide depolarize the membrane potential by blocking KATP channels. The resulting Ca2+ influx via L-type channels is coupled to Ca2+-induced Ca2+ release from the ER and insulin secretion. Membrane depolarization (this study) and Ca2+ influx (Mogami et al., 2003; Jacobo et al., 2009) is also apparently coupled to activation of a phospholipase C activity. When membrane potential depolarization is prevented with diazoxide, the unique ability of gliclazide to activate PLC via a mechanism that is partially mediated by Gαi/O is unmasked. The observation that PTx only partially inhibits gliclazide-stimulated PLC activity in the presence of diazoxide suggests that another G protein (presumably Gαq/11) is also involved. We hypothesize that gliclazide is an agonist at an as of yet unidentified G protein-coupled receptor. (B) model for EPAC-mediated potentiation of sulfonylurea action. Binding of cAMP to EPAC2 activates PLC-ε via Rap1 (Dzhura et al., 2010), leading to an influx of Ca2+ that can greatly amplify Ca2+-induced Ca2+ release and insulin secretion stimulated by sulfonylureas. TRPC channels are proposed as potential effectors of EPAC2 /Rap1 activation based upon their sensitivity to 2-APB and activation via a PLC-dependent mechanism.
The concentrations at which 8-pCPT-2′-O-Me-cAMP-AM began to potentiate insulin secretion (2 μM) and the first phase of the Ca2+ transient stimulated by sulfonylureas (5 μM) were an order of magnitude below the lowest concentration of 8-pCPT-2′-O-Me-cAMP-AM that activated phospholipase C as detected by the IP1 assay (50 μM). A previous study reported activation of phospholipase C activity in INS-1 cells by 10 μM 8-pCPT-2′-O-Me-cAMP-AM as detected by the translocation of a pleckstrin homology domain/GFP fusion (Leech et al., 2010b). Our data argue that concentrations as low as 2 μM of 8-pCPT-2′-O-Me-cAMP-AM stimulate PLC activity, which is sufficient to stimulate a rise in intracellular Ca2+ concentration, but below the level of detection of the IP1 HTRF assay. The greater sensitivity of fluorescent Ca2+ indicators compared with the IP1 HTRF assay in detecting the activation of phospholipase C was previously reported (Liu et al., 2008). The 8-pCPT-2′-O-Me-cAMP-AM-stimulated Ca2+ rise is sensitive to both 2-APB and removal of extracellular Ca2+, suggesting that influx of Ca2+ via a TRP channel may be involved (Fig. 10B). Indeed, inclusion of 5 μM 8-pCPT-2′-O-Me-cAMP in the pipette solution during voltage-clamp experiments activated an NMDG conductance in the plasma membrane of INS-1 cells (Fig. 7C). Interestingly, several members of the TRP superfamily of cation channels are reported to conduct NMDG, including TRPC (Hillyard et al., 2010), TRPA (Banke et al., 2010), and TRPV (Chung et al., 2008). TRPC channel subtypes are attractive candidates for this activity because they are blocked by 2-APB (Birnbaumer, 2009) and many are positively regulated by PLC activity (Beech, 2012). Interestingly, TRPC1 and TRPC4 are expressed in INS-1 cells and rat β-cells (Li and Zhang, 2009), and TRPC4 is expressed in βTC3 cells (Qian et al., 2002).
In summary, our data show that insulin secretion and Ca2+ transients stimulated by gliclazide or tolbutamide are potentiated equally by the EPAC selective cAMP analog 8-pCPT-2′-O-Me-cAMP in INS-1 cells. However, gliclazide, but not tolbutamide, exhibits KATP channel-independent stimulation of phospholipase C activity that is mediated, in part, by a Gαi/O-dependent mechanism. Uncovering differences in activities between gliclazide and other sulfonylureas is of therapeutic relevance because gliclazide has shown some clinical advantages over other second generation drugs. Our data also suggest a potential role for activation of a 2-APB-sensitive Ca2+ influx in the EPAC-dependent potentiation of sulfonylurea-stimulated insulin secretion in INS-1 cells. It will be of interest to further characterize this Ca2+ flux and identify the channel by which it is conducted.
Supplementary Material
Abbreviations
- 2-APB
2-aminoethoxydiphenylborate
- BIS
bisindolylmaleimide I
- Carbachol-2
(trimethylazaniumyl)ethyl carbamate chloride
- diazoxide
7-chloro-3-methyl-4H-1,2,4-benzothiadiazine-1,1-dione
- EPAC
exchange protein directly activated by cAMP
- ESCA
EPAC-selective cAMP analog
- gliclazide
1-[(4-methylbenzene)sulfonyl]-3-{octahydrocyclopenta[c]pyrrol-2-yl}urea
- IP1
inositol monophosphate
- nicardipine
3-{2-[benzyl(methyl)amino]ethyl} 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate
- 8-pCPT
2′-O-Me-cAMP-AM-para-chlorophenylthio-2′-O-methyladenosine-3′-5′-cyclic monophosphate, acetoxymethyl ester
- Rp-cAMPs
adenosine-3′-5′-cyclic monophosphorothioate, Rp-isomer
- SERCA
sarcoplasmic/endoplasmic reticulum Ca2+-ATPase
- tolbutamide
3-butyl-1-[(4-methylbenzene)sulfonyl]urea
- TRP
transient receptor potential
- U73122
1-[6{[(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]- amino}hexyl]-1H-pyrrole-2,5-dione
- U73343
1-[6-{[(17β)-3-methoxyestra-1,3,5[10]-trien-17-yl]amino}hexyl]-2,5-pyrrolidinedione
Authorship Contributions
Participated in research design: Hockerman, Jarrard, Wang, Guerra.
Conducted experiments: Jarrard, Wang, Guerra, Salyer, Soderling, Pratt, Lange, Broderick.
Performed data analysis: Hockerman, Jarrard, Wang, Pratt, Guerra.
Wrote or contributed to the writing of the manuscript: Hockerman.
Footnotes
The work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01 DK064736] to G.H.H.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Babenko AP, Aguilar-Bryan L, Bryan J. (1998) A view of sur/KIR6.X, KATP channels. Annu Rev Physiol 60:667–687 [DOI] [PubMed] [Google Scholar]
- Banke TG, Chaplan SR, Wickenden AD. (2010) Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. Am J Physiol Cell Physiol 298:C1457–C1468 [DOI] [PubMed] [Google Scholar]
- Barg S, Ma XS, Eliasson L, et al. (2001) Fast exocytosis with few Ca(2+) channels in insulin-secreting mouse pancreatic B cells. Biophys J 81:3308–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ. (2012) Integration of transient receptor potential canonical channels with lipids. Acta Physiol (Oxf) 204:227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L. (2009) The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca(2+) concentrations. Annu Rev Pharmacol Toxicol 49:395–426 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. (2002) 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16:1145–1150 [DOI] [PubMed] [Google Scholar]
- Burnstock G, Novak I. (2012) Purinergic signalling in the pancreas in health and disease. J Endocrinol 213(2):123–141 [DOI] [PubMed] [Google Scholar]
- Chepurny OG, Kelley GG, Dzhura I, Leech CA, Roe MW, Dzhura E, Li X, Schwede F, Genieser HG, Holz GG. (2010) PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab 298:E622–E633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AE, Selheim F, de Rooij J, et al. (2003) cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem 278:35394–35402 [DOI] [PubMed] [Google Scholar]
- Chung MK, Güler AD, Caterina MJ. (2008) TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci 11:555–564 [DOI] [PubMed] [Google Scholar]
- Cook DL, Hales CN. (1984) Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature 311:271–273 [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Marini P, Matias I, Moriello AS, Starowicz K, Cristino L, Nigam S, Di Marzo V. (2007) Mechanisms for the coupling of cannabinoid receptors to intracellular calcium mobilization in rat insulinoma beta-cells. Exp Cell Res 313:2993–3004 [DOI] [PubMed] [Google Scholar]
- Ding WG, Gromada J. (1997) Protein kinase A-dependent stimulation of exocytosis in mouse pancreatic beta-cells by glucose-dependent insulinotropic polypeptide. Diabetes 46:615–621 [DOI] [PubMed] [Google Scholar]
- Dunne MJ, Petersen OH. (1986) Intracellular ADP activates K+ channels that are inhibited by ATP in an insulin-secreting cell line. FEBS Lett 208:59–62 [DOI] [PubMed] [Google Scholar]
- Dzhura I, Chepurny OG, Kelley GG, et al. (2010) Epac2-dependent mobilization of intracellular Ca²+ by glucagon-like peptide-1 receptor agonist exendin-4 is disrupted in β-cells of phospholipase C-ε knockout mice. J Physiol 588:4871–4889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson L, Ma X, Renström E, et al. (2003) SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol 121:181–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster SD, Chung WJ, Zhai R, Cases-Langhoff C, Voss B, Garner AM, Kaempf U, Kindler S, Gundelfinger ED, Garner CC. (2000) Piccolo, a presynaptic zinc finger protein structurally related to bassoon. Neuron 25:203–214 [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T, Seino S. (2002) Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2. Piccolo complex in cAMP-dependent exocytosis. J Biol Chem 277:50497–50502 [DOI] [PubMed] [Google Scholar]
- Grill V, Cerasi E. (1978) Interacting effects of sulfonylureas and glucose on cyclic AMP metabolism and insulin release in pancreatic islets of the rat. J Clin Invest 61:1346–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop LC. (1992) Sulfonylureas in NIDDM. Diabetes Care 15:737–754 [DOI] [PubMed] [Google Scholar]
- Hallcher LM, Sherman WR. (1980) The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem 255:10896–10901 [PubMed] [Google Scholar]
- Hillyard SD, Willumsen NJ, Marrero MB. (2010) Stretch-activated cation channel from larval bullfrog skin. J Exp Biol 213:1782–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth A, Szabadkai G, Várnai P, Arányi T, Wollheim CB, Spät A, Enyedi P. (1998) Voltage dependent calcium channels in adrenal glomerulosa cells and in insulin producing cells. Cell Calcium 23:33–42 [DOI] [PubMed] [Google Scholar]
- Jacobo SM, Guerra ML, Hockerman GH. (2009) Cav1.2 and Cav1.3 are differentially coupled to glucagon-like peptide-1 potentiation of glucose-stimulated insulin secretion in the pancreatic beta-cell line INS-1. J Pharmacol Exp Ther 331:724–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen CH, Gislason GH, Andersson C, Ahlehoff O, Charlot M, Schramm TK, Vaag A, Abildstrøm SZ, Torp-Pedersen C, Hansen PR. (2010) Effects of oral glucose-lowering drugs on long term outcomes in patients with diabetes mellitus following myocardial infarction not treated with emergent percutaneous coronary intervention—a retrospective nationwide cohort study. Cardiovasc Diabetol 9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. (2003) Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem 278:8279–8285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. (1998) A family of cAMP-binding proteins that directly activate Rap1. Science 282:2275–2279 [DOI] [PubMed] [Google Scholar]
- Leech CA, Chepurny OG, Holz GG. (2010a) Epac2-dependent rap1 activation and the control of islet insulin secretion by glucagon-like peptide-1. Vitam Horm 84:279–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech CA, Dzhura I, Chepurny OG, Schwede F, Genieser HG, Holz GG. (2010b) Facilitation of ß-cell K(ATP) channel sulfonylurea sensitivity by a cAMP analog selective for the cAMP-regulated guanine nucleotide exchange factor Epac. Islets 2:72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Bowe JE, Jones PM, Persaud SJ. (2010) Expression and function of cannabinoid receptors in mouse islets. Islets 2:293–302 [DOI] [PubMed] [Google Scholar]
- Li F, Zhang ZM. (2009) Comparative identification of Ca2+ channel expression in INS-1 and rat pancreatic beta cells. World J Gastroenterol 15:3046–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Renier G. (2009) The oral anti-diabetic agent, gliclazide, inhibits oxidized LDL-mediated LOX-1 expression, metalloproteinase-9 secretion and apoptosis in human aortic endothelial cells. Atherosclerosis 204:40–46 [DOI] [PubMed] [Google Scholar]
- Lin M, Aladejebi O, Hockerman GH. (2011) Distinct properties of amlodipine and nicardipine block of the voltage-dependent Ca2+ channels Ca v 1.2 and Ca v 2.1 and the mutant channels Ca v 1.2/Dihydropyridine insensitive and Ca v 2.1/Dihydropyridine sensitive. Eur J Pharmacol 670:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Jacobo SM, Hilliard N, Hockerman GH. (2006) Differential modulation of Cav1.2 and Cav1.3-mediated glucose-stimulated insulin secretion by cAMP in INS-1 cells: distinct roles for exchange protein directly activated by cAMP 2 (Epac2) and protein kinase A. J Pharmacol Exp Ther 318:152–160 [DOI] [PubMed] [Google Scholar]
- Liu K, Titus S, Southall N, Zhu P, Inglese J, Austin CP, Zheng W. (2008) Comparison on functional assays for Gq-coupled GPCRs by measuring inositol monophospate-1 and intracellular calcium in 1536-well plate format. Curr Chem Genomics 1:70–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CJ, Zhou Q, Shyng SL, et al. (2012) Some cannabinoid receptor ligands and their distomers are direct-acting openers of SUR1 K(ATP) channels. Am J Physiol Endocrinol Metab 302:E540–E551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami H, Zhang H, Suzuki Y, Urano T, Saito N, Kojima I, Petersen OH. (2003) Decoding of short-lived Ca2+ influx signals into long term substrate phosphorylation through activation of two distinct classes of protein kinase C. J Biol Chem 278:9896–9904 [DOI] [PubMed] [Google Scholar]
- Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. (2009) Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem 284:1514–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian F, Huang P, Ma L, Kuznetsov A, Tamarina N, Philipson LH. (2002) TRP genes: candidates for nonselective cation channels and store-operated channels in insulin-secreting cells. Diabetes 51 (Suppl 1):S183–S189 [DOI] [PubMed] [Google Scholar]
- Renier G, Mamputu JC, Serri O. (2003) Benefits of gliclazide in the atherosclerotic process: decrease in monocyte adhesion to endothelial cells. Metabolism 52(8, Suppl 1)13–18 [DOI] [PubMed] [Google Scholar]
- Rorsman P, Trube G. (1985) Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch 405:305–309 [DOI] [PubMed] [Google Scholar]
- Satoh J, Takahashi K, Takizawa Y, Ishihara H, Hirai M, Katagiri H, Hinokio Y, Suzuki S, Tsuji I, Oka Y. (2005) Secondary sulfonylurea failure: comparison of period until insulin treatment between diabetic patients treated with gliclazide and glibenclamide. Diabetes Res Clin Pract 70:291–297 [DOI] [PubMed] [Google Scholar]
- Schmid-Antomarchi H, De Weille J, Fosset M, Lazdunski M. (1987) The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem 262:15840–15844 [PubMed] [Google Scholar]
- Schramm TK, Gislason GH, Vaag A, et al. (2011) Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J 32:1900–1908 [DOI] [PubMed] [Google Scholar]
- Seino S, Chen L, Seino M, Blondel O, Takeda J, Johnson JH, Bell GI. (1992) Cloning of the alpha 1 subunit of a voltage-dependent calcium channel expressed in pancreatic beta cells. Proc Natl Acad Sci USA 89:584–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. (2004) Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem 279:7956–7961 [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, et al. (2007) Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA 104:19333–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thore S, Dyachok O, Tengholm A. (2004) Oscillations of phospholipase C activity triggered by depolarization and Ca2+ influx in insulin-secreting cells. J Biol Chem 279:19396–19400 [DOI] [PubMed] [Google Scholar]
- Van Obberghen E, Somers G, Devis G, Ravazzola M, Malaisse-Lagae F, Orci L, Malaisse WJ. (1975) Dynamics of insulin release and microtubular-microfilamentous system. VII. Do microfilaments provide the motive force for the translocation and extrusion of beta granules? Diabetes 24:892–901 [DOI] [PubMed] [Google Scholar]
- Wang Y, Sugita S, Sudhof TC. (2000) The RIM/NIM family of neuronal C2 domain proteins. Interactions with Rab3 and a new class of Src homology 3 domain proteins. J Biol Chem 275:20033–20044 [DOI] [PubMed] [Google Scholar]
- Wollheim CB, Blondel B, Trueheart PA, Renold AE, Sharp GW. (1975) Calcium-induced insulin release in monolayer culture of the endocrine pancreas. Studies with ionophore A23187. J Biol Chem 250:1354–1360 [PubMed] [Google Scholar]
- Yada T, Hamakawa N, Yaekura K. (1995) Two distinct modes of Ca2+ signalling by ACh in rat pancreatic beta-cells: concentration, glucose dependence and Ca2+ origin. J Physiol 488:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Katoh M, Shibasaki T, Minami K, Sunaga Y, Takahashi H, Yokoi N, Iwasaki M, Miki T, Seino S. (2009) The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 325:607–610 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.