Abstract
We evaluated the efficacy, potency, and selectivity of the three most commonly used adenylate cyclase (AC) inhibitors in a battery of cell lines constructed to study signaling via three discrete cAMP sensors identified in neuroendocrine cells. SQ22,536 [9-(tetrahydrofuryl)-adenine] and 2′,5′-dideoxyadenosine (ddAd) are effective and potent AC inhibitors in HEK293 cells expressing a cAMP response element (CRE) reporter gene, and MDL-12,330A [cis-N-(2-phenylcyclopentyl)azacyclotridec-1-en-2-amine hydrochloride] is not. Neuroscreen-1 (NS-1) cells were used to assess the specificity of the most potent AC inhibitor, SQ22,536, to block downstream cAMP signaling to phosphorylate CREB (via PKA); to activate Rap1 (via Epac); and to activate ERK signaling leading to neuritogenesis (via the newly described neuritogenic cAMP sensor NCS). SQ22,536 failed to inhibit the effects of cAMP analogs 8-Br-cAMP and 8-CPT-2′-O-Me-cAMP on PKA-mediated CREB activation/phosphorylation and Epac-mediated Rap1 activation, indicating that it does not inhibit these cAMP pathways beyond the level of AC. On the other hand, SQ22,536, but not ddAd, inhibited the effects of cAMP analogs 8-Br-cAMP and 8-CPT-cAMP on ERK phosphorylation and neuritogenesis, indicating that it acts not only as an AC blocker, but also as an inhibitor of the NCS. The observed off-target actions of SQ22,536 are specific to cAMP signaling: SQ22,536 does not block the actions of compounds not related to cAMP signaling, including ERK induction by PMA, and ERK activation and neuritogenesis induced by NGF. These data led us to indicate a second target for SQ22,536 that should be considered when interpreting its effects in whole cell and in vivo experiments.
Introduction
In neuronal and neuroendocrine cells, a variety of ligands, such as neurotransmitters and hormones, signal via activation of G protein coupled receptors (GPCRs) coupled to Gsα. These receptors activate adenylate cyclases (ACs), the family of enzymes that generate cAMP (Gilman, 1995). Until the late twentieth century, the only known sensor for cAMP was protein kinase A (PKA), which has been shown to transduce an array of cAMP-regulated cognitive (Greengard, 2001; Kandel, 2001), metabolic (Sutherland, 1972), developmental (Kao et al., 2002), cell growth (Gottesman and Fleischmann, 1986), and cell survival (Insel et al., 2012) processes. Since then, other cAMP sensors have been identified and have come into focus as noncanonical (i.e., PKA independent) cAMP signaling molecules (Kopperud et al., 2003). The most prominent of these are the Rap guanine nucleotide exchange factors (GEFs) Epacs 1 and 2, both of which activate the small G protein Rap1 via GDP-GTP exchange (de Rooij et al., 1998; Kawasaki et al., 1998). Cyclic nucleotide-gated ion channels activated by cAMP have also been identified but appear to be restricted in their expression to cells specialized for sensory transduction (Nakamura and Gold, 1987; Matulef and Zagotta, 2003).
Working in neuroendocrine chromaffin, PC12, and neuroscreen-1 (NS-1) cells, we identified a cAMP-dependent signaling pathway for cellular differentiation, transcription of neuroendocrine-specific genes, and neurite extension (Emery and Eiden, 2012; Hamelink et al., 2002; Ravni et al., 2008). This signaling pathway requires sequential activation of a neuritogenic cAMP sensor (NCS) and the MAP kinase ERK, and it is wholly independent of both PKA and Epac. NS-1 cells allow for the study of all three pharmacologically and functionally distinguishable neuroendocrine cAMP signaling pathways: PKA-dependent CREB phosphorylation, Epac-dependent Rap1 activation, and ERK phosphorylation/neuritogenesis through the NCS.
In addition to the multiple signaling cascades activated by cAMP described above, the fact that its synthesis from ATP can be accomplished by 10 different isoforms of adenylate cyclase represents an additional level of complexity in cAMP signaling. ACs 1-9 are membrane-bound, and AC10 is soluble. ACs 1-9 differ in their pharmacological properties and anatomic localization (Sunahara et al., 1996). Only AC1, AC3, and AC8 are stimulated by calcium/calmodulin (Cali et al., 1994; Choi et al., 1992). AC2, AC4, AC5, AC6, and AC7 are stimulated by Gβγ subunits (Chen et al., 1997; Gao et al., 2007; Tang and Gilman, 1991). Only AC5 and AC6 are inhibited by both calcium (Chiono et al., 1995) and Giα (Taussig et al., 1993). Only AC9 is unresponsive to forskolin (Premont et al., 1996).
There are numerous compounds that inhibit AC in vitro, but only a handful of these are suitably potent, cell-permeable, and AC-specific for use in studies of intact cells or tissue systems. Among the latter, lipophilic P-site (purine site) inhibitors of AC are most commonly used (Seifert et al., 2012). The nucleosides 9-(tetrahydrofuryl)-adenine (SQ22,536) and 2′,5′-dideoxyadenosine (ddAd) were among the first identified inhibitors of AC (Haslam et al., 1978), and both are frequently used for this purpose in intact cells and tissues. Although we are not aware of any reports detailing nonspecific actions of either ddAd or SQ22,536, both compounds are commonly applied at concentrations ranging from micromolar to millimolar. Moreover, despite their wide use as AC inhibitors, their potencies and efficacies to actually inhibit cAMP elevation in intact cells are generally not reported (Seifert et al., 2012).
The lactamimide MDL-12,330A is another commonly used AC inhibitor for studies in whole cells and in vivo, with a proposed mechanism of action distinct from P-site inhibition. There are several known limitations of MDL-12,330A, including enhancement of AC activity in the CNS of at least one species, freshwater snails (Ferretti et al., 1996). MDL-12,330A also has several reported nonspecific effects, including phosphodiesterase inhibition (Hunt and Evans, 1980), glycine transport inhibition in retinal glial cells (Gadea et al., 1999), and inhibition of calcium entry (van Rossum et al., 2000).
In light of the intense interest in the multiple modes of cAMP signaling that can now be distinguished physiologically, we tested the potency, specificity, and efficacy of the three most commonly used AC inhibitors in a battery of intact cells amenable to high-throughput pharmacological analysis, in which all three of the major cAMP signaling pathways can be examined. Our studies indicate that the most potent of these AC inhibitors, SQ22,536, also inhibits the major cAMP sensor responsible for GPCR-initiated neuritogenesis in neuroendocrine cells.
Materials and Methods
Cell Culture.
Solutions used for cell culture were obtained from Invitrogen (Carlsbad, CA), unless otherwise noted, and cells were confirmed to be mycoplasma-free. HEK293 CRE-luc 2P GloResponse luciferase reporter cells (Promega, San Luis Obispo, CA) were cultured in DMEM supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and 50 μg/ml hygromycin B. Neuroscreen-1 (NS-1) cells (Cellomics, Pittsburgh, PA) were used between passages 5 and 11 for all experiments and were grown on plates coated with collagen I from rat tail as described previously (Emery and Eiden, 2012). NS-1 cells were cultured in RPMI 1640 medium supplemented with 10% horse serum, 5% heat-inactivated fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Drugs and Reagents.
PACAP-38 was purchased from Phoenix Pharmaceuticals (Mountain View, CA). Cholera toxin (NaN3-free), forskolin, H-89, MDL-12,330A, and PMA were purchased from Calbiochem (San Diego, CA) and SQ22,536 from Tocris Cookson (Ellisville, MO). Nerve growth factor (NGF) and 2′, 5′-dideoxyadenosine (ddAd) were from Sigma (St. Louis, MO). Cyclic AMP analogs 8-Br-cAMP, 8-CPT-cAMP, and 8-CPT-2′-O-Me-cAMP (007) were purchased from Biolog Life Science Institute (Bremen, Germany). Most drugs were diluted in culture media, with the exceptions noted below. Forskolin, phorbol-12-myristate-13-acetate (PMA), and H-89 were solubilized in DMSO to yield a final DMSO concentration of 0.01%. In all experiments using forskolin, PMA, or H-89, 0.01% DMSO was also applied to all other cells. MDL-12,330A was solubilized in DMSO, yielding a final solution of 0.3% DMSO. In only those assays using MDL, 0.3% DMSO was applied to all other cells. For all experiments, cells were plated and, after 12–18 hours, were treated with 10× solutions of inhibitors or vehicle. After incubation for 30 minutes, 10× solutions of drugs were added as indicated.
CRE-Luciferase Assays.
HEK293 CRE-luc2P GloResponse luciferase reporter cells (Promega) were transduced with retroviral vectors expressing rat PAC1hop receptors, as described previously (Holighaus et al., 2011). Individual cell lines were obtained by limiting dilution cloning, and a clonal PAC1-expressing line was propagated and used for CRE luciferase assays, which were performed according to the manufacturer’s protocol. In brief, HEK293 CRE-luc2P cells were plated in 96-well plates (10,000 cells in 80 μl media per well) in assay media (DMEM supplemented with 1% fetal bovine serum). One day after plating, cells were treated with AC inhibitors (10 μl in assay media/well) for 30 minutes, followed by agonists (10 μl in assay media/well), and were incubated for 4 hours. Luciferase activity was determined after the addition of 100 μl/well Bright-Glo Luciferase Assay Reagent (Promega). Luminescence (RLU) was measured in a Victor3 microtiter plate reader (Perkin Elmer, Waltham, MA) after 2 minutes of agitation at room temperature.
Cyclic AMP Measurements.
Cyclic AMP was measured in NS-1 cells, as described previously (Emery and Eiden, 2012). In brief, NS-1 cells were seeded and grown overnight in 96-well plates. The next day, cells were pretreated for 20 minutes in media containing the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (0.5 mM) with or without SQ22,536. After pretreatment with inhibitors, cells were stimulated with agonists, added as 10× solutions, for an additional 20 minutes. Intracellular cAMP was then assayed using the cAMP Biotrak enzyme immunoassay kit (Amersham Biosciences, Piscataway, NJ) according to the manufacturer’s protocol for measurement of nonacetylated cAMP.
Neurite Outgrowth Assays.
NS-1 cells, grown in 6-well plates, were treated for 48 hours, as indicated. Images of cells were randomly acquired on a computer-assisted microscope using a 20× objective. In each field, neurites were measured and cells were counted by a blinded observer using NIS Elements BR Software (Nikon). Data from neurite outgrowth assays are expressed as mean neurite length per cell (μm).
Western Blotting.
Western blotting was performed essentially as described previously (Emery and Eiden, 2012). In brief, NS-1 cells grown in 12-well plates were treated as indicated for 10 minutes, followed by collection in lysis buffer (150 mM NaCl, 50 mM Tris-HCl, 1% NP-40, and 1 mM EDTA) with Halt protease and phosphatase inhibitor cocktails (Pierce Biotechnology, Rockford, IL). Protein concentrations were determined using BioRad DC Protein Assays (BioRad, Hercules, CA), and 20 µg samples were electrophoresed on 12% polyacrylamide Bis-Tris gels, which were electroblotted onto nitrocellulose membranes (Invitrogen). Membranes were incubated in blocking buffer containing 2% nonfat milk in Tris-buffered saline with 0.05% Tween 20 (TBST) for 2 hours at room temperature. After blocking, blots were incubated overnight at 4◦C with a 1:1000 dilution of antibodies raised against phosphorylated p44/42 MAP kinase (ERK), total p44/42 MAP kinase (ERK), phospho-CREB (Ser133), or total CREB (all from Cell Signaling Technology, Beverly, MA). After five washes in TBST, membranes were incubated with HRP-conjugated secondary antibodies (1:5000 in blocking buffer) and again washed five times in TBST. Membranes were then incubated with Super Signal West Pico Chemiluminescence Substrate (Pierce) for 5 minutes, and immunoreactive bands were photographed (AlphaImager, San Jose, CA). Bound phospho-p44/42 MAP kinase or phospho-CREB antibodies were removed with Restore Western Blot Stripping Buffer (Pierce) before incubating the same blot with either total p44/42 MAP kinase or total CREB antibodies. Images were quantified using ImageJ (Wayne Rasband, NIH, Bethesda, MD; http://imagej.nih.gov/ij/).
Rap1 Activation Assays.
Rap1 activation was measured using the Active Rap1 Pull-Down and Detection Kit (Pierce, Catalog #16120) according to the manufacturer’s instructions. NS-1 cells were grown in 6-well plates overnight and treated as indicated for 10 minutes, followed by lysis using the provided buffer. GTP-bound Rap1 was affinity purified from 500-μg samples by incubation in a solution of 20 μg of GST-RalGDS-RBD in slurry containing 50% glutathione resin for 1 hour at 4◦C. In parallel, 20 μg of protein was not subjected to affinity purification and served as an index of total Rap1 levels for each sample. Samples were then centrifuged through the provided spin cups, washed three times with lysis buffer, dissolved in reducing sample buffer, vortexed, and boiled for 5 minutes. Affinity purified samples were then analyzed by Western blots, which were probed with an antibody against Rap1 at a dilution of 1:1000 (Upstate Biotechnology, Lake Placid, NY). Total cellular protein from each sample was electrophoresed on a separate gel and probed for total Rap1 to account for possible differences in Rap1 content between samples.
CREB Reporter Gene Assays.
CREB activation was monitored using the PathDetect CREB trans-Reporting System luciferase assay (Agilent Technologies, Santa Clara, CA). NS-1 cells, grown in 24-well plates, were transfected with the reporter plasmid pFR-Luc (400 ng) and pFA2-CREB expression plasmid (20 ng) for the Gal4-CREB fusion protein activating pFR-Luc in response to Ser133 phosphorylation of Gal4-CREB using Lipofectamine 2000 (Invitrogen). One day after transfection, cells were pretreated with SQ22,536 as indicated for 30 minutes, followed by stimulation with agonists for 6 hours. For those assays measuring inhibition of catalytically active PKA, cells were transfected as described above with the addition of the plasmid pFC-PKA (20 ng) or a negative control plasmid pFC2-dbd (20 ng). For these experiments, cells were treated with H-89 or SQ22,536 immediately after transfection and were incubated for 48 hours. At the conclusion of all experiments, cells were lysed, and 20 μl of lysate was added to 100 μl of the provided luciferase assay reagent in opaque 96-well plates. Light emission was immediately measured using a Victor3 microtiter plate reader.
Elk Reporter Gene Assays.
To obtain a cell-based system in which gene transcription is reported from a stably integrated, low copy number gene locus, NS-1 Elk-luc cells were constructed by sequentially transducing NS-1 cells with the pLNCFA2-Elk and pBABE-Luc retroviral expression vectors. The pLNCFA2-Elk retroviral plasmid was made by cleaving pFA2-ELK-1 plasmid (Stratagene, La Jolla, CA) with the restriction enzymes SnaB1 and HindIII (New England Biolabs, Ipswich, MA) to generate a 1.1-kb fragment that was then ligated to the 3.8-kb fragment of pLNCX plasmid (Clontech, Mountain View, CA) generated by digesting this plasmid with SnaBI and HindIII. Retroviral vectors were generated by transfecting this recombinant retroviral genome expression plasmid together with a murine retroviral gagpol plasmid and a VSV-G plasmid into 293T cells, as previously described (Farrell et al., 2002). The pBABE-Luc retroviral expression plasmid was constructed by digesting the pBABE plasmid with BamHI, blunting, and then digesting with SalI. A SalI site was introduced at the 3′ end of the luciferase open reading frame in the pFR-Luc plasmid (Stratagene) with use of the QuikChange Site-Directed Mutagenesis Kit (Stratagene), and this plasmid was then cleaved with HindIII. HindIII-generated cleavage overhangs were blunted with T4 ligase (New England Biolabs) and the digested pFR-Luc plasmid was ligated to the pBABE retroviral expression plasmid after cleavage of the latter with HindIII, blunting of the overhang, and digestion with SalI. The integrity of the transgenes contained in these retroviral plasmids was verified by sequencing. NS-1 cells transduced with pLNCFA2-Elk were selected with 500 µg/ml G418, after which they were exposed to pBABE-Luc vectors and selected with 1 µg/ml puromycin, to create the NS-1 Elk-luc cell line. Cells were seeded in collagen I–coated 96-well plates in 80 μl of complete media per well. The following day, the cells were treated with SQ22,536 or vehicle at a volume of 10 μl. After incubation for 30 minutes, drugs (10 μl) were added and cells were incubated for 5 hours, at the conclusion of which 100 μl/well of Bright-Glo Luciferase Assay Reagent (Promega) was added, according to the manufacturer’s instructions. Luminescence was determined after 2 minutes of agitation at room temperature.
Calculations and Statistics.
SigmaPlot, version 11.0 (Systat), was used for all calculations and statistics. Significance testing was done by two-factor analysis of variance (ANOVA), followed by Bonferroni-corrected t tests, for multiple comparisons. Statistical significance was deemed at P < 0.05. In dose-response experiments, IC50 values were obtained from curves fit to data using four parameter logistic regressions.
Results
SQ22,536, 2′, 5′-dideoxyadenosine (ddAd), and MDL-12,330A represent the prototypical cell-permeable AC inhibitors used in experimental pharmacology. We decided to recertify these compounds as bona fide AC inhibitors by measuring their respective efficacies and potencies to inhibit cAMP-dependent reporter gene induction in HEK293 cells in response to AC activation by forskolin and a Gs-coupled GPCR.
Efficacy and Potency of Cell-Permeable AC Inhibitors in HEK293 Cells.
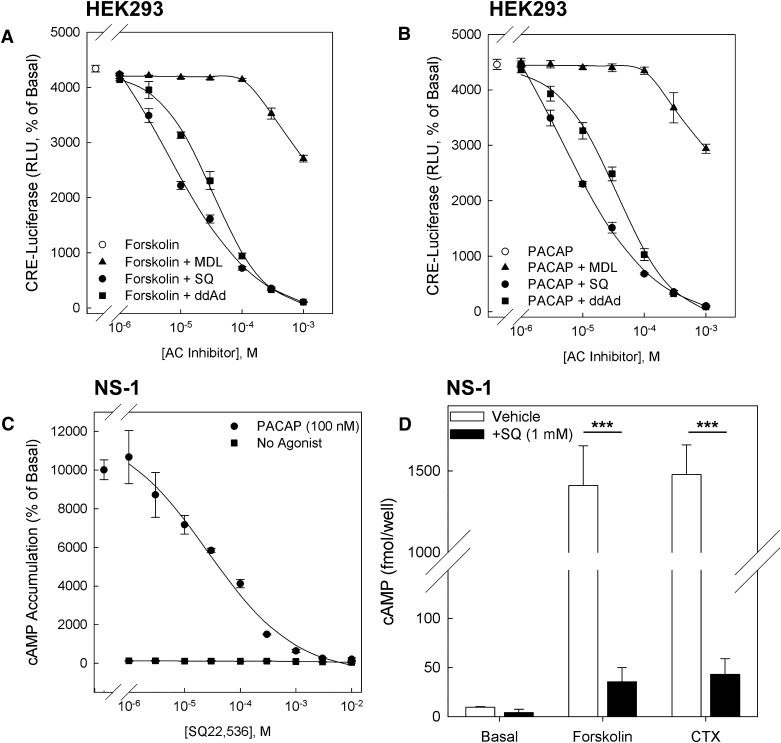
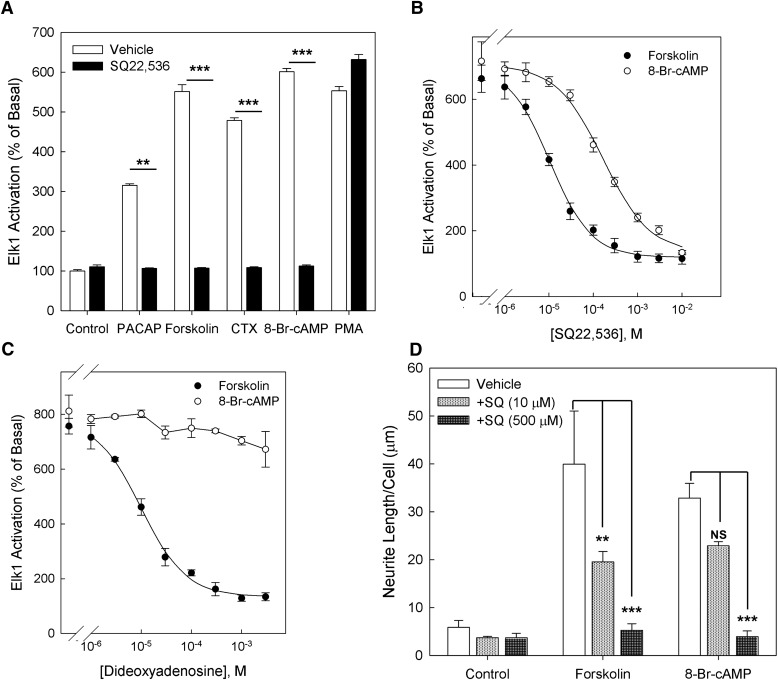
We evaluated the efficacy and potency of three reported AC inhibitors (MDL-12,330A, SQ22,536, and ddAd) to inhibit the effect of the AC activator forskolin (25 μM) to induce a cAMP-dependent reporter gene. HEK293 CRE-luciferase Glo-Response cells were pretreated with graded concentrations of AC inhibitors (1 μM to 1 mM) for 30 minutes, followed by stimulation of AC by forskolin (25 μM) for 4 hours. As seen in Fig. 1A, both SQ22,536 and ddAd effectively inhibited the effect of forskolin with respective IC50 values of 5 μM and 33 μM. MDL-12,330A significantly inhibited the effect of forskolin when applied at 1 mM. To confirm that these compounds also inhibit GPCR-dependent AC activation, we stably transduced HEK293 CRE-luciferase Glo-Response cells with a vector expressing the rat PAC1hop receptor, a GPCR selectively activating Gs and Gq-dependent pathways after occupancy by the neuropeptide ligand pituitary adenylate cyclase-activating polypeptide (PACAP-38) (Pisegna and Wank, 1996). PAC1 receptor–expressing HEK293 CRE-luciferase Glo-Response cells pretreated with AC inhibitors were stimulated with PACAP-38 (100 nM). As seen in Fig. 1B, preincubation with graded concentrations of SQ22,536, MDL-12,330A, and ddAd revealed that both SQ22,536 and ddAd effectively inhibited PACAP-induced reporter gene activation with approximate IC50 values of 5 μM and 35 μM, respectively. At high concentrations, MDL-12,330A inhibited the effect of PACAP-38; however, significant inhibition was again only observed at 1 mM. Because a concentration of MDL-12,330A sufficient to confer greater than 50% inhibition could not be achieved while maintaining a concentration of DMSO that is compatible with normal cell function, we excluded this compound from further analysis.
Fig. 1.
Evaluation of cell-permeable AC inhibitors. (A) HEK293 cells stably expressing CRE-luciferase were pretreated with AC inhibitors MDL, SQ22,536, and ddAd for 30 minutes. Cells were then treated with inhibitors and forskolin (25 μM) for 4 hours, after which luciferase activity was measured. (B) HEK293 CRE-luc cells were transduced with retroviral vectors expressing the rat PAC1hop receptor and treated with PACAP-38 (100 nM) in the presence of AC inhibitors. Data are normalized to basal response from untreated control cells, and data points represent means from triplicate determinations ± S.E.M. (C) In NS-1 cells, cAMP accumulation induced by 100 nM PACAP in the presence of graded concentrations of SQ22,536. Data points represent the mean from triplicate measurements ± S.E.M. (D) In NS-1 cells, SQ22,536 (1 mM) inhibits cAMP generation due to treatment with Gs activator cholera toxin (CTX; 50 μg/ml) or AC activator forskolin (25 μM). ***P < 0.001 comparing cells treated with same agonist and either vehicle or SQ22,536 (Bonferroni).
Efficacy and Potency of SQ22,536 in Neuroscreen-1 Cells.
SQ22,536 was the most potent inhibitor of cAMP-dependent activity in HEK293 cells, and we therefore confirmed that SQ22,536 also effectively inhibits cAMP elevation in neuroendocrine NS-1 cells. NS-1 cells, a subclone of PC12 cells, which express AC isoforms AC3, AC4, AC6, AC7, and AC9 (X. Lu and L.E. Eiden, unpublished observations), were used to measure the effect of graded concentrations of SQ22,536 on cAMP accumulation in response to treatment with 100 nM PACAP-38, the concentration approximating its EMAX to stimulate cAMP elevation in NS-1 cells via native PAC1 receptors (Emery and Eiden, 2012). As seen in Fig. 1C, SQ22,536 caused dose-dependent inhibition of PACAP-induced cAMP elevation, demonstrating that it is an effective AC inhibitor in NS-1 cells. To confirm that the inhibitory effects of SQ22,536 are not limited to a GPCR first messenger, such as PACAP-38, we tested whether SQ22,536 also inhibits cAMP elevation caused by activation of Gs by cholera toxin (CTX; 50 μg/ml) and direct activation of AC by forskolin (25 μM). As seen in Fig. 1D, SQ22,536 (1 mM) effectively inhibited cAMP elevation after treatment with PACAP-38, CTX, or forskolin, confirming that it is an effective AC inhibitor in NS-1 cells.
SQ22,536 Inhibits cAMP-Induced Neuritogenesis in NS-1 Cells.
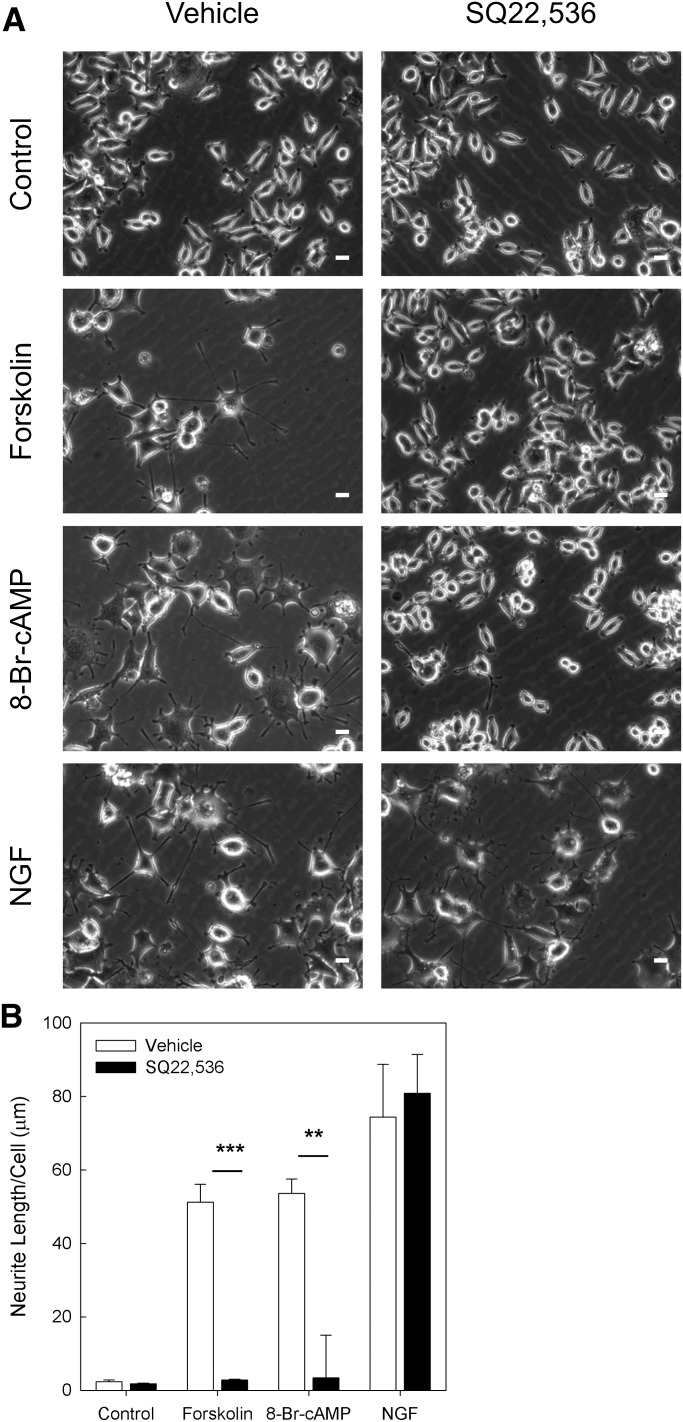
We previously demonstrated that cAMP elevation is sufficient for neuritogenesis in NS-1 cells (Emery and Eiden, 2012). We therefore next tested whether SQ22,536 used at 1 mM, a concentration at which it effectively inhibits cAMP accumulation in NS-1 cells, also inhibits cAMP-induced neuritogenesis. As seen in Fig. 2, treatment for 48 hours with forskolin (25 μM), the cell-permeable cAMP analog 8-Br-cAMP (500 μM) or nerve growth factor (NGF; 100 ng/ml) caused significant increases in neurite length. SQ22,536 (1 mM) inhibited neuritogenesis due to treatment with either forskolin or 8-Br-cAMP, and it had no effect on NGF-induced neuritogenesis. Pharmacological actions of cAMP analogs, such as 8-Br-cAMP, should occur independently of AC. Therefore, these data suggest that SQ22,536 also inhibits an additional target situated downstream of AC. NGF induces neuritogenesis in neuroendocrine cells via a cAMP-independent mechanism (Guroff et al., 1981; Richter-Landsberg and Jastorff, 1986; Damon et al., 1990; Ginty et al., 1991). As seen in Fig. 2, NGF-induced neuritogenesis was not affected by SQ22,536, indicating that this compound does not nonselectively inhibit proteins required for differentiation or neurite extension. Taken together, these data demonstrate that SQ22,536 exerts independent inhibitory effects on both AC and at least one additional signaling protein that is activated downstream of cAMP, whereas ddAd is an AC inhibitor qua AC inhibitor. Accordingly, we next investigated whether a known cAMP sensor is sensitive to inhibition by SQ22,536.
Fig. 2.
SQ22,536 inhibits cAMP-dependent neuritogenesis. (A) Representative micrographs from neurite outgrowth assays. NS-1 cells were treated for 48 hours with neuritogenic compounds forskolin (25 μM), 8-Br-cAMP (500 μM), or NGF (100 ng/ml) in the absence or presence of 1 mM SQ22,536 (scale bar = 20 µm). (B) Quantification of three independent neurite outgrowth assays. Bars represent mean neurite length per cell, and error bars represent S.E.M. SQ22,536 significantly inhibited neuritogenesis due to treatment with either forskolin (25 μM) or 8-Br-cAMP (500 μM), but did not affect neuritogenesis due to treatment with NGF (100 ng/ml). Statistical significance determined by pairwise Bonferroni-corrected t test comparisons of the effect of SQ22,536 within each condition (**P < 0.01, ***P < 0.001).
SQ22,536 Affects PKA- and Epac-Dependent cAMP Signaling Only at the Level of AC.
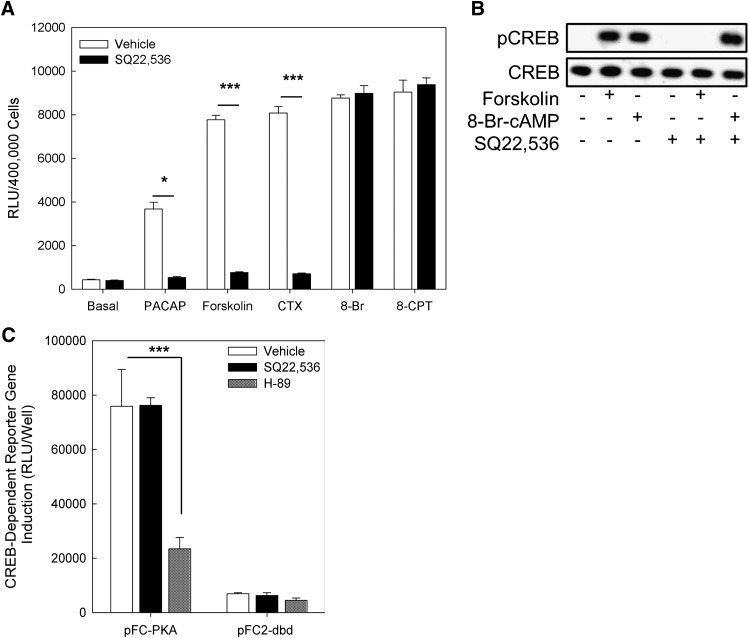
PKA is the canonical immediate effector in cAMP signaling and regulates a variety of cellular processes by reversible phosphorylation of numerous protein substrates, including Ser133 of the transcription factor CREB. To efficiently monitor the activation of CREB due to treatment with various pharmacological agents, NS-1 cells were transiently transfected with a Gal4/CREB fusion protein and a reporter gene sensitive to binding of the fusion protein that yields a luminescent signal in response to transactivation. As shown in Fig. 3A, PACAP-38 (100 nM), forskolin (25 μM), CTX (50 μg/ml), 8-Br-cAMP (500 μM), and 8-CPT-cAMP (500 μM) caused significant increases in CREB reporter gene transactivation, as reported elsewhere (Emery and Eiden, 2012). The effects of the cAMP-stimulating agents PACAP-38, forskolin, and CTX were sensitive to inhibition by SQ22,536, whereas the effects of cAMP analogs 8-Br-cAMP and 8-CPT-cAMP were not. These data indicate that SQ22,536 does not inhibit PKA. To confirm these results using a complementary approach, we measured the inhibitory effect of SQ22,536 on forskolin and 8-Br-cAMP-induced CREB phosphorylation at Ser133 by Western blot (Fig. 3B). In these experiments, although SQ22,536 (1 mM) completely blocked the effect of forskolin (25 μM), it failed to inhibit the effect of 8-Br-cAMP (500 μM), indicating that SQ22,536 does not inhibit the canonical cAMP sensor PKA. To further confirm that SQ22,536, applied at 1 mM, does not inhibit PKA, we cotransfected NS-1 cells with a Gal4/CREB fusion protein, a reporter gene (pFR-Luc), along with either the catalytic subunit of PKA (pFC-PKA) or a negative control plasmid (pFC2-dbd). As shown in Fig. 3C, H-89 (30 μM) significantly inhibited CREB reporter gene activation caused by expression of catalytically active PKA, whereas SQ22,536 had no effect on PKA-induced CREB induction; hence, SQ22,536 does not inhibit PKA.
Fig. 3.
SQ22,536 inhibits PKA-dependent canonical cAMP signaling by its action at AC. (A) CREB reporter gene transactivation assays. NS-1 cells were transfected with a reporter gene responsive to a transfected Gal4-CREB fusion protein. CREB activity was measured after 6 hours of treatment with cAMP elevating or mimicking agents PACAP-38 (100 nM), forskolin (25 μM), cholera toxin (CTX; 50 μg/ml), 8-Br-cAMP (8-Br; 500 μM), or 8-CPT-cAMP (8-CPT; 500 μM) in the absence or presence of SQ22,536 (1 mM). All agents tested significantly increase CREB activity (ANOVA P < 0.001). SQ22,536 significantly inhibited the effects of the cAMP-elevating agents PACAP, forskolin, and CTX on CREB activation, although it failed to inhibit the effects of cAMP analogs 8-Br-cAMP or 8-CPT-cAMP. Bonferroni, *P < 0.05, ***P < 0.001. Bars represent means from 6 independent measurements, and error bars correspond to the S.E.M. (B) Representative Western blot of CREB phosphorylation at Ser133 (pCREB) and total CREB (CREB) in NS-1 cells treated for 10 minutes with forskolin (25 μM) or 8-Br-cAMP (500 μM) ± SQ22,536 (1 mM), n = 3 with similar results. (C) PKA inhibition assays. NS-1 cells were cotransfected with a Gal4/CREB fusion protein, the pFR-Luc reporter gene, and either catalytically active PKA (pFC-PKA) or a negative control plasmid (pFC2-dbd). After transfection, cells were exposed to either vehicle (0.01% DMSO), SQ22,536 (1 mM), or H-89 (30 μM) for 48 hours. In cells expressing pFC-PKA, H-89 significantly inhibited CREB activation (Bonferroni, ***P < 0.001), whereas SQ22,536 had no effect. Bars represent 6 separate determinations, with error bars corresponding to the S.E.M.
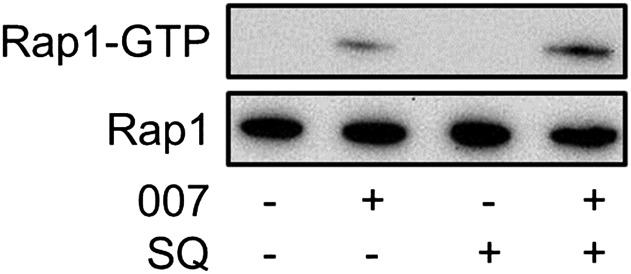
Because SQ22,536 does not inhibit canonical cAMP signaling downstream of AC, we next tested the hypothesis that SQ22,536 may have secondary inhibitory activity on the Rap GEF Epac, a noncanonical cAMP sensor. To this end, we measured Rap1 activation in response to treatment with the Epac-selective cAMP analog 8-CPT-2′-O-Me-cAMP (007). As shown in Fig. 4, treatment with 007 (100 μM) for 5 minutes caused an increase in Rap1 activation, which is a well-characterized cellular consequence of Epac activation. Epac-dependent Rap1 activation was not inhibited by pretreatment with 1 mM SQ22,536, indicating that although SQ22,536 inhibits AC and possibly an additional cAMP-related signaling molecule, Epac signaling downstream of AC is SQ22,536 insensitive.
Fig. 4.
SQ22,536 fails to inhibit Epac-mediated signaling. Representative Western blots showing both affinity purified GTP-bound Rap1 (Rap1-GTP) and total cellular Rap1 from NS-1 cells after treatment with the selective Epac activator 8-CPT-2′-O-Me-cAMP (007; 100 μM) for 10 minutes in the absence or presence of SQ22,536 (SQ; 1 mM). Experiment was repeated with identical results (n = 3).
SQ22,536 Specifically Inhibits cAMP-Dependent ERK Phosphorylation and Elk Induction.
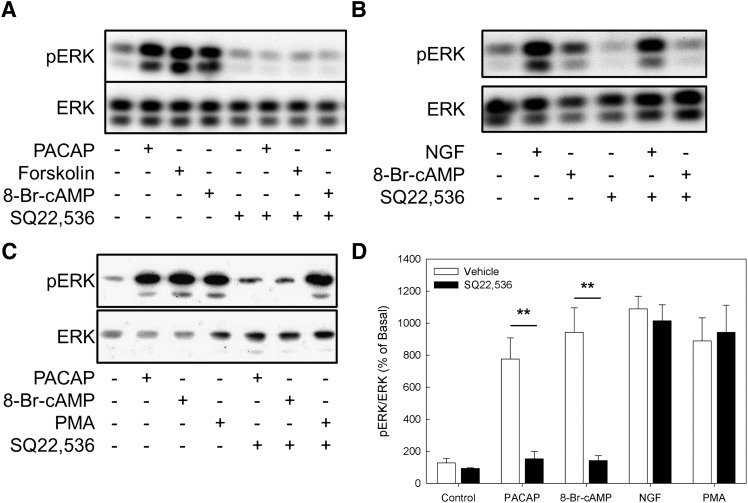
We previously reported that a noncanonical cAMP sensor, termed the NCS, mediates cAMP-dependent ERK phosphorylation and resultant neuritogenesis in NS-1 cells (Emery and Eiden, 2012). Because SQ22,536 inhibited the effect of 8-Br-cAMP on neuritogenesis and failed to inhibit the effects of cAMP analogs on processes dependent on PKA and Epac, we next assessed whether SQ22,536 inhibits NCS-dependent ERK activation. As shown in Fig. 5A, ERK was robustly phosphorylated after treatment for 10 minutes with PACAP-38 (100 nM), forskolin (25 μM), or 8-Br-cAMP (500 μM). Thus, ERK activation both upstream and downstream of AC was inhibited by SQ22,536.
Fig. 5.
SQ22,536 inhibits cAMP-dependent ERK phosphorylation, but not NGF or PMA-induced ERK phosphorylation. (A) Representative Western blot showing ERK phosphorylation in response to treatment with PACAP-38 (100 nM), forskolin (25 μM), and 8-Br-cAMP (500 μM) ± SQ22,536 (SQ; 1 mM). (B) Representative Western blot from NS-1 cells treated with 8-Br-cAMP (500 μM) or NGF (100 ng/ml) ± SQ22,536 (1 mM). (C) Representative Western blot of ERK phosphorylation following treatment with PACAP (100 nM), 8-Br-cAMP (500 μM), and PMA (100 nM) ± SQ22,536 (1 mM). (D) Quantification of blots from all experiments. Bars represents the mean from three or more independent experiments, and error bars correspond to the S.E.M. Data are presented as the ratio of phosphorylated ERK to total ERK and are expressed as a percentage of basal ERK phosphorylation as determined from untreated controls from each experiment. PACAP-38 (100 nM), 8-Br-cAMP (500 μM), NGF (100 ng/ml), and PMA (100 nM) all significantly increased ERK phosphorylation (ANOVA P < 0.001). SQ22,536 blocked enhancement of ERK phosphorylation by PACAP-38 and 8-Br-cAMP (Bonferroni, **P < 0.01), whereas it failed to significantly affect ERK phosphorylation due to either NGF or PMA.
To test whether SQ22,536 directly inhibits a component of the MAP kinase pathway, we measured ERK phosphorylation in cells treated with NGF, which causes cAMP-independent ERK phosphorylation, in the absence or presence of SQ22,536 (Fig. 5B). As shown in Fig. 5B, NGF-induced ERK phosphorylation was not sensitive to SQ22,536, suggesting that this compound does not directly inhibit the MEK/ERK signaling cassette activated by NGF. Furthermore, we compared the inhibitory effects of SQ22,536 on ERK phosphorylation resulting from treatment with the PKC activator phorbol myristate acetate (PMA; 100 nM) and with PACAP-38 and 8-Br-cAMP. As shown in Fig. 5C, although SQ22,536 blocked ERK phosphorylation caused by PACAP and 8-Br-cAMP, it did not affect PMA-dependent ERK phosphorylation, which occurs via PKC-mediated activation of the MEK/ERK signaling cassette.
Data from all ERK phosphorylation assays are summarized in Fig. 5D. SQ22,536 significantly inhibited ERK phosphorylation stimulated by PACAP, forskolin, or 8-Br-cAMP, and did not affect NGF or PMA-dependent ERK phosphorylation. Taken together, these results indicate that SQ22,536 has at least one additional target besides AC, its reported mechanism of action, but that SQ22,536 acts specifically within this cAMP-related signaling pathway.
ERK activation is the only known downstream target of cAMP signaling through the NCS (Emery and Eiden, 2012), and the transcription factor Elk-1 is activated by phosphorylation by ERK and underlies the induction of numerous ERK-dependent genes. To measure the inhibitory effect of SQ22,536 on Elk-1 induction, we generated a cell line (termed NS-1 Elk-luc) from NS-1 cells retrovirally transduced to express a Gal4/Elk-1 fusion protein and a luciferase reporter gene sensitive to Gal4/Elk. As shown in Fig. 6A, ERK-activating agents PACAP (100 nM), forskolin (25 μM), CTX (50 μg/ml), 8-Br-cAMP (500 μM), and PMA (100 nM) all caused significant increases in Elk-1 transactivation. The effects of cAMP-elevating agents PACAP, forskolin, CTX, and the cAMP analog 8-Br-cAMP were significantly inhibited by SQ22,536 (1 mM), however the effect of PMA was insensitive to inhibition by SQ22,536. These findings are consistent with those obtained by measurement of ERK phosphorylation (Fig. 5) and neuritogenesis (Fig. 2), indicating that SQ22,536 specifically inhibits cAMP-dependent Elk activation, both at the level of AC and its secondary target, the NCS.
Fig. 6.
SQ22,536 inhibits cAMP-dependent Elk activation. (A) NS-1 cells stably expressing an Elk/Gal4 fusion protein and a reporter gene sensitive to fusion protein transactivation (NS-1 Elk-luc) were treated for 6 hours with PACAP (100 nM), forskolin (25 μM), CTX (50 μg/ml), 8-Br-cAMP (500 μM), or PMA (100 nM) ± SQ22,536 (1 mM). All agents tested significantly increased Elk transactivation relative to untreated control cells (analysis of variance, P < 0.001). SQ22,536 inhibited Elk activation due to treatment with PACAP, forskolin, CTX, or 8-Br-cAMP (Bonferroni, **P < 0.01, ***P < 0.001), whereas it failed to significantly ERK phosphorylation due to either NGF or PMA. Data are expressed as a percentage of basal (vehicle-treated controls), with bars representing means from triplicate determinations + S.E.M. (B) The effect of graded concentrations of SQ22,536 on Elk transactivation due to treatment with forskolin (25 μM) or 8-Br-cAMP (500 μM). Data points are means from triplicate determinations, and error bars represent S.E.M. (C) Dose-dependent inhibition of forskolin-induced Elk activation by the AC inhibitor 2′,5′-dideoxyadenosine, which failed to significantly inhibit 8-Br-cAMP-induced Elk activation. (D) Summary of data from neurite outgrowth assays where NS-1 cells were treated with forskolin (25 μM) or 8-Br-cAMP (500 μM) in the absence or presence of SQ22,536 (10 μM or 500 μM). Bars represent means from triplicate determinations + S.E.M. Forskolin and 8-Br-cAMP caused significant increases in neurite length (ANOVA P < 0.001). SQ22,536 caused dose-dependent inhibition of neuritogenesis: Bonferroni-corrected post hoc comparisons of cells treated with agonists + SQ to cells treated with agonists + vehicle. NS, not significant. **P < 0.01, ***P < 0.001.
To assess whether there is a detectable difference in the potency of SQ22,536 to inhibit AC versus the NCS, we compared Elk-1 activation caused by treatment with forskolin (AC-dependent) and 8-Br-cAMP (AC-independent) in the presence of graded concentrations of SQ22,536 (Fig. 6B). SQ22,536 caused a dose-dependent inhibition of Elk-1 activation after treatment with either forskolin (25 μM) or 8-Br-cAMP (500 μM). However, SQ22,5536 more potently inhibited forskolin-induced Elk activation (IC50 = 10 μM) than 8-Br-cAMP-induced Elk activation (IC50 = 170 μM). These data indicate that SQ22,536 inhibits two distinct targets with different apparent affinities; it is more potent at AC, its reported target, and less potent at its secondary target, a signaling molecule that is activated by cAMP and located upstream of the MAP kinase pathway.
We previously reported that the P-site AC inhibitor 2′, 5′-dideoxyadenosine (ddAd) fails to inhibit 8-Br-cAMP-dependent ERK phosphorylation and neuritogenesis in NS-1 cells when applied at 100 μM, a concentration at which it effectively inhibits cAMP elevation (Emery and Eiden, 2012). However, we wanted to address the possibility that inhibition of the NCS is a common property of P-site AC inhibitors when applied at greater concentrations. To this end, we measured Elk-1 activation after treatment with forskolin (25 μM) and 8-Br-cAMP (500 μM) in a range of concentrations of ddAd (Fig. 6C). Although ddAd inhibited forskolin-induced Elk-1 transactivation (IC50 = 10 μM) with a similar potency to SQ22,536, it failed to significantly attenuate 8-Br-cAMP-induced Elk-1 transactivation even at concentrations as high as 3 mM, indicating that the NCS is not a secondary target that all AC inhibitors share in common.
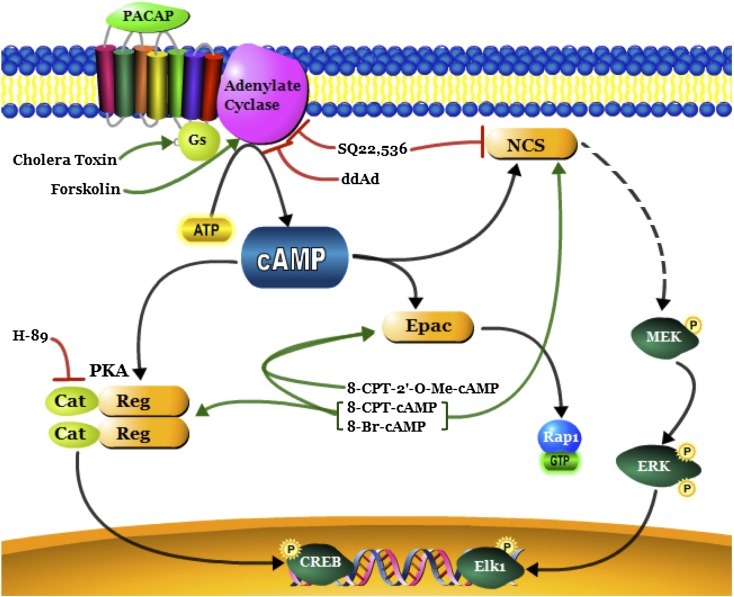
On the basis of the results that SQ22,536 inhibits Elk-1 induction by forskolin and 8-Br-cAMP at different potencies, we measured neurite outgrowth in response to treatment with forskolin (25 μM) and 8-Br-cAMP (500 μM) in the presence of different concentrations of SQ22,536 (10 μM or 500 μM). As shown in Fig. 6D, both forskolin and 8-Br-cAMP caused significant increases in neurite outgrowth. SQ22,536, applied at 10 μM, significantly inhibited the neuritogenic effect of forskolin, whereas 8-Br-cAMP was not significantly inhibited. At a greater concentration (500 μM), SQ22,536 significantly inhibited neurite elongation due to either forskolin or 8-Br-cAMP. The data, taken together (summarized in Fig. 7), indicate that SQ22,536 has two targets in cAMP signaling: AC and the NCS.
Fig. 7.
Schematic representation of the role of SQ22,536 in cAMP signaling. In NS-1 cells, cAMP activates at least three sensors (depicted in orange): PKA, Epac, and the neuritogenic cAMP sensor (NCS). Green arrows represent pharmacological activators, red arrows represent inhibitors, and black arrows represent signaling pathway linkages. Dashed lines indicate additional signaling components that have not been determined. Cat, catalytic subunit; Reg, regulatory subunit.
Discussion
It was assumed through most of the last century that cAMP signaling within mammalian cells proceeded mainly, if not exclusively, through activation of PKA (Kuo and Greengard, 1969). More recently, it has been appreciated that at least three major cAMP-dependent signaling pathways exist in rodent and human cells. A large number of molecular biological and pharmacological investigations have been devoted to parsing the relative contributions of PKA, Epac, and now NCS in cAMP-dependent signaling in mammalian, especially neuroendocrine, cells. These investigations have been fraught with the twin difficulties of establishing both (1) that a particular pathway is, in fact, cAMP-dependent and (2) that the pathway, if cAMP-dependent, relies on either canonical (PKA-dependent) or noncanonical (cAMP sensors other than PKA) signaling. Obviously, reagents for selective stimulation or inhibition of the various isoforms of adenylate cyclase have become increasingly central to these efforts. There is a plethora of information about the pharmacological properties of various AC inhibitors in vitro, and the three used in the present studies exhibit activity at varying concentrations against ATP cyclohydrolyzing activities of a wide variety of AC isoforms (see Seifert, et al., 2012). However, basic pharmacological investigations focused on the selectivity, specificity, and potency of inhibitors of adenylate cyclase relevant to understanding cAMP signaling in intact cells have been sparse, and the information obtained to date surprisingly limited.
In this study, we evaluated the efficacies and potencies of the AC inhibitors MDL-12,330A, SQ22,536, and ddAd to inhibit cAMP-dependent activity using human and rat cell lines in which signaling through various AC isoforms to each of the three major cAMP-dependent pathways can be conveniently monitored. Of importance, the lines that we used feature expression of gene and signaling protein reporters in a stable, low copy number format, which preserves the approximate stoichiometry of cellular signaling components that exists in vivo.
Using HEK293 cells, we found that SQ22,536 and ddAd each effectively inhibit forskolin and PACAP-dependent AC activity. SQ22,536 is approximately seven times more potent than ddAd. We previously reported that ddAd is an effective and specific AC inhibitor at 100 μM in neuroendocrine NS-1 cells (Emery and Eiden, 2012). However, the usefulness of ddAd is limited by the fact that its potency varies considerably among cell types. In cortical neurons, for example, ddAd applied at concentrations greater than 500 μM fails to completely inhibit PACAP-induced cAMP elevation (Holighaus et al., 2012). Whether this is attributable to differential cell penetration of these compounds into neurons, compared with neuroendocrine cell lines, has not been determined. Thus, AC inhibitors with greater potency for all AC isoforms, but preservation of specificity for AC, compared with other ATP- and cAMP-binding proteins, are needed tools for use in studies of cAMP-mediated signaling, particularly in neuroendocrine cells with multiple defined cAMP signaling pathways causing diverse cellular responses (Emery and Eiden, 2012; Hamelink et al., 2002; Vaudry et al., 2002; Ravni et al., 2008)
The present studies indicate that SQ22,536 is a more potent AC inhibitor than is ddAd but has an additional downstream target that is related to the intracellular actions of cAMP. Specifically, SQ22,536 blocks the action of two distinct targets involved in cAMP-related signaling: AC, as previously reported, and the neuritogenic cAMP sensor or NCS, a signaling component linking cAMP elevation to activation of ERK (Emery and Eiden, 2012). The NS-1 Elk-luc stable cell line created to conduct these studies provides a rapid readout for multiple downstream components of the cAMP signaling pathway initiated via activation of the NCS. This cell line confers several advantages for pharmacological studies using neuroendocrine cells: unlike the parent PC12 cell line, NS-1 Elk-luc cells can be easily grown and treated in 96-well plates. Moreover, transactivation of recombinant Elk-1 results from activation of endogenous cellular proteins, including the PAC1 GPCR, AC, Gs, and PKC, ultimately converging on the MEK-ERK signaling dyad. Using NS-1 Elk-luc cells, we were able to demonstrate the ability of SQ22,536 to inhibit 8-Br-cAMP phosphorylation of ERK leading to Elk-1 activation, but without inhibiting cAMP-independent activation of ERK or Elk-1 and without inhibiting PKA- or Epac-dependent signaling initiated by 8-Br-cAMP and other cAMP analogs, as measured by CREB activation/phosphorylation and Rap1 activation, respectively. The fact that activation of native PAC1 receptors by PACAP resulted in a robust and consistent activation of Elk-1 through the NCS, and was inhibited by SQ22,536 at two discrete points in this signaling pathway, suggests that the NS-1 Elk-luc cell line may be particularly useful for high-throughput screening for additional pharmacological agents with greater specificity for both AC and the NCS, without effects on other signaling proteins, including MEK, ERK, or Elk-1 itself.
Knowledge of the NCS inhibitory activity of SQ22,536 should significantly limit confusion about its actions on whole-cell signaling pathways and enable better exploitation of its differential inhibition of isoforms within the AC protein family (Seifert et al., 2012). Most notably, there are substantial differences in the reported potencies of SQ22,536 to inhibit the activities of recombinant AC5 and AC6, with respective IC50 values of 2 μM and 360 μM (Johnson et al., 1997; Onda et al., 2001). Of interest, AC5 and AC6 share highly homologous amino acid sequences and appear to be otherwise similar in their pharmacological properties. These large differences in affinity between AC isoforms may account for the high concentrations of SQ22,536 commonly applied in studies using whole cells, because different cell types express different combinations of AC isoforms. Our observation that SQ22,536 is more potent in HEK293 cells than in NS-1 cells may be explained by the patterns of AC isoform expression in these two types of cells: HEK293 cells express only mRNAs corresponding to AC3, AC6, and AC9 (Zagranichnaya et al., 2005). PC12 cells, the parent cell line from which NS-1 cells were subcloned, express mRNAs encoding AC3, AC4, AC6, AC7, and AC9 (X. Lu and L.E. Eiden, unpublished observations). In fact, considerable effort has been dedicated to the development of isoform-specific AC inhibitors, including those directed either to the P-site (Dessauer et al., 1999; Seifert et al., 2012) or to the diterpene (forskolin) binding site (Pinto et al., 2008), for potential clinical use in heart failure, neurodegeneration, and neuropathic pain (Pierre et al., 2009).
In contrast to SQ22,536, the P-site inhibitor ddAd did not inhibit the effect of 8-Br-cAMP on Elk transactivation, nor does it inhibit ERK phosphorylation or neuritogenesis in response to treatment of NS-1 cells with 8-Br-cAMP or dibutyryl-cAMP (Emery and Eiden, 2012). This suggests that ddAd is AC-specific, at least within the context of cAMP signaling. We anticipate that the clarification of the specificity, potency, and efficacy of SQ22,536 and ddAd in intact cells, reported here, will be helpful in elucidating the contributions of canonical versus noncanonical cAMP signaling relevant to signaling physiology, pathophysiology, and drug and molecular probe discovery in the immediate future. Specifically, the use of a lipophilic cAMP analog in the absence or presence of SQ22,536 should represent a novel approach to probe the involvement of the NCS in cAMP-mediated signaling in a variety of cell types.
Acknowledgments
This work was performed as part of a drug screening program in collaboration with Dr. Marc Ferrer of the National Center for Advancing Translational Sciences (NCATS). The authors acknowledge the shared use of NCATS reagents under an NIMH-NCATS collaborative agreement. The authors thank Dr. Tomris Mustafa (NIMH), for helpful comments about the manuscript, and James Nagle and Deborah Kauffman at the National Institute of Neurologic Disorders and Stroke sequencing facility.
Abbreviations
- 8-Br-cAMP
8-bromoadenosine 3′,5′-cyclic monophosphate
- 8-CPT-cAMP
8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate
- 8-CPT-2′-O-Me-cAMP
8- (4-chlorophenylthio)- 2′-O-methyladenosine 3′, 5′-cyclic monophosphate
- AC
adenylate cyclase
- ANOVA
analysis of variance
- CRE
cAMP response element
- CREB
cAMP response element-binding protein
- CTX
cholera toxin
- ddAd
2′,5′-dideoxyadenosine
- GEF
guanine nucleotide exchange factor
- GPCR
G protein-coupled receptor
- H-89
N-[2-[[3-(4-bromophenyl)-2-propenyl]amino]ethyl]-5-isoquinolinesulfonamide dihydrochloride
- MDL-12,330A
cis-N-(2-phenylcyclopentyl)azacyclotridec-1-en-2-amine hydrochloride
- NCS
neuritogenic cAMP sensor
- NGF
nerve growth factor
- NS-1
neuroscreen-1
- P-site
purine site
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PKA
protein kinase A
- PMA
phorbol-12-myristate-13-acetate
- SQ22,536
9-(tetrahydrofuryl)-adenine
Authorship Contributions
Participated in research design: Emery and L. E. Eiden.
Conducted experiments: Emery.
Contributed new reagents or analytical tools: M. V. Eiden.
Performed data analysis: Emery and L. E. Eiden.
Wrote or contributed to the writing of the manuscript: Emery, M. V. Eiden, L. E. Eiden.
Footnotes
This work was funded by the Intramural Research Program of the National Institutes of Health [National Institute of Mental Health] [Projects 1ZIAMH002386 and 1ZIAMH002592].
References
- Cali JJ, Zwaagstra JC, Mons N, Cooper DM, Krupinski J. (1994) Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem 269:12190–12195 [PubMed] [Google Scholar]
- Chen Y, Weng G, Li J, Harry A, Pieroni J, Dingus J, Hildebrandt JD, Guarnieri F, Weinstein H, Iyengar R. (1997) A surface on the G protein beta-subunit involved in interactions with adenylyl cyclases. Proc Natl Acad Sci USA 94:2711–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiono M, Mahey R, Tate G, Cooper DM. (1995) Capacitative Ca2+ entry exclusively inhibits cAMP synthesis in C6-2B glioma cells. Evidence that physiologically evoked Ca2+ entry regulates Ca(2+)-inhibitable adenylyl cyclase in non-excitable cells. J Biol Chem 270:1149–1155 [DOI] [PubMed] [Google Scholar]
- Choi EJ, Xia Z, Storm DR. (1992) Stimulation of the type III olfactory adenylyl cyclase by calcium and calmodulin. Biochemistry 31:6492–6498 [DOI] [PubMed] [Google Scholar]
- Damon DH, D’Amore PA, Wagner JA. (1990) Nerve growth factor and fibroblast growth factor regulate neurite outgrowth and gene expression in PC12 cells via both protein kinase C- and cAMP-independent mechanisms. J Cell Biol 110:1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJT, Verheijen MHG, Cool RH, Nijman SM, Wittinghofer A, Bos JL. (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474–477 [DOI] [PubMed] [Google Scholar]
- Dessauer CW, Tesmer JJ, Sprang SR, Gilman AG. (1999) The interactions of adenylate cyclases with P-site inhibitors. Trends Pharmacol Sci 20:205–210 [DOI] [PubMed] [Google Scholar]
- Emery AC, Eiden LE. (2012) Signaling through the neuropeptide GPCR PAC₁ induces neuritogenesis via a single linear cAMP- and ERK-dependent pathway using a novel cAMP sensor. FASEB J 26:3199–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell KB, Ting YT, Eiden MV. (2002) Fusion-defective gibbon ape leukemia virus vectors can be rescued by homologous but not heterologous soluble envelope proteins. J Virol 76:4267–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti ME, Sonetti D, Pareschi MC, Buzzi M, Colamussi ML, Biondi C. (1996) MDL 12330A inhibits the non-neuronal adenylyl cyclase from the freshwater snail Planorbarius corneus, but the neuronal enzyme is activated by this compound. Neurosci Lett 207:191–194 [DOI] [PubMed] [Google Scholar]
- Gadea A, López E, López-Colomé AM. (1999) The adenylate cyclase inhibitor MDL-12330A has a non-specific effect on glycine transport in Müller cells from the retina. Brain Res 838:200–204 [DOI] [PubMed] [Google Scholar]
- Gao X, Sadana R, Dessauer CW, Patel TB. (2007) Conditional stimulation of type V and VI adenylyl cyclases by G protein betagamma subunits. J Biol Chem 282:294–302 [DOI] [PubMed] [Google Scholar]
- Gilman AG. (1995) Nobel Lecture. G proteins and regulation of adenylyl cyclase. Biosci Rep 15:65–97 [DOI] [PubMed] [Google Scholar]
- Ginty DD, Glowacka D, DeFranco C, Wagner JA. (1991) Nerve growth factor-induced neuronal differentiation after dominant repression of both type I and type II cAMP-dependent protein kinase activities. J Biol Chem 266:15325–15333 [PubMed] [Google Scholar]
- Gottesman MM, Fleischmann RD. (1986) The role of cAMP in regulating tumour cell growth. Cancer Surv 5:291–308 [PubMed] [Google Scholar]
- Greengard P. (2001) The neurobiology of slow synaptic transmission. Science 294:1024–1030 [DOI] [PubMed] [Google Scholar]
- Guroff G, Dickens G, End D, Londos C. (1981) The action of adenosine analogs on PC12 cells. J Neurochem 37:1431–1439 [DOI] [PubMed] [Google Scholar]
- Hamelink C, Lee HW, Chen Y, Grimaldi M, Eiden LE. (2002) Coincident elevation of cAMP and calcium influx by PACAP-27 synergistically regulates vasoactive intestinal polypeptide gene transcription through a novel PKA-independent signaling pathway. J Neurosci 22:5310–5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam RJ, Davidson MM, Desjardins JV. (1978) Inhibition of adenylate cyclase by adenosine analogues in preparations of broken and intact human platelets. Evidence for the unidirectional control of platelet function by cyclic AMP. Biochem J 176:83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holighaus Y, Mustafa T, Eiden LE. (2011) PAC1hop, null and hip receptors mediate differential signaling through cyclic AMP and calcium leading to splice variant-specific gene induction in neural cells. Peptides 32:1647–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holighaus Y, Weihe E, Eiden LE. (2012) STC1 induction by PACAP is mediated through cAMP and ERK1/2 but not PKA in cultured cortical neurons. J Mol Neurosci 46:75–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt NH, Evans T. (1980) RMI 12330A, an inhibitor of cyclic nucleotide phosphodiesterases and adenylate cyclase in kidney preparations. Biochim Biophys Acta 613:499–506 [DOI] [PubMed] [Google Scholar]
- Insel PA, Zhang L, Murray F, Yokouchi H, Zambon AC. (2012) Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol (Oxf) 204:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Désaubry L, Bianchi G, et al. (1997) Isozyme-dependent sensitivity of adenylyl cyclases to P-site-mediated inhibition by adenine nucleosides and nucleoside 3′-polyphosphates. J Biol Chem 272:8962–8966 [DOI] [PubMed] [Google Scholar]
- Kandel ER. (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294:1030–1038 [DOI] [PubMed] [Google Scholar]
- Kao HT, Song HJ, Porton B, Ming GL, Hoh J, Abraham M, Czernik AJ, Pieribone VA, Poo MM, Greengard P. (2002) A protein kinase A-dependent molecular switch in synapsins regulates neurite outgrowth. Nat Neurosci 5:431–437 [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. (1998) A family of cAMP-binding proteins that directly activate Rap1. Science 282:2275–2279 [DOI] [PubMed] [Google Scholar]
- Kopperud R, Krakstad C, Selheim F, Døskeland SO. (2003) cAMP effector mechanisms. Novel twists for an ‘old’ signaling system. FEBS Lett 546:121–126 [DOI] [PubMed] [Google Scholar]
- Kuo JF, Greengard P. (1969) Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3′,5′-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci USA 64:1349–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulef K, Zagotta WN. (2003) Cyclic nucleotide-gated ion channels. Annu Rev Cell Dev Biol 19:23–44 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Gold GH. (1987) A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature 325:442–444 [DOI] [PubMed] [Google Scholar]
- Onda T, Hashimoto Y, Nagai M, et al. (2001) Type-specific regulation of adenylyl cyclase. Selective pharmacological stimulation and inhibition of adenylyl cyclase isoforms. J Biol Chem 276:47785–47793 [DOI] [PubMed] [Google Scholar]
- Pierre S, Eschenhagen T, Geisslinger G, Scholich K. (2009) Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov 8:321–335 [DOI] [PubMed] [Google Scholar]
- Pinto C, Papa D, Hübner M, Mou TC, Lushington GH, Seifert R. (2008) Activation and inhibition of adenylyl cyclase isoforms by forskolin analogs. J Pharmacol Exp Ther 325:27–36 [DOI] [PubMed] [Google Scholar]
- Pisegna JR, Wank SA. (1996) Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J Biol Chem 271:17267–17274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Matsuoka I, Mattei MG, Pouille Y, Defer N, Hanoune J. (1996) Identification and characterization of a widely expressed form of adenylyl cyclase. J Biol Chem 271:13900–13907 [DOI] [PubMed] [Google Scholar]
- Ravni A, Vaudry D, Gerdin MJ, Eiden MV, Falluel-Morel A, Gonzalez BJ, Vaudry H, Eiden LE. (2008) A cAMP-dependent, protein kinase A-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Mol Pharmacol 73:1688–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Landsberg C, Jastorff B. (1986) The role of cAMP in nerve growth factor-promoted neurite outgrowth in PC12 cells. J Cell Biol 102:821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Lushington GH, Mou TC, Gille A, Sprang SR. (2012) Inhibitors of membranous adenylyl cyclases. Trends Pharmacol Sci 33:64–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara RK, Dessauer CW, Gilman AG. (1996) Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol 36:461–480 [DOI] [PubMed] [Google Scholar]
- Sutherland EW. (1972) Studies on the mechanism of hormone action. Science 177:401–408 [DOI] [PubMed] [Google Scholar]
- Tang WJ, Gilman AG. (1991) Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science 254:1500–1503 [DOI] [PubMed] [Google Scholar]
- Taussig R, Iñiguez-Lluhi JA, Gilman AG. (1993) Inhibition of adenylyl cyclase by Gi alpha. Science 261:218–221 [DOI] [PubMed] [Google Scholar]
- van Rossum DB, Patterson RL, Ma HT, Gill DL. (2000) Ca2+ entry mediated by store depletion, S-nitrosylation, and TRP3 channels. Comparison of coupling and function. J Biol Chem 275:28562–28568 [DOI] [PubMed] [Google Scholar]
- Vaudry D, Stork PJ, Lazarovici P, Eiden LE. (2002) Signaling pathways for PC12 cell differentiation: making the right connections. Science 296:1648–1649 [DOI] [PubMed] [Google Scholar]
- Zagranichnaya TK, Wu X, Danos AM, Villereal ML. (2005) Gene expression profiles in HEK-293 cells with low or high store-operated calcium entry: can regulatory as well as regulated genes be identified? Physiol Genomics 21:14–33 [DOI] [PubMed] [Google Scholar]