Abstract
Some situations require one to quickly stop an initiated response. Recent evidence suggests that rapid stopping engages a mechanism that has diffuse effects on the motor system. For example, stopping the hand dampens the excitability of the task-irrelevant leg. However, it is unclear whether this ‘global suppression’ could apply across wider motor modalities. Here we tested whether stopping speech leads to suppression of the task-irrelevant hand. We used Transcranial Magnetic Stimulation over the primary motor cortex with concurrent electromyography from the hand. We found that when speech was successfully stopped the motor evoked potential from the task-irrelevant hand was significantly reduced compared to when the participant failed to stop speaking, or responded on non stop signal trials, or compared to baseline. This shows that when speech is quickly stopped, there is a broad suppression across the motor system. This has implications for the neural basis of speech control and stuttering.
Keywords: inhibitory control, speech, motor evoked potential, stop signal task
1. Introduction
Many situations require the rapid stopping of initiated actions. For example, one must withhold one's initiated step into the street when a car suddenly appears, and one must withhold one's initiated verbal comment when the person whom one is about to refer to suddenly appears. In the laboratory, stopping has been studied using the stop signal task. On each trial, participants initiate a response, and sometimes, when a signal occurs, they are required to stop it (Logan, Cowan, & Davis, 1984; Verbruggen & Logan, 2008). Several studies have shown that stopping is accompanied by suppression of non task-related effectors, such as the task-irrelevant muscle of the same hand (Coxon, Stinear, & Byblow, 2006; Leocani, Cohen, Wassermann, Ikoma, & Hallett, 2000; Sohn, Wiltz, & Hallett, 2002; van den Wildenberg et al., 2010), the homologous muscle of the task-irrelevant hand (Coxon et al., 2006) and even the task-irrelevant leg (Badry et al., 2009; Greenhouse, Oldenkamp, & Aron, 2011; Majid, Cai, George, Verbruggen, & Aron, 2011). This indicates that rapid stopping has a diffuse suppression effect over the motor system. While this has been termed ‘global suppression’ (Aron & Verbruggen, 2008), it is unclear how broadly it extends since the above studies have only examined the limbs. Here we aimed to test a putative global suppression mechanism by using two very different effector systems: vocal and manual.
We used transcranial magnetic stimulation (TMS) to probe the corticospinal excitability of the task-irrelevant hand muscle while participants performed a vocal version of the standard stop-signal task (Fig 1a). Participants prepared to name a letter “T” or “D” on each trial. Occasionally, they tried to stop their vocal response when the letter changed color (a stop signal). Concurrent electromyography (EMG) was recorded from the right hand, which is task-irrelevant. We delivered TMS over the contralateral motor cortex and measured the motor evoked potential (MEP, an index of corticospinal excitability) from the hand at specific points in the trial. The global suppression hypothesis predicts that when the participant successfully stops speech, then the MEP of the task-irrelevant hand will be smaller than for trials in which participants did not successfully stop, and also compared to the pre-trial baseline (Badry et al., 2009; Greenhouse et al., 2011; Majid et al., 2011). The alternative hypothesis is that stopping speech has no effect on the task-irrelevant hand.
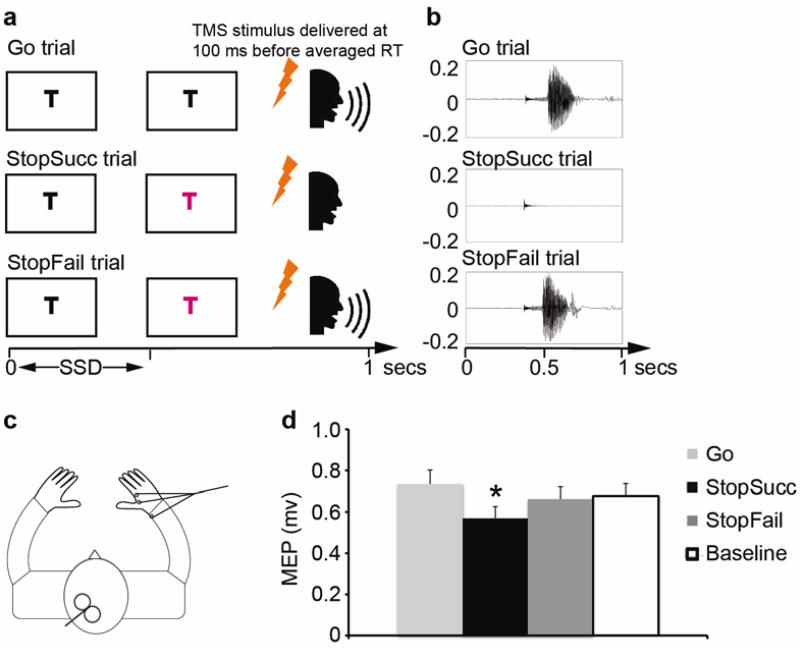
Figure 1.
a. A vocal version of the stop-signal task: participants named a letter “T” or “D” (a go signal) on each trial; on 33% of trials, the letter turned red (a stop signal) and participants tried to cancel the vocal response. TMS stimulation was given at 100 ms before average RT on TMS trials. b. Examples of the sound wave for the Go, StopSucc and StopFail trials with TMS stimulation. Note that TMS stimulation induced a sharp but weak audio signal around 400 ms whereas the human sound wave had a large amplitude and long duration. c. A figure-of-eight coil was placed over the left primary motor cortex and EMG was recorded from the right hand. d. MEP on the StopSucc trials was significantly smaller than on the Go, StopFail and baseline TMS trials (all ps < 0.05).
2. Material and Methods
2.1. Participants
Sixteen adults participated in this study (aged 19-27 yrs, 11 female, 2 left handed). Three participants were excluded from the group analysis because of noisy EMG or over-saturated MEPs. All provided consent according to an Institutional Review Board at the University of California, San Diego, and passed TMS safety screening.
2.2. Task paradigm
In each trial, a letter “T” or “D” in white (a go signal) was presented. Participants were instructed to say the letter as quickly as possible. In 33% of trials, the letter turned red (a stop signal) shortly after the go signal (a Stop trial). Participants tried to withhold their vocal response. The interval between the go and stop signal is called the stop signal delay (SSD). SSD values began at 150 ms and were adjusted dynamically, increasing 50 ms for a successful stop and decreasing 50 ms for a failed stop. Two separate staircases were used for the two letters. The response window was 1 second. The inter-trial-interval (ITI) was jittered between 3 and 3.5 seconds.
Participants performed 5 blocks of the task. In the first block, there were 60 Go and 30 Stop trials without TMS. Behavioral data from this block were used to calculate the mean Go RT. In so doing we could set the TMS stimulation time for the second block at mean Go RT – 100ms (Badry et al., 2009; Majid et al., 2011). This approach assumes that the speed of stopping (stop-signal reaction time, SSRT) is in the range of 150 to 250 ms (Xue, Aron, & Poldrack, 2008), and the race between the go and stop processes is tied; if so, then this stimulation timing should ensure that the TMS pulse is delivered during the stopping process. Subsequent blocks (2 through 5) all included three types of stimulation trials: TMS trials (48 in each block: 32 Go and 16 Stop trials) in which stimulation was given 100 ms before the mean Go RT, noTMS trials (24 in each block: 16 Go and 8 Stop trials) in which no stimulation was given, and baseline TMS trials (6 in each block: 4 Go and 2 Stop trials) in which stimulation was given 200 ms before the go signal (i.e. in the Inter-Trial-Interval). The probability of the stop signal was the same for the three stimulation trial types, so the presence/absence of TMS pulses did not carry any information about whether the trial was a go or stop. After each block, the stimulation time was updated based on the mean Go RT of all completed noTMS trials in the prior blocks. Because we did not know whether TMS stimulation would speed or delay speech, we expected it would be more reliable to estimate the stimulation time using noTMS trials rather than TMS trials.
2.3. Voice input and sound thresholding
Vocal responses were collected through a microphone, which was connected to the experiment computer. An in-house built voice key was made to capture participants' vocal output and measure the onset of vocal response using Psychtoolbox-3 (www.psychtoolbox.org). To differentiate participants' vocal responses from the TMS stimulation, we recorded the sound waves for the letters “T” and “D” and also for TMS stimulation. These sound waves were differentiable in amplitude, duration and shape (Fig 1b). As the amplitude of the TMS sound wave was much smaller than the human voice, a threshold was chosen to trigger the voice key to the voice alone.
2.4. EMG recording
Surface EMG was recorded from the first dorsal interosseous of the right hand (Fig 1c) via 10-mm-diameter Ag-AgCl hydrogel electrodes (Medical Supplies Inc, Newbury Park, CA). A ground electrode was placed over the right radius. The EMG signal was amplified via a Grass QP511 Quad AC Amplifier (Grass Technologies, West Warwick, RI), with a band-pass filter between 30 Hz and 1 kHz and a notch filter at 60 Hz. A CED Micro 1401 mk II acquisition system was used to sample data at 2kHz. Data were displayed and recorded using CED Signal v4 (Cambridge Electronic Design, Cambridge, UK).
2.5. TMS and hotspotting
TMS stimulation was generated with a MagStim 200-2 system (MagStim, Whitland, UK) and a 70-mm figure-of-eight coil. A ‘hotspotting’ procedure was performed to identify the stimulation locus and intensity. The coil was first placed 5 cm lateral and 2 cm anterior to the vertex and repositioned to where the largest MEPs were observed consistently (Fig 1c). Then we measured resting motor threshold which was defined as the minimum stimulation intensity required to induce 0.1 mV peak-to-peak amplitude MEP in 5 out of 10 consecutive stimulations (Rossini et al., 1994). Next, the maximum MEP size was determined by increasing stimulus intensity in 2% increments until the MEP amplitude no longer increased or until stimulation intensity reached 160% of resting motor threshold. Finally, the TMS stimulus intensity was adjusted to produce an MEP that was approximately half of the maximum MEP amplitude. This was the intensity used during the experiment (50 ± 7% maximum stimulator output, which was ∼110% of resting motor threshold: 45 ± 5%).
2.6. MEP analysis
An EMG sweep started 200 ms before stimulation. MEPs were identified from EMG using in house software developed in Matlab (Mathworks, Natick, MA). Trials were excluded if the root mean square EMG in the 100 ms before the TMS stimulus was greater than 0.01 mV. Mean peak-to-peak amplitude of MEPs was calculated for Go trials, successful Stop trials (StopSucc) and failed Stop trials (StopFail) and baseline trials. The top and bottom 10% of MEPs in each type of trials were trimmed (Cai, Oldenkamp, & Aron, 2011; Majid et al., 2011). The global suppression hypothesis predicts smaller MEPs for StopSucc trials than for Go, StopFail and even baseline TMS trials (Badry et al., 2009; Greenhouse et al., 2011; Majid et al., 2011). To test this prediction, paired t-tests were conducted between StopSucc vs. Go, StopSucc vs. StopFail, and StopSucc vs. baseline TMS trials. Multiple comparisons were corrected using the Holm-Bonferroni method (Levin, 1996).
3. Results
3.1. Behavior
Behavioral data are summarized in Table 1. On Go trials, the mean RT was 566 ± 30 ms for TMS and 569 ± 31 ms for noTMS trials. This was not a significant difference, t(12) < 1. For stop trials, the speed of stopping (SSRT) for TMS trials was 207 ± 16ms and for noTMS was 214 ± 21ms. This was not a significant difference, t(12) < 1. Go RT in our study is relatively longer compared to a previous report (Xue et al., 2008). The elongated RT might result from the combination of TMS effect and the stop-signal task. It is possible that some participants applied a waiting strategy. However, we do not think this could possibly affect our main results, e.g. MEP difference for StopSucc vs. StopFail. The fact that behavior was not different for TMS and noTMS trials indicates that TMS over the hand territory of the primary motor cortex has no effect on speech behavior (i.e. there was no startle or distraction) and thus validates our approach of adjusting TMS stimulation with respect to mean Go RT on noTMS trials.
Table 1.
Behavioral measures.
| noTMS | TMS | T | Sig. | |
|---|---|---|---|---|
| Go ACC (%) | 93(3) | 91(3) | 1.76 | 0.10 |
| Go RT (ms) | 569(31) | 566(30) | 0.37 | 0.72 |
| Stop ACC (%) | 50(2) | 48(2) | 0.36 | 0.73 |
| Stop SSRT (ms) | 214(21) | 207(16) | 0.90 | 0.39 |
Go ACC: the accuracy in Go trials; Go RT: the reaction time (RT) in Go trials; Stop ACC: the accuracy in Stop trials; SSRT: stop-signal reaction time (Go RT minus SSD) (Verbruggen & Logan, 2008)
3.2. MEP
Figure 1d shows the MEP data. As predicted, MEPs were significantly smaller on StopSucc than Go, t(12) = 6.08, p < 0.001, Cohen's d = 1.69. This difference was evident for every participant. Further, MEPs on StopSucc were significantly smaller than StopFail, t(12) = 2.57, p = 0.024, Cohen's d = 0.72; and MEPs on StopSucc were also smaller than baseline TMS trials, t(12) = 2.79, p = 0.016, Cohen's d = 0.78. All three tests were significant after the Holm-Bonferroni correction. Notably, analysis of EMG in the 100 ms before TMS showed that the hand muscle was equally at rest in all three conditions before stimulation was given: for the above three comparisons, all ps > 0.12.
Note: further analyses were done to compare Go and StopFail and baseline conditions. MEPs for Go were higher than for StopFail (p=0.04), but this was not significant after multiple comparison correction. MEPs for Go were not higher than baseline (p=0.18), nor were MEPs higher for StopFail than Baseline (p=0.78).
4. Discussion
We measured the corticospinal excitability of the task-irrelevant hand while participants performed a vocal stop signal task. We found that when participants successfully stopped their speech, there was reduced corticospinal excitability of the task-irrelevant hand compared to when they failed to stop, or compared to when they responded on Go trials, or compared to the pre-trial baseline. This extends previous findings that stopping the hand reduces the corticospinal excitability of the task-irrelevant leg (Badry et al., 2009; Greenhouse et al., 2011; Majid et al., 2011) by showing that stopping, at least in this basic paradigm, also has global suppressive effects across response modalities, in this case across vocal and manual motor systems.
An alternative explanation of the MEP reduction from the task-irrelevant hand on successful stop trials could relate to nonspecific brain arousal arising from ‘odd ball’ saliency, orienting or detection of the stop signal. However, we regard this generalized arousal account as very unlikely for several reasons. First, this account should predict that StopFail is different from Go and baseline trials because StopFail is an error trial that should be as arousing or even more so than StopSucc [Note: StopFail trials activate widespread autonomic/error-detection brain regions including the anterior cingulate cortex] (Li et al., 2008). Yet we did not see any difference between StopFail and Go or baseline trials; whereas we did see that StopSucc was different from all of StopFail, Go and baseline trials (also see Majid et al., 2011). Second, there is independent behavioral evidence for global suppression during successful stop trials. When subjects need to stop one manual response rapidly while continuing with another, the continuing response is severely delayed (Aron & Verbruggen, 2008; Coxon, Stinear, & Byblow, 2007). Thus, the ‘global suppression’ observed in the MEP in this and other studies (Badry et al., 2009; Majid et al., 2011) has its counterpart in behavior; which again speaks against a mere arousal account for the MEP findings. Third, we have shown, in a different stop paradigm, that successful stopping of one hand does not lead to MEP suppression of the leg (‘global suppression’) when there is a requirement to stop selectively (i.e. to continue with another hand); but it does lead to leg suppression if the subject has to stop both hands (Majid et al., 2011). This shows that whether there is MEP suppression or not from a task irrelevant effect is not simply related to whether stopping is successful and again speaks against a mere arousal account for the current finding. Taken together, we suggest that the reduced MEPs of the task-irrelevant muscle on successful stop trials is caused by an active suppression mechanism rather than nonspecific arousal induced by saliency, orienting or detection of stop signals.
The current results have implications for better understanding the neural mechanisms of how speech is stopped, and also perhaps for stuttering. Whereas much research with manual stopping points to a three-way network between the pre supplementary motor area, the right inferior frontal cortex and the basal ganglia (perhaps specifically the subthalamic nucleus) (Aron, Behrens, Smith, Frank, & Poldrack, 2007; Chambers, Garavan, & Bellgrove, 2009; Eagle et al., 2008; King et al., 2011; Schmidt et al., 2011), less data speaks to the neural basis for stopping speech. A single fMRI study did show that stopping speech also activated the pre-supplementary motor area and the right inferior frontal cortex (and both these loci overlapped with activation for stopping manual responses), but vocal stopping activation was not evident in the basal ganglia (Xue et al., 2008). Consistent with the importance of the pre-supplementary motor area and the right inferior frontal cortex for stopping speech, macrostimulation of either of these regions in human patients produces speech arrest (Fried et al., 1991; Luders et al., 1988; Swann et al., In Press). Together with previous studies (Badry et al., 2009; Greenhouse et al., 2011; Majid et al., 2011), the current finding that stopping speech leads to corticospinal suppression of the task-irrelevant hand is consistent with the idea that simple stopping recruits a global mechanism with very broad effects on the motor system. This could relate to the recruitment of the subthalamic nucleus. First, this nucleus has been implicated in stopping by fMRI, lesion and neurophysiological studies (Aron & Poldrack, 2006; Eagle et al., 2008; Schmidt et al., 2011). Second, it has been hypothesized that the STN sends a ‘massive pulse’ to the pallidum, thus affecting the motor system generally (Gillies & Willshaw, 1998; Mink, 1996; Nambu, Tokuno, & Takada, 2002). It is interesting in this regard that stutterers showed hyperactivity in the pontomesencephalic region, including the subthalamic nucleus (Watkins, Smith, Davis, & Howell, 2008) and that stimulation of the subthalamic nucleus also produces dysarthria and makes stuttering worse in some Parkinson's patients (Burghaus et al., 2006; Toft &; Dietrichs, 2011). Interestingly, some types of stuttering have also been associated with ‘freezing’ or ‘body immobility’ (Alm, 2004), which could be a sign of global motor suppression.
5. Conclusion
In summary, we showed that stopping speech dampens the corticospinal excitability of the task-irrelevant hand. This suggests that global suppression is not limited to the control of limbs, but it also has a much broader effect across wider motor modalities, including vocal and limb motor systems. We speculate that when people rapidly stop speech, it is possibly implemented through a fronto-basal ganglia pathway, perhaps via the subthalamic nucleus, and this could explain the global suppression effect. We expect that the current methodology could be used to further explore the stopping of speech, both globally and selectively, as well as the relation between stopping speech and other actions (such as gestures). The methodology could also be useful for studies of induced and pathological stuttering, and the relation with whole-body immobility.
We measured corticospinal excitability of the task-irrelevant hand while subjects stopped speech.
When speech was stopped, the task irrelevant-hand was suppressed.
The rapid stopping of speech induces global suppression across the motor system.
Acknowledgments
This work was supported by NIH NIDA Grant DA026452.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alm PA. Stuttering, emotions, and heart rate during anticipatory anxiety: a critical review. J Fluency Disord. 2004;29(2):123–133. doi: 10.1016/j.jfludis.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27(14):3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26(9):2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Verbruggen F. Stop the presses: dissociating a selective from a global mechanism for stopping. Psychological science. 2008;19(11):1146–1153. doi: 10.1111/j.1467-9280.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- Badry R, Mima T, Aso T, Nakatsuka M, Abe M, Fathi D, et al. Suppression of human cortico-motoneuronal excitability during the Stop-signal task. Clin Neurophysiol. 2009;120(9):1717–1723. doi: 10.1016/j.clinph.2009.06.027. [DOI] [PubMed] [Google Scholar]
- Burghaus L, Hilker R, Thiel A, Galldiks N, Lehnhardt FG, Zaro-Weber O, et al. Deep brain stimulation of the subthalamic nucleus reversibly deteriorates stuttering in advanced Parkinson's disease. J Neural Transm. 2006;113(5):625–631. doi: 10.1007/s00702-005-0341-1. [DOI] [PubMed] [Google Scholar]
- Cai W, Oldenkamp C, Aron AR. A proactive mechanism for selective suppression of response tendencies. Journal of Neuroscience. 2011;31(16):5965–5969. doi: 10.1523/JNEUROSCI.6292-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33(5):631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow W. Selective inhibition of movement. J Neurophysiol. 2007;97(3):2480–2489. doi: 10.1152/jn.01284.2006. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol. 2006;95(6):3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C, Hutcheson DM, Lehmann O, Shah AP, Robbins TW. Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb Cortex. 2008;18(1):178–188. doi: 10.1093/cercor/bhm044. [DOI] [PubMed] [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, et al. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11(11):3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies AJ, Willshaw DJ. A massively connected subthalamic nucleus leads to the generation of widespread pulses. Proc Biol Sci. 1998;265(1410):2101–2109. doi: 10.1098/rspb.1998.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse I, Oldenkamp CL, Aron AR. Stopping a response has globalor non-global effects on the motor system depending on preparation. J Neurophysiol. 2011 doi: 10.1152/jn.00704.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AV, Linke J, Gass A, Hennerici MG, Tost H, Poupon C, et al. Microstructure of a three-way anatomical network predicts individual differences inresponse inhibition: A tractography study. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123(Pt 6):1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Levin B. On the Holm, Simes, and Hochberg multiple test procedures. Am J Public Health. 1996;86(5):628–629. doi: 10.2105/ajph.86.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Yan P, Chao HH, Sinha R, Paliwal P, Constable RT, et al. Error-specific medial cortical and subcortical activity during the stop signal task: a functional magnetic resonance imaging study. Neuroscience. 2008;155(4):1142–1151. doi: 10.1016/j.neuroscience.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10(2):276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Luders H, Lesser RP, Dinner DS, Morris HH, Wyllie E, Godoy J. Localization of cortical function: new information from extraoperative monitoring of patients with epilepsy. Epilepsia. 1988;29(2):S56–65. doi: 10.1111/j.1528-1157.1988.tb05799.x. [DOI] [PubMed] [Google Scholar]
- Majid DS, Cai W, George JS, Verbruggen F, Aron AR. Transcranial Magnetic Stimulation Reveals Dissociable Mechanisms for Global Versus Selective Corticomotor Suppression Underlying the Stopping of Action. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43(2):111–117. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Dodani S, Leventhal DK, Pettibone JR, Case AC, Patil PG, et al. Soceity for Neurosceince. Washington, D.C: 2011. Subthalamic nucleus activity during action initiation and suppression in rats and humans. [Google Scholar]
- Sohn YH, Wiltz K, Hallett M. Effect of volitional inhibition on cortical inhibitory mechanisms. J Neurophysiol. 2002;88(1):333–338. doi: 10.1152/jn.2002.88.1.333. [DOI] [PubMed] [Google Scholar]
- Swann NC, Cai W, Pieters T, Claffey MP, George JS, Conners C, et al. roles for the presupplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. Neuroimage. doi: 10.1016/j.neuroimage.2011.09.049. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft M, Dietrichs E. Aggravated stuttering following subthalamic deep brain stimulation in Parkinson's disease--two cases. BMC Neurol. 2011;11:44. doi: 10.1186/1471-2377-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg WP, Burle B, Vidal F, van der Molen MW, Ridderinkhof KR, Hasbroucq T. Mechanisms and dynamics of cortical motor inhibition in the stop-signal paradigm: a TMS study. J Cogn Neurosci. 2010;22(2):225–239. doi: 10.1162/jocn.2009.21248. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn Sci. 2008;12(11):418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131(Pt 1):50–59. doi: 10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex. 2008;18(8):1923–1932. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]