Abstract
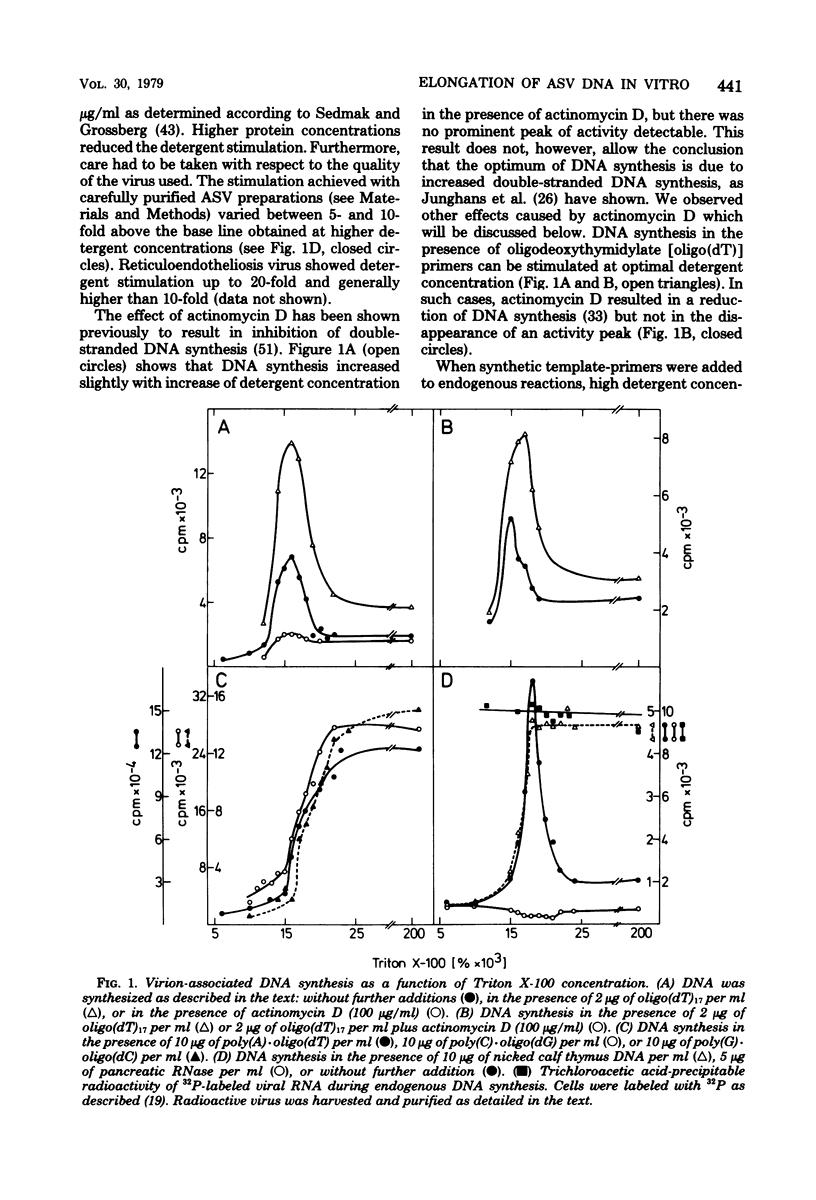
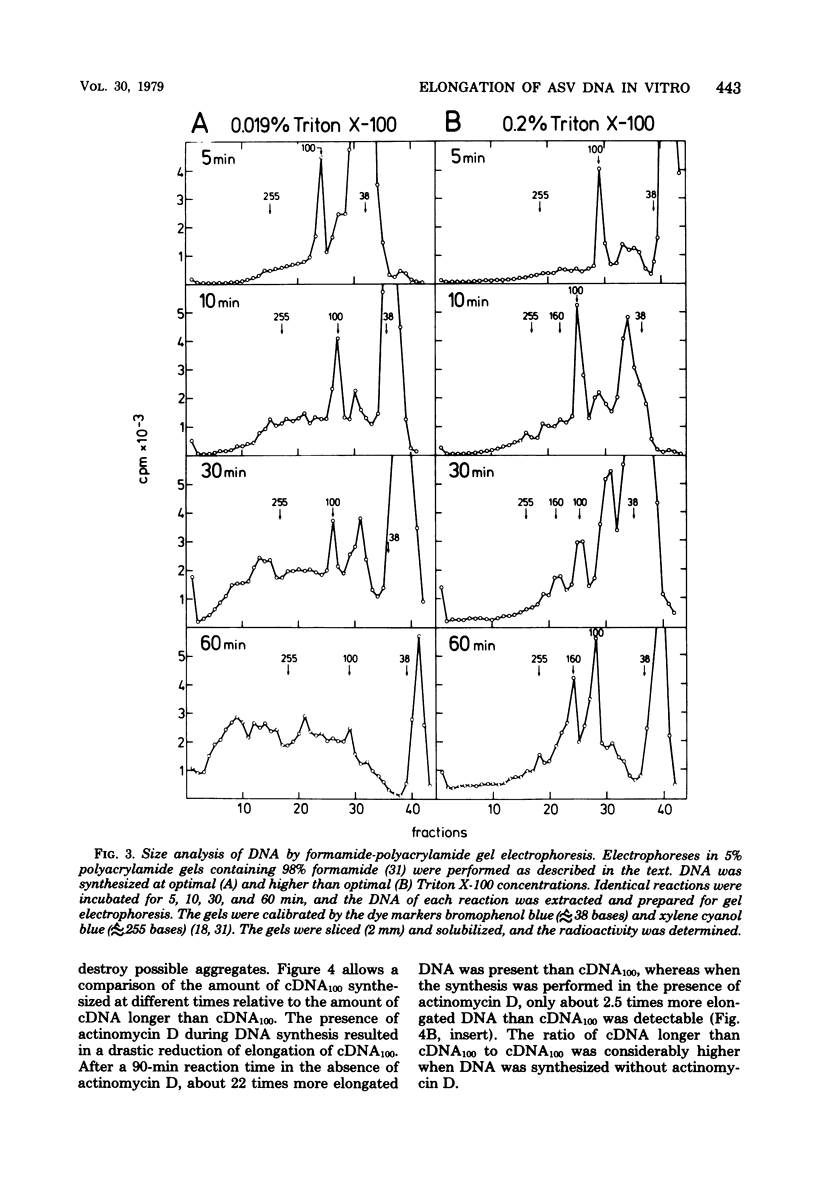
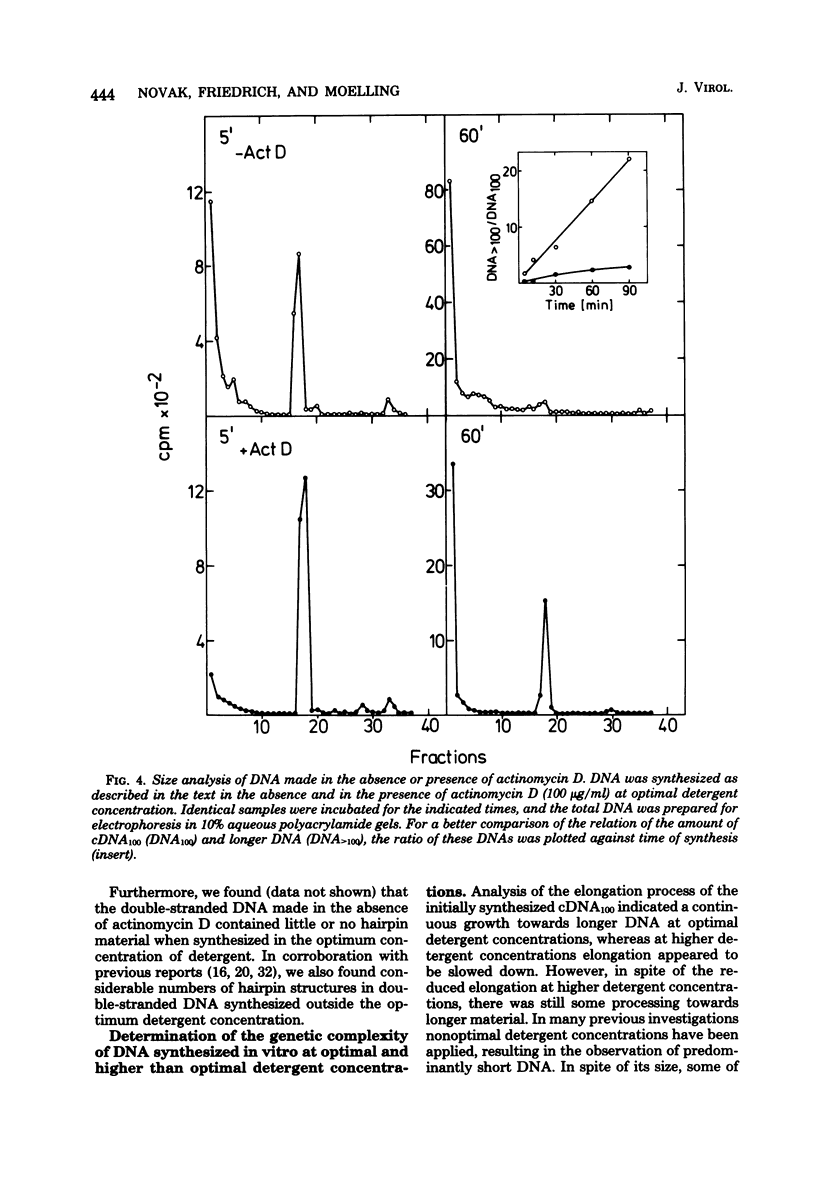
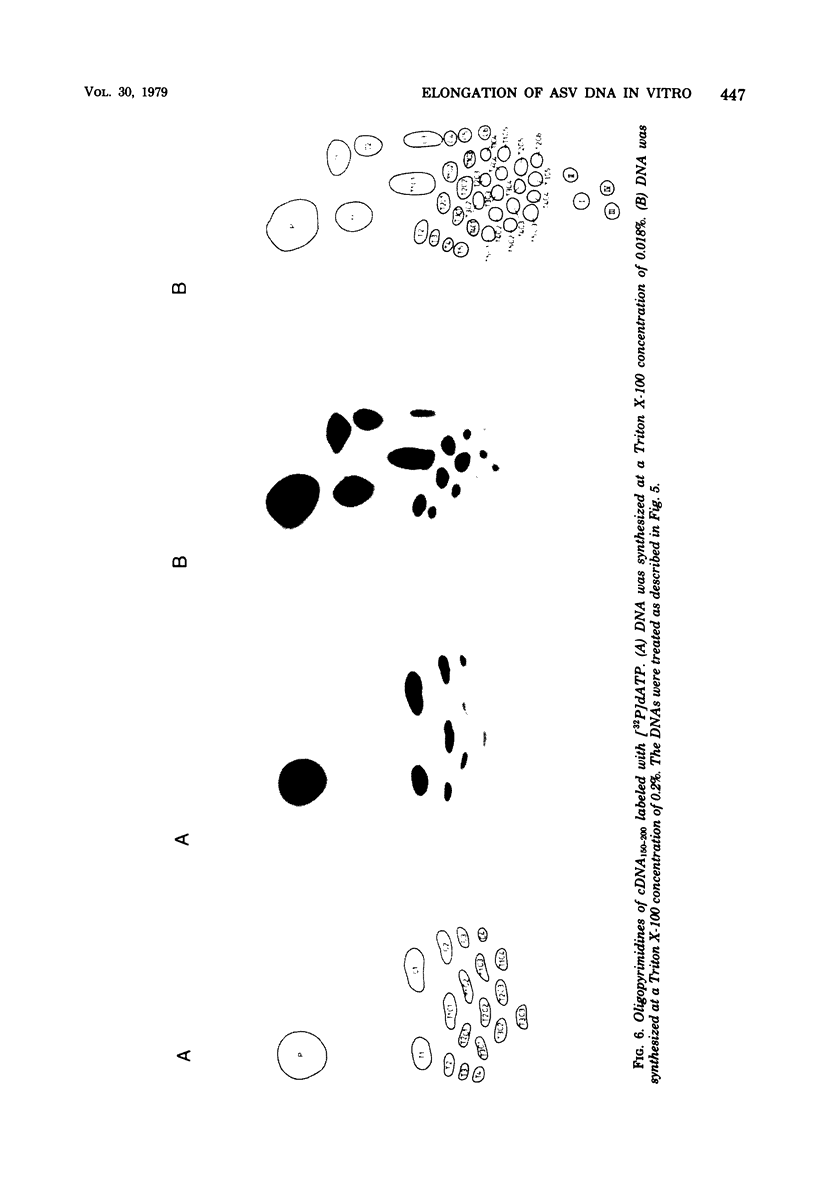
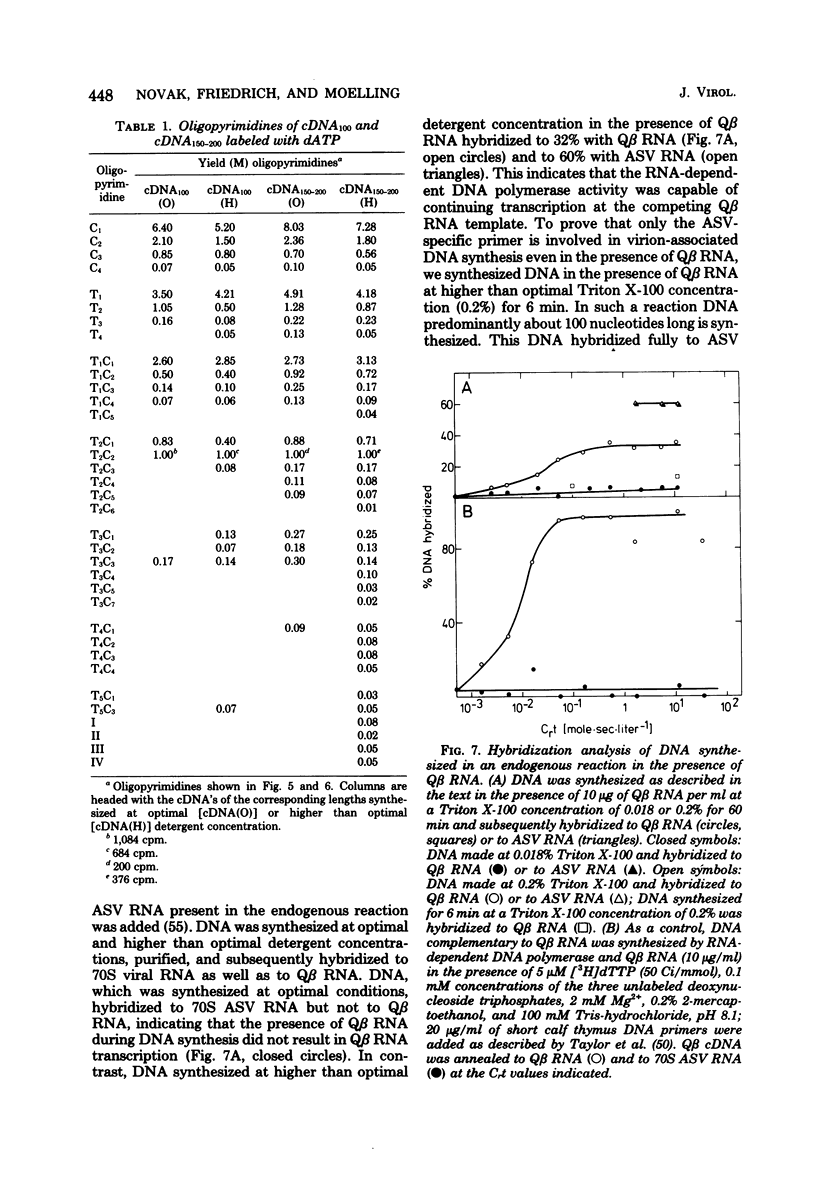
RNA-dependent DNA synthesis in a virion-associated reaction has been described as being dependent upon the detergent concentration used for disruption of the virion. In this study, the Triton X-100 concentration was found to affect the elongation of the initially synthesized DNA complementary to the last approximately 100 nucleotides at the 5' end of the RNA (cDNA100). Whereas elongation of cDNA100 increased with time of incubation at the optimal detergent concentration, this process was retarded at higher detergent concentrations. At the optimal detergent concentration, elongated DNA was of low chemical complexity, indicating that extension of cDNA100 occurred at a unique site on the RNA. Higher than optimal detergent concentrations resulted in nonspecific elongation and in DNA of high chemical complexity. This was shown by oligopyrimidine tract analysis. Furthermore, actinomycin D was observed to inhibit the elongation of cDNA100 at the optimal detergent concentration. The nature of the elongation process was elucidated by analysis of DNA synthesized in a virion-associated reaction in the presence of bacteriophage Qbeta RNA. At the optimal detergent concentration DNA complementary only to avian sarcoma virus RNA was synthesized, whereas at higher concentrations DNA was copied from both avian sarcoma virus and Qbeta RNA. We conclude that the elongation mechanism of cDNA100 is affected by the detergent concentration and elongation is unspecific at higher than optimal detergent concentrations. The mechanism by which the nonionic detergent stimulates DNA synthesis has not yet been resolve. We assume that other factors in addition to DNA polymerase are involved in elongation of cDNA100.
Full text
PDF


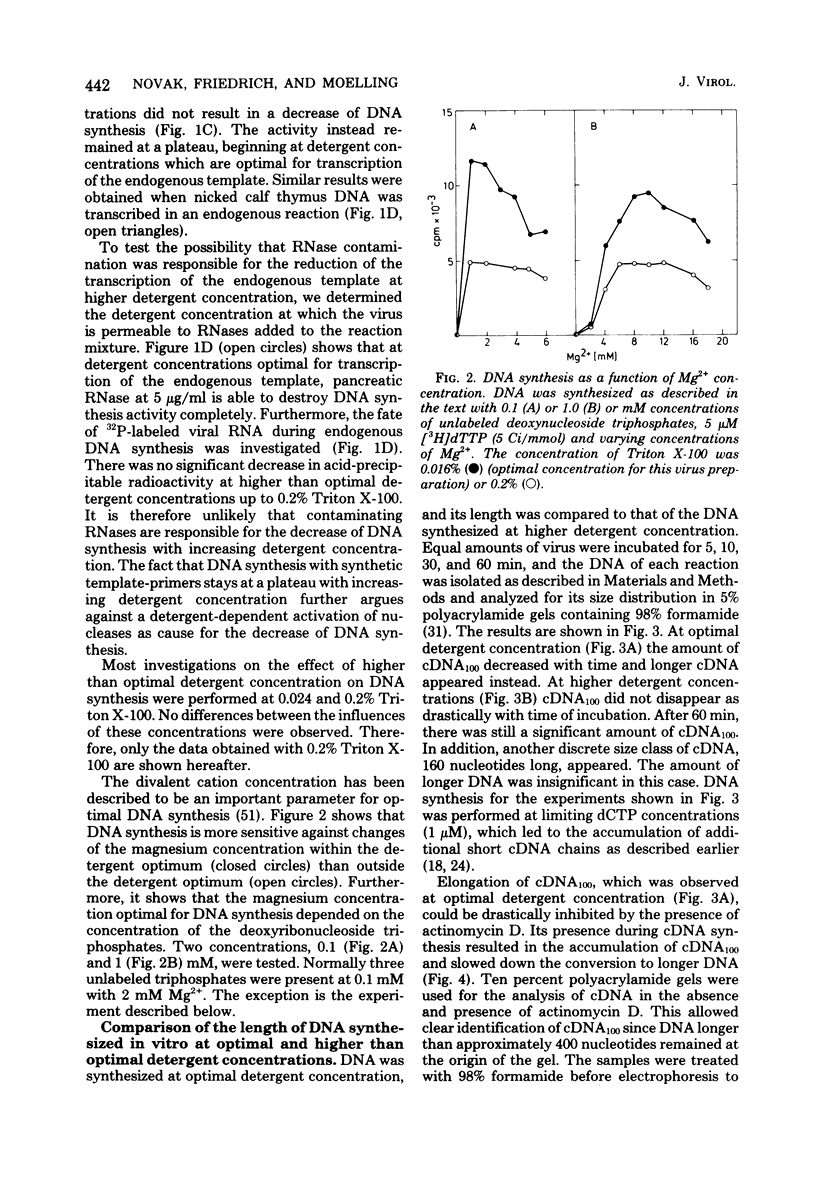






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Ruprecht R., Simpson R. W., Spiegelman S. Deoxyribonucleic acid polymerase of Rous sarcoma virus: reaction conditions and analysis of the reaction product nucleic acids. J Virol. 1971 Nov;8(5):730–741. doi: 10.1128/jvi.8.5.730-741.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Cashion L. M., Joho R. H., Planitz M. A., Billeter M. A., Weissmann C. Initiation sites of Rous sarcoma virus RNA-directed DNA synthesis in vitro. Nature. 1976 Jul 15;262(5565):186–190. doi: 10.1038/262186a0. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Hageman T. C., Maxam A. M., Haseltine W. A. Structure of the genome of Moloney murine leukemia virus: a terminally redundant sequence. Cell. 1978 Apr;13(4):761–773. doi: 10.1016/0092-8674(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Haseltine W. A. Terminal redundancy and the origin of replication of Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1908–1912. doi: 10.1073/pnas.74.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Temin H. M. Hybridization of Rous sarcoma virus deoxyribonucleic acid polymerase product and ribonucleic acids from chicken and rat cells infected with Rous sarcoma virus. J Virol. 1972 May;9(5):766–775. doi: 10.1128/jvi.9.5.766-775.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Dierks P., Cahill J. F., Faras A. J., Parsons J. T. Terminally repeated sequences in the avian sarcoma virus RNA genome. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2389–2393. doi: 10.1073/pnas.74.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Dierks P., Parsons J. T., Faras A. J. RNase H hydrolysis of the 5' terminus of the avian sarcoma virus genome during reverse transcription. Nature. 1978 Mar 9;272(5649):181–184. doi: 10.1038/272181a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. Avian retrovirus RNA-directed DNA synthesis: transcription at the 5' terminus of the viral genome and the functional role for the viral terminal redundancy. Virology. 1978 May 15;86(2):297–311. doi: 10.1016/0042-6822(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Faras A. J. In vitro transcription of DNA from the 70S RNA of Rous sarcoma virus: identification and characterization of various size classes of DNA transcripts. J Virol. 1975 Nov;16(5):1220–1228. doi: 10.1128/jvi.16.5.1220-1228.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Bromley P. A., Spahr P. F. Extensive in vitro transcription of rous sarcoma virus RNA by avian myeloblastosis virus DNA polymerase and concurrent activation of the associated RNase H. J Virol. 1977 Sep;23(3):659–668. doi: 10.1128/jvi.23.3.659-668.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Bromley P. A., Spahr P. F. New procedure for the direct analysis of in vitro reverse transcription of Rous sarcoma virus RNA. J Virol. 1977 Apr;22(1):118–129. doi: 10.1128/jvi.22.1.118-129.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Canaani E. Complementarity between Rous sarcoma virus (RSV) RNA and the in vitro-synthesized DNA of the virus-associated DNA polymerase. Virology. 1970 Nov;42(3):783–788. doi: 10.1016/0042-6822(70)90325-9. [DOI] [PubMed] [Google Scholar]
- Faras A. J., Taylor J. M., Levinson W. E., Goodman H. M., Bishop J. M. RNA-directed DNA polymerase of Rous sarcoma virus: initiation of synthesis with 70 S viral RNA as template. J Mol Biol. 1973 Sep 5;79(1):163–183. doi: 10.1016/0022-2836(73)90277-5. [DOI] [PubMed] [Google Scholar]
- Friedrich R., Kung H. J., Baker B., Varmus H. E., Goodman H. M., Bishop J. M. Characterization of DNA complementary to nucleotide sequences at the 5'-terminus of the avian sarcoma virus genome. Virology. 1977 Jun 1;79(1):198–215. doi: 10.1016/0042-6822(77)90345-2. [DOI] [PubMed] [Google Scholar]
- Friedrich R., Morris V. L., Goodman H. M., Bishop J. M., Varmus H. E. Differences between genomes of two strains of mouse mammary tumor virus as shown by partial RNA sequence analysis. Virology. 1976 Jul 15;72(2):330–340. doi: 10.1016/0042-6822(76)90162-8. [DOI] [PubMed] [Google Scholar]
- Fujinaga K., Parsons J. T., Beard J. W., Beard D., Green M. Mechanism of carcinogenesis by RNA tumor viruses. 3. Formation of RNA, DNA complex and duplex DNA molecules by the DNA polymerase (s) of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1432–1439. doi: 10.1073/pnas.67.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., McDonnell J. P., Levinson W., Quintrell N., Fanshier L., Bishop J. M. Deoxyribonucleic acid polymerase associated with Rous sarcoma virus and avian myeloblastosis virus: properties of the enzyme and its product. J Virol. 1970 Nov;6(5):589–598. doi: 10.1128/jvi.6.5.589-598.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Green M., Gerard G. F. RNA-directed DNA polymerase--properties and functions in oncogenic RNA viruses and cells. Prog Nucleic Acid Res Mol Biol. 1974;14(0):187–334. [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Duesberg P. H., Knight C. A. In vitro synthesis of full-length DNA transcripts of Rous sarcoma virus RNA by viral DNA polymerase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4895–4899. doi: 10.1073/pnas.72.12.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J. P., Smith R. E., Dierks P., Parsons J. T., Collett M. S., Faras A. J. In vitro transcription of reconstituted 35s RNA.tRNAtrp template.primer complexes by the avian oncornavirus DNA polymerase. Effect of temperature on the size of the DNA transcripts. Virology. 1978 Mar;85(1):28–42. doi: 10.1016/0042-6822(78)90409-9. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V. Fractionation and sequences of the large pyrimidine oligonucleotides from bacteriophage fd DNA. J Mol Biol. 1972 Feb 28;64(1):87–102. doi: 10.1016/0022-2836(72)90322-1. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K. Further characterization of the Friend murine leukemia virus reverse transcriptase-RNase H complex. J Virol. 1976 May;18(2):418–425. doi: 10.1128/jvi.18.2.418-425.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Gelderblom H., Pauli G., Friis R. A comparative study of the avian reticuloendotheliosis virus: relationship to murine leukemia virus and viruses of the avian sarcoma-leukosis complex. Virology. 1975 Jun;65(2):546–557. doi: 10.1016/0042-6822(75)90059-8. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S., Kacian D. L. Synthesis of full-length DNA copies of avian myeloblastosis virus RNA in high yields. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2840–2843. doi: 10.1073/pnas.74.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintrell N., Fanshier L., Evans B., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase(s) of Rous sarcoma virus: effects of virion-associated endonuclease on the enzymatic product. J Virol. 1971 Jul;8(1):17–27. doi: 10.1128/jvi.8.1.17-27.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Synthesis of long, representative DNA copies of the murine RNA tumor virus genome. J Virol. 1975 Jan;17(1):168–174. doi: 10.1128/jvi.17.1.168-174.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Smotkin D., Baltimore D., Weinberg R. A. In vitro synthesis of infectious DNA of murine leukaemia virus. Nature. 1977 Sep 8;269(5624):122–126. doi: 10.1038/269122a0. [DOI] [PubMed] [Google Scholar]
- Scolnick E., Rands E., Aaronson S. A., Todaro G. J. RNA-dependent DNA polymerase activity in five RNA viruses: divalent cation requirements. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1789–1796. doi: 10.1073/pnas.67.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Bernstein E. H. Production and purification of large amounts of Rous sarcoma virus. Appl Microbiol. 1973 Mar;25(3):346–353. doi: 10.1128/am.25.3.346-353.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskus K. A., Collett M. S., Faras A. J. Initiation of DNA synthesis by the avian oncornavirus RNA-directed DNA polymerase: structural and functional localization of the major species of primer RNA on the oncornavirus genome. Virology. 1976 May;71(1):162–168. doi: 10.1016/0042-6822(76)90102-1. [DOI] [PubMed] [Google Scholar]
- Stoll E., Billeter M. A., Palmenberg A., Weissmann C. Avian myeloblastosis virus RNA is terminally redundant: implications for the mechanism of retrovirus replication. Cell. 1977 Sep;12(1):57–72. doi: 10.1016/0092-8674(77)90185-4. [DOI] [PubMed] [Google Scholar]
- Taylor J. M. An analysis of the role of tRNA species as primers for the transcription into DNA of RNA tumor virus genomes. Biochim Biophys Acta. 1977 Mar 21;473(1):57–71. doi: 10.1016/0304-419x(77)90007-5. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Tokiwa F. Effects of surface active compounds on thermal denaturation of DNA. J Am Oil Chem Soc. 1977 Dec;54(12):585–586. doi: 10.1007/BF03027641. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Kung H. J., Oppermann H., Smith V. C., Bishop J. M., Shank P. R. Kinetics of synthesis, structure and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978 Mar 25;120(1):55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Levinson W. E., Bishop J. M. Extent of transcription by the RNA-dependent DNA polymerase of Rous sarcoma virus. Nat New Biol. 1971 Sep 1;233(35):19–21. doi: 10.1038/newbio233019a0. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Avian tumor viruses. Adv Virus Res. 1965;11:293–385. doi: 10.1016/s0065-3527(08)60549-7. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Feix G. Replication of viral RNA. XI. Synthesis of viral "minus" strands in vitro. Proc Natl Acad Sci U S A. 1966 May;55(5):1264–1268. doi: 10.1073/pnas.55.5.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Cetta A. On the stimulation of viral DNA polymerase activity by nonionic detergent. Biochemistry. 1975 Feb 25;14(4):789–795. doi: 10.1021/bi00675a022. [DOI] [PubMed] [Google Scholar]