Abstract
4-(2-Butyl-6,7-dichloro-2-cyclopentyl-indan-1-on-5-yl) oxobutyric acid (DCPIB) was identified as the selective blocker of volume-regulated anion channels (VRAC). VRAC are permeable to small inorganic and organic anions, including the excitatory neurotransmitter glutamate. In recent years DCPIB has been increasingly used for probing the physiologic and pathologic roles of VRAC and was found to potently suppress pathologic glutamate release in cerebral ischemia. Because ischemic glutamate release can be mediated by a plethora of mechanisms, in this study we explored the selectivity of DCPIB toward the majority of previously identified glutamate transporters and permeability pathways. l-[3H]glutamate, d-[3H]aspartate, and l-[14C]cystine were used to trace amino acid release and uptake. We found that in addition to its well-characterized effect on VRAC, DCPIB potently inhibited glutamate release via connexin hemichannels and glutamate uptake via the glutamate transporter GLT-1 in rat glial cells. In contrast, DCPIB had no direct effect on vesicular glutamate release from rat brain synaptosomes or the cystine/glutamate exchange in astrocytes. The compound did not affect the astrocytic glutamate transporter GLAST, nor did it block glutamate release via the P2X7/pannexin permeability pathway. The ability of DCPIB to directly block connexin hemichannels was confirmed using a gene-specific siRNA knockdown approach. Overall, our data demonstrate that DCPIB influences several glutamate transport pathways and that its effects on VRAC in vivo should be verified using additional pharmacological controls.
Introduction
The majority of mammalian cells responds to cellular swelling with increases in swelling-activated Cl− currents, ICl,swell, which play critical roles in regulation of cell volume but are also thought to be involved in apoptosis, regulation of membrane potential, and release of physiologically active molecules (Lang et al., 1998; Mongin and Orlov, 2001; Hoffmann et al., 2009). ICl,swell are mediated by the ubiquitously expressed volume-regulated anion channels (VRAC), which are also termed volume-sensitive outwardly rectifying (VSOR) Cl− channels or volume-sensitive organic osmolyte/anion channels (VSOAC) (Strange et al., 1996; Nilius et al., 1997; Okada, 1997). Throughout this manuscript we use the acronym VRAC. Despite extensive research efforts, the molecular nature of VRAC remains unknown, and therefore functional significance of these channels is evaluated by studying the effects of pharmacological inhibitors and correlating physiologic phenomena with macroscopic whole cell Cl− currents (Okada, 2006; Hoffmann et al., 2009). Essentially all commonly used pharmacological inhibitors poorly discriminate between different Cl− channels. However, several years ago the ethacrinic acid derivative DCPIB was found to selectively block swelling-activated Cl− currents (Decher et al., 2001). This compound is now increasingly used for probing the involvement of VRAC in physiologic and pathologic processes (see for example Best et al., 2004; Abdullaev et al., 2006; Harrigan et al., 2008; Rosenberg et al., 2010; Min et al., 2011; Sato et al., 2011).
Recently, DCPIB was found to potently protect brain tissue against experimental ischemic damage in a rat model of middle cerebral artery occlusion (Zhang et al., 2008). The neuroprotective effects of DCPIB and the other less selective VRAC blocker, tamoxifen, were ascribed to inhibition of pathologic glutamate release via VRAC (Feustel et al., 2004; Zhang et al., 2008). Buildup of glutamate in the extracellular space causes excessive activation of neuronal Ca2+-permeable glutamate receptor channels of the NMDA family (N-methyl-d-aspartateis the selective agonist of thesereceptors), leading to cytosolic Ca2+ overload and neuronal death (Choi, 1992). On the basis of potent reductions of ischemic brain damage by DCPIB and tamoxifen, VRAC have been suggested to represent a new and promising therapeutic target in stroke (Kimelberg, 2005; Mongin and Kimelberg, 2005; Mongin, 2007).
It should be noted, however, that pathologic glutamate release in stroke and ischemia is mediated by a number of mechanisms. In addition to opening of VRAC, disruption of transmembrane [Na+] and [K+] gradients causes reversal of glial Na+/K+-dependent glutamate transporters, which unload glutamate to the extracellular space (Szatkowski et al., 1990; Rossi et al., 2000). One of these transporters, GLT-1, represents a major source of pathologic accumulation of glutamate in the ischemic core (Seki et al., 1999). Additionally, results obtained in cellular and ex vivo models suggest that reversal of the cystine/glutamate heteroexchanger (system xC− or xCT), opening of connexin hemichannels, and/or activation of purinergic P2X7 receptor channels may represent alternative routes for ischemic glutamate release (Duan et al., 2003; Ye et al., 2003; Fogal et al., 2007). The cystine/glutamate heteroexchanger releases glutamate to the extracellular space and takes cystine into the cytosol, to sustain synthesis of the major cellular antioxidant glutathione, which protects cells against oxidative stress and promotes cell survival (Bannai, 1986). However, under pathologic circumstances, release of glutamate via this transporter by astrocytes or microglial cells may contribute to neuronal toxicity (Takeuchi et al., 2006; Fogal et al., 2007). Connexin hemichannels are formed by unpaired gap junctions created by membrane proteins of the connexin family. A number of factors, which are related to ischemia and inflammation, such as metabolic depletion and/or oxidative stress, can lead to opening of hemichannels, allowing for release of small cytosolic molecules, including glutamate (reviewed in Orellana et al., 2009). Finally, long-term activation of P2X7 purinergic receptors also leads to opening of large permeability pores capable of releasing glutamate (Duan et al., 2003). This latter process is now thought to be mediated by the P2X7 receptor-dependent activation of the pannexin permeability pathway, formed by pannexin proteins, which have moderate homology to the insect gap junction molecules innexins (Pelegrin and Surprenant, 2006; Iglesias et al., 2009).
On the basis of the facts discussed above and in view of several recent reports of strong pharmacological “cross-sensitivity” between VRAC and connexin hemichannels (Benfenati et al., 2009;Ye et al., 2009), we decided to test if DCPIB discriminates between VRAC and other glutamate release pathways. The effects of DCPIB on several major plasma membrane glutamate transporters and permeability pathways were explored in primary cultures of rat astrocytes and microglial cells. The results presented here are critical for interpretation of the physiologic effects and neuroprotective properties of DCPIB in vivo.
Materials and Methods
Reagents and Antibodies.
DCPIB, dihydrokainic acid (DHK), dl-threo-β-benzyloxyaspartic acid (TBOA), and N-[2-[[2-[(2-hydroxyethyl)amino]ethyl]amino]-5-quinolinyl]-2-tricyclo[3.3.1.13,7]dec-1-ylacetamide dihydrochloride (AZ 10606120 dihydrochloride) were purchased from R&D Systems/Tocris (Ellisville, MO). N-ethylmaleimide (NEM), 18α-glycyrrhetinic acid (18-αGA), l-serine-O-sulfate (l-SOS), dl-homocysteine, l-cystine, and 2′(3′)-O-(4-benzoylbenzoyl)adenosine-5′-triphosphate tri(triethylammonium) salt (BzATP) were purchased from Sigma-Aldrich (St. Louis, MO). Acivicin (α-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). l-[3H]glutamate, d-[3H]aspartate, and l-[14C]cystine were purchased from PerkinElmer/New England Nuclear (Boston, MA). Rabbit polyclonal antibody to Connexin-43 (cat. # C6219) was obtained from Sigma-Aldrich; monoclonal mouse antibody to actin (cat. # ab3280) and mouse monoclonal antibody against human Bestrophin-1 (cat. # ab2182) were purchased from AbCam (Cambridge, MA). Three gene-specific siRNA constructs targeting rat connexin-43 mRNA were from Qiagen-USA (Valencia, CA). The target sequences were 5′-AACAGTGCACATGTAACTAAT-3′ (Rn_Gja1_1, annotated within the text as siCx43-1), 5′-CAGGTAAGCTTCCCTGGTCTA-3′ (Rn_Gja1_5, annotated siCx43-5), and 5′-TGCCGCAATTACAACAAGCAA-3′ (Rn_Gja1_7, annotated siCx43-7). As a negative control we used Alexa-488 conjugated AllStars negative control siRNA from Qiagen (cat # 1027292).
Primary Cultures of Glial Cells.
Primary cultures of rat astrocytes and microglial cells were prepared from neonatal cortical tissue as previously described (Hyzinski-Garcia et al., 2011). All animal procedures in this work were carried in adherence to the Guide for the Care and Use of Laboratory Animals as adopted by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Albany Medical College. One-day-old Sprague-Dawley rat pups were euthanized by rapid decapitation. The cerebral cortices were dissected from the meninges, hippocampi, and basal ganglia. Cells were extracted from minced tissue using three incubations in a solution containing a 1:1 mix of the recombinant protease TrypLE at 37°C (Invitrogen, Carlsbad, CA) and optimized minimal essential medium (OptiMEM, Invitrogen) supplemented with bovine pancreatic DNase I (1 mg/ml, Sigma). Dissociated cells collected from the second and the third extractions were transferred into minimal essential medium (MEM) containing 10% heat-inactivated horse serum (HIHS) and 50 U/ml penicillin plus 50 µg/ml streptomycin (Pen/Strep). Cells were plated on poly-l-lysine coated T75 culture flasks (Techno Plastic Products, TPP, Trasadingen, Switzerland) at a density of 250,000 cells/flask for pure astroglial cultures or 1–1.3 million cells/flask to produce mixed glial cultures. Cultures were grown to confluence for 2–3 weeks in MEM/HIHS + Pen/Strep at 37°C in a humidified atmosphere of 5% CO2/95% air. Astrocytes were removed from substrate by treating a confluent monolayer with TrypLE, replated, and used for glutamate transport experiments as described below. Microglial cells were purified from mixed glial cultures by 5–15 minutes shaking on a titer plate shaker, replated, and used for glutamate transport experiments within 24 hours as described below. Purity of cell cultures was verified using immunostaining with monoclonal antibodies for the astrocyte marker glial fibrillary acidic protein (GFAP) and the microglial marker OX-42 (CD11b). These procedures confirmed that astrocytic cultures were of more than 95% purity, and microglial cultures were more than 98% pure.
Preparation of Rat Brain Synaptosomes.
Presynaptic nerve endings (synaptosomes) were prepared from the forebrains of male Sprague-Dawley rats (150–180 g) according to the method of Hajos (1975) with modifications described elsewhere (Rudkouskaya et al., 2010). Forebrain tissue was homogenized by hand in 0.32 M sucrose containing 5 mM HEPES (pH 7.4) using a Potter-Elvehjem tissue grinder with Teflon pestle. Synaptosomes were isolated by centrifugation using several sucrose density gradients. Final synaptosomal pellets were resuspended at a concentration of ∼2 mg protein/ml in basal medium containing (in mM): 135 NaCl, 3.8 KCl, 1.2 MgSO4, 1.3 CaCl2; 1.2 KH2PO4, 10 d-glucose, and 10 HEPES (pH adjusted to 7.4 with NaOH). Synaptosomes were further preincubated for 1 hour at 37°C with constant agitation to restore their metabolic status and transmembrane ionic gradients prior to use in neurotransmitter release experiments.
Measurements of Vesicular Glutamate Release in Synaptosomes.
Vesicular glutamate release was measured using l-[3H]glutamate as a radiotracer according to the previously described procedure (Rudkouskaya et al., 2010). Synaptosomes were loaded with l-[3H]glutamate (10 µCi/ml) at 37°C for 30 minutes with constant agitation. To terminate loading and remove extracellular isotope, synaptosomes were washed in ice-cold medium S containing (in mM): 243 sucrose; 3.8 KCl, 1.2 MgSO4, 1.2 KH2PO4, 10 HEPES, 10 d-glucose (pH 7.4). Synaptosomes were then pelleted by 20-minute centrifugation at 9000 g (2°C). The resulting pellets were stored on ice for not more than 2 hours. Immediately before transport measurements, synaptosomes were resuspended in 8 ml ice-cold medium S. To initiate neurotransmitter release, 400-µl aliquots of l-[3H]glutamate-loaded synaptosomes were injected into tubes, containing 4.5 ml of one of the three following media: basal medium (for composition, see Preparation of Rat Brain Synaptosomes), high [K+], or Ca2+-free high [K+] medium. In high [K+] medium, K+ concentration was elevated to 43 mM (to yield final [K+]o=40 mM) by an equimolar replacement of Na+, leaving all other components the same. The composition of Ca2+-free high [K+] medium was similar, except 1.3 mM CaCl2 was replaced with 1.3 mM MgCl2 and 50 µM EGTA was added. After 5 minutes incubation at 37°C, the neurotransmitter efflux was terminated by rapid vacuum filtration through the GF/B filters (Whatman-GE Healthcare, Florham Park, NJ). The filters were incubated overnight in 4 ml of the Ecoscint A scintillation cocktail (National Diagnostics, Atlanta, GA) and counted for radioactivity remaining in synaptosomes. Fractional release of l-[3H]glutamate was calculated in relationship to total isotope content measured at time 0. Vesicular release was calculated as the difference between l-[3H]glutamate content in synaptosomes exposed to the high K+ media without or with Ca2+. In our previous study we verified the vesicular nature of Ca2+-activated l-[3H]glutamate release by its sensitivity to botulinum toxin and N-ethylmaleimide (Rudkouskaya et al., 2010).
Amino Acid Efflux Assays.
Glutamate release was measured in cultured rat astrocytes using the nonmetabolized analog of l-[3H]glutamate, d-[3H]aspartate, which is taken inside glial cells by all Na+-dependent plasma membrane glutamate transporters and generally released via the same transport and permeability pathways as l-[3H]glutamate. Two exceptions from this rule are vesicular glutamate transporters (VGLUT1-3) and the plasmalemmal glutamate/cysteine heteroexchanger (xCT) that do not transport d-aspartate (Patel et al., 2004; Reimer and Edwards, 2004). Therefore, vesicular glutamate release was measured with L-[3H]glutamate, whereas the xCT activity was detected as uptake of l-[14C]cystine.
We used two complementary approaches to quantify rates of glutamate release via various transporters. In the majority of experiments, efflux was quantified using a Lucite perfusion chamber containing a depression on the bottom to accommodate 18 × 18 mm glass coverslips (Carolina Biologic, Burlington, NC). Astrocytes plated on coverslips and grown to confluence were preloaded overnight with d-[3H]aspartate (4 µCi/ml, ∼250 nM). Extracellular isotope was washed with basal medium (for composition, see Preparation of Rat Brain Synaptosomes), and coverslips were transferred into the Lucite chamber. Cells were perfused with experimental media as indicated in Results and figure legends at a rate of ∼1.2 ml/min, and 1-minute fractions were collected using a TriCarb 1900TR liquid scintillation analyzer fraction collector (PerkinElmer). At the end of each experiment, astrocytes on coverslips were lysed using a solution of 2% sodium dodecylsulfate plus 8 mM EDTA (SDS+EDTA). Radioactivity in all fractions was calculated using a Tri-Carb 1900TR liquid scintillation analyzer (PerkinElmer) after addition of 3 ml of Ecoscint A scintillation cocktail (National Diagnostics). The fractional release rate was calculated in relation to isotope content in cells at each consecutive minute using a custom Excel-based program.
In addition, dose-response experiments for various pharmacological inhibitors were done using cells grown on 12-well culture plates (TPP). Astrocytes were preloaded with d-[3H]aspartate (4 µCi/ml, ∼250 nM) for 3 h, washed from extracellular isotope, and incubated in 1 ml of basal medium for 10 minutes at 37°C. After this initial incubation, medium was collected and replaced with 1 ml of experimental media containing various ion transporter blockers as indicated in Results and figure legends. Experimental media containing released d-[3H]aspartate were collected, and cells were lysed using SDS+EDTA to collect the remaining isotope. Isotope efflux rates during 10- or 20-minute periods were quantified using scintillation counting (see preceding paragraph) through dividing extracellular [3H] counts by the total [3H] counts collected in media and cell lysates.
Glutamate Uptake Assay.
Uptake rates by glia-specific glutamate transporters were measured using l-[3H]glutamate as a radiotracer (1 µCi/ml supplemented with unlabeled L-glutamate to the total glutamate concentration of 2 µM) in the absence or presence of glutamate transporter inhibitors. Astrocytes grown to confluence in MEM+HIHS, or freshly prepared 50%–70% confluent cultures of microglial cells plated in OptiMEM plus serum supplement B27 (Invitrogen), were washed from culture media and preincubated in HEPES-buffered Basal medium for 30 minutes at 37°C. Basal medium was then replaced with 1 ml of media containing l-[3H]glutamate and indicated concentrations of transport inhibitors. l-[3H]Glutamate uptake proceeded for 20 minutes at 37°C. The transport reaction was terminated by adding 1 ml of ice-cold Basal medium, following three washes with the same medium at 2°C. Cells were then lysed in SDS+EDTA, and glutamate uptake rates were calculated by measuring [3H] content in cell lysates and normalizing isotope uptake to specific activity of l-[3H]glutamate in incubation media and protein content in wells.
Measurements of Cystine/Glutamate Exchange Activity.
Activity of xCT was measured in cultured astrocytes using l-[14C]cystine as a radiotracer according to the technique proposed by Jackman et al. (2010) with several modifications. Rates of the l-[14C]cystine uptake were determined in Na+-free LiCl medium to suppress activity of Na+-dependent amino acid transporters, which was additionally supplemented with the γ-glutamyl transpeptidase inhibitor 0.5 mM acivicin. Composition of the LiCl medium was similar to that of the basal medium, except NaCl was replaced with equimolar LiCl and pH was adjusted with CsOH. Confluent astrocyte cultures grown in 6-well plates were washed from culture media, and 0.1 µCi/ml l-[14C]cystine (∼1 µM) was added to each well in the presence or absence of the tested pharmacological compounds. Cystine uptake was terminated 30 minutes later by adding 2 ml of ice-cold LiCl medium followed by three washes with the same LiCl medium. The amount of accumulated l-[14C]cystine was determined by liquid scintillation counting in cell lysates. Nonspecific l-[14C]cystine binding was determined in the presence of 1 mM unlabeled l-cystine and was subtracted from all uptake values. Rates of uptake were calculated by normalizing specific uptake values to the protein content determined by a bicinchoninic acid assay (Thermo Scientific/Pierce, Rockford, IL) according to the manufacturer’s instructions and using bovine serum albumin as a standard.
siRNA Knockdowns.
To downregulate expression of Cx43 protein, we used three unique gene-specific siRNA constructs targeting different regions of Cx43 mRNA (for target sequences, see Reagents and Antibodies). Astrocytes grown in 60-mm Petri dishes or on glass coverslips to ∼70% confluence were transferred to serum-free OptiMEM and transfected with 100 nM of one of the Cx43 siRNAs or negative control nonsense siRNA using Lipofectamine RNAiMAX transfection agent (2.75 µl/ml). After approximately 4 hours of incubation with siRNA/Lipofectamine complexes, equal volumes of MEM+HIHS were added to each well or 60-mm dish. Twenty-four hours after transfection, media were completely changed to MEM+HIHS. The transfection efficacy was evaluated 48 hours posttransfection using a quantitative RT-PCR approach and 72 hours posttransfection by Western blot analysis. All functional glutamate transport experiments were carried 72 hours posttransfection.
Transient Expression of Bestrophin-1.
Primary astrocytic cultures or HEK293 cells were transiently transfected with either canonical human Best1 (OriGene Technologies, Rockville, MD, cat. # SC310244), which was subcloned in pCDNA3 plasmid (Invitrogen), or empty pcDNA3 as a negative control, using 3 μg cDNA per one well of 6-well plate and Lipofectamine 2000 transfecting agent (Invitrogen). To determine transfection efficiency, astrocytes or HEK293 cells were cotransfected with eGFP (Lonza, Allendale, NJ; 200 ng/well). After 48 hours, astrocytic cultures were used in either amino acid efflux assays as described above or lysed for Western blot analysis.
Western Blot Analysis of Protein Expression.
Protein expression levels were determined in astrocyte cell lysates. Cells plated in 60-mm dishes and transfected with gene-specific or negative control siRNAs were lysed in 500 µl of 2% SDS plus 8 mM EDTA. Small aliquots were taken for protein analysis, and the remaining protein lysates were mixed with reducing Laemmli buffer and frozen for further analysis. The protein concentration was determined using a bicinchoninic acid assay. Proteins were separated on 10% polyacrylamide Pierce Precise Protein gels, and transferred onto polyvinylidene fluoride (PVFD) membrane (BioRad, Hercules, CA). The membranes were blocked in 5% non-fat milk in Tris-buffered saline containing 0.1% Tween (TBS-T) and then incubated overnight at 4°C with primary anti-Cx43 or anti-Bestrophin-1 antibodies diluted (1:1000) in 5% milk in TBS-T. The membranes were washed five times with TBS-T and incubated with secondary anti-rabbit or anti-mouse immunoglobulin antibody (1:10,000) at room temperature for 2 hours. After additional washes, protein immunoreactivity was visualized using ECL Plus Western blot detection reagent (GE Healthcare, Piscataway, NJ) and luminescent image analyzer LAS-4000 (FujiFilm Medical Systems, Stamford, CT). Equal protein loading was additionally verified by stripping membranes and reprobing them with monoclonal anti-actin antibody (1:1000) following the same detection procedures after secondary anti-mouse immunoglobulin antibody (1:10,000).
Electrophysiology.
Whole cell electrophysiological recordings of swelling-activated Cl− currents were performed in cultured astrocytes as previously described (Abdullaev et al., 2006). Astrocytes plated on glass coverslips were treated with 300 µM dibutyryl-cyclicAMP. The resulting change in cell morphology of initially very flat cells strongly increased the probability of achieving and maintaining the whole cell configuration during the ∼30-minute experiment. Whole cell recordings were performed at room temperature with borosilicate glass electrodes fabricated using a micropipette puller (P-87, Sutter Instruments, Novato, CA) and had a resistance of 3-3.5 MΩ when filled with pipette solution. Currents were recorded using an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Series resistance was always ≤15 MΩ. The pipette solution contained (in mM): 110 CsCl, 1 MgSO4, 1 Na2-ATP, 15 Na-HEPES, 10 HEPES, and 1 EGTA (pH 7.3). The isoosmotic bath solution consisted of (in mM): 110 CsCl, 2 CaCl2, 1 MgSO4, 5 glucose, 10 HEPES, and 60 mannitol (pH 7.4). Hypoosmotic medium was prepared by removing mannitol from isoosmotic solution. Development of Cl− currents was monitored by applying ±40-mV pulses from the holding potential of 0 mV. pCLAMP software (version 9.2 Axon Instruments) was used for pulse control command, data acquisition, and analysis.
Statistical Analysis.
Data are presented as the mean values ± S.E.M. Statistical significance was determined using Student’s t test and one-way or two-way ANOVA with Tukey or Dunnett’s post hoc analysis for multiple comparisons, as appropriate. In few cases, when indicated, sets of data with nonparametric distribution were analyzed using the Wilcoxon matched pairs test. All data were analyzed with Origin 8.1 (Origin Laboratories, Northampton, MA) and Prism 5 (GraphPad Software, San Diego, CA). P values less than 0.05 were considered significant.
Results
DCPIB Does Not Directly Block Vesicular Glutamate Release in Synaptosomes.
Because the major source of extracellular glutamate under physiologic conditions is vesicular release, we started with testing DCPIB’s effect on this release mechanism in rat brain synaptosomes preloaded with l-[3H]glutamate. DCPIB has an ∼50 μM solubility limit in physiologic solutions and completely blocks VRAC at 20 μM (Decher et al., 2001). Therefore, we chose 20 μM as the maximal concentration for all experiments in this study. When 20 μM DCPIB was added during depolarization with high K+, it produced no effect on the Ca2+-dependent (vesicular) component of neurotransmitter release (Fig. 1A). However, when synaptosomes were incubated with DCPIB 30 minutes prior to and during stimulation with high K+, the Ca2+-dependent release was reduced by ∼50% (Fig. 1B). As a positive control, we pretreated synaptosomes with N-ethylmaleimide, which inhibits synaptic neurotransmitter release via modification of the NSF fusion factor and depletion of intracellular ATP (Rudkouskaya et al., 2010). As in our previous study, N-ethylmaleimide inhibited the Ca2+-dependent l-[3H]glutamate release by ∼85% (Fig. 1B). Overall, these results suggest that DCPIB does not have a direct effect on vesicular release machinery, but may influence it indirectly after long exposure.
Fig. 1.
Acute and chronic effects of DCPIB on vesicular release of glutamate from rat brain synaptosomes. Synaptosomes were preloaded with l-[3H]glutamate and then injected into basal or high K+ media containing 0 or 1.2 mM [Ca2+]o. Vesicular neurotransmitter release was determined as the Ca2+-dependent component of K+-stimulated l-[3H]glutamate release (see Materials and Methods for details). (A) acute effect of 20 µM DCPIB was measured by adding this compound into the release assay media only. n = 5 or 6 for each condition. (B) chronic effect of 20 µM DCPIB was tested by exposing synaptosomes to this agent 30 minutes prior to and during glutamate release assay. As a positive control for inhibition of vesicular release, synaptosomes were preincubated with 100 µM N-ethylmaleimide (NEM). n = 5 for control and DCPIB; n = 3 for NEM. ***P < 0.001 versus control.
DCPIB Inhibits the Glial Glutamate Transporter GLT-1, but Not GLAST.
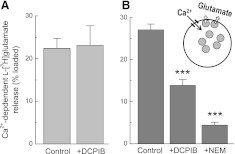
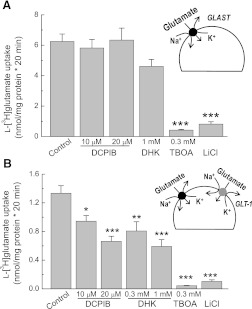
Subsequently, we tested the effects of DCPIB on the major glutamate transporters in astroglial cells, GLAST and GLT-1 (Danbolt, 2001). In the absence of neuronal stimulation or certain differentiation factors, astrocytes in culture predominantly express GLAST but not GLT-1 (Swanson et al., 1997). Consistent with previous literature findings on the major contribution of GLAST, in our experiments, astroglial glutamate uptake was potently suppressed with the broad spectrum glutamate transporter blocker TBOA, but not with the selective GLT-1 inhibitor DHK (Fig. 2A). Glutamate uptake was also inhibited by substitution of extracellular Na+ with Li+, confirming the Na+-dependent nature of the measured transport (Fig. 2A). DCPIB did not affect GLAST-mediated glutamate uptake at the maximal tested concentration of 20 μM (Fig. 2A).
Fig. 2.
DCPIB blocks the glial glutamate transporter GLT-1 but does not affect activity of GLAST. (A) uptake of l-[3H]glutamate was measured in primary astrocyte cultures to determine the effects of DCPIB on activity of the glutamate transporter GLAST. In addition to DCPIB, the GLT-1-specific inhibitor dihydrokainic acid (DHK), a broad spectrum glutamate transporter inhibitor, dl-threo-β-benzyloxyaspartic (TBOA), and Na+-free Li+-containing medium were used as positive controls. n = 18 for control, n = 9–12 for other groups. ***P < 0.001 versus control. (B) identical experiments were performed in primary microglial cells to measure the activity of GLT-1, which was determined as the DHK-sensitive component of l-[3H]glutamate uptake. n = 33 for controls, n = 9–12 for all other groups. *P < 0.05, **P < 0.01, ***P < 0.001 versus control.
To measure the effect of DCPIB on the activity of GLT-1, we took advantage of the known fact that primary microglial cells express this type of glutamate transporter (Nakajima et al., 2001). In our hands, glutamate uptake in pure microglial cultures was inhibited by ∼50% with the GLT-1 blocker DHK and nearly completely suppressed with the broad spectrum glutamate transporter inhibitor TBOA (Fig. 2B). These results are quantitatively indistinguishable from the original findings of Nakajima et al. (2001). DCPIB (20 μM) suppressed glutamate uptake to the same extent as 1 mM DHK, strongly suggesting that the GLT-1 transporter is completely blocked by this tested concentration (Fig. 2B). The effects of DCPIB and DHK were not additive (data not shown). The estimated IC50 for DCPIB at GLT-1 was ∼10 μM (Fig. 2B). However, the precise determination of GLT-1 sensitivity will have to be tested in GLT-1 heteroexpression systems to exclude contributions of other glutamate transporters present in microglia.
DCPIB Weakly Inhibits xCT.
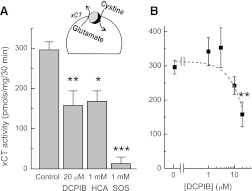
We next tested if DCPIB affects the cystine/glutamate heteroexchanger. This type of transport is thought to contribute to tonic glutamate release in vivo and, under pathologic circumstances, may play a role in excitotoxic injury of neuronal cells (Melendez et al., 2005; Fogal et al., 2007). To determine activity of xCT, we measured astrocytic uptake of l-[14C]cystine under Na+-free conditions and in the presence of the γ-glutamyl transpeptidase inhibitor acivicin. The resulting uptake was nearly completely suppressed by the xCT blocker 1 mM l-SOS and was reduced by ∼50% in the presence of 1 mM dl-homocysteine (Fig. 3A). These results were quantitatively similar to the previous literature reports for the xCT-expressing cell lines, confirming that the activity we measured was mediated by the cystine/glutamate heteroexchanger (Patel et al., 2004). In our experiments, DCPIB inhibited xCT activity only at the highest tested concentration (∼50% at 20 µM; Fig. 3B). When the effect of DCPIB on xCT-mediated l-[14C]cystine uptake was tested over a shorter period of time (5 minutes), it was completely ineffective, whereas the positive control l-SOS was still able to inhibit the cystine transport of (Supplemental Fig. 1). These latter results suggest that DCPIB likely influences the xCT activity indirectly, perhaps via decreasing the intracellular l-glutamate levels, as shown in our previous study (Hyzinski-Garcia et al., 2011).
Fig. 3.
DCPIB partially inhibits the cystine/glutamate antiporter (xCT). (A) xCT activity was measured in primary cultured astrocytes as uptake of l-[14C]cystine. To block activity of the Na+-dependent amino acid transporters and γ-glutamyl transpeptidase, these assays were performed in Na+-free media (Na+ substituted with Li+) and in the presence of 0.5 mM acivicin. The xCT inhibitors, dl-homocysteine (HCA) and l-serine-O-sulfate (l-SOS), were used as positive controls. n = 8 for control; n = 4-6 for other groups. *P < 0.05, **P < 0.01, ***P < 0.01 versus control. (B) a dose-response curve of DCPIB’s effects on the xCT activity. n = 3–8. **P < 0.01 versus control.
Comparison of the Effects of DCPIB on VRAC and Connexin Hemichannels.
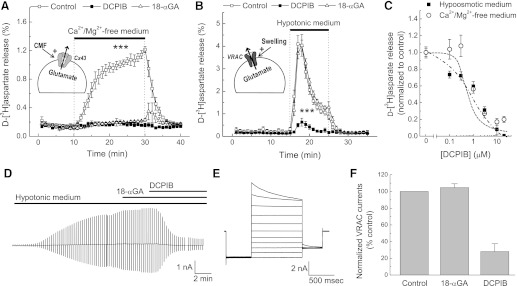
Recent studies found that many commonly used VRAC inhibitors, such as NPPB, IAP-94, and tamoxifen, potently block connexin hemichannels (Ye et al., 2009). Vice versa, some of the frequently used gap junction inhibitors, carbenoxolone and 18β-glycyrrhetinic acid (18-αGA) strongly suppress activity of VRAC (Benfenati et al., 2009; Ye et al., 2009). Therefore, it was critical to test if DCPIB affects connexin-mediated glutamate release. To trigger connexin hemichannel opening, we superfused primary cultured astrocytes with Ca2+/Mg2+-free (CMF) medium. Application of CMF caused sustained d-[3H]aspartate release, which was completely suppressed by the connexin hemichannel blocker 18-αGA (10 µM; Fig. 4A). In contrast to its effects on connexins, 18-αGA did not affect hypoosmotic activation of glutamate release via VRAC (Fig. 4B). DCPIB dose dependently blocked the connexin-mediated release under the CMF conditions with an IC50 of ∼1 µM (Fig. 4, A and C). Predictably, DCPIB potently inhibited glutamate release via VRAC with an apparent IC50 of ∼1 µM, which was consistent with our previous findings and close to the published IC50 of 4 µM for VRAC activity measured using an electrophysiology approach (Decher et al., 2001; Abdullaev et al., 2006).
Fig. 4.
DCPIB blocks both volume-regulated anion channels (VRAC) and connexin (Cx) hemichannels. (A) comparative effects of 10 μM 18α-glycyrrhetinic acid (18-αGA) and 20 μM DCPIB on Cx-mediated glutamate (d-[3H]aspartate) release from cultured astrocytes superfused with Ca2+/Mg2+-free (CMF) medium. n = 3. ***P < 0.001, CMF versus other groups. (B) comparative effects of 10 μM 18-αGA and 20 μM DCPIB on VRAC-mediated glutamate (d-[3H]aspartate) release from cultured astrocytes superfused with hypotonic medium (40% reduction of medium osmolarity by lowering [NaCl]). n = 5. ***P < 0.001, DCPIB versus other groups. (C) a dose response curve of DCPIB’s effects on Cx (CMF)- or VRAC-mediated (Hypo) glutamate release; n = 6–21. (D) a representative trace showing the effects of 20 μM DCPIB and 10 μM 18-αGA on VRAC currents measured using the whole cell electrophysiology approach in primary astrocytes exposed to hypoosmotic medium (see Materials and Methods for details). (E) representative recording of VRAC currents in response to step voltage commands in the range of −100 to +100 mV, applied in 20-mV increments. (F) normalized VRAC current densities in the presence of 20 μM DCPIB (n = 2) and 10 μM 18-αGA (n = 4) in the experiments presented in (D). Effects of inhibitors were measured when VRAC currents were saturated under hypoosmotic conditions.
To further demonstrate that DCPIB has a direct effect on connexin hemichannels, we performed several additional controls. First, we increased tonicity of CMF medium to reduce astrocytic cell volume and block VRAC by adding 150 mM mannitol. Addition of mannitol had no significant effect on CMF-induced glutamate release (∼15% inhibition, data not shown), but completely blocked VRAC (data not shown). Second, we additionally validated the ability of 18-αGA to discriminate between connexins and VRAC using an electrophysiological approach. Whole cell Cl− currents were dramatically increased in cells subjected to hypoosmotic medium (Fig. 4D). Contribution of VRAC to these currents was verified by biophysical analysis. Moderate outward rectification and time-dependent inactivation at positive potential (Fig. 4E) are the signature characteristics of VRAC (Okada, 1997). 18-αGA at the concentration of 10 µM did not affect VRAC currents (Fig. 4, D and F). As in our previous study (Abdullaev et al., 2006), DCPIB potently suppresses swelling-activated Cl− currents when added alone or in combination with 18-αGA (Fig. 4, D and F).
Finally, we downregulated expression of the major astrocytic connexin, Cx43, using gene-specific siRNA constructs. Three different siRNAs reduced Cx43 mRNA levels by 80%–90% as verified by quantitative RT-PCR (Fig. 5A) and Cx43 protein levels by >90% as verified by Western blot analysis (Fig. 5B). In Western blotting, two major immunopositive bands were detected at ∼43–47 kDa, which was close to the predicted molecular mass of Cx43 protein (Fig. 5B). The presence of multiple Cx43 bands can be explained by protein phosphorylation (see for example Musil and Goodenough, 1991). The most effective Cx43 siRNA (siCx43-1) was used in glutamate release assays. It inhibited the CMF-stimulated glutamate efflux by >90%, in a manner that was indistinguishable from DCPIB (Fig. 5C).
Fig. 5.
siRNA knockdown experiments confirm that DCPIB inhibits glutamate release via connexin-43 (Cx43) hemichannels. (A) qRT-PCR analysis of relative mRNA expression of Cx43 and GAPDH (negative control) in astrocytes treated with three independent siRNA constructs (Cx43-1, Cx43-5, Cx43-7). Expression levels were normalized to housekeeping gene RPL13a and compared with a scrambled negative control siRNA (NC) or untransfected cells (WT). n = 3 independent transfections. P < 0.001 versus NC siRNA-treated cells. (B) Western blot analysis of Cx43 immunoreactivity in sister cultures transfected on the same day as in (A). Representative of three independent transfections, framed box below main Western blot shows immunoreactivity on the same membranes that were stripped and probed with anti-actin antibody (loading control). (C) efflux of preloaded d-[3H]aspartate in cultured astrocytes transfected with Cx43-1 construct or negative control (NC) siRNA. One of the curves shows effect of 20 μM DCPIB on d-[3H]aspartate in cells transfected with NC. n = 7–14. ***P < 0.001 NC siRNA-treated cells versus other two groups, Wilcoxon matched pairs non-parametric test.
Altogether, the results presented in this section strongly affirm that Cx43 hemichannels represent a molecular target for DCPIB. Furthermore, both glutamate release measurements and electrophysiological assays establish 18-αGA as a pharmacological tool allowing discrimination between connexins and VRAC.
DCPIB Potentiates Glutamate Release Associated with Activation of P2X7 Receptors.
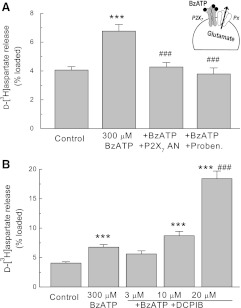
Literature data suggest that activation of the P2X7 purinergic receptor channels can trigger glutamate release from astrocytes (Duan et al., 2003). Recent studies indicate that the P2X7 receptor does not form a glutamate-permeable pore itself, but rather activates a separate permeability pathway formed by pannexins (Pelegrin and Surprenant, 2006; Iglesias et al., 2009). To test the impact of DCPIB on the P2X7/pannexin complexes, we used the P2X7 agonist BzATP. This compound induced a modest increase in efflux of d-[3H]aspartate from cultured astrocytes, and such an effect was completely suppressed by the selective P2X7 inhibitor AZ 10606120 (Fig. 6A). To test for downstream involvement of pannexins, we used 2 mM probenecid that discriminates between the pannexin and connexin permeability pathways (Silverman et al., 2008). Probenicid completely prevented the BzATP-stimulated d-[3H]aspartate efflux, confirming that it is mediated by pannexins (Fig. 6A). When DCPIB was added in conjunction with BzATP, we found a dose-dependent increase in d-[3H]aspartate efflux, which was statistically significant at the DCPIB concentration of 20 µM (Fig. 6B). These results suggest that DCPIB can potentiate P2X7/pannexin complexes via an unknown mechanism.
Fig. 6.
DCPIB potentiates glutamate release via the P2X7 receptor (P2X7)/pannexin (Pnx) permeability pathway. (A) release of glutamate (d-[3H]aspartate) was measured in astrocytes treated with the P2X7 agonist 300 μM 4-benzoylbenzyl adenosine (BzATP), the P2X7 antagonist 300 nM AZ 10606120 dihydrochloride (P2X7 AN), and the Pnx channel blocker 2 mM probenecid. n = 9–18 per group. ***P < 0.001 versus control; ###P < 0.001 versus BzATP. (B) effects of 3, 10, and 20 μM DCPIB on the BzATP-induced d-[3H]aspartate release from astrocyte cultures. n = 6-18 per group. ***P < 0.001 versus control; ###P < 0.001 versus 300 BzATP; n = 6–18.
DCPIB Inhibits the ATP-Stimulated Glutamate Release in Astrocytes.
Collapse of cellular metabolism during ischemia causes strong elevations of intracellular levels of free Ca2+ in neuronal and non-neuronal cells. Several recent studies identified the Bestrophin-1 (Best1) protein as the endogenous Ca2+-activated Cl− channel, which is responsible for release of γ-aminobutyric acid (GABA) from rodent astroglial cells in vitro and in vivo (Park et al., 2009; Lee et al., 2010). The Best1-mediated anion currents are transiently activated by agonists for numerous phospholipase C-linked G-protein-coupled receptors, including metabotropic purinergic receptors (Park et al., 2009). Therefore, to test if astrocytes release glutamate via the Best1 permeability pathway and if such a pathway is sensitive to DCPIB, we exposed astrocytic cultures to 100 µM ATP, a treatment that is known to cause glutamate/d-[3H]aspartate release via stimulation of several types of metabotropic P2Y receptors (Mongin and Kimelberg, 2002, 2003). As seen in Fig. 7A, ATP caused transient stimulation of d-[3H]aspartate release that was strongly inhibited by DCPIB, consistent with the previous findings from this and other laboratories (see for example Abdullaev et al., 2006; Heacock et al., 2006; Liu et al., 2009). These data do not necessarily point to activation of Best1 channels because ATP was previously shown to activate the DCPIB-sensitive VRAC channels (Mongin and Kimelberg, 2002; Liu et al., 2009; Akita et al., 2011). VRAC can be regulated by various G-protein-coupled receptors even in the absence of cell swelling (reviewed in Fisher et al., 2010). To discriminate between VRAC and Ca2+-activated Cl− channels we tested if the ATP-stimulated amino acid release was sensitive to niflumic acid. At the concentrations of ≤100 μM, niflumic acid potently blocks Ca2+-activated Cl− channels, including the Best1 channels, but has very weak effects on VRAC (Pedersen et al., 1998; Abdullaev et al., 2006). In our hands, 100 μM niflumic acid inhibited the ATP-stimulated d-[3H]aspartate release by ∼80% (Fig. 7B), therefore suggesting that such release is at least partially mediated by an anion channel other than VRAC. In addition to Best1, niflumic acid also potently blocks Ca2+-activated Cl− channels of the anoctamin/TMEM16 family (Verkman and Galietta, 2009). Therefore, to directly test if Best1 contributes to the niflumic acid-sensitive component of amino acid transport, we transiently overexpressed human Best1 protein in primary rat astrocytes and HEK293 cells as control. We expected Best1 overexpression to strongly increase ATP-stimulated glutamate release in glia. In HEK293 cells, transfection with Best1 caused appearance of Ca2+-activated Cl− currents that were sensitive to niflumic acid (Y.H. Kuo, I.F. Abdullaev, and A.A. Mongin, unpublished observations). In astrocytes, heteroexpression of Best1 did not affect ATP-stimulated d-[3H]aspartate release, as compared with the wild-type or negative control cells (Supplemental Fig. 2). Unfortunately, these results were inconclusive because, although Western blot analysis found heteroexpressed hBest1 protein HEK293 cells, we were unable to detect Best1 upregulation in astrocytes (Supplemental Fig. 2). Overall, the results presented in this section suggest that DCPIB blocks the ATP-stimulated and niflumic acid-sensitive glutamate release pathway, the molecular nature of which remains uncertain (see Discussion).
Fig. 7.
DCPIB strongly suppresses ATP-induced glutamate release from cultured astrocytes. (A) effect of 20 μM DCPIB on glutamate (d-[3H]aspartate) release induced by stimulation with 100 μM ATP. DCPIB was present in the perfusion medium 5 minutes before and during application of ATP. n = 8 per group. ***P < 0.001, DCPIB versus control. (B) effect of the blocker of Ca2+-activated Cl− channels 100 μM niflumic acid on d-[3H]aspartate release induced by stimulation with 100 μM ATP. Niflumic acid was present in the perfusion medium 5 minutes before and during application of ATP. n = 5–7 per group. ***P < 0.001, niflumic acid versus control.
Discussion
The major finding of this work is that DCPIB, which is marketed as the selective inhibitor of VRAC, strongly affects two other glutamate transport pathways, namely connexin hemichannels formed by Cx43 and the glia specific glutamate transporter GLT-1. In addition, extended exposures to high concentrations of DCPIB (20 μM for ≥ 30 min) weakly suppress the activity of the cystine/glutamate exchanger (xCT) and vesicular glutamate release, both effects likely occurring via an indirect mechanism. Other physiologically and pathologically relevant glutamate transporters were either not directly affected by DCPIB (GLAST) or moderately potentiated (P2X7/pannexin complexes). The graphic summary of our findings is presented in Fig. 8. The newly identified pharmacological properties of DCPIB are important for understanding ischemia pathology and deciphering mechanisms responsible for neuroprotective actions of this compound in animal stroke models.
Fig. 8.
Summary of DCPIB’s effects on physiologically and pathologically relevant glutamate release pathways in neural cells. Note that for the reasons of simplicity all transporters and release mechanisms are collected in one cartoon cell. DCPIB-sensitive transport routes are depicted in red. Transporters, which are insensitive to DCPIB or potentiated by this compound, are depicted in blue. Red arrows illustrate directionality of glutamate transport under physiologic conditions. CaCC, Ca2+-activated Cl− channels, the molecular identity of which is uncertain (see Discussion).
DCPIB was originally identified as a highly selective VRAC blocker on the basis of its effects on an array of chloride channels tested using an electrophysiological approach (Decher et al., 2001). Because the molecular identity of VRAC has yet to be identified, DCPIB represents a highly useful pharmacological tool in studies examining physiologic and pathologic roles of VRAC (see Introduction for references). Two VRAC blockers, tamoxifen and DCPIB, have been found to potently reduce ischemic brain damage and additionally inhibited the intra-ischemic release of the excitatory neurotransmitter glutamate in several rodent models of stroke (Kimelberg et al., 2000, 2003; Feustel et al., 2004; Zhang et al., 2008). Because, tamoxifen and DCPIB potently block swelling-activated glutamate release via VRAC in vitro (Abdullaev et al., 2006), the simplest interpretation of the aforementioned in vivo data is that VRAC is largely responsible for both pathologic glutamate release and tissue damage in stroke. The strength of this conclusion was built on the assumed selectivity of DCPIB. The data obtained in the present study call for reevaluation of the importance of VRAC in cerebral ischemia. In particular, the effects of DCPIB may be determined by inhibition of connexin hemichannels. As seen in Figs. 4 and 5, DCPIB has a similar IC50 for both VRAC and connexin hemichannels composed by the major astrocytic connexin Cx43. This may not be surprising because substantial pharmacological “crossreactivity” between VRAC and Cx hemichannel blockers, including Cx inhibition by tamoxifen, has been reported in several studies (Benfenati et al., 2009; Ye et al., 2009). As previously found by Ye et al. (2009), 18-αGA discriminates between VRAC and Cx permeability pathways and inhibits only connexins. We validated this finding using two approaches: electrophysiology and glutamate release assays. In our hands, 10 μM 18-αGA completely blocked Cx hemichannels but had no effect on VRAC.
Another glutamate transporter strongly suppressed by DCPIB, is the glial GLT-1, which plays a major role in maintenance of low extracellular glutamate levels (Rothstein et al., 1996). Our experiments in microglial cells indicate that DCPIB potently blocks GLT-1 at 20 μM. Interestingly, it had no effect on another glial glutamate transporter, GLAST. Thus, DCPIB appears to discriminate between GLT-1 and GLAST when applied at concentrations much lower than submillimolar concentrations for the commonly used GLT-1 inhibitor, DHK. In the context of brain physiology, perfusion of DCPIB in vivo may impair glutamate uptake by acting at the GLT-1 transporter. At least under normal physiologic conditions, DCPIB does not appear to affect extracellular glutamate homeostasis, because perfusion of this compound via a microdialysis probe does not increase microdialysate glutamate levels (Zhang et al., 2008). In severe ischemia, GLT-1 has been reported to work in “reverse” mode and release glutamate to the extracellular space because of disrupted membrane ionic gradients (Seki et al., 1999). We believe that the neuroprotective properties of DCPIB in ischemia are unlikely related to inhibition of GLT-1. In the ischemic penumbra, the clinically relevant area of less severe ischemia, DCPIB and tamoxifen suppress pathologic glutamate release, whereas the GLT-1 blocker DHK produces the opposite effect (Feustel et al., 2004).
We found no direct effect of DCPIB on vesicular glutamate release and weak inhibition of the cystine/glutamate heteroexchanger at the maximal tested concentration (20 μM). The latter effect is likely indirect because it cannot be seen during short incubation periods. Paradoxically, DCPIB increased glutamate release associated with the P2X7 receptor-induced activation of pannexins, but only at the highest concentration tested. Several studies proposed a role for the pannexin permeability pathway in ischemic damage and death of neuronal cells in vitro and in vivo (Thompson et al., 2006; Bargiotas et al., 2011). Therefore, DCPIB can theoretically exacerbate ischemic brain damage by promoting pannexin channel opening. Yet, keeping in mind the potent neuroprotective actions of DCPIB in rodent stoke, its actions on pannexins appear to be insignificant. Either DCPIB does not reach high enough levels in the ischemic brain or the contribution of pannexins to stroke pathology is minor.
Another potential target for DCPIB is the Ca2+-activated Cl− channels, which may be activated in ischemia by sustained elevations in intracellular [Ca2+]. The original study on DCPIB selectivity found no effect of this compound on the Ca2+-activated Cl− currents, but this was tested only in one cell type, calf pulmonary artery endothelial cells (Decher et al., 2001). Two recent publications identified Best1 as the endogenous Ca2+-activated Cl− channel that is responsible for release of γ-aminobutyric acid from rodent astroglial cells in vitro and in vivo (Park et al., 2009; Lee et al., 2010). The Best1 channel is also permeable to glutamate (Park et al., 2009). In our experiments, stimulation of primary astrocytes with ATP triggered release of the glutamate analog d-[3H]aspartate via a pathway sensitive to niflumic acid, which was also strongly inhibited by DCPIB. The niflumic acid sensitivity clearly discriminated this release pathway from VRAC channels. VRAC can be also activated in a Ca2+-sensitive manner by ATP and other G-protein-coupled receptors but they are largely insensitive to niflumic acid (Pedersen et al., 1998; Mongin and Kimelberg, 2002; Fisher et al., 2010; Akita et al., 2011). The niflumic acid sensitivity, however, is not sufficient to infer contribution of Best1 because members of a different family of the Ca2+-activated Cl− channels, anoctamins or TMEM16, are also potently blocked by this compound (Verkman and Galietta, 2009). Our attempts to verify that ATP-stimulated glutamate release is mediated by Best1 were inconclusive. We were unable to overexpress functional human Best1 in rat astrocytes to link this protein to glial glutamate release. Thus at this stage, Best1 can be considered as an additional candidate target for DCPIB, but further work is needed to test this hypothesis and ascertain relevance of Best1 to stroke pathology.
In summary, DCPIB blocks several glutamate transport pathways, and therefore its effects on glutamate release in vivo cannot be automatically ascribed to its actions at VRAC. Our present findings call for additional experiments clarifying the role of VRAC in stroke pathology. In particular, by comparing the effects of DCPIB and 18α-glycyrrhetinic acid on pathologic glutamate release and tissue damage, it will be possible to elucidate the relative pathologic importance of VRAC and connexin hemichannels in stroke.
Supplementary Material
Acknowledgments
We thank Dr. Iskandar F. Abdullaev for performing electrophysiological experiments included in this work, María C. Hyzinski-García for preparation of primary glial cultures and technical support, and Mashal Shaikh for contribution to preliminary experiments leading to this study.
Abbreviations
- BzATP
4-benzoylbenzyl adenosine
- CMF
Ca2+/Mg2+-free media
- Cx
connexin
- 18-αGA
18α-glycyrrhetinic acid
- DCPIB
4-(2-butyl-6,7-dichloro-2-cyclopentyl-indan-1-on-5-yl) oxobutyric acid
- DHK
dihydrokainate
- HIHS
heat-inactivated horse serum
- MEM
minimal essential medium
- NEM
N-ethylmaleimide
- NSF
N-ethylmaleimide-sensitive fusion factor
- Pen/Strep
penicillin plus streptomycin
- SDS
sodium dodecyl sulfate
- l-SOS
l-serine-O-sulfate
- TBOA
dl-threo-β-benzyloxyaspartic acid
- VRAC
volume-regulated anion channels
- xCT
cystine/glutamate antiporter
Authorship Contributions
Participated in research design: Bowens, Mongin.
Conducted experiments: Bowens, Dohare.
Contributed new reagents or analytical tools: Kuo.
Performed data analysis: Bowens, Dohare, Mongin.
Wrote or contributed to the writing of the manuscript: Bowens, Mongin.
Footnotes
This work was supported in part by the National Institutes of Health National Institute for Neurologic Disorders and Stroke [Grant R01 NS061953], and by Albany Medical College Graduate Studies Program.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
This work was presented in part at the 2011 Annual Meeting of the Society for Neuroscience and published in a preliminary form [Bowens N and Mongin AA (2011) DCPIB, a selective blocker of volume-regulated anion channels, blocks connexin 43 hemichannels and affects several other glutamate release pathways. Program No. 251.06. 2011 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience 2011].
References
- Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. (2006) Pharmacological comparison of swelling-activated excitatory amino acid release and Cl- currents in cultured rat astrocytes. J Physiol 572:677–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita T, Fedorovich SV, Okada Y. (2011) Ca2+ nanodomain-mediated component of swelling-induced volume-sensitive outwardly rectifying anion current triggered by autocrine action of ATP in mouse astrocytes. Cell Physiol Biochem 28:1181–1190 [DOI] [PubMed] [Google Scholar]
- Bannai S. (1986) Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem 261:2256–2263 [PubMed] [Google Scholar]
- Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, Penuela S, von Engelhardt J, Monyer H, Schwaninger M. (2011) Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci USA 108:20772–20777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati V, Caprini M, Nicchia GP, Rossi A, Dovizio M, Cervetto C, Nobile M, Ferroni S. (2009) Carbenoxolone inhibits volume-regulated anion conductance in cultured rat cortical astroglia. Channels (Austin) 3:323–336 [DOI] [PubMed] [Google Scholar]
- Best L, Yates AP, Decher N, Steinmeyer K, Nilius B. (2004) Inhibition of glucose-induced electrical activity in rat pancreatic beta-cells by DCPIB, a selective inhibitor of volume-sensitive anion currents. Eur J Pharmacol 489:13–19 [DOI] [PubMed] [Google Scholar]
- Choi DW. (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276 [DOI] [PubMed] [Google Scholar]
- Danbolt NC. (2001) Glutamate uptake. Prog Neurobiol 65:1–105 [DOI] [PubMed] [Google Scholar]
- Decher N, Lang HJ, Nilius B, Brüggemann A, Busch AE, Steinmeyer K. (2001) DCPIB is a novel selective blocker of I(Cl,swell) and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol 134:1467–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. (2003) P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci 23:1320–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feustel PJ, Jin Y, Kimelberg HK. (2004) Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke 35:1164–1168 [DOI] [PubMed] [Google Scholar]
- Fisher SK, Heacock AM, Keep RF, Foster DJ. (2010) Receptor regulation of osmolyte homeostasis in neural cells. J Physiol 588:3355–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogal B, Li J, Lobner D, McCullough LD, Hewett SJ. (2007) System x(c)- activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury. J Neurosci 27:10094–10105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós F. (1975) An improved method for the preparation of synaptosomal fractions in high purity. Brain Res 93:485–489 [DOI] [PubMed] [Google Scholar]
- Harrigan TJ, Abdullaev IF, Jourd’heuil D, Mongin AA. (2008) Activation of microglia with zymosan promotes excitatory amino acid release via volume-regulated anion channels: the role of NADPH oxidases. J Neurochem 106:2449–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heacock AM, Dodd MS, Fisher SK. (2006) Regulation of volume-sensitive osmolyte efflux from human SH-SY5Y neuroblastoma cells following activation of lysophospholipid receptors. J Pharmacol Exp Ther 317:685–693 [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Lambert IH, Pedersen SF. (2009) Physiology of cell volume regulation in vertebrates. Physiol Rev 89:193–277 [DOI] [PubMed] [Google Scholar]
- Hyzinski-García MC, Vincent MY, Haskew-Layton RE, Dohare P, Keller RW, Jr, Mongin AA. (2011) Hypo-osmotic swelling modifies glutamate-glutamine cycle in the cerebral cortex and in astrocyte cultures. J Neurochem 118:140–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. (2009) Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci 29:7092–7097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman NA, Uliasz TF, Hewett JA, Hewett SJ. (2010) Regulation of system x(c)(-)activity and expression in astrocytes by interleukin-1β: implications for hypoxic neuronal injury. Glia 58:1806–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK. (2005) Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia 50:389–397 [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Feustel PJ, Jin Y, Paquette J, Boulos A, Keller RW, Jr, Tranmer BI. (2000) Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport 11:2675–2679 [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Jin Y, Charniga C, Feustel PJ. (2003) Neuroprotective activity of tamoxifen in permanent focal ischemia. J Neurosurg 99:138–142 [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. (1998) Functional significance of cell volume regulatory mechanisms. Physiol Rev 78:247–306 [DOI] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. (2010) Channel-mediated tonic GABA release from glia. Science 330:790–796 [DOI] [PubMed] [Google Scholar]
- Liu HT, Akita T, Shimizu T, Sabirov RZ, Okada Y. (2009) Bradykinin-induced astrocyte-neuron signalling: glutamate release is mediated by ROS-activated volume-sensitive outwardly rectifying anion channels. J Physiol 587:2197–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. (2005) Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther 314:139–147 [DOI] [PubMed] [Google Scholar]
- Min XJ, Li H, Hou SC, He W, Liu J, Hu B, Wang J. (2011) Dysfunction of volume-sensitive chloride channels contributes to cisplatin resistance in human lung adenocarcinoma cells. Exp Biol Med (Maywood) 236:483–491 [DOI] [PubMed] [Google Scholar]
- Mongin AA. (2007) Disruption of ionic and cell volume homeostasis in cerebral ischemia: The perfect storm. Pathophysiology 14:183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. (2002) ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes. Am J Physiol Cell Physiol 283:C569–C578 [DOI] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. (2003) Is autocrine ATP release required for activation of volume-sensitive chloride channels? J Neurophysiol 90:2791–2792, author reply 2792–2793 [DOI] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. (2005) Astrocytic swelling in neuropathology, in Neuroglia (Kettenmann H, Ransom BR. eds) pp 550–562, Oxford University Press, Oxford, New York [Google Scholar]
- Mongin AA, Orlov SN. (2001) Mechanisms of cell volume regulation and possible nature of the cell volume sensor. Pathophysiology 8:77–88 [DOI] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. (1991) Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol 115:1357–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Tohyama Y, Kohsaka S, Kurihara T. (2001) Ability of rat microglia to uptake extracellular glutamate. Neurosci Lett 307:171–174 [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. (1997) Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68:69–119 [DOI] [PubMed] [Google Scholar]
- Okada Y. (1997) Volume expansion-sensing outward-rectifier Cl- channel: fresh start to the molecular identity and volume sensor. Am J Physiol 273:C755–C789 [DOI] [PubMed] [Google Scholar]
- Okada Y. (2006) Cell volume-sensitive chloride channels: phenotypic properties and molecular identity. Contrib Nephrol 152:9–24 [DOI] [PubMed] [Google Scholar]
- Orellana JA, Sáez PJ, Shoji KF, Schalper KA, Palacios-Prado N, Velarde V, Giaume C, Bennett MV, Sáez JC. (2009) Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid Redox Signal 11:369–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Oh SJ, Han KS, Woo DH, Park H, Mannaioni G, Traynelis SF, Lee CJ. (2009) Bestrophin-1 encodes for the Ca2+-activated anion channel in hippocampal astrocytes. J Neurosci 29:13063–13073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Warren BA, Rhoderick JF, Bridges RJ. (2004) Differentiation of substrate and non-substrate inhibitors of transport system xc(-): an obligate exchanger of L-glutamate and L-cystine. Neuropharmacology 46:273–284 [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Prenen J, Droogmans G, Hoffmann EK, Nilius B. (1998) Separate swelling- and Ca2+-activated anion currents in Ehrlich ascites tumor cells. J Membr Biol 163:97–110 [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer RJ, Edwards RH. (2004) Organic anion transport is the primary function of the SLC17/type I phosphate transporter family. Pflugers Arch 447:629–635 [DOI] [PubMed] [Google Scholar]
- Rosenberg D, Kartvelishvily E, Shleper M, Klinker CM, Bowser MT, Wolosker H. (2010) Neuronal release of D-serine: a physiological pathway controlling extracellular D-serine concentration. FASEB J 24:2951–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. (2000) Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403:316–321 [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, et al. (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16:675–686 [DOI] [PubMed] [Google Scholar]
- Rudkouskaya A, Sim V, Shah AA, Feustel PJ, Jourd’heuil D, Mongin AA. (2010) Long-lasting inhibition of presynaptic metabolism and neurotransmitter release by protein S-nitrosylation. Free Radic Biol Med 49:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Numata T, Saito T, Ueta Y, Okada Y. (2011) V₂ receptor-mediated autocrine role of somatodendritic release of AVP in rat vasopressin neurons under hypo-osmotic conditions. Sci Signal 4:ra5. [DOI] [PubMed] [Google Scholar]
- Seki Y, Feustel PJ, Keller RW, Jr, Tranmer BI, Kimelberg HK. (1999) Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke 30:433–440 [DOI] [PubMed] [Google Scholar]
- Silverman W, Locovei S, Dahl G. (2008) Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 295:C761–C767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. (1996) Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol 270:C711–C730 [DOI] [PubMed] [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. (1997) Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci 17:932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowski M, Barbour B, Attwell D. (1990) Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature 348:443–446 [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. (2006) Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281:21362–21368 [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Zhou N, MacVicar BA. (2006) Ischemia opens neuronal gap junction hemichannels. Science 312:924–927 [DOI] [PubMed] [Google Scholar]
- Verkman AS, Galietta LJ. (2009) Chloride channels as drug targets. Nat Rev Drug Discov 8:153–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Oberheim N, Kettenmann H, Ransom BR. (2009) Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia 57:258–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. (2003) Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 23:3588–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Feustel PJ, Kimelberg HK. (2008) DCPIB, a specific inhibitor of volume regulated anion channels (VRACs), reduces infarct size in MCAo and the release of glutamate in the ischemic cortical penumbra. Exp Neurol 210:514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.