Abstract
Gram-negative bacterial endotoxin lipopolysaccharide (LPS) triggers the production of inflammatory cytokines, reactive oxygen species (ROS), and prostaglandins (PGs) by pulmonary macrophages. Here, we investigated if ROS influenced PGs production in response to LPS treatment in mouse bone marrow-derived macrophages (BMDM). We observed that pretreatment of BMDM with two structurally unrelated ROS scavengers, MnTMPyP and EUK-134, not only prevented LPS-induced ROS accumulation, but also attenuated the LPS-induced PGD2, but not PGE2, production. Conversely LPS-induced PGD2, but not PGE2, production, was potentiated with the cotreatment of BMDM with H2O2. These data suggest that ROS differentially regulate PGD2 and PGE2 production in BMDM. In addition, selective inhibition of the ROS generator NADPH oxidase (NOX) using either pharmacologic inhibitors or its p47phox subunit deficient mouse BMDM also attenuated LPS-induced PGD2, but not PGE2 production, suggesting the critical role of NOX-generated ROS in LPS-induced PGD2 production in BMDM. We further found that both hematopoietic PGD synthase (H-PGDS) siRNA and its inhibitor HQL-79, but not lipocalin PGDS (L-PGDS) siRNA and its inhibitor AT-56, significantly attenuated LPS-induced PGD2 production, suggesting that H-PGDS, but not L-PGDS, mediates LPS-induced PGD2 production in BMDM. Furthermore, data from our in vitro cell-free enzymatic studies showed that coincubation of the recombinant H-PGDS with either MnTMPyP, EUK-134, or catalase significantly decreased PGD2 production, whereas coincubation with H2O2 significantly increased PGD2 production. Taken together, our results show that LPS-induced NOX-generated ROS production differentially and specifically regulates the H-PGDS-mediated production of PGD2, but not PGE2, in mouse BMDM.
Introduction
Cyclooxygenase (COX) converts arachidonic acid to PGH2, which is the precursor of distinct PGs. PGs are a group of lipid compounds derived from fatty acids in nearly every cell type (Funk, 2001; Cao et al., 2008) and mediate a variety of important physiologic functions in vivo (McAdam et al., 2000; Burleigh et al., 2002). For example, PGE2 promotes tumor initiation, progression, and metastasis (Samuelsson et al., 2007), whereas PGD2 triggers asthmatic responses (Matsuoka et al., 2000). Both PGE2 and PGD2 are converted from PGH2 by various isoforms of PGE synthase (PGES) and PGDS (Samuelsson et al., 2007; Yu et al., 2011), respectively. We recently showed that BMDM express all three types of PGES including cytosolic PGES (cPGES), microsomal PGES-1 (mPGES-1), and mPGES-2, as well as H-PGDS (Xiao et al., 2012). H-PGDS is a cytosolic, GSH-dependent enzyme that catalyzes the isomerization of PGH2 to PGD2, whereas L-PGDS is an N-glycosylated, GSH-independent protein (Urade and Eguchi, 2002). HQL-79 and AT-56 are reported isoform-selective PGDS inhibitors interdicting H-PGDS- and L-PGDS-mediated PGD2 production both in vivo and in vitro, respectively (Matsushita et al., 1998a,b; Irikura et al., 2009).
ROS are chemical reactive molecules containing oxygen generated during normal and disease-related metabolic processes (Xiao et al., 2002; Bedard and Krause, 2007), including three major species superoxide (O2•−), hydroxyl radical (•OH), and hydrogen peroxide (H2O2). Several major ROS-generating systems in different tissues have been identified, including NOX (Xiao et al., 2002; Cross and Segal, 2004), mitochondrial electron transport chain (METC) (Balaban et al., 2005), and nitric oxide synthase (NOS) (Xia et al., 1998). METC inhibitors, rotenone and antimycin A, have been shown to induce mitochondrial ROS generation from mitochondria complex I and complex III, respectively (Li et al., 2003). The NOS-selective inhibitor l-NAME prevented NOS-produced NO or ROS generation (Kim et al., 2007). ROS have been reported to play an important role in carcinogenesis (Benhar et al., 2002), cardiac myocyte hypertrophy (Amin et al., 2001), and augmented airway obstruction in asthma (Henricks and Nijkamp, 2001). We have shown that in BMDM, LPS induces both generation of ROS and PGs, which are important mediators in host defense (Park and Christman, 2006; Xiao et al., 2010). MnTMPyP is a membrane-permeable and nontoxic superoxide dismutase (SOD)/catalase mimetic that efficiently scavenges ROS (i.e., O2•− and H2O2) both in vivo and in vitro (Amin et al., 2001; Xiao et al., 2002; Zhao et al., 2011). EUK-134 is another structurally unrelated and membrane-permeable synthetic SOD/catalase mimetic that has been commonly used as a scavenger for intracellular ROS and peroxynitrite (Rong et al., 1999). Catalase catalyzes the decomposition of H2O2 to water and oxygen (Yu et al., 2006).
NOX is a membrane-bound multisubunit enzyme complex (Geiszt and Leto, 2004; Lambeth, 2004; Bedard and Krause, 2007) that contains two transmembrane subunits gp91phox and p22phox and at least four cytosolic subunits p47phox, p67phox, p40phox, and Rac. Currently, six additional isoforms (Nox1, 3, 4, and 5 and Duox1 and 2) of the NOX catalytic subunit gp91phox (Nox2) have been reported (Shiose et al., 2001; Geiszt and Leto, 2004; Lambeth, 2004). The primary enzymatic activity of NOX is to generate superoxide (O2•−) by transferring a single electron from NADPH/NADH to O2 (Leto, 1999). The NOX-generated O2•− radicals are rapidly converted to H2O2 by intracellular SOD, which is further decomposed by catalase to water and oxygen. Diphenylene iodonium (DPI) is a commonly used NOX-selective inhibitor, although it also inhibits other flavin protein-containing enzymes including NOS or METC (Bedard and Krause, 2007; Jaquet et al., 2009). The membrane-permeable protein tyrosine phosphatase inhibitor phenylarsine oxide (PAO) is also reported as a potent NOX2-selective inhibitor (Xiao et al., 2002; Jaquet et al., 2009).
We and others have previously shown that LPS induced production of both PGD2 and PGE2 in macrophages via the expression of COX-2 (Park and Christman, 2006). We also recently showed that LPS stimulated the activation and expression of the NOX enzyme and PGs production in BMDM (Zhao et al., 2010). ROS are known to play important roles in carcinogenesis (Benhar et al., 2002) and asthma (Henricks and Nijkamp, 2001) , which are mediated by PGE2 (Samuelsson et al., 2007) and PGD2 (Matsuoka et al., 2000), respectively. However, it is unclear if LPS-induced production of PGs is functionally connected with NOX-dependent ROS generation in BMDM. Here, we have explored the potential regulatory roles and signaling mechanisms of ROS in LPS-induced PGs production in BMDM.
Materials and Methods
Animals.
The p47phox-deficient mice (10—12 weeks old) were kindly provided from Dr. Steven M. Holland (National Institutes of Health, Bethesda, MD). Age- and sex-matched wild-type (WT) C57BL/6 male mice from Harlan were used with the p47phox-deficient mice in paired experiments. Mice were housed at the University of Illinois at Chicago animal facility in a temperature-controlled room with a 12:12-hour light-dark cycle and were given standard chow and bottle water. All procedures and protocols using mice were approved by the University of Illinois at Chicago animal care committee and complied with the Animal Welfare Act.
Materials.
Hanks’ balanced salt solution, fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Invitrogen (Carlsbad, CA). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Mediatech (Manassas, VA). Lysis buffer was purchased from Cell Signaling Technology (Danvers, MA). Laemmli sample buffer was purchased from Bio-Rad Laboratories (Richmond, CA). Polyvinylidene difluoride (PVDF) membrane was purchased from Amersham Pharmacia Biotech (Little Chalfont, Buckinghamshire, UK). SuperSignal chemiluminescent substrate solution and enhanced chemiluminescence solution were purchased from Thermo Fisher Scientific (Rockford, IL). Amaxa Mouse Macrophage Nuclefector Kit (catalog # VPA-1009) was purchased from Lonza (Walkersville, MD), and siRNAs were purchased from Thermo Dharmacon (Lafayette, CO). The primary antibody for L-PGDS (M-17), actin, and peroxidase-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The primary antibodies for COX-2, H-PGDS and L-PGDS (catalog # 10004344), human recombinant H-PGDS, human recombinant L-PGDS, human recombinant mPGES-1, GSH, EUK-134, HQL-79, AT-56, PGH2, PGD2, PGE2, d4-PGD2, and d4-PGE2 were purchased from Cayman Chemicals (Ann Arbor, MI). Isoluminol, NADPH, zymosan A (zymosan), LPS, rotenone, antimycin A, catalase, H2O2, Tris base, hydrogen peroxide, citric acid, and EDTA were purchased from Sigma-Aldrich (St. Louis, MO). MnTMPyP, DPI, PAO, l-NAME, and formic acid were purchased from EMD Chemicals (San Diego, CA). Hydrochloric acid and butylated hydroxytoluene (BHT) were purchased from Thermo Fisher Scientific. Purified water was prepared using a Millipore (Billerica, MA) Milli-Q purification system. All organic solvents were HPLC grade or better and were purchased from Thermo Fisher, and all other chemicals and solvents were ACS reagent grade, unless stated otherwise.
BMDM Isolation and Culture.
BMDM were isolated from WT C57BL/6 or p47phox-deficient mice as we previously described (Yu et al., 2011). Briefly, after mice were euthanized, bone marrow was flushed from the rear femurs. The cells were washed and resuspended in DMEM medium containing 10% endotoxin-free FBS and 10% (v/v) L929 cell-conditioned medium as a biologic source of macrophage colony-stimulating factor. The medium was then replenished at day 4 in culture, and the nonadherent cells were removed. The adherent bone marrow cells were used for experiments after day 7 in culture, corresponding to a mature macrophage phenotype.
Western Blot Assay.
Western blot method was similar as we previously described (Xiao et al., 2002; Cao et al., 2008). Briefly, BMDM were harvested in protein lysis buffer and sonicated for 10 seconds to shear genomic DNA. Protein concentration was determined by the Bradford assay. Equal amounts of the denatured proteins in Laemmli sample buffer were subjected to SDS-PAGE and transferred to a PVDF membrane, blocked with 5% nonfat dry milk, then incubated with primary antibody at 4°C overnight. Protein was detected with horseradish peroxidase-conjugated secondary antibody using SuperSignal enhanced chemiluminescent method.
Supernatant Sample Preparation.
Supernatant sample preparation was similar to what we previously described (Cao et al., 2008; Yu et al., 2011). Briefly, a 500-μl aliquot of supernatant was spiked together with d4-PGE2 and d4-PGD2 as surrogate standards. Next, citric acid and BHT were added to prevent free radical-catalyzed peroxidation. Prostaglandins were extracted by adding hexane/ethyl acetate (50:50, v/v). The upper organic phase was collected and the extraction procedure was repeated and then the organic phases were combined and evaporated to dryness. Immediately before analysis using liquid chromatography-tandem mass spectrometry (LC-MS-MS), each extract was reconstituted in methanol/water (50:50, v/v). Standards for calibration curves and quality control measurements were prepared by spiking 500-μl aliquots of cell culture medium with measured amounts of PGD2 and PGE2. These standards were then processed as described above. The concentrations of PGD2 and PGE2 in these standards ranged from 0.1 to 1000 ng/ml.
siRNA Transfection.
Primary BMDM were transfected with either ON-TARGET plus control siRNA (25 nM) or siRNAs for H-PGDS or L-PGDS (25 nM) using the Amaxa Mouse Macrophage Nuclefector kit. After 48 hours post-transfection, BMDM were stimulated by LPS (1 μg/ml) for 16 hours.
ROS Detection by Chemiluminescence assay.
The isoluminol-enhanced chemiluminescent assay for ROS detection in BMDM was similar to the previous reports (Dahlgren and Karlsson, 1999; Maeda et al., 2010). Briefly, primary cultured BMDM were seeded into 96-well culture plate (E&K Scientific, Santa Clara, CA) at 5 × 104/well in triplicate and primed with 100 ng/ml LPS for 16 hours prior to ROS measurement. The culture medium was then replaced with phenol red- and serum-free DMEM for subsequent ROS measurement. After preincubation with 50 M isoluminol, 40 U/ml HRP, and 100 μM NADPH at 37°C in the dark for 5 minutes, the BMDM were stimulated with either LPS (1 µg/ml) or the known phagocyte ROS stimulus zymosan (200 μg/ml). Chemiluminescence signals of each well were continuously recorded for 2 hours post-stimulation in a BioTek Synergy 2 multi-detection microplate reader (BioTek, Winooski, VT), and the averaged net increase of the plateau chemiluminescence signals was used to quantify the ROS production in each sample.
The In Vitro Cell-Free Enzyme Assay.
Equal amount of recombinant PG synthase, including mPGES-1 and H-PGDS from Cayman Chemicals, was prepared in Tris•HCl buffer (pH 8.0 at 37°C) on ice in the presence of enzyme cofactor GSH. When necessary, an aliquot of MnTMPyP, catalase, or H2O2 was added into the appropriate sample and preincubated at 37°C for 10 minutes. The reaction was initiated by adding the enzyme substrate PGH2, followed by incubation for 30 minutes at 37°C. Identical amounts of PGD2 and PGE2 were added in separate tubes as quantitative controls. Mixture of d4-PGD2 and d4-PGE2 was added as internal standards. The reaction was terminated by adding HCl; sample was extracted using hexane/ethyl acetate (50:50, v/v), and the organic phase was removed, evaporated to dryness, and reconstituted in methanol/water (50:50, v/v) immediately prior to quantitative analysis using LC-MS-MS (Yu et al., 2011).
Mass Spectrometry.
For the quantitative analysis of PGD2 and PGE2, HPLC separations were carried out using a Shimadzu (Columbia, MD) Prominence HPLC system with a Waters (Milford, MA) XTerra MS C18 (2.1 mm × 50 mm, 3.5 μm) analytical column and a 5-minute isocratic mobile phase consisting of acetonitrile/aqueous 0.1% formic acid (37:63, v/v) at a flow rate of 200 μl/min. The HPLC system was interfaced to a Thermo-Finnigan (San Jose, CA) TSQuantum triple quadruple mass spectrometer that was operated using negative ion electrospray. Isomeric PGD2 and PGE2 were measured using a SRM transition of m/z 351 to m/z 271, and the SRM transition of m/z 355 to m/z 275 was selected for the internal standards d4-PGE2 and d4-PGD2 (Cao et al., 2010, 2011; Yu et al., 2011).
Statistical Analysis.
Samples were run in triplicate (unless stated otherwise), and values were expressed as mean ± S.E.M. Statistical significance was assessed using either student t test or one-way analysis of variance, and P values <0.05 were considered significantly different.
Results
LPS and Zymosan Induced ROS Generation in BMDM.
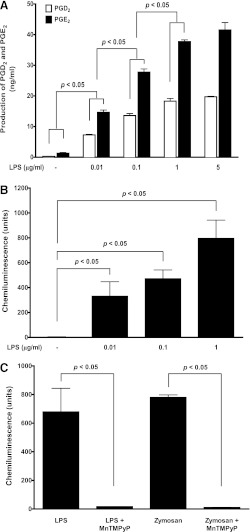
The Gram-negative bacterial endotoxin LPS is known to selectively activate Toll-like receptor 4 (TLR4) in the mammalian immune system and triggers the secretion of inflammatory cytokines and PGs in macrophages (Azim et al., 2007). As shown in Fig. 1A, LPS treatment concentration dependently (0.01, 0.1, 1, 5 µg/ml, 16 hours) stimulated both PGD2 and PGE2 production in BMDM. Because 1 and 5 µg/ml LPS treatment showed similar plateau levels of PGs production, we thus used 1 µg/ml LPS treatment in the following studies. Similarly, LPS treatment also concentration dependently (0.01, 0.1, 1 µg/ml) induced ROS generation in BMDM (Fig. 1B).
Fig. 1.
LPS concentration dependently stimulated production of both PGs and ROS in BMDM. (A) primary cultured WT BMDM were treated with LPS for 16 hours, and the concentrations of PGD2 and PGE2 in culture medium were determined using LC-MS-MS. LPS concentration dependently (0.01, 0.1, 1, and 5 µg/ml) stimulated both PGD2 and PGE2 production in BMDM (n = 3). P < 0.05 represents significant differences in productions of both PGE2 and PGD2 versus their individual counterparts between the compared LPS concentrations. (B) the primed WT BMDM was treated with LPS for ROS measurement using isoluminol-enhanced chemiluminescence assay. LPS concentration-dependently (0.01, 0.1, and 1 µg/ml, n = 3) stimulated ROS generation in BMDM as measured by increased chemiluminescent signals. (C) both LPS-induced and the phagocyte ROS stimulus zymosan-induced ROS generation in BMDM were completely prevented by pretreatment of BMDM with the ROS scavenger MnTMPyP (50 μM, 0.5 hours prior to stimulation, n = 6).
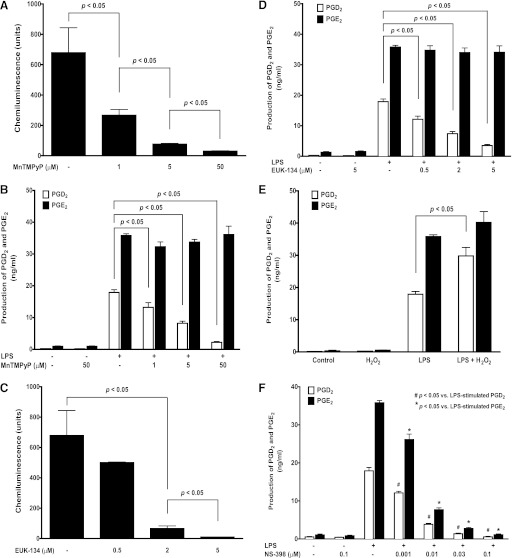
Although LPS is known to stimulate ROS production in neutrophils, the reports of LPS-induced ROS generation in macrophages have been controversial in the literature (Szefler et al., 1989; Pfeiffer et al., 2007; Deschacht et al., 2010; Maeda et al., 2010). In contrast, zymosan consistently stimulates ROS generation in macrophages (Russwurm et al., 1994; Bramble and Anderson, 1998) and thus was used as a positive ROS stimulus in our studies. Like zymosan stimulation, LPS significantly induced ROS generation in BMDM to a level similar to that of zymosan (Fig. 1, B and C), which was completely abolished by two structurally unrelated ROS scavengers MnTMPyP (50 µM; Fig. 1C) or EUK-134 (5 µM; Fig. 2C), suggesting that the ROS scavenger MnTMPyP (50 µM) and EUK-134 (5 µM) pretreatment could efficiently and completely prevent LPS-stimulated ROS generation in BMDM.
Fig. 2.
ROS selectively regulated LPS-induced production of PGD2, but not PGE2, in BMDM. Primary cultured WT BMDM were stimulated with 1 μg/ml LPS for detection the production of PGs (16 hours) and ROS using LC-MS-MS or chemiluminescence assay, respectively. The pretreatment of BMDM with the ROS scavenger MnTMPyP not only concentration dependently (1, 5, and 50 µM, 0.5 hour) prevented the LPS-induced ROS generation (n = 8) (A), but also the LPS-induced (n = 9) production of PGD2, but not PGE2 (B). Similarly, another structurally unrelated ROS scavenger EUK-134 also concentration dependently (0.5, 2, and 5 µM, 0.5 hour, n = 3) prevented LPS-induced ROS generation (C) and the production of PGD2, but not PGE2, in BMDM (D). Conversely, coincubation of BMDM with (0.3 mM, n = 4) enhanced LPS-induced production of PGD2, but not PGE2, in BMDM, whereas H2O2 treatment alone (16 hours) does not increase PGs production in BMDM (E). In contrast, COX-2-selective inhibitor NS-398 pretreatment (0.5 hour) concentration dependently (0.001 to 0.1 μM), but nonselectively inhibited both LPS-induced PGE2 and PGD2 production in BMDM to the same extent at each tested concentration (F).
ROS Regulated PGD2 Production in BMDM.
We previously reported that LPS induced both PGD2 and PGE2 production in macrophages via TLR4 pathway (Park and Christman, 2006; Xiao et al., 2012). In the current studies, we found that pretreatment of BMDM with the ROS scavenger MnTMPyP not only concentration dependently attenuated the LPS-stimulated ROS generation (Fig. 2A), but also significantly and selectively decreased LPS-induced PGD2 production by about 85% at 50 µM (Fig. 2B), whereas the LPS-induced PGE2 production was not affected (Fig. 2B). Similarly, pretreatment of BMDM with another structurally unrelated ROS scavenger EUK-134 also concentration dependently (0.5, 2, 5 µM) attenuated the LPS-stimulated ROS generation (Fig. 2C) and production of PGD2, but not PGE2 (Fig. 2D). Conversely, when increasing the overall cellular ROS level by directly adding H2O2 (0.3 mM) in BMDM, the LPS-induced production of PGD2, but not PGE2, was selectively and significantly enhanced by over 65%. In contrast, H2O2 treatment alone could not induce any detectable PGs production in BMDM (Fig. 2E). When BMDM were pretreated with a COX-2-selective inhibitor NS-398 (0.5 hours), LPS induced both PGE2 and PGD2 production were concentration dependently (0.001 to 0.1 μM), but nonselectively inhibited to the same extent at each tested concentration (Fig. 2F). This result is completely different from our above observation using ROS scavengers or H2O2 (only production of PGD2, but not PGE2, was affected), suggesting that the observed ROS effect on PG production is not likely acting on the COX-2 enzyme.
NOX Regulated PGD2 Production in BMDM.
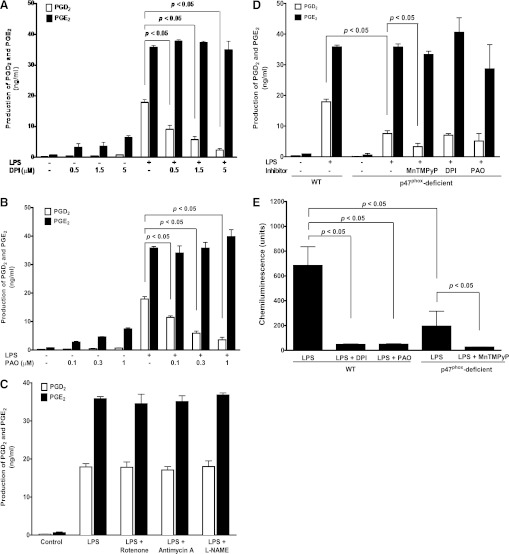
To further investigate the potential ROS source in BMDM, we next used a series of selective inhibitors targeting various reported potential ROS generating systems in our studies, including NOX (DPI and PAO), METC (rotenone and antimycin A), and NOS (l-NAME). Among the above pharmacological inhibitors, both DPI (0.5, 1.5, 5 µM; Fig. 3A) and PAO (0.1, 0.3, 1 µM; Fig. 3B) pretreatment of BMDM concentration dependently inhibited LPS-induced production of PGD2, but not PGE2, similar to that seen with the MnTMPyP or EUK-134 pretreatment. The LPS-induced PGD2 production was reduced by about 87% with 5 µM DPI pretreatment (Fig. 3A), whereas 1 µM PAO inhibited LPS-induced PGD2 production by around 80% (Fig. 3B). Because DPI may also inhibit other potential ROS-generating enzymes containing flavin protein (i.e., NOS or mitochondrial enzymes), we next tested the effects of selective inhibitors for NOS or mitochondria on LPS-induced PGD2 production. In contrast, pretreatment of BMDM with mitochondrial inhibitor rotenone (1 µM) and antimycin A (1 µM) or the NOS inhibitor l-NAME (1 mM) had no effect on LPS-induced production of either PGD2 or PGE2 (Fig. 3C), thus excluding the possibility of mitochondria and NOS as the potential ROS generators in BMDM regulating LPS-stimulated PGD2 production. Taken together, these data suggested that NOX, but not mitochondria or NOS, was the potential ROS generator in response to the LPS treatment in mouse BMDM.
Fig. 3.
NOX-generated ROS selectively regulated LPS-induced production of PGD2, but not PGE2, in BMDM. Primary cultured BMDM from either WT or p47phox-deficient mice were treated with 1 μg/ml LPS for 16 hours, and the concentrations of PGD2 and PGE2 in culture medium were determined using LC-MS-MS. (A-C) LPS-induced production of PGD2, but not PGE2, in WT BMDM was concentration dependently inhibited by NOX inhibitors DPI (0.5, 1.5, and 5 μM, n = 10; A) or PAO (0.1, 0.3, and 1 μM, n = 12; B), but not affected by METC inhibitors rotenone (1 μM, n = 3; C) and antimycin A (1 μM, n = 3; C), or NOS inhibitor l-NAME (1 mM, n = 3; C). (D) LPS-induced production of PGD2, but not PGE2, in p47phox-deficient BMDM was significantly decreased compared with that in WT BMDM (n = 4). The LPS-induced production of PGD2, but not PGE2, was selectively further inhibited by MnTMPyP (50 μM, n = 5) in p47phox-deficient BMDM, whereas DPI (0.5 μM) or PAO (0.1 μM) did not affect the production of either PGD2 or PGE2 in p47phox-deficient BMDM. (E) inhibition of NOX by pretreatment of WT BMDM with DPI (0.5 µM, 0.5 hours, n = 6), PAO (0.1 µM, 0.5 hour, n = 6), or using p47phox-deficient BMDM (n = 5) attenuated LPS-induced ROS generation as measured by chemiluminescence assay. LPS-induced low-level ROS generation in p47phox-deficient BMDM was further prevented by MnTMPyP pretreatment (50 μM, 0.5 hour, n = 5).
To confirm our above finding using pharmacological inhibitors, we next used a molecular approach to test our hypothesis in our studies. NOX is a multisubunit enzyme that requires the presence of a critical cytosolic subunit p47phox for its enzyme activity of ROS generation. The p47phox-deficient transgenic mice showed significantly attenuated ability of NOX-mediated ROS production in neutrophils (Bäumer et al., 2008; Leto et al., 2009). Similar to the above inhibitory effects observed with MnTMPyP, EUK-134, DPI, and PAO, the LPS-induced production of PGD2, but not PGE2, was significantly lowered by about 60% in BMDM from the p47phox-deficient mice compared with that from the WT mice, whereas DPI and PAO showed no further inhibitory effect on LPS-induced PGD2 production in p47phox-deficient BMDM (Fig. 3D). This result from the p47phox-deficient transgenic mouse confirmed that NOX/p47phox was involved in regulating LPS-induced PGD2 production.
Both DPI and PAO inhibited the LPS-induced ROS generation in WT BMDM (Fig. 3E), confirming their inhibitory effects on NOX-mediated ROS production in response to LPS stimulation. The LPS-induced ROS generation in p47phox-deficient BMDM decreased by about 70% (Fig. 3E), indicating that in response to LPS stimulation, the p47phox-deficient BMDM still could generate a lower but yet significant amount of ROS, which was completely abolished by the ROS scavenger MnTMPyP (Fig. 3E). Similarly, the LPS-induced production of PGD2, but not PGE2, was attenuated by about 60% in p47phox-deficient BMDM (Fig. 3D).
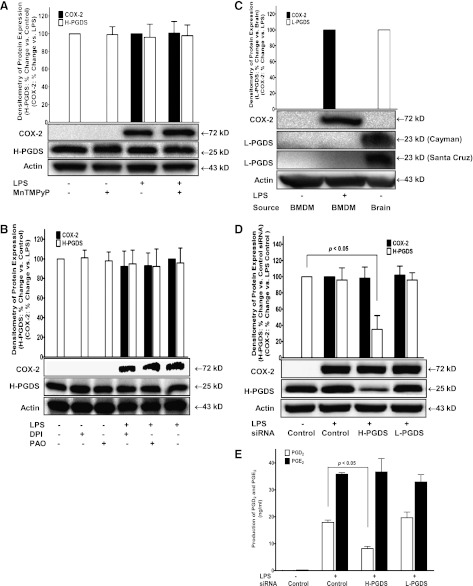
H-PGDS Mediates LPS-Induced PGD2 Production in BMDM.
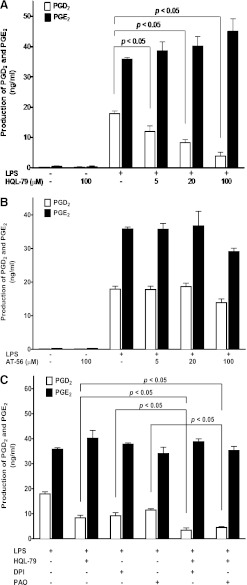
LPS stimulation is known to generate PGs in BMDM via the expression and activation of COX-2 enzyme (Park and Christman, 2006; Xiao et al., 2012). Two isoforms of PGD synthases have been reported, namely H-PGDS and L-PGDS (Matsushita et al., 1998a,b; Irikura et al., 2009). Our data showed that pretreatment of BMDM with MnTMPyP (50 µM; Fig. 4A), DPI (5 µM), or PAO (1 µM; Fig. 4B) had no effect on either H-PGDS or LPS-induced COX-2 expression, suggesting that the inhibitory effect of MnTMPyP, DPI, and PAO on PGD2 production was not via the inhibition of COX-2 or H-PGDS enzyme expression. In addition, using two different isoform-selective antibodies targeting for mouse L-PGDS isomerase with the mouse brain tissue as a positive control, our Western blot results indicated no detectable L-PGDS protein expression in mouse BMDM (Fig. 4C), suggesting that L-PGDS was not likely to be the PGDS isoform that mediated LPS-induced PGD2 production. To further identify the PGDS isomerases involved in this signaling pathway, siRNAs for both H-PGDS and L-PGDS, H-PGDS-selective inhibitor HQL-79, and L-PGDS-selective inhibitor AT-56 were used. Selective inhibition of H-PGDS protein expression using its siRNA (Fig. 4D) significantly attenuated LPS-induced PGD2 production by about 55% in BMDM (Fig. 4E), whereas siRNA for L-PGDS did not show any inhibitory effect on LPS-induced PGD2 production (Fig. 4E). Additionally, the H-PGDS-selective inhibitor HQL-79 concentration dependently (5, 20, 100 µM) attenuated LPS-induced PGD2 production in BMDM (Fig. 5A), whereas the L-PGDS-selective inhibitor AT-56 (5, 20, 100 µM) had no such effect (Fig. 5B). Furthermore, additive effects on the inhibition of LPS-induced PGD2 production were observed after co-pretreatment of BMDM with HQL-79 (20 µM) and DPI (0.5 µM) or PAO (0.1 µM; Fig. 5C), suggesting the roles of both NOX and H-PGDS in regulation of LPS-induced PGD2 production. Taken together, our data suggested that NOX regulated the enzymatic function of H-PGDS that mediated LPS-induced PGD2 production in mouse BMDM.
Fig. 4.
H-PGDS, but not L-PGDS, mediated LPS-induced PGD2 production in BMDM. (A and B) primary cultured WT BMDM were treated with LPS (1 μg/ml) with or without ROS/NOX inhibitors pretreatment (0.5 hour) including 50 µM MnTMPyP (A), 1.5 µM DPI (B), and 1 µM PAO (B). The protein expressions of COX-2 and H-PGDS in BMDM were determined by Western blot using actin as protein loading control. LPS treatment did not change the protein expression of H-PGDS, but significantly induced COX-2 expression (n = 3). LPS-induced expressions of COX-2 and H-PGDS were not affected by any of the above inhibitors. (C) mouse brain tissue lysate was used as a positive control for L-PGDS protein expression that was detected using two different antibodies (Cayman and Santa Cruz) specific for mouse L-PGDS. In contrast, the expression of L-PGDS protein was not detectable in mouse BMDM. (D and E) primary cultured WT BMDM were transfected with either control siRNA or siRNA for H- or L-PGDS for 48 hours prior to LPS treatment. The concentrations of PGD2 and PGE2 were determined using LC-MS-MS. The protein expression of H-PGDS by Western blot (D) and LPS-induced PGD2 production (E) were selectively attenuated by H-PGDS siRNA (n = 3), but not by L-PGDS siRNA (n = 2), whereas siRNA for either H- or L-PGDS had no effect on LPS-induced PGE2 production. Densitometric quantification of the relative protein expression of COX-2, H-PGDS, or L-PGDS (all normalized to its actin expression) determined by Western blots were also shown in A-D.
Fig. 5.
The H-PGDS-selective inhibitor HQL-79, but not the L-PGDS-selective inhibitor AT-56, attenuated LPS-induced PGD2 production in BMDM. Primary cultured WT BMDM were treated with 1 μg/ml LPS for 16 hours in the presence of various concentrations of HQL-79 (A) or AT-56 (B). The concentrations of PGD2 and PGE2 were determined using LC-MS-MS. (A) HQL-79 concentration dependently (5, 20, and 100 μM) inhibited LPS-induced production of PGD2, but not PGE2, in BMDM (n = 6); (B) AT-56 did not affect LPS-induced either PGD2 or PGE2 production in BMDM (n = 3); (C) co-pretreatment (0.5 hour) of WT BMDM with HQL-79 (20 µM) and either DPI (0.5 µM) or PAO (0.1 µM) showed additive and selective inhibitory effects on LPS-induced production of PGD2, but not PGE2, in BMDM (n = 3).
ROS Directly Regulate H-PGDS-Mediated PGD2 Production In Vitro.
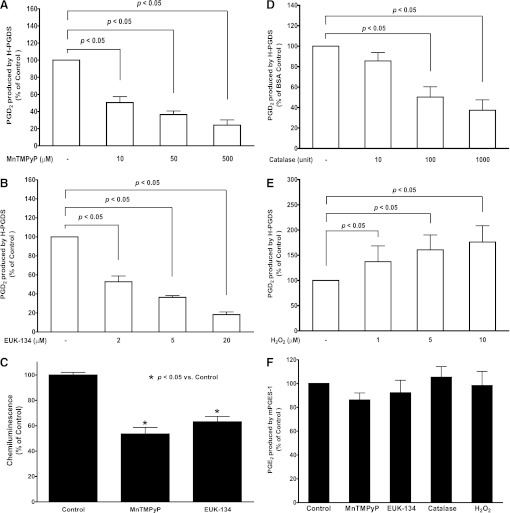
To test if ROS could directly regulate H-PGDS-mediated PGD2 production in vitro, ROS scavenger MnTMPyP, EUK-134, catalase, and H2O2 were used in our in vitro cell-free enzymatic assay. Coincubation of recombinant enzyme H-PGDS and its substrate PGH2 with either MnTMPyP (10, 50, 500 µM; Fig. 6A), EUK-134 (2, 5, 20 µM; Fig. 6B), or catalase (10, 100, 1000 units; Fig. 6D) showed concentration-dependent attenuation of H-PGDS-mediated PGD2 production, whereas coincubation with H2O2 (1, 5, 10 µM) significantly promoted H-PGDS-mediated PGD2 production (Fig. 6E). In contrast, coincubation of recombinant enzyme mPGES-1 and its substrate PGH2 with MnTMPyP, EUK-134, catalase, or H2O2 showed no significant effect on PGE2 production under the same experimental conditions in vitro (Fig. 6F). Because there is no other cytosolic factors or enzymes present in this simple cell-free reaction system except for the recombinant isomerase (i.e., either H-PGDS or mPGES-1) and its substrate PGH2 in PBS buffer, these in vitro cell-free enzyme assay results confirmed that the ROS level or redox state surrounding the H-PGDS enzyme directly affect its enzymatic capability of PGD2 production. Unlike mPGES-1, an adequate basal level of ROS is required for H-PGDS activity of PGD2 production in vitro. To strengthen our conclusion, we further determined the basal oxidant level in the above enzyme reaction mixture, which showed significant decrease of ROS signals (i.e., superoxide) when MnTMPyP or EUK-134 was added into the reaction mixture (Fig. 6C), suggesting the changes of ROS level or redox state in the reaction mixture by ROS scavengers.
Fig. 6.
ROS directly regulated H-PGDS-mediated PGD2 production in cell-free enzymatic assay in vitro. Recombinant enzymes H-PGDS (0.1 unit) or mPGES-1 (3 units) were preincubated with MnTMPyP, EUK-134, catalase, H2O2, or equal volume of vehicle for 10 minutes at 37°C in test tubes in vitro prior to the addition of the enzyme substrate PGH2 (2 μM). The concentrations of PGD2 and PGE2 in each sample buffer were determined after the reaction using LC-MS-MS. Coincubation of H-PGDS with either MnTMPyP (n = 8; A), EUK-134 (n = 3; B), or catalase (n = 7; D) attenuated H-PGDS-mediated PGD2 production in a concentration dependent manner, whereas coincubation with H2O2 (n = 4; E) concentration dependently enhanced H-PGDS-mediated PGD2 production. In contrast, mPGES-1-mediated PGE2 production was not affected by coincubation with MnTMPyP (50 μM), EUK-134 (5 μM), catalase (100 units), or H2O2 (10 μM, n = 3; F). The basal oxidant level in the reaction mixture was also determined using isoluminol-enhanced chemiluminescence assay. Either MnTMPyP (50 μM) or EUK-134 (5 μM) significantly decreased the basal oxidant levels in the reaction mixture (n = 3; C).
Discussion
In our studies, we showed that inhibition of LPS-stimulated ROS production in BMDM also selectively inhibited the production of PGD2, but not its isomer PGE2. LPS-induced PGD2 production in BMDM was mediated via H-PGDS isomerase, but not L-PGDS. LPS-induced H-PGDS-mediated PGD2 production was sensitive to and dependent on the NOX-generated ROS in BMDM. In contrast, the LPS-induced PGE2 production in BMDM was ROS independent.
To our knowledge, this is the first report of the role of ROS in differential regulation of LPS-induced PGD2 and PGE2 production. The novel finding of our study is that the modulation of intracellular ROS levels in macrophages could selectively regulate LPS-induced production of PGD2, but not PGE2. Therefore, it is impossible that the ROS or any NOX/ROS inhibitors exert their selective effects on PGD2 production via the modification of COX-2 enzyme in BMDM. If there is any modifications of the COX-2 protein expression or its enzyme activity by the above inhibitors or H2O2, the production of both PGE2 and PGD2 would accordingly change uniformly in the same direction as shown in Fig. 2F with the COX-2 selective inhibitor NS-398, but not unilaterally with only one product PGD2, as COX-2 is the common upstream rate-limiting PGs synthase for both PGE2 and PGD2. Thus this ROS effect must occur further downstream from the COX-2 enzyme and is only specific for the PGD2 signaling pathway. Although previous reports have not shown a consensus on whether ROS could regulate COX-2 expression in different cell types (Feng et al., 1995), in our hands, LPS-induced ROS production clearly had no inhibitory effect on either COX-2 protein expression or its enzyme activity in BMDM as shown by the unaffected downstream product PGE2 generation.
Previous reports indicated that ROS generated from NOX played important roles in regulating the expression of several proinflammatory genes in macrophages (Sanlioglu et al., 2001; Hsu and Wen, 2002). In our studies, both NOX inhibitors DPI and PAO significantly attenuated the LPS-induced production of PGD2, but not PGE2, suggesting the role of NOX as the ROS generator in response to LPS stimulation in WT BMDM. Although both DPI and PAO are known to have other NOX-unrelated potential side effects in cells (i.e., inhibition of flavin proteins by DPI or proteases by PAO), the inhibitory effect of DPI on ROS and PGD2 productions in BMDM was not likely achieved via nonspecific inhibition of other flavin-containing ROS generators because the METC inhibitors rotenone, antimycin A, and NOS inhibitor l-NAME showed absolutely no effect on LPS-induced PGs production, thus eliminating the possibility of mitochondria or NOS as potential ROS generators in this signaling pathway. Furthermore, the LPS-induced production of ROS and PGD2, but not PGE2, in p47phox-deficient BMDM was also significantly attenuated compared with that in WT BMDM, confirming the role of NOX/p47phox in LPS-induced production of ROS and PGD2. Taken together, our data from DPI, PAO, p47phox-deficient BMDM and the negative results from METC and NOS inhibitors, collectively confirmed the critical role of NOX as the main ROS source in BMDM in response to LPS stimulation.
Interestingly, although the LPS-induced production of PGD2 and ROS was significantly attenuated in p47phox-deficient BMDM, the production of neither PGD2 nor ROS was completely prevented and was further attenuated by MnTMPyP pretreatment. It was reported that the lack of p47phox subunit is sufficient to inactivate the p47phox-dependent NOX2 isoform enzyme activity in neutrophils. However, we found that there was still a low level of LPS-induced ROS and PGD2 production in p47phox-deficient BMDM, suggesting that unlike neutrophils, other non-p47phox-dependent and low-level expression NOX isoforms (i.e., NOX1 or NOX4; L. Xiao's laboratory unpublished data) or other potential ROS-generating systems in macrophages might compensate for the loss of NOX2/p47phox system and contribute to the residual ROS production in p47phox-deficient BMDM.
To understand the ROS-dependent signaling mechanism of PGD2 generation, we first identified the PGDS isoform(s) that mediated LPS-induced PGD2 production. Between the two cloned PGDS isoforms, L-PGDS was not detected in mouse BMDM with or without LPS stimulation, and inhibition of L-PGDS by either AT-56 or its siRNA failed to prevent LPS-induced PGD2 production in BMDM. Therefore, the potential role of L-PGDS in LPS-induced PGD2 production in BMDM is eliminated. In contrast, we found strong H-PGDS expression in BMDM, and H-PGDS-selective inhibitor HQL-79 or its siRNA significantly attenuated LPS-induced PGD2 production. In addition, co-pretreatment of BMDM with HQL-79 and either DPI or PAO showed additive inhibitory effects on LPS-induced PGD2 production, suggesting that NOX-regulated LPS-induced PGD2 production via H-PGDS isoform. Taken together, these data confirmed that H-PGDS mediated LPS-induced PGD2 production in BMDM.
To determine the mechanism of ROS regulation on H-PGDS, an in vitro cell-free enzyme assay was conducted. Scavenging ROS in the H-PGDS reaction system by either SOD/catalase mimetics MnTMPyP and EUK-134 or catalase concentration dependently attenuated H-PGDS-mediated PGD2 production, which was significantly enhanced by the addition of H2O2. In contrast, these reagents have no inhibitory effect on PGE2 production mediated by mPGES-1. These results indicated that the ROS levels could directly affect the in vitro enzyme activity of H-PGDS, but not mPGES-1, and a certain amount of ROS is required to maintain H-PGDS enzyme activity. We showed that MnTMPyP or EUK-134 could further decrease the ROS level in the enzyme reaction mixture, confirming the presence of a basal level of oxidants (i.e., superoxide) in this enzyme reaction solution. These enzyme assay data strongly supported our findings that intracellular ROS regulate LPS-induced PGD2 production of via H-PGDS, and a basal level of ROS is required to maintain H-PGDS enzyme activity.
Although our enzyme assay results confirmed that ROS could directly modify H-PGDS enzyme activity in vitro, the precise and complete molecular mechanism of ROS regulation on H-PGDS-mediated PGD2 production in macrophages is still not entirely defined. Our data did not completely exclude the possibility that ROS may also work through other intracellular signaling intermediates (e.g., kinases) to modify H-PGDS enzyme activity in macrophages in addition to the above confirmed mechanism of direct ROS interaction with H-PGDS. The potential mechanisms of ROS regulate H-PGDS-mediated, but not L-PGDS-mediated, PGD2 production in BMDM may result from the differences between H-PGDS and L-PGDS as follows. (1) The enzyme activity of H-PGDS is glutathione (GSH) dependent, whereas L-PGDS enzyme activity does not require GSH (Urade and Eguchi, 2002). GSH is a well known antioxidant protein with its thiol groups acting as reducing agents, preventing damage to important cellular enzymes or components caused by ROS. GSH reduces disulfide bonds formed within cytoplasmic proteins to cysteines and is thus converted to its oxidized form glutathione disulfide (GSSG), which can be reduced back to GSH by glutathione reductase, using NADPH as an electron donor. It is possible that the NOX inhibitors or ROS scavengers changed the cytosolic ratio of GSH/GSSG and thus affected the enzyme activity of H-PGDS. (2) H-PGDS and L-PGDS may have different sensitivities to redox environment. Because the L-PGDS is not detectable in our system, thus the different sensitivities of the two isomerases to redox environment are not likely to be the main mechanism of ROS-regulated PGD2 production in BMDM. (3) ROS may facilitate the formation of a hydrogen bond that is a required for H-PGDS activation (Uchida et al., 2010). Our in vitro enzyme assay results strongly suggested the critical role of ROS-(H-PGDS) interaction in modulation of H-PGDS activity, suggesting ROS may serve as an indispensable cofactor for maintaining the normal enzyme configuration and activity of H-PGDS, whereas depletion of ROS would decrease or abolish its enzyme activity.
In our studies, MnTMPyP (50 µM) inhibited PGD2 production more efficiently in BMDM (∼85% inhibition) compared with that from the cell-free enzyme assay (∼60% inhibition). This could be explained by the nonenzymatic conversion of PGH2 in aqueous solution. We recently reported the spontaneous conversion of PGH2 to PGE2 (42.7%) and PGD2 (24.2%) without the presence of PGES or PGDS enzymes in the cell-free enzyme assay (Yu et al., 2011). These findings suggested that unlike in macrophages, a large portion of PGD2 was spontaneously converted from PGH2 independent of H-PGDS enzyme activity in this cell-free enzyme assay and thus would not be affected by the changes of its surrounding ROS levels (i.e., MnTMPyP concentrations). Currently, it is still unclear if the spontaneous conversion of PGH2 to PGD2 in vitro also occurs in live cells (i.e., BMDM). However, this observation could explain why MnTMPyP reduced the PGD2 production by 60% in the cell-free enzyme assay, but more efficiently blocked PGD2 production in BMDM.
In conclusion, our results, for the first time, showed that LPS-induced PGD2 production in BMDM was mediated by H-PGDS that required NOX-derived ROS to maintain its proper enzymatic function. ROS could directly modulate the H-PGDS enzyme activity of PGD2 production in vitro, but not the mPGES-1 enzyme activity of PGE2 production. Our findings not only illustrate the critical role of intracellular ROS in differential regulation of PGs production in macrophages, but also implicate a potential new therapeutic strategy in selectively regulating PGD2 production in the treatment of PGs-involved diseases by regulating the intracellular ROS levels.
Acknowledgments
The authors thank Dr. Tong Zhou for help in reviewing the statistical analysis of our data and Dr. Sherene Thomas and Jean Fitzpatrick for technical support and assistance on this project.
Abbreviations
- AT-56
4-(5H-dibenzo[a,d]cyclohepten-5-ylidene)-1-[4-(2H-tetrazol-5-yl)butyl]-piperidine
- BHT
butylated hydroxytoluene
- BMDM
bone marrow-derived macrophages
- COX
cyclooxygenase
- cPGES
cytosolic PGE synthase
- DMEM
Dulbecco’s modified Eagle’s medium
- DPI
diphenylene iodonium
- EUK-134
chloro[[2,2'-[1, 2-ethanediylbis[(nitrilo-κN)methylidyne]]bis[6-methoxyphenolato-κO]]]-manganese
- FBS
fetal bovine serum
- H-PGDS
hematopoietic PGD synthase
- HPLC
high-performance liquid chromatography
- HQL-79
4-(diphenylmethoxy)-1-[3-(1H-tetrazol-5-yl)]propyl-piperidine
- LC-MS-MS
liquid chromatography-tandem mass spectrometry
- l-NAME
l-NG-nitroarginine methyl ester
- L-PGDS
lipocalin PGD synthase
- LPS
lipopolysaccharide
- METC
mitochondrial electron transport chain
- MnTMPyP
Mn(III)-terakis-(1-methyl-4-pyridyl)-porphyrin pentachloride
- mPGES-1
microsomal PGE synthase-1
- NOS
nitric oxide synthase
- NOX
NADPH oxidase
- PAO
phenylarsine oxide
- PG
prostaglandin
- PVDF
polyvinylidene difuoride
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- SRM
selected reaction monitoring
- TLR4
Toll-like receptor 4
- zymosan
zymosan A
Authorship Contributions
Participated in research design: Zhao G, Yu, Zhao Q, Xiao.
Conducted experiments: Zhao G, Yu, Deng, Li, Xiao.
Contributed new reagents or analytic tools: Christman, Zhao Q, Joo, van Breemen, Xiao.
Performed data analysis: Zhao G, Yu, Xiao.
Wrote or contributed to the writing of the manuscript: Yu, Zhao G, van Breemen, Zhao Q, Christman, Xiao.
Footnotes
This work was supported in part by National Institutes of Health [Grants 1R01-HL083218, 3R01-HL083218-01A2S1, P50-AT000155, and 5R01-HL075557] to L.X., R.B.v.B., or J.W.C.; Merit Review [Grant 1I01BX000108] to J.W.C. from Jesse Brown VA Medical Center; Campus Research Board [Grant S06-118] and Faculty Scholarship Support Grants to L.X. from the University of Illinois at Chicago.
References
- Amin JK, Xiao L, Pimental DR, Pagano PJ, Singh K, Sawyer DB, Colucci WS. (2001) Reactive oxygen species mediate alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol 33:131–139 [DOI] [PubMed] [Google Scholar]
- Azim AC, Cao H, Gao X, Joo M, Malik AB, van Breemen RB, Sadikot RT, Park GY, Christman JW. (2007) Regulation of cyclooxygenase-2 expression by small GTPase Rac2 in bone marrow macrophages. Am J Physiol Lung Cell Mol Physiol 293:L668–L673 [DOI] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. (2005) Mitochondria, oxidants, and aging. Cell 120:483–495 [DOI] [PubMed] [Google Scholar]
- Bäumer AT, Ten Freyhaus H, Sauer H, Wartenberg M, Kappert K, Schnabel P, Konkol C, Hescheler J, Vantler M, Rosenkranz S. (2008) Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem 283:7864–7876 [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313 [DOI] [PubMed] [Google Scholar]
- Benhar M, Engelberg D, Levitzki A. (2002) ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep 3:420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramble LH, Anderson RS. (1998) A comparison of the chemiluminescent response of Crassostrea virginica and Morone saxatilis phagocytes to zymosan and viable Listonella anguillarum. Dev Comp Immunol 22:55–61 [DOI] [PubMed] [Google Scholar]
- Burleigh ME, Babaev VR, Oates JA, Harris RC, Gautam S, Riendeau D, Marnett LJ, Morrow JD, Fazio S, Linton MF. (2002) Cyclooxygenase-2 promotes early atherosclerotic lesion formation in LDL receptor-deficient mice. Circulation 105:1816–1823 [DOI] [PubMed] [Google Scholar]
- Cao H, Xiao L, Park GY, Wang X, Azim AC, Christman JW, van Breemen RB. (2008) An improved LC-MS/MS method for the quantification of prostaglandins E(2) and D(2) production in biological fluids. Anal Biochem 372:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Yu R, Choi Y, et al. (2010) Discovery of cyclooxygenase inhibitors from medicinal plants used to treat inflammation. Pharmacol Res 61:519–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Yu R, Tao Y, Nikolic D, van Breemen RB. (2011) Measurement of cyclooxygenase inhibition using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 54:230–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AR, Segal AW. (2004) The NADPH oxidase of professional phagocytes—prototype of the NOX electron transport chain systems. Biochim Biophys Acta 1657:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C, Karlsson A. (1999) Respiratory burst in human neutrophils. J Immunol Methods 232:3–14 [DOI] [PubMed] [Google Scholar]
- Deschacht M, Horemans T, Martinet W, Bult H, Maes L, Cos P. (2010) Comparative EPR study of different macrophage types stimulated for superoxide and nitric oxide production. Free Radic Res 44:763–772 [DOI] [PubMed] [Google Scholar]
- Feng L, Xia Y, Garcia GE, Hwang D, Wilson CB. (1995) Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-alpha, and lipopolysaccharide. J Clin Invest 95:1669–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CD. (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294:1871–1875 [DOI] [PubMed] [Google Scholar]
- Geiszt M, Leto TL. (2004) The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem 279:51715–51718 [DOI] [PubMed] [Google Scholar]
- Henricks PAJ, Nijkamp FP. (2001) Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther 14:409–420 [DOI] [PubMed] [Google Scholar]
- Hsu HY, Wen MH. (2002) Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem 277:22131–22139 [DOI] [PubMed] [Google Scholar]
- Irikura D, Aritake K, Nagata N, Maruyama T, Shimamoto S, Urade Y. (2009) Biochemical, functional, and pharmacological characterization of AT-56, an orally active and selective inhibitor of lipocalin-type prostaglandin D synthase. J Biol Chem 284:7623–7630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. (2009) Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal 11:2535–2552 [DOI] [PubMed] [Google Scholar]
- Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ. (2007) Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem 282:13736–13745 [DOI] [PubMed] [Google Scholar]
- Lambeth JD. (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4:181–189 [DOI] [PubMed] [Google Scholar]
- Leto TL. (1999) Inflammation Basic Principles and Clinical Correlates (Gallin JI, Snyderman R. eds) pp 769–787, Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- Leto TL, Morand S, Hurt D, Ueyama T. (2009) Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal 11:2607–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. (2003) Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 278:8516–8525 [DOI] [PubMed] [Google Scholar]
- Maeda K, Sakonju I, Kanda A, Suzuki T, Kakuta T, Shimamura S, Okano S, Takase K. (2010) Priming effects of lipopolysaccharide and inflammatory cytokines on canine granulocytes. J Vet Med Sci 72:55–60 [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Hirata M, Tanaka H, et al. (2000) Prostaglandin D2 as a mediator of allergic asthma. Science 287:2013–2017 [DOI] [PubMed] [Google Scholar]
- Matsushita N, Aritake K, Takada A, et al. (1998a) Pharmacological studies on the novel antiallergic drug HQL-79: II. Elucidation of mechanisms for antiallergic and antiasthmatic effects. Jpn J Pharmacol 78:11–22 [DOI] [PubMed] [Google Scholar]
- Matsushita N, Hizue M, Aritake K, et al. (1998b) Pharmacological studies on the novel antiallergic drug HQL-79: I. Antiallergic and antiasthmatic effects in various experimental models. Jpn J Pharmacol 78:1–10 [DOI] [PubMed] [Google Scholar]
- McAdam BF, Mardini IA, Habib A, Burke A, Lawson JA, Kapoor S, FitzGerald GA. (2000) Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. J Clin Invest 105:1473–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park GY, Christman JW. (2006) Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol 290:L797–L805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer ZA, Guerra AN, Hill LM, Gavala ML, Prabhu U, Aga M, Hall DJ, Bertics PJ. (2007) Nucleotide receptor signaling in murine macrophages is linked to reactive oxygen species generation. Free Radic Biol Med 42:1506–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y, Doctrow SR, Tocco G, Baudry M. (1999) EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc Natl Acad Sci USA 96:9897–9902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russwurm S, Krause S, Finkelberg L, Rühling K, Schauer U, Lösche W. (1994) Generation of reactive oxygen species and activity of platelet-activating factor acetylhydrolase in human monocyte-derived macrophages. Thromb Res 74:505–514 [DOI] [PubMed] [Google Scholar]
- Samuelsson B, Morgenstern R, Jakobsson PJ. (2007) Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev 59:207–224 [DOI] [PubMed] [Google Scholar]
- Sanlioglu S, Williams CM, Samavati L, Butler NS, Wang G, McCray PB, Jr, Ritchie TC, Hunninghake GW, Zandi E, Engelhardt JF. (2001) Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J Biol Chem 276:30188–30198 [DOI] [PubMed] [Google Scholar]
- Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. (2001) A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem 276:1417–1423 [DOI] [PubMed] [Google Scholar]
- Szefler SJ, Norton CE, Ball B, Gross JM, Aida Y, Pabst MJ. (1989) IFN-gamma and LPS overcome glucocorticoid inhibition of priming for superoxide release in human monocytes. Evidence that secretion of IL-1 and tumor necrosis factor-alpha is not essential for monocyte priming. J Immunol 142:3985–3992 [PubMed] [Google Scholar]
- Uchida Y, Urade Y, Mori S, Kohzuma T. (2010) UV resonance Raman studies on the activation mechanism of human hematopoietic prostaglandin D(2) synthase by a divalent cation, Mg(2+). J Inorg Biochem 104:331–340 [DOI] [PubMed] [Google Scholar]
- Urade Y, Eguchi N. (2002) Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostaglandins Other Lipid Mediat 68-69:375–382 [DOI] [PubMed] [Google Scholar]
- Xia Y, Tsai AL, Berka V, Zweier JL. (1998) Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem 273:25804–25808 [DOI] [PubMed] [Google Scholar]
- Xiao L, Ornatowska M, Zhao G, Cao H, Yu R, Deng J, Li Y, Zhao Q, Sadikot R, Christman JW. (2012) Lipopolysaccharide-induced expression of microsomal prostaglandin E synthase-1 mediates late-phase PGE2 production in bone marrow derived macrophages. PLoS One, doi:10.1371/journal.pone.0050244, In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB. (2002) Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol 282:C926–C934 [DOI] [PubMed] [Google Scholar]
- Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke EH, Lenardo M. (2006) Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA 103:4952–4957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Xiao L, Zhao G, Christman JW, van Breemen RB. (2011) Competitive enzymatic interactions determine the relative amounts of prostaglandins E2 and D2. J Pharmacol Exp Ther 339:716–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Thomas S, Kowalsky G, Christman JW, Qian F, Levitan I, Deng J, Xiao L. (2010) Lipopolysaccharide stimulation up-regulates the expression of NADPH oxidase in mouse bone marrow-derived macrophages (Abstract). Am J Respir Crit Care Med 181:A1277 [Google Scholar]
- Zhao MM, Xu MJ, Cai Y, Zhao G, Guan Y, Kong W, Tang C, Wang X. (2011) Mitochondrial reactive oxygen species promote p65 nuclear translocation mediating high-phosphate-induced vascular calcification in vitro and in vivo. Kidney Int 79:1071–1079 [DOI] [PubMed] [Google Scholar]