Abstract
Unlike the majority of G protein–coupled receptors, the prostaglandin E2 (PGE2) E-prostanoid 3 (EP3) receptor binds agonist with high affinity that is insensitive to the presence of guanosine 5[prime]-O-(3-thio)triphosphate (GTPγS). We report the identification of mutations that confer GTPγS sensitivity to agonist binding. Seven point mutations were introduced into the conserved motif in the second extracellular loop (ECII) of EP3, resulting in acquisition of GTP-sensitive agonist binding. One receptor mutation W203A was studied in detail. Loss of agonist binding was observed on intact human embryonic kidney 293 cells expressing the W203A receptor, conditions where high GTP levels are present; however, high affinity binding [3H]PGE2 was observed in broken cell preparations washed free of GTP. The [3H]PGE2 binding of W203A in broken cell membrane fractions was inhibited by addition of GTPγS (IC50 21 ± 1.8 nM). Taken together, these results suggest that the wild-type EP3 receptor displays unusual characteristics of the complex coupled equilibria between agonist-receptor and receptor–G protein interaction. Moreover, mutation of ECII can alter this coupled equilibrium from GTP-insensitive agonist binding to more conventional GTP-sensitive binding. This suggests that for the mutant receptors, ECII plays a critical role in linking the agonist bound receptor conformation to the G protein nucleotide bound state.
Introduction
The arachidonic acid metabolite prostaglandin E2 (PGE2) mediates diverse physiologic responses via its interactions with specific seven transmembrane G protein-coupled receptors (GPCRs). Four distinct PGE2 receptor subtypes, upon the basis of ligand selectivity and signal transduction pathways activated, have been classified as E-prostanoid 1 (EP1), EP2, EP3, and EP4 (Coleman et al., 1994; Hata and Breyer, 2004). Physiologic evidence suggests that the rabbit EP3 receptor signals via inhibition of cyclic AMP (cAMP) generation in vivo (Sonnenburg et al., 1990), and the cloned rabbit, mouse, bovine, and human receptors were shown to signal through this pathway when expressed in cell culture systems (Sugimoto et al., 1992; Namba et al., 1993;Audoly and Breyer, 1997a). Additional alternate EP3-evoked signal transduction pathways have been described (Sugimoto et al., 1992, 1993; Irie et al., 1993; Namba et al., 1993; Negishi et al., 1993a,b), including a pertussis toxin-insensitive pathway that increased cAMP response elements (CRE) reporter activity (Audoly et al., 1999).
Ligand binding to GPCRs is a coupled equilibrium dependent on both the ligand, agonist, antagonist, or inverse agonist as well as the interaction of the receptor with G proteins. The classic ternary complex model describes the interconversion of the inactive conformation of the receptor R to the active conformation of the receptor R* facilitated by the presence of G proteins and modulated by the presence of GTP or GTP analogs (De Lean et al., 1980; Samama et al., 1993). Agonist binds with high affinity to the active R* state, and a concomitant exchange of guanosine diphosphate (GDP) for GTP on the associated G protein initiates the intracellular signaling cascade. In the ternary complex model, addition of nonhydrolyzable GTP analogs such as guanosine 5[prime]-O-(3-thio)triphosphate (GTPγS) causes an affinity shift of agonist from a high-affinity state to a low-affinity state. Classic studies by De Lean et al. (1980) showed that the β-adrenergic receptor has both high- and low-affinity sites differing in affinity by 100-fold. Addition of GTP analogs dramatically shifted the population of receptors to the low-affinity state for the β-adrenergic receptor, leading to a loss of radiolabeled agonist binding (Williams and Lefkowitz, 1977). While this model explains the behavior of some classes of GPCRs, the behavior of other GPCRs does not easily fit this model. Several GPCRs have been shown to have little or no affinity shift upon the addition of nonhydrolyzable GTP analogs, including the histamine H3 receptor, the PACAP receptor, and the melatonin Mel1A receptor (Roka et al., 1999; Hann et al., 2004; Muller et al., 2007). For these receptors, agonist binding is insensitive to the presence of nonhydrolyzable GTP analogs.
The agonist affinity for recombinant mouse prostaglandin EP3 receptor was reported to be relatively insensitive to the presence of GTPγS binding and found to increase or decrease the affinity for PGE2, depending on the splice variant tested (Sugimoto et al., 1993). The changes in affinity were quite modest, resulting in only a 2- or 3-fold change in agonist affinity. A central question is whether the EP3 receptor displays true high- and low-affinity binding, whether the high-affinity agonist binding is dependent on the presence of G proteins, and what the consequences of dissociation of the G protein are on agonist binding.
To address these questions, we compared the agonist binding and signaling properties of wild-type (WT) and mutant EP3 receptors. Analysis of the amino acid sequences of the cloned prostanoid receptors have identified regions of characteristic, conserved amino acid sequences, including a sequence of eight amino acid residues clustered in the amino-terminal portion of the second extracellular loop (ECII), including an invariant triplet Trp-Cys-Phe (Pierce et al., 1995; Audoly and Breyer, 1997a). Mutation of ECII of the EP3 receptor leads to gain-of-function of C1 methyl ester ligands and may affect receptor-ligand interactions either directly or indirectly. Here, we demonstrate that mutation of conserved residues in ECII alters GTP sensitivity and agonist-evoked signal transduction. These results suggest that the conformation of ECII is critical in sensing the nucleotide bound state of the G protein.
Materials and Methods
Expression of the EP3 Receptor in Cell Culture.
Mutation of the hemagglutinin (HA)-tagged EP3 receptor and plasmid generation was previously described elsewhere (Audoly and Breyer, 1997a). The mutant nomenclature has been updated from the original report to agree with current standards; for example, WA203 is now designated W203A. Human embryonic kidney (HEK)293 cells (5 × 106 cells) plated at approximately 50% confluence were transfected with 3 μg of the receptor cDNA of interest and 3 μg of pCRE/lacZ plasmid (a kind gift from Dr. R. Cone, Vanderbilt University) (Chen et al., 1995) using lipofectamine-2000 (Invitrogen, Carlsbad, CA). Six hours after the addition of DNA-Lipofectamine complex, the medium was aspirated and replaced with Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen), containing 10% fetal bovine serum (FBS). For reporter assays, 24 hours after transfection cells were plated in 96-well plates at a density of 5 × 104 cells/well in 100-μl DMEM/10% FBS/20 μM indomethacin containing 5 mM sodium butyrate, and incubated an additional 12 to 16 hours, at which point the cells had reached confluence. In some cases, polyclonal cell lines were selected from cells transiently transfected with plasmids bearing WT or mutant EP3 cDNAs. Briefly, transfected cells were cultured for 3 to 4 days in DMEM medium with 10% FBS, and upon reaching confluence, 600 μg/ml G418 antibiotic was added for selection. The G418 level was gradually reduced to 500 μg/ml over several days, and then cell lines were maintained in medium containing 400 μg/ml G418 thereafter. Monoclonal lines were isolated from polyclonal pools by limiting dilution.
Flow Cytometry Analysis.
Cell surface expression levels of receptors in the 77A WT and W203A mutants were analyzed by flow cytometry using a FACSAria Cell Sorting system (BD Biosciences, San Jose, CA). The EP3 receptor fused with HA-tag on the extreme N terminus of the receptor was detected by using monoclonal anti-HA antibody (Cell Signaling Technology, Beverly, MA) with phycoerythrin (PE) conjugated anti-mouse secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA) and sorted by fluorescence intensity. Cells incubated with secondary antibody alone served as controls.
Cell Surface ELISA.
HEK293 monoclonal 77A #47 WT (clone 47), W203A #102 (clone 102), and vector #5 (clone 5) cells were plated into poly-l-lysine coated 24-well plate (200,000 cells/well) in DMEM complete medium containing 5 mM sodium butyrate and 20 μM indomethacin and were cultured for 18 to 24 hours in CO2 incubator at 37°C. All subsequent incubations were performed at 23°C. Cells were then washed three times with Tris-buffered saline (TBS), and wells were blocked with 1% bovine serum albumin (BSA)/TBS for 30 minutes and then incubated with mouse monoclonal anti-HA primary antibody (1:1000) (Cell Signaling Technology) for 1 hour. Cells were washed three times with TBS and were again blocked with 1% BSA/TBS for 15 minutes. Cells were incubated with horseradish peroxidase (HRP) conjugated anti-mouse secondary antibody (1:1000) (Jackson Immunoresearch Laboratories) in 1% BSA/TBS for 1 hour, and washed 3 times with TBS; the plate was then developed with hydrogen peroxide and 2,2′-azino-bis-[3-ethylbenzoline-6-sulfonic acid] (ABTS; Bio-Rad Laboratories, Hercules, CA). The reaction was terminated by addition of 2% oxalic acid, and the absorbance was measured at 415 nm.
Confocal Immunocytochemistry.
Monoclonal WT, W203A mutant and vector cells were plated into glass-bottom chamber slides in DMEM medium containing 5 mM sodium butyrate and 20 μM indomethacin, and they were cultured 16 hours at 37°C in the CO2 incubator. Cells were washed with PBS, fixed with 4% paraformaldehyde at room temperature for 10 minutes, and permeabilized with 0.2% Triton X-100 (Sigma-Aldrich, Saint Louis, MO) for 5 minutes at room temperature. Wells were washed with PBS, blocked with 5% normal goat serum and 1% BSA in PBS for 40–60 minutes at 23°C, and then washed twice with TBS. Double staining was performed using antibodies specific for either endoplasmic reticulum (ER) (Abcam, Cambridge, MA), antirecycling endosomes (Lapierre et al., 2007), or anti-Golgi (Abcam) and colocalized with anti-HA (1:100 dilution) antibodies in 1% BSA in TBS and incubated 16 hours at 4°C. Wells were washed three times for 5 minutes each with TBS. Fluorescently labeled Alexa-488 (for ER, endosome and Golgi) and Alexa-568 (for HA) secondary antibodies (1:500) (Invitrogen) were incubated in 1% BSA/TBS for 30 to 45 minutes at room temperature in the dark. Wells were washed 3 times with TBS and mounted using Vectashield mounting medium (Vector Laboratories, Burlingame, CA) in low lighting. Images were obtained using a Zeiss Inverted LSM510 microscope (Carl Zeiss Microscopy, Thornwood, NY).
Cell Surface Biotinylation.
Cell surface biotinylation was performed according to the manufacturer’s instructions (Pierce cell-surface protein isolation kit; Thermo Fisher Scientific, Rockford, IL). An equal number of monoclonal WT, W203A, and vector cells were plated in T75 cell culture flasks in DMEM medium containing 5 mM sodium butyrate and 20 μM indomethacin; they were cultured overnight at 37°C in the CO2 incubator. Cell surface biotinylation was performed using Sulfo-NHS-SS-Biotin for 15 minutes at 4°C, and the reaction was stopped by the addition of quenching solution supplied by the company. Collected cells were solubilized with lysis buffer and the lysate was centrifuged to eliminate the insoluble fraction. The supernatant with biotinylated proteins was incubated with immobilized NeutrAvidin gel slurry for 60 minutes at room temperature, and the beads were then extensively washed with wash buffer containing protease inhibitors. Proteins were eluted with SDS-PAGE sample buffer containing 50 mM dithiothreitol for 60 minutes at room temperature and used the eluted sample for Western blot analysis.
Western Blot Analysis.
Biotinylated protein samples collected from monoclonal WT, W203A and vector cells were analyzed for cell-surface expression of glycosylated EP3 receptors. In some cases, the biotinylated WT and mutant samples were treated with endoglycosidase H (Endo H), which cleaves the chitobiose core of high mannose and hybrid oligosaccharides from N-linked glycoproteins or with N-glycosidase F (PNGase F), which cleaves between the innermost GlcNAc and asparagine residues of high mannose, hybrid, and complex oligosaccharides from N-linked glycoproteins. Equal amounts of biotinylated or enzyme treated samples were loaded into 10% SDS-PAGE. Resolved proteins were transferred onto a nitrocellulose membrane. The filter was then incubated overnight with anti-HA mouse monoclonal (1:200) primary antibody at 4°C. The blot was washed with ice-cold Tris-buffered saline/Tween 20 (TBST), and incubated with HRP-conjugated anti-mouse secondary antibody (1:2000) for 60 minutes at room temperature and then developed with ECL reagent (PerkinElmer Life and Analytical Sciences, Waltham, MA).
To validate the cell-surface selectivity of the biotinylation, Western blot analysis was performed in the biotinylated samples of Rho-GDI, one of the predominantly expressed cytosolic proteins. Lysate collected from untreated (nonbiotinylated) cells served as a negative control. Samples were resolved in 10% SDS-PAGE, and the proteins were transferred onto a nitrocellulose membrane. The filter was incubated with anti-Rho-GDI rabbit polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA) primary antibody (1:250) overnight at 4°C. Similarly, control and urea treated samples were resolved in 10% SDS-PAGE to detect Gαi protein using a 1:2500 dilution of rabbit anti-Gαi antibody (Carlson et al., 1989) and incubated overnight with 3% milk in TBST at 4°C. Filters were then washed with ice-cold TBST, and incubated with HRP conjugated anti-rabbit secondary antibody (1:2000) (Jackson Immunoresearch Laboratories) for 60 minutes at room temperature. The blots were developed with ECL reagent.
Radioligand Binding on Intact Cells.
Monoclonal 77A WT, W203A, and vector transfected HEK293 cells were plated into a poly-l-lysine coated 24-well plate in DMEM medium containing 10% FBS, 5 mM sodium butyrate, and 20 μM indomethacin and were cultured for 18 to 24 hours in a CO2 incubator at 37°C. Cells were washed once with ice-cold PBS and incubated with 200 μl of 2 nM [3H]PGE2 (PerkinElmer Life and Analytical Sciences) in the presence or absence of (Z)-7-[(1R,2R,3R)-3-hydroxy-2-[(E,3R)-3-hydroxy-4-(phenoxy)but-1-enyl]-5-oxocyclopentyl]-N-methyl sulfonyl hept-5-enamide (sulprostone; 5 μM) for 2 hours at 4°C. For saturation-binding isotherms, increasing concentrations of [3H]PGE2 was incubated with whole cells in the presence or absence of sulprostone (5 μM). Wells were then washed 3 times with ice-cold PBS, and the cells were then lysed with 150-μl 1 N sodium hydroxide. Lysates were counted in the liquid scintillation counter.
Radioligand Binding on Isolated Membranes.
Membranes from stable HEK293 cell lines transfected with 77A WT, W203A, or empty vector were prepared after incubation of the cells with 5 mM sodium butyrate for 24 hours. After hypotonic lysis, the lysate was layered on a 60% sucrose cushion and centrifuged at 150,000g for 1 hour at 4°C. The membrane fraction collected and the protein concentration were determined by the BCA assay (Thermo Fisher Scientific). For saturation-binding isotherm experiments, 2-μg membrane proteins were incubated with varying concentrations of [3H]PGE2, and the reactions were stopped by filtration through glass-fiber filters, as described previously elsewhere (Breyer et al., 1994). In some cases, membranes were fractionated on a sucrose gradient to separate plasma membranes from light vesicle fractions, as described elsewhere (Toews et al., 1984; Wang et al., 1997). Briefly, membranes were layered on a discontinuous sucrose density gradient consisting of 1.7 ml of 15% sucrose, 4.5 ml of 30% sucrose, and 2 ml of 60% sucrose and were centrifuged at 175,000g for 1 hour at 4°C. Fractions of 0.8 ml were collected from the top of the tube, and 20 μl of each of the fraction was used to determine [3H]PGE2 binding in the presence or absence of 5 μM sulprostone. In some studies, ligand binding was performed in the presence of varying concentrations of GTPγS (Sigma-Aldrich, St. Louis, MO).
In Vitro GTP Sensitivity Assay.
To dissociate receptor-associated G proteins, cell membranes collected from WT and W203A were incubated with 4 M urea in HEPES buffer (15 mM HEPES, 5 mM EDTA, 5 mM EGTA, pH 7.6) for 15 minutes and were centrifuged 32,000 rpm for 1 hour at 4°C. Membranes were resuspended in HEPES buffer and centrifuged again to eliminate urea from the membrane preparation. Equal volumes of control and urea-treated membranes collected from WT and W203A were analyzed for one point [3H]PGE2 binding in the presence or absence of 5 μM sulprostone. In some studies, urea-washed membranes were preincubated with GDP (10 μM) and heterotrimeric G proteins (Gαi1, 10 μg; Gβγ, 10 μg) and tested for ligand binding in the presence or absence of GTPγS (10 μM).
CRE Reporter Assay.
Transfected cells were plated in DMEM/10% FBS/20 μM indomethacin (2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetic acid) containing 5 mM sodium butyrate in 96-well plates at 5 × 104 cells per well. Sulprostone dissolved at varying concentrations in OPTI-MEM containing 20 μM indomethacin were added to cells and incubated for an additional 6 hours at 37°C in 5% CO2. Medium was aspirated and β-activity was measured as described using chlorophenol red β-d-galactopyranoside (Roche Applied Science, Indianapolis, IN) as a substrate (Konig et al., 1991). Plates were incubated for 6 hours, and the absorbance read was measured at 570 nm.
Intracellular Cyclic AMP Assay.
Intracellular cAMP ([cAMP]i) levels were determined as described elsewhere (Sheffler and Conn, 2008). Monoclonal HEK293 expressing 77A WT, W203A, or empty vector (60,000 cells/well) were plated into poly-l-lysine coated 24-well plate in complete DMEM medium containing 5 mM sodium butyrate and 20 μM indomethacin and were cultured for 16 to 18 hours in CO2 incubator at 37°C. The media were replaced with serum-free DMEM containing 20 mM HEPES and incubated at 37°C for 2 hours, pretreated with 0.5 mM IBMX in stimulation buffer (DMEM containing 15 mM HEPES, 0.025% ascorbic acid) at 37°C for another 30 minutes, and then stimulated with either vehicle or 1 μM isoproterenol for another 20 minutes. The reaction was terminated by the addition of ice-cold 3% trichloroacetic acid (TCA) and incubated at least 2 hours at 4°C. Competition binding was performed incubating 15-μl TCA extract, 35 μl of 15.71 nM 3[H]cAMP, and 500 μl of assay buffer (100 mM Tris-HCl, pH 7.4; 100 mM NaCl and 5 mM EDTA) containing 0.2 μg/ml cAMP-binding protein isolated from bovine adrenal cortices. The binding reaction was incubated on ice for 2 hours, and the reaction was terminated by vacuum filtration over GF/F filters and counted in a liquid scintillation counter.
Data Analysis.
All data are presented as the mean ± S.E.M. of at least three independent experiments. Statistical analyses were performed using the Student’s t test. A two-tailed value of P < 0.05 was considered statistically significant. KD and Bmax values for saturation isotherm radioligand-binding experiments were calculated based on a one-site binding model using Prism software (GraphPad Software, Inc., San Diego, CA). EC50 values for CRE signal transduction experiments were calculated based on a sigmoidal dose-response model using Prism software (GraphPad Software, Inc., San Diego, CA).
Results
Agonist Affinity to the Rabbit EP3 Receptor Is Insensitive to GTP Analogs.
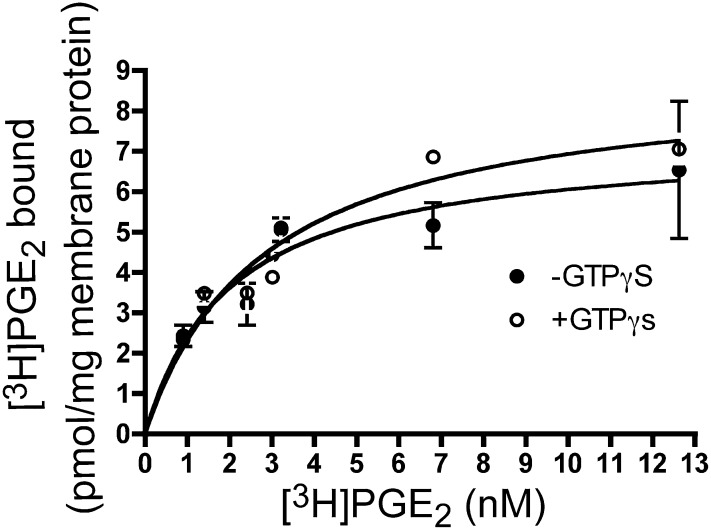
The rabbit receptor has not been characterized with respect to GTP sensitivity of agonist binding. As seen in Fig. 1, saturation isotherm binding experiments on total membrane fractions revealed single-site high-affinity specific binding of [3H]PGE2. Addition of GTPγS did not result in a statistically significant change in ligand-binding affinity (KD WT = 1.6 ± 0.3 nM, KD WT, +GTPγS = 2.6 ± 0.6 nM, N.S.) (Fig. 1). Thus, similar to findings reported for the mouse EP3 receptors, agonist binding to the rabbit EP3 receptor is insensitive to the presence of GTP analogs.
Fig. 1.
The effect of GTPγS on agonist affinity of the rabbit EP3 77A receptor. Saturation isotherm analysis was performed in monoclonal cell membranes of the WT EP3 receptor in the absence (●) or presence (○) of 100 μM GTPγS. Data presented are from a single experiment performed in duplicate, and are representative of three independent experiments.
Loss of Signal Transduction upon Mutation of ECII.
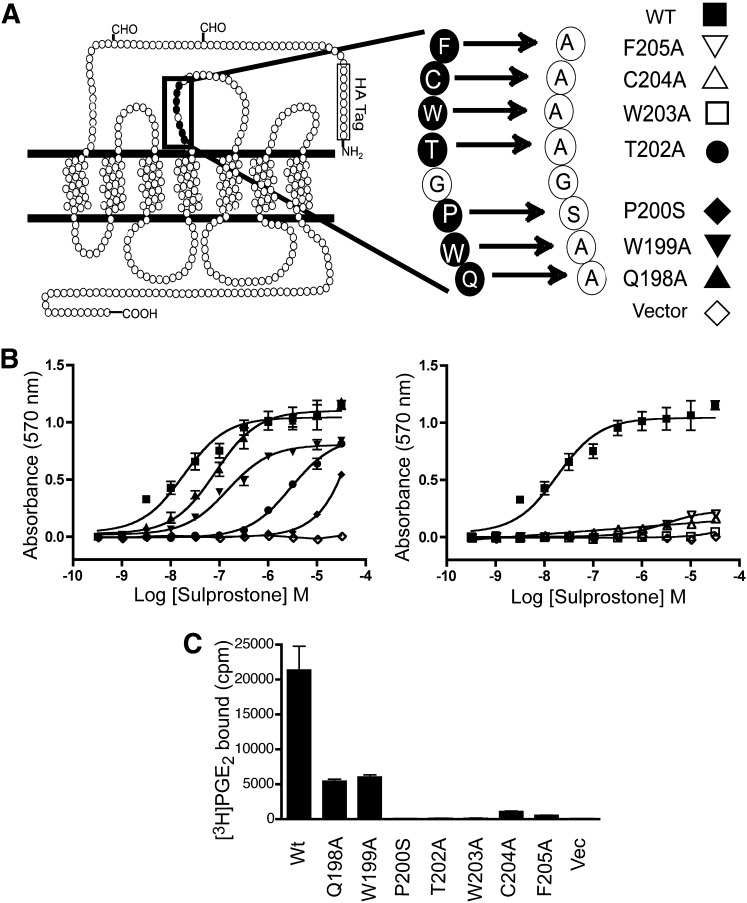
The ability of the WT rabbit EP3 receptor 77A splice variant to evoke an agonist-dependent increase in CRE reporter activity was compared with that of receptors bearing a series of point mutations in the conserved sequence in the ECII (Fig. 2, A and B). HEK293 cells transiently transfected with WT EP3 receptor and stimulated with the EP3 agonist sulprostone increased CRE reporter activity in a dose-dependent manner, with an EC50 of 12.1 ± 3.8 nM, consistent with previous published findings (Audoly et al., 1999). Each point mutation tested caused a reduction in the EC50 for reporter activation. In some cases, the reduction was modest, such as the receptors bearing the point mutations Q198A (EC50 = 68.8 ± 39 nM; P < 0.0001), W199A (EC50 = 93.6 ± 46 nM; P = 0.0008), or more dramatic as in T202A (EC50 = 2.2 ± 1.1 μM; P < 0.0001). In the remaining mutations, the cells transfected with EP3 receptors bearing P200S, W203A, C204A, and F205A substitutions, the response to sulprostone in the CRE reporter assay was minimal or absent at agonist concentrations up to 30 μM. This is despite the fact that each of these receptors, with the exception of P200S, has been previously demonstrated to bind the agonist sulprostone with affinity equal to or greater than that observed for the WT receptor in total cell membranes (Audoly and Breyer, 1997a). In the case of P200S, the receptor binds sulprostone with an affinity of 5.1 nM, 5-fold weaker than WT (Audoly and Breyer, 1997a), yet no CRE reporter activity was observed at concentrations up to 1 μM, and only modest stimulation was observed at 30 μM.
Fig. 2.
Mutation and signal transduction of ECII of the rabbit EP3 77A receptor. (A) a stretch of amino acids in the ECII is highly conserved among prostaglandin receptors. Selected residues were individually mutated to alanine as indicated, except for the proline at position 200, which was mutated to serine, the residue found in the FP receptor. (B) analysis of CRE reporter signal transduction was performed in cells expressing EP3 WT or receptors with mutation at seven other positions in the ECII. Left panel: HEK293 cells were transiently transfected with EP3 77A WT (▪), Q198A (▴), W199A (▾), P200S (♦), T202A (●), or pRc/CMV empty vector (◇). Right panel: W203A (□), C204A (▵), F205A (▿). In the right panel, the WT and vector curves are replotted from the left panel for comparison. (C) a whole-cell binding assay was performed in HEK293 cells transiently transfected with cDNA encoding the WT or mutant EP3 receptor. Intact cells were incubated with [3H]PGE2 in the presence or absence of 5 μM sulprostone, then washed, lysed with 1-N NaOH, and counted in scintillation fluid. The data shown in (B) are from a single experiment performed in triplicate and are representative of six to eight individual experiments. The data shown in (C) represent the mean ± S.E.M. of at least three independent experiments performed in duplicate.
Because these mutant receptors bind agonists in broken-cell membrane preparations but do not signal in the reporter assay, we tested whether radiolabeled agonist binding could be detected on intact cells. HEK293 cells transiently transfected with the mutant receptors displayed reduced radioligand binding in intact cells as compared with the WT EP3 receptor, and for several mutant receptors no binding was detected including receptors bearing the P200S, T202A, and W203A substitutions (Fig. 2C).
Intact Cells Stably Expressing W203A Receptor Neither Bind Agonist nor Evoke Reporter Signaling.
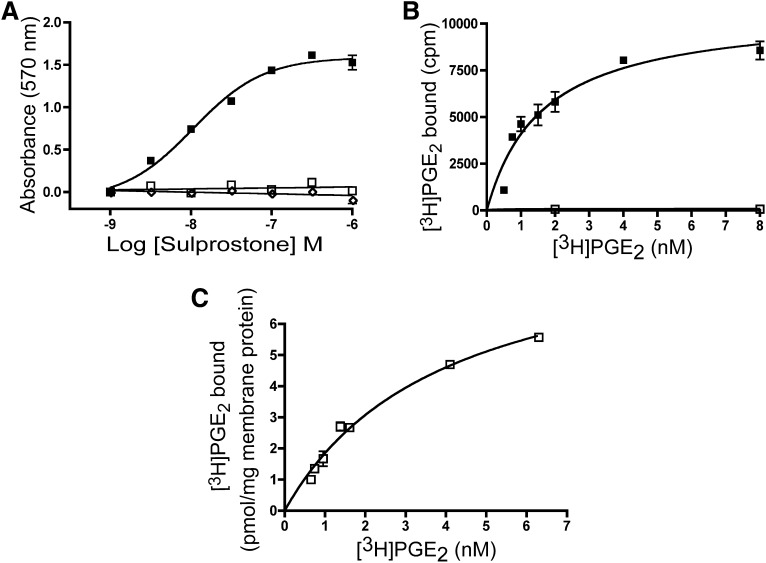
Receptor signaling can be affected by differences in cellular expression level, causing changes in receptor spareness or reserve. To more carefully characterize the properties of the mutant EP3 receptors, monoclonal stable cell lines were established from polyclonal pools so that clones with matching receptor density could be identified, eliminating differences in receptor reserve in these studies (Fig. 3). A monoclonal cell line expressing the WT EP3 receptor displayed an EC50 value (77A WT clone #47) of 7.2 ± 2.2 nM while the mutant W203A (clone #102) does not display detectable stimulation of the CRE reporter in response to treatment with the agonist sulprostone (Fig. 3A), in agreement with what was observed for the transiently transfected receptor cDNA clones.
Fig. 3.
Receptor expression and signal transduction in monoclonal stable lines. (A) analysis of CRE-mediated signal transduction in monoclonal cell lines. Monoclonal cell lines WT (▪), mutant W203A (□), and vector (◇) were stimulated with the indicated doses of sulprostone. (B) saturation isotherm analysis of intact cell-surface binding of WT monoclonal cells expressing WT (▪) or the W203A mutant EP3 receptor (□) incubated with [3H]PGE2 in the presence or absence of 5 μM sulprostone. (C) saturation isotherm analysis performed in monoclonal cell membranes of W203A (□). Data presented in (A) are from a single experiment performed in triplicate and are representative of seven independent experiments. Data presented in (B) are combined from four independent experiments. Data presented in (C) are from a single experiment performed in duplicate and are representative of three independent experiments.
Saturation-binding isotherms using the intact cells expressing the EP3 WT receptor displayed a single high-affinity binding site for [3H]PGE2 (KD WT = 2.0 ± 0.2 nM; n = 3) very similar to what was observed in broken-cell assays (Fig. 1). In contrast, cells stably expressing W203A did not display cell-surface binding (Fig. 3B). Saturation-binding isotherm studies on total broken-cell membrane fractions revealed single-site high-affinity specific binding for [3H]PGE2 while membranes prepared from monoclonal cell lines expressing W203A receptor had a slightly weaker affinity (KD WT = 1.6 ± 0.3 nM and KD W203A = 3.6 ± 0.2 nM; P = 0.001) (Fig. 3C). The receptor density was not statistically significantly different between WT and W203A expressing cells (Bmax W203A = 10.9 ± 1.8 pmol and Bmax WT = 5.9 ± 0.4 pmol; P > 0.05) in the broken cell assay, suggesting that the absence of signal transduction in the CRE reporter assay is not due to decreased receptor expression per se. Thus, W203A neither binds agonists nor signals through the CRE pathway in intact cells, although high-affinity radioligand binding is observed in membrane fractions.
Mutant W203A Is Glycosylated and Traffics to the Cell Surface.
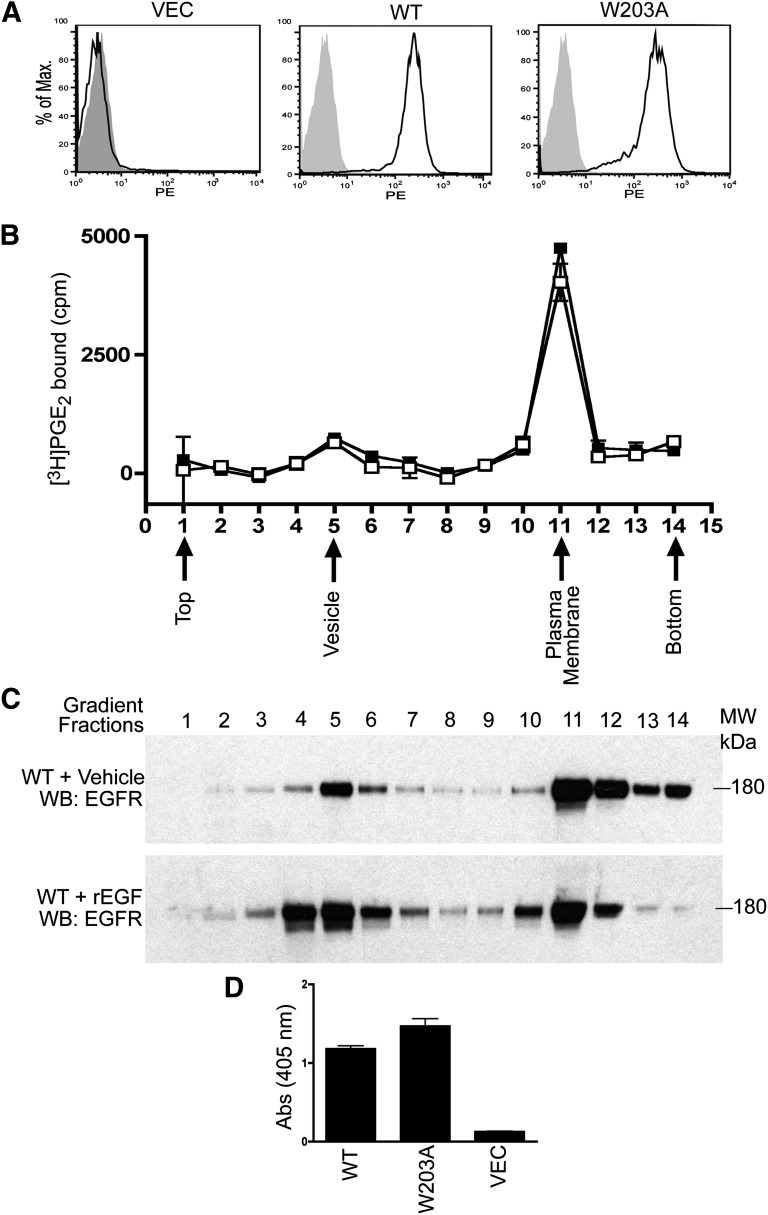
A trivial explanation for the phenotype of the W203A mutation would be that it prevented or diminished cell-surface expression of the receptor. To test this hypothesis, monoclonal stable cell lines were analyzed for cell-surface protein expression, using antibodies to the N-terminal HA tag; FACS analysis demonstrated that both the WT and W203A receptor were expressed on the cell surface (Fig. 4A), consistent with the W203A receptor efficiently trafficking to the cell surface. Similar results were observed in the polyclonal stable pools, arguing that this phenotype is not an artifact of the monoclonal line (data not shown).
Fig. 4.
Cellular expression patterns of WT and W203A are indistinguishable. (A) flow cytometry analysis was performed in monoclonal WT, W203A, and empty vector control. Cells were treated with anti-HA monoclonal primary antibody and detected with PE-conjugated secondary antibody. The PE fluorescence of cells treated with secondary antibody only (gray) is shown for comparison. (B) specific [3H]PGE2 binding to sucrose gradient membrane fractions was determined by incubation with 4 nM [3H]PGE2 in the presence or absence of sulprostone (5 μM). Membrane fractions were purified from total membranes prepared from WT (▪) or mutant W203A (□) HEK293 cells. (C) WT cells were treated with or without recombinant EGF, and the membrane was collected by sucrose gradient centrifugation. Equal volumes of collected fractions were loaded to perform Western blot analysis. (D) cell-surface ELISA of HEK293 cells expressing WT, W203A, or vector. Data shown in (A) and (D) are from a single experiment and are representative of three independent experiments. Data shown in (B) are from five independent experiments performed in duplicate. Data shown in (C) are from a single experiment and are representative of two independent experiments.
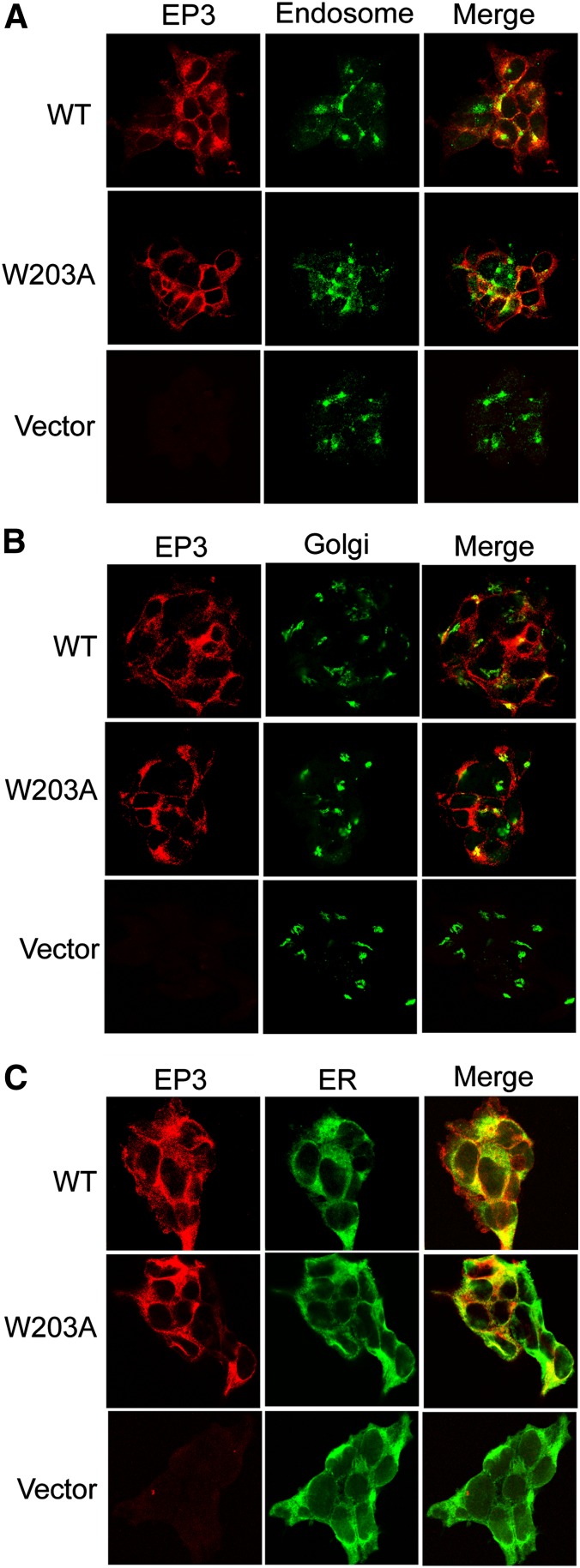
One possibility is that correctly folded W203A receptor is present only in internal “light vesicle” compartments, and the receptor expressed at the cell-surface plasma membrane may not be competent to bind ligand. To test this hypothesis, we fractionated the cells expressing each receptor into plasma membrane/Golgi/ER and light vesicle fractions using a discontinuous sucrose gradient (Toews et al., 1984; Wang et al., 1997). We assayed fractionated membranes and found an indistinguishable distribution of the EP3 WT and W203A mutant receptors in the sucrose gradient (Fig. 4B). Fractions containing plasma membrane or light vesicles were identified using antibodies to the EGF receptor on control cells with or without pretreatment with EGF to internalize the EGF receptor (Hertel et al., 1985) (Fig. 4C). Greater than 85% of the [3H]PGE2 radioligand binding was found in the dense membrane fraction of cells expressing either the EP3 WT or the W203A receptor proteins. This supports the notion that W203A receptor expressed at the cell surface is competent to bind PGE2, and is not rapidly internalized into light vesicles. Consistent with this finding, a cell-surface enzyme-linked immunosorbent assay (ELISA) using antibodies directed to the N-terminal HA tag on nonpermeabilized cells gave similar signals in this quantitative assay (Fig. 4D). We confirmed the cell-surface expression of the receptors on the monoclonal lines by confocal microscopy and evaluated both WT and mutant receptor transport to the plasma membrane using specific antibodies to colocalize ER, recycling endosomes, and Golgi compartments with EP3. The observed colocalization of ER, recycling endosome, and Golgi with EP3 in permeabilized cells suggests that both WT and mutant receptors were properly trafficked to the plasma membrane (Fig. 5, A, B, and C).
Fig. 5.
Confocal localization of EP3 WT and W203A demonstrates cell-surface expression. Confocal microscopy analyses reveal EP3 receptor colocalization with markers of recycling endosomes, Golgi, and ER. WT, W203A, and vector EP3 receptors were targeted for anti-HA using Alexa-568 (red), and colocalized with markers of endosomes (A), Golgi (B), and ER (C) using Alexa-488 (green). Empty vector stable clones display Alexa-488 positive for endosomes (A), Golgi (B), and ER (C). Data shown are representative of two independent experiments examined under four high-power fields of each.
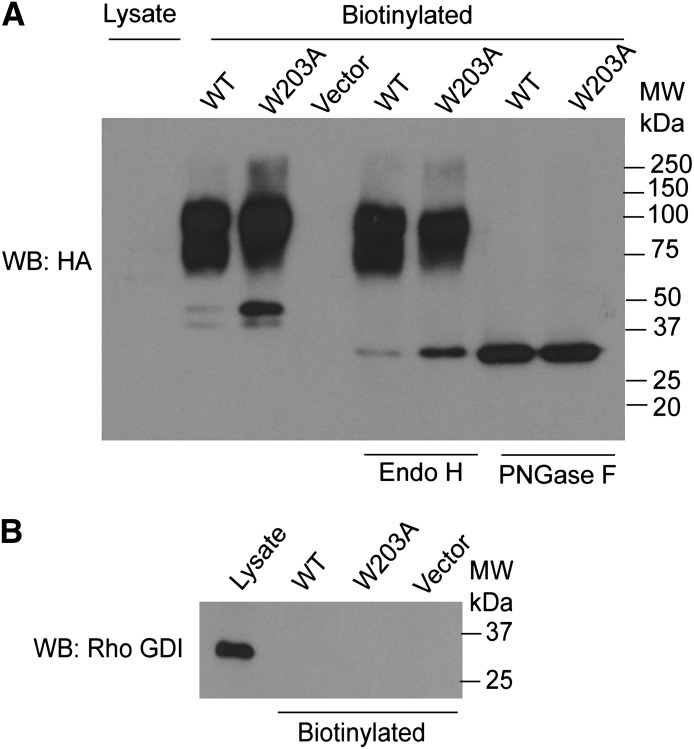
To test the posttranslational processing of the receptors at the plasma membrane, we performed cell-surface biotinylation analysis for glycosylation. The extent of receptor glycosylation was assessed by treating the biotinylated samples with glycosidase Endo H and PNGase F, which cleave off the core or complex oligosaccharides from N-linked glycoproteins, respectively. Analysis of cell-surface biotinylated proteins confirmed that both WT and W203A cells expressed fully glycosylated receptors on the surface of the cells, and both WT and mutant receptors displayed similar patterns of glycosylation (Fig. 6). Taken together with antibody staining studies, these data provide compelling evidence that the correctly folded and processed receptor is found at the cell-surface plasma membrane for both WT and W203A receptors.
Fig. 6.
Glycosylation and cell-surface expression of WT and W203A. (A) cell-surface biotinylated samples of WT, W203A, and vector clones were analyzed for receptor glycosylation. Western blot analysis indicates that comparable levels of fully glycosylated WT and mutant receptors are expressed at the cell surface. Treating glycosylated mature receptors with glycosidase enzymes Endo H cleaves the chitobiose core of high mannose and some hybrid oligosaccharides from N-linked glycoproteins. Treatment of samples with PNGase F cleaves between the innermost GlcNAc and asparagine residues of high mannose, hybrid, and complex oligosaccharides from N-linked glycoproteins. (B) Western blot analysis of Rho-GDI, an abundant cytosolic protein, as a control for cytosolic contamination. Data shown in (A) are from a single experiment and are representative of four independent experiments; data in (B) are representative of three independent experiments.
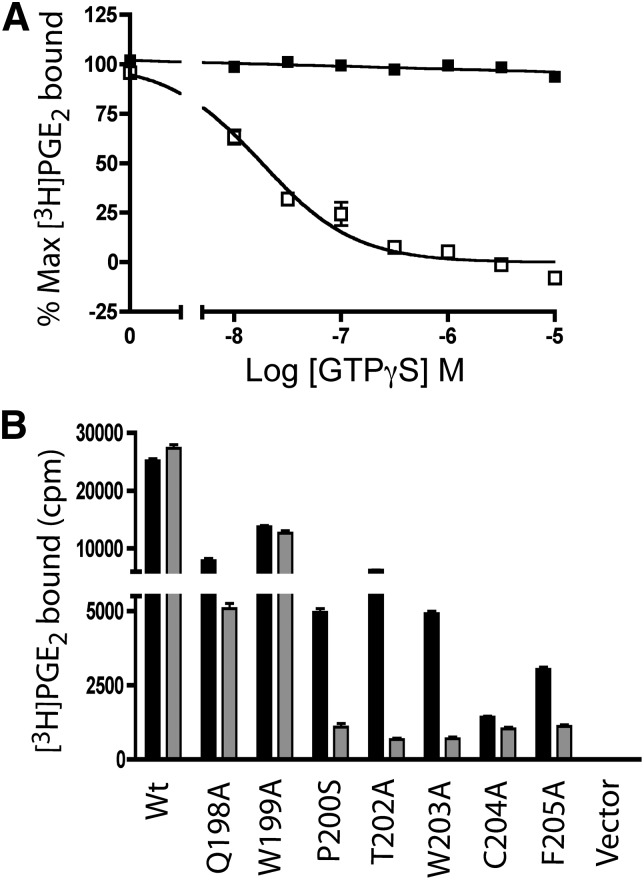
Agonist Binding to W203A Receptors Is GTPγS Sensitive.
To test the hypothesis that the agonist binding of W203A was sensitive to the high intracellular GTP concentrations, [3H]PGE2 binding was performed on broken-cell membranes in the presence of increasing concentrations of GTPγS. No change in [3H]PGE2 binding was observed with concentrations of GTPγS up to 10 μM in WT, whereas the agonist binding to W203A was significantly inhibited by the addition of GTPγS (Fig. 7A). The GTPγS insensitivity of the WT receptor agonist-binding observed in these studies is consistent with results observed in the saturation-binding isotherm studies (Fig. 1). Agonist binding to W203A receptor was decreased with an EC50 value of 21 ± 1.8 nM, and complete inhibition of [3H]PGE2 binding was observed at 1 μM GTPγS. Because we had observed decreased cell-surface radioligand binding for each of the transiently expressed mutant receptors (Fig. 2C), the GTPγS sensitivity of agonist binding of these mutated receptors was determined. As shown in Fig. 7B, the receptors that displayed robust CRE signaling, WT and W199A, demonstrated GTPγS insensitive agonist binding, and Q198A had an intermediate reduction in agonist binding in the presence of GTPγS. In contrast, P200S, T202A, W203A, and F205A, which had reduced or absent signaling, each displayed profound reductions in agonist binding in the presence of GTPγS. Radioligand binding for receptor C204A was too low to assess the effect of GTPγS addition.
Fig. 7.
GTPγS inhibits agonist binding in the W203A receptor. (A) membranes prepared from stable HEK293 clones expressing WT (▪) and W203A (□) were incubated with 4 nM [3H]PGE2 in the presence of varying concentrations of GTPγS. (B) one point binding analysis of membranes prepared from HEK293 cells transiently transfected with WT or mutant EP3 receptors. Specific binding of membranes was determined by incubation with [3H]PGE2 in the presence or absence of sulprostone (5 μM) (solid bars), and compared with the specific binding in the presence of 5 μM GTPγS (gray bars). Data are from a single experiment performed in duplicate and are representative of three or four independent experiments.
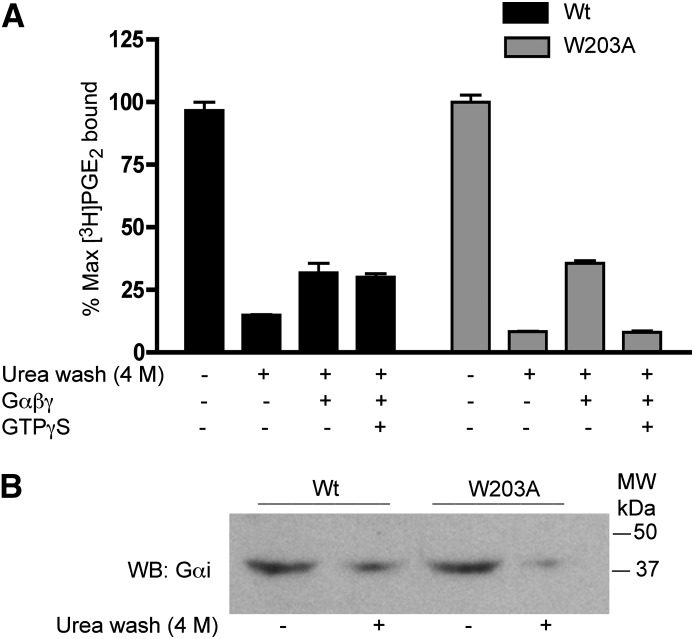
The change in GTPγS sensitivity of the EP3 receptor is consistent with an altered interaction of the receptor with G protein. To test this hypothesis, G proteins were stripped from the membranes by washing with 4 M urea. As shown in Fig. 8A, urea-washed membranes prepared from cells expressing either WT and W203A receptors showed a loss of [3H]PGE2 binding. The loss of radioligand binding with urea washing suggests that the low-affinity R state that would be expected in the absence of G proteins is “nontrappable”; that is, it has a low affinity for PGE2 and a fast dissociation rate that is not detectable in filtration assays. Urea-washed membranes were reconstituted with purified heterotrimeric Gi protein, which partially restored radioligand binding. GTPγS agonist-binding sensitivity was observed in the reconstituted W203A-expressing membranes, while the membranes expressing the EP3 WT were insensitive to the addition of GTPγS. These data demonstrate that there is an intrinsic difference in the receptor G protein interaction and the response to GTPγS induced by mutations in the ECII region.
Fig. 8.
The effect of urea washing on EP3 agonist affinity. (A) control and urea (4 M) washed membranes prepared from monoclonal cells expressing the EP3 WT (black bars) and W203A mutant (gray bars) were analyzed for [3H]PGE2 (4 nM) binding. Urea-washed membranes were incubated with GDP (10 μM), Gαi1 (10 μg), and Gβγ (10 μg) in the presence or absence of GTPγS (10 μM). Data are from a single experiment performed in duplicate and representative of three independent experiments. (B) Western blot analysis was performed in control and urea-washed membranes to determine the level of Gαi protein using an anti-Gαi1 antibody. This Western blot is representative of three experiments.
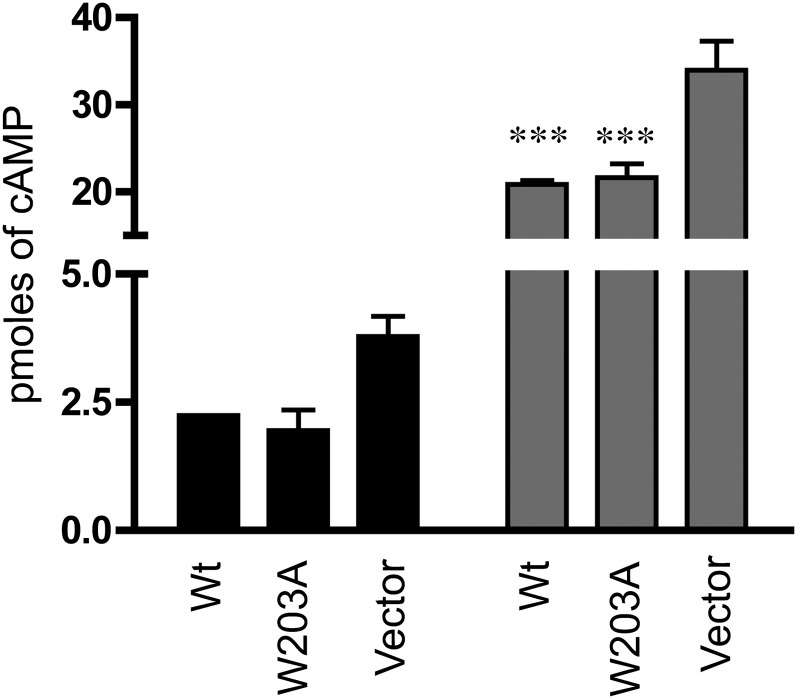
Receptors displaying GTP-insensitive agonist binding have been described as being “tightly coupled constitutively active”—that is, as having high agonist affinity binding due to the receptor being tightly associated with the G protein in the R* state, even in the absence of ligand. To test the constitutive activity of the receptor, cells expressing the EP3 receptor were transiently transfected with the Gs-coupled β2AR and stimulated with the β2AR agonist isoproterenol. Cells expressing the EP3 receptor had statistically significantly lower [cAMP]i levels when compared with vector-transfected stable cell lines, consistent with a tightly coupled constitutively active state for this receptor. Cells expressing the W203A receptor had [cAMP]i levels indistinguishable from WT, suggesting that although they have GTPγS-sensitive agonist binding there is no decrease in constitutive activity (Fig. 9).
Fig. 9.
Constitutive activity of EP3 WT is not decreased in W203A. HEK293 cells stably transfected with EP3 WT, W203A, or empty vector were transiently transfected with human β2AR and were stimulated with isoproterenol. The [cAMP]i level was determined as described in the text at baseline (black bars = N.S.; P = 0.092 one-way ANOVA) or with isoproterenol stimulation (gray bars). Isoproterenol-stimulated cells expressing either EP3 WT or W203A had a significantly reduced stimulation of [cAMP]i as compared with the vector transfected cells. ***P < 0.0001, one-way ANOVA. Data are averaged from three independent experiments, each performed in triplicate.
Discussion
We describe here the interaction of the family A PGE2 EP3 receptor with G proteins and GTP. The rabbit EP3 receptor has at least five splice variants—72A, 74A, 77A, 80A, and NT—each of which can activate the CRE reporter with similar potency (Breyer et al., 1994; Audoly et al., 1999). These splice variants are identical except in the C-terminal intracellular sequence. The 77A splice variant of EP3 was studied in detail.
The EP3 receptor has a high-affinity state with nanomolar affinity and a low-affinity state that is below the limit of detection of vacuum filtration. Mutation of ECII alters the coupled equilibrium of agonist binding and GTP-G protein interaction, making the receptor at least two orders of magnitude more sensitive to the presence of GTPγS. This suggests a key role for ECII in sensing the nucleotide bound state of the G protein in the cytoplasm.
Agonist affinity at the WT rabbit EP3 receptor is insensitive to the addition of GTPγS at concentrations up to 100 μM. This is consistent with observations made with endogenous EP3 receptors in rabbit kidney membranes (Sonnenburg et al., 1990). In contrast, physical dissociation of the G protein with urea leads to a loss of agonist binding, as has been previously described elsewhere for the adenosine receptor (May et al., 2005). The fact that PGE2 binding could be recovered, in part, by reconstitution with exogenously added purified G protein argues against urea-mediated disruption of the EP3 receptor structure per se, and suggests that the receptor-agonist affinity in the absence of G protein is below the level of detection.
Mutations in the ECII region of the EP3 receptor were identified, which confer allosteric modulation of agonist binding by GTP and GTP analogs via interaction with the G protein. Addition of low concentrations of GTP analogs reduced the agonist affinity of the receptor below the limit of detection using vacuum filtration. As a result, agonist binding of these mutant receptors was undetectable in intact cells where high intracellular concentrations of GTP are present; as a consequence, signal transduction was reduced or absent. These data suggest that the ECII region plays a key role in the receptor response to G proteins and GTP occupancy.
Activation of GPCRs requires alteration of the interhelical constraints that stabilize the inactive state to form a new set of contacts in the activated state. The free energy for this activation process comes from binding of the ligand, which in turn results in activation of the G protein (Kenakin, 1997;Gether and Kobilka, 1998; Lefkowitz, 2000; Akal-Strader et al., 2002). Although classic studies have focused on the role of the transmembrane helices in the binding of the agonist (Dohlman et al., 1987, 1988; Audoly and Breyer, 1997b), several studies have suggested a role for ECII in binding ligands. Studies reported here support a role for ECII in the interplay between ligand binding and the conversion of the receptor from the inactive (“R”) to the activated (“R*”) state. These studies suggest that, in addition to binding ligands, ECII may play a crucial role in the allosteric modulation of agonist binding by G proteins and GTP.
Recent elucidation of GPCR structures has provided extraordinary insight into the molecular details of receptor-ligand interaction as well as receptor G protein interaction (Cherezov et al., 2007; Rasmussen et al., 2007, 2011; Warne et al., 2008; Rosenbaum et al., 2011). Nonetheless, structural studies by their nature provide incomplete understanding of the basis of signal transduction. In general, the exofacial ligand-binding surface of the receptor does not appear to undergo large conformational changes upon binding an agonist as compared with the intracellular surface, which interacts and undergoes larger conformational changes, particularly of transmembrane helices V and IV (for review, see Lebon et al., 2012). Our data support the hypothesis that for the EP receptors ECII is an important sensor of agonist binding from the exofacial to intracellular surfaces. In the structures solved thus far, ECII has been found in a variety of conformations, including α-helices and β sheets. In some cases, ECII appears to make direct contact with bound ligands, whereas in other structures it does not (for review, see Peeters et al., 2011). It may be that, like the S1P receptor, the ECII of the lipid-liganded EP3 forms a tight lid over the ligand-binding pocket (Hanson et al., 2012). For the adenosine A2a receptor, there is evidence for direct ECII-agonist interaction, and significant movement of EC regions and transmembrane helices may transmit conformational changes that lead to signal transduction (Xu et al., 2011). Thus, there does not appear to be a universal role for ECII in receptor function, and structural studies will be required to understand the exact role of ECII in EP3.
Random mutagenesis of ECII in the C5a receptor has identified mutations that resulted in constitutively active receptors, consistent with a role for this region in interconversion of the R and R* states (Klco et al., 2005). It is interesting to note that random mutagenesis of the ECII region of the M3 muscarinic receptor has identified a class of mutations that were inactive or displayed reduced activity in signal transduction in intact cells, though they retained agonist binding in membrane preparations (Scarselli et al., 2007). Those investigators concluded that these mutations are important in signal transduction. In light of the findings described here, their results might also be consistent with alterations in GTP sensitivity of the agonist binding of the receptor. These mutations might then be unable to bind ligands in intact cells while retaining binding in broken-membrane preparations. It would be of interest to know whether they have altered GTP sensitivity in their ECII mutations of the M3 receptor as well.
Naturally occurring mutations in the EC loop regions of GPCRs may also affect ligand binding via directly perturbing the receptor–ligand interaction or by altering the coupled equilibrium interaction of the GPCR and the G protein. For example, naturally occurring mutations of the P2Y12 receptor have been described that affect activation but not ligand binding (Cattaneo, 2011). Mutations in the EC domains may play an important role in human disease.
The rabbit EP3 receptor displays high levels of constitutive activity, as has been observed for the EP3 receptor characterized in other species (Negishi et al., 1996; Hasegawa et al., 1997; Hizaki et al., 1997; Jin et al., 1997). It has been suggested that the tight G protein binding is associated with both GTP-insensitive agonist binding as well as constitutive activity. Our results suggest that these phenotypes are not necessarily linked, as the W203A mutation retains its constitutive activity despite its GTP-sensitive agonist binding. Constitutive activity has important implications in the physiology of the EP3 receptor. We and others have shown that the multiple PG receptors have functionally antagonistic actions. For example, the EP1 and EP3 receptors have pressor effects in vivo whereas the EP2 and EP4 receptors act as vasodepressors. Blockade of prostanoid biosynthesis would eliminate all ligand-evoked EP receptor signaling, but differences in constitutive activity of receptors may result in alterations in the balance of receptor action. A high level of EP3 constitutive activity in the absence of opposing EP2 and EP4 depressor effects might result in dominant EP3 effects, which would be expected to elevate blood pressure upon PG blockade by nonsteroidal anti-inflammatory drugs.
In summary, our study suggests that for the EP3 receptor, the ECII region can alter GTP effects on agonist binding and thus on intracellular signaling. These effects may be easily overlooked in systems where membrane fraction rather than intact cell binding is assessed. Because the ECII region is conserved among the eight PG receptors in this family, it seems plausible that this region may play a critical role for other members this family. Indeed, mutation of residues in the ECII region of the human EP2 and EP4 receptors leads to a complete loss of agonist binding in broken-cell membrane preparations (Stillman et al., 1998, 1999). It may also hold true for other family A GPCRs in general. These studies support a key role for the ECII region in triggering the conformational changes accompanying agonist-induced receptor activation and in determining the changes in receptor-agonist affinity in response to changes in the nucleotide bound state of the G protein in the cytoplasm.
Acknowledgments
The authors thank the Vanderbilt University Core Facility for flow cytometry, the VUMC Cell Imaging Shared Resource for the analysis of confocal images, David Manning for providing the anti-Gi antibody, Dr. Myron Toews for insightful discussions, and Drs. Chuck Sanders, Nathan Gilbert, and Tina Iverson for critical reading for the manuscript.
Abbreviations
- BSA
bovine serum albumin
- cAMP
cyclic AMP
- [cAMP]i
intracellular cyclic AMP
- CRE
cAMP response elements
- DMEM
Dulbecco’s modified Eagle’s medium
- EC
extracellular loop
- Endo H
endoglycosidase H
- EP
E-prostanoid
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- GDP
guanosine diphosphate
- GPCR
G protein-coupled receptor
- GTPγS
guanosine 5[prime]-O-(3-thio)triphosphate
- HA
hemagglutinin
- HEK
human embryonic kidney
- HRP
horseradish peroxidase
- PE
phycoerythrin
- PGE2
prostaglandin E2
- PNGase F
N-glycosidase F
- TBS
Tris-buffered saline
- TBST
Tris-buffered saline/Tween 20
- WT
wild-type
Authorship Contributions
Participated in research design: Natarajan, Hata, Hamm, Zent, Breyer.
Conducted experiments: Natarajan.
Contributed new reagents or analytic tools: Hamm.
Wrote or contributed to the writing of the manuscript: Natarajan, Hata, Breyer.
Footnotes
This work was supported by the National Institutes of Health, [Grants DK46205, DK37097, P50GM015431, 2P01DK065123, DK075594, and DK9921], Merit Awards from the Department of Veterans Affairs [Grant IBX000616A], and an American Heart Association established investigator award.
References
- Akal-Strader A, Khare S, Xu D, Naider F, Becker JM. (2002) Residues in the first extracellular loop of a G protein-coupled receptor play a role in signal transduction. J Biol Chem 277:30581–30590 [DOI] [PubMed] [Google Scholar]
- Audoly L, Breyer RM. (1997a) The second extracellular loop of the prostaglandin EP3 receptor is an essential determinant of ligand selectivity. J Biol Chem 272:13475–13478 [DOI] [PubMed] [Google Scholar]
- Audoly L, Breyer RM. (1997b) Substitution of charged amino acid residues in transmembrane regions 6 and 7 affect ligand binding and signal transduction of the prostaglandin EP3 receptor. Mol Pharmacol 51:61–68 [DOI] [PubMed] [Google Scholar]
- Audoly LP, Ma L, Feoktistov I, de Foe SK, Breyer MD, Breyer RM. (1999) Prostaglandin E-prostanoid-3 receptor activation of cyclic AMP response element-mediated gene transcription. J Pharmacol Exp Ther 289:140–148 [PubMed] [Google Scholar]
- Breyer RM, Emeson RB, Tarng JL, Breyer MD, Davis LS, Abromson RM, Ferrenbach SM. (1994) Alternative splicing generates multiple isoforms of a rabbit prostaglandin E2 receptor. J Biol Chem 269:6163–6169 [PubMed] [Google Scholar]
- Carlson KE, Brass LF, Manning DR. (1989) Thrombin and phorbol esters cause the selective phosphorylation of a G protein other than Gi in human platelets. J Biol Chem 264:13298–13305 [PubMed] [Google Scholar]
- Cattaneo M. (2011) Molecular defects of the platelet P2 receptors. Purinergic Signal 7:333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Shields TS, Stork PJ, Cone RD. (1995) A colorimetric assay for measuring activation of Gs- and Gq-coupled signaling pathways. Anal Biochem 226:349–354 [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, et al. (2007) High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318:1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. (1994) International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46:205–229 [PubMed] [Google Scholar]
- De Lean A, Stadel JM, Lefkowitz RJ. (1980) A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem 255:7108–7117 [PubMed] [Google Scholar]
- Dohlman HG, Bouvier M, Benovic JL, Caron MG, Lefkowitz RJ. (1987) The multiple membrane spanning topography of the beta 2-adrenergic receptor. Localization of the sites of binding, glycosylation, and regulatory phosphorylation by limited proteolysis. J Biol Chem 262:14282–14288 [PubMed] [Google Scholar]
- Dohlman HG, Caron MG, Strader CD, Amlaiky N, Lefkowitz RJ. (1988) Identification and sequence of a binding site peptide of the beta 2-adrenergic receptor. Biochemistry 27:1813–1817 [DOI] [PubMed] [Google Scholar]
- Gether U, Kobilka BK. (1998) G protein-coupled receptors. II. Mechanism of agonist activation. J Biol Chem 273:17979–17982 [DOI] [PubMed] [Google Scholar]
- Hann V, Shenton FC, Chazot PL. (2004) GTP-insensitive agonist binding to native and recombinant H(3) receptors. Inflamm Res 53 (Suppl 1):S67–S68 [DOI] [PubMed] [Google Scholar]
- Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, et al. (2012) Crystal structure of a lipid G protein-coupled receptor. Science 335:851–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H, Negishi M, Katoh H, Ichikawa A. (1997) Two isoforms of prostaglandin EP3 receptor exhibiting constitutive activity and agonist-dependent activity in Rho-mediated stress fiber formation. Biochem Biophys Res Commun 234:631–636 [DOI] [PubMed] [Google Scholar]
- Hata AN, Breyer RM. (2004) Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther 103:147–166 [DOI] [PubMed] [Google Scholar]
- Hertel C, Coulter SJ, Perkins JP. (1985) A comparison of catecholamine-induced internalization of beta-adrenergic receptors and receptor-mediated endocytosis of epidermal growth factor in human astrocytoma cells. Inhibition by phenylarsine oxide. J Biol Chem 260:12547–12553 [PubMed] [Google Scholar]
- Hizaki H, Hasegawa H, Katoh H, Negishi M, Ichikawa A. (1997) Functional role of carboxyl-terminal tail of prostaglandin EP3 receptor in Gi coupling. FEBS Lett 414:323–326 [DOI] [PubMed] [Google Scholar]
- Irie A, Sugimoto Y, Namba T, Harazono A, Honda A, Watabe A, Negishi M, Narumiya S, Ichikawa A. (1993) Third isoform of the prostaglandin-E-receptor EP3 subtype with different C-terminal tail coupling to both stimulation and inhibition of adenylate cyclase. Eur J Biochem 217:313–318 [DOI] [PubMed] [Google Scholar]
- Jin J, Mao GF, Ashby B. (1997) Constitutive activity of human prostaglandin E receptor EP3 isoforms. Br J Pharmacol 121:317–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. (1997) Differences between natural and recombinant G protein-coupled receptor systems with varying receptor/G protein stoichiometry. Trends Pharmacol Sci 18:456–464 [DOI] [PubMed] [Google Scholar]
- Klco JM, Wiegand CB, Narzinski K, Baranski TJ. (2005) Essential role for the second extracellular loop in C5a receptor activation. Nat Struct Mol Biol 12:320–326 [DOI] [PubMed] [Google Scholar]
- König M, Mahan LC, Marsg JW, Fink JS, Brownstein MJ. (1991) Method for identifying ligands that bind to cloned G(s)- or G(i)-coupled receptors. Mol Cell Neurosci 2:331–337 [DOI] [PubMed] [Google Scholar]
- Lapierre LA, Avant KM, Caldwell CM, Ham AJ, Hill S, Williams JA, Smolka AJ, Goldenring JR. (2007) Characterization of immunoisolated human gastric parietal cells tubulovesicles: identification of regulators of apical recycling. Am J Physiol Gastrointest Liver Physiol 292:G1249–G1262 [DOI] [PubMed] [Google Scholar]
- Lebon G, Warne T, Tate CG. (2012) Agonist-bound structures of G protein-coupled receptors. Curr Opin Struct Biol 22:482–490 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. (2000) The superfamily of heptahelical receptors. Nat Cell Biol 2:E133–E136 [DOI] [PubMed] [Google Scholar]
- May LT, Sexton PM, Christopoulos A. (2005) Effects of urea pretreatment on the binding properties of adenosine A1 receptors. Br J Pharmacol 146:1119–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JM, Debaigt C, Goursaud S, Montoni A, Pineau N, Meunier AC, Janet T. (2007) Unconventional binding sites and receptors for VIP and related peptides PACAP and PHI/PHM: an update. Peptides 28:1655–1666 [DOI] [PubMed] [Google Scholar]
- Namba T, Sugimoto Y, Negishi M, Irie A, Ushikubi F, Kakizuka A, Ito S, Ichikawa A, Narumiya S. (1993) Alternative splicing of C-terminal tail of prostaglandin E receptor subtype EP3 determines G-protein specificity. Nature 365:166–170 [DOI] [PubMed] [Google Scholar]
- Negishi M, Hasegawa H, Ichikawa A. (1996) Prostaglandin E receptor EP3gamma isoform, with mostly full constitutive Gi activity and agonist-dependent Gs activity. FEBS Lett 386:165–168 [DOI] [PubMed] [Google Scholar]
- Negishi M, Namba T, Sugimoto Y, Irie A, Katada T, Narumiya S, Ichikawa A. (1993a) Opposite coupling of prostaglandin E receptor EP3C with Gs and G(o). Stimulation of Gs and inhibition of G(o). J Biol Chem 268:26067–26070 [PubMed] [Google Scholar]
- Negishi M, Sugimoto Y, Hayashi Y, Namba T, Honda A, Watabe A, Narumiya S, Ichikawa A. (1993b) Functional interaction of prostaglandin E receptor EP3 subtype with guanine nucleotide-binding proteins, showing low-affinity ligand binding. Biochim Biophys Acta 1175:343–350 [DOI] [PubMed] [Google Scholar]
- Peeters MC, van Westen GJ, Li Q, IJzerman AP. (2011) Importance of the extracellular loops in G protein-coupled receptors for ligand recognition and receptor activation. Trends Pharmacol Sci 32:35–42 [DOI] [PubMed] [Google Scholar]
- Pierce KL, Gil DW, Woodward DF, Regan JW. (1995) Cloning of human prostanoid receptors. Trends Pharmacol Sci 16:253–256 [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, et al. (2011) Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature 469:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, et al. (2007) Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature 450:383–387 [DOI] [PubMed] [Google Scholar]
- Roka F, Brydon L, Waldhoer M, Strosberg AD, Freissmuth M, Jockers R, Nanoff C. (1999) Tight association of the human Mel(1a)-melatonin receptor and G(i): precoupling and constitutive activity. Mol Pharmacol 56:1014–1024 [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi HJ, Devree BT, Sunahara RK, et al. (2011) Structure and function of an irreversible agonist-β(2) adrenoceptor complex. Nature 469:236–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. (1993) A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem 268:4625–4636 [PubMed] [Google Scholar]
- Scarselli M, Li B, Kim SK, Wess J. (2007) Multiple residues in the second extracellular loop are critical for M3 muscarinic acetylcholine receptor activation. J Biol Chem 282:7385–7396 [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Conn PJ. (2008) Allosteric potentiators of metabotropic glutamate receptor subtype 1a differentially modulate independent signaling pathways in baby hamster kidney cells. Neuropharmacology 55:419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg WK, Zhu JH, Smith WL. (1990) A prostaglandin E receptor coupled to a pertussis toxin-sensitive guanine nucleotide regulatory protein in rabbit cortical collecting tubule cells. J Biol Chem 265:8479–8483 [PubMed] [Google Scholar]
- Stillman BA, Audoly L, Breyer RM. (1998) A conserved threonine in the second extracellular loop of the human EP2 and EP4 receptors is required for ligand binding. Eur J Pharmacol 357:73–82 [DOI] [PubMed] [Google Scholar]
- Stillman BA, Breyer MD, Breyer RM. (1999) Importance of the extracellular domain for prostaglandin EP(2) receptor function. Mol Pharmacol 56:545–551 [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Namba T, Honda A, Hayashi Y, Negishi M, Ichikawa A, Narumiya S. (1992) Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. J Biol Chem 267:6463–6466 [PubMed] [Google Scholar]
- Sugimoto Y, Negishi M, Hayashi Y, Namba T, Honda A, Watabe A, Hirata M, Narumiya S, Ichikawa A. (1993) Two isoforms of the EP3 receptor with different carboxyl-terminal domains. Identical ligand binding properties and different coupling properties with Gi proteins. J Biol Chem 268:2712–2718 [PubMed] [Google Scholar]
- Toews ML, Waldo GL, Harden TK, Perkins JP. (1984) Relationship between an altered membrane form and a low affinity form of the beta-adrenergic receptor occurring during catecholamine-induced desensitization. Evidence for receptor internalization. J Biol Chem 259:11844–11850 [PubMed] [Google Scholar]
- Wang J, Zheng J, Anderson JL, Toews ML. (1997) A mutation in the hamster alpha1B-adrenergic receptor that differentiates two steps in the pathway of receptor internalization. Mol Pharmacol 52:306–313 [DOI] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. (2008) Structure of a beta1-adrenergic G-protein-coupled receptor. Nature 454:486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LT, Lefkowitz RJ. (1977) Slowly reversible binding of catecholamine to a nucleotide-sensitive state of the beta-adrenergic receptor. J Biol Chem 252:7207–7213 [PubMed] [Google Scholar]
- Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC. (2011) Structure of an agonist-bound human A2A adenosine receptor. Science 332:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]