Abstract
Nasonia (Hymenoptera: Pteromalidae) is a genus of parasitoid wasps which is fast emerging as a model system for evolutionary, genetic, developmental and host-endosymbiont interaction studies. Here we report a new species, Nasonia oneida, distinguish its behavioral, genetic and morphological features and characterize its pre-mating and post-mating isolation with the other Nasonia species. Phylogenetic analyses indicate that N. oneida is the sister species to N. giraulti with its own uniquely distinct cuticular hydrocarbon profiles, behavioral characteristics and subtle morphological differences. An important characteristic of N. oneida is the strong mate discrimination shown by the females against all the other Nasonia species. A genetic analysis of this phenotype by interspecies hybridization indicates that this strong discriminating phenotype is recessive. A formal species description of N. oneida is also provided.
Introduction
The parasitoid wasp Nasonia has been used for genetic research for over fifty years (Whiting, 1967) and with the recent genome sequencing for three species (Werren et al., 2009), methods for systemic RNAi (Lynch & Desplan, 2006), and additional genetic tools, is now emerging as a genetic model system (Werren & Loehlin, in press). Evolutionary genetic studies of Nasonia have been conducted on sex-ratio (Werren, 1980; Skinner, 1982), interspecific differences in morphology (Weston et al., 1999; Gadau et al., 1999; Clark et al., in review), hybrid breakdown (Breeuwer & Werren, 1995; Gadau et al., 1999, 2002; Niehuis et al., 2008; Clark et al., in review), host-endosymbiont interactions (Breewuer et al., 1992; Bordenstein et al., 2001, 2006), courtship and mating behaviour (Beukeboom & van den Assem, 2002; Velthuis et al., 2004; Burton-Chellew et al., 2007) and early development (Lynch et al., 2006; Rosenberg et al., 2009). Any additional species in the genus would broaden and strengthen this model system, especially for evolutionary genetic studies.
Until the 1990s only one species was described in the genus, the cosmopolitan N. vitripennis, which is a generalist parasitoid attacking a variety of calyptrate flies in the families Sarcophagidae, Muscidae and Calliphoridae. Darling and Werren (1990) subsequently described two additional sibling species (N. giraulti and N. longicornis) indigenous to North America. N. longicornis is found in the western United States and N. giraulti in the northeastern United States and both are specialists on the calliphorid genus Protocalliphora (bird blowflies). Here we describe a fourth species of Nasonia, N. oneida. We present morphological, genetic and behavioral features of N. oneida and also comment on the population genetics of this new species and its sister species N. giraulti.
Materials and Methods
Nasonia Strains Used
Nasonia are usually collected from bird nests where the wasps parasitize the blowfly pupae which in turn parasitize altricial nestlings. Nests were collected following the fledgling of the nestlings and the blowfly pupae were then sorted from the nest. These were then placed individually in vials and emergence of parasitoids, if any, were recorded. Emerging Nasonia or other parasitoids were collected from them for further analysis. N. oneida was first obtained in 2005 from Brewerton, New York, USA, and subsequently in 2008 from Springville, which is 295 kilometers southwest from Brewerton. Initially, this species was thought to be N. giraulti because the males of N. oneida have large forewings, which distinguishes N. giraulti from its sympatric relative N. vitripennis, where males have small forewings (Darling & Werren, 1990). Moreover, N. oneida and N. giraulti have very similar Wolbachia and mitochondria, reflecting introgression and subsequent sweep of cytotype (Raychoudhury et al., 2009). Thus, N. oneida isolates from the field were initially believed to be N. giraulti until other biological features became apparent. Initial behavioral observations revealed pre-mating discrimination with a standard N. giraulti strain. This led to the discovery of additional phenotypic characteristics of N. oneida and additional field collections, confirming it as a distinct species.
Eleven different strains of N. oneida have been collected from two locations in New York State. The standard laboratory strains of N. oneida are NONY11/36 (O1), which is from Brewerton, New York (USA), while NONYSP1C (O2) is the strain obtained from Springville, New York (USA). Both of these strains were used for behavioural observations against standard laboratory strains from the other three species. Since, in our preliminary analysis N. giraulti appeared to be a very close relative of N. oneida we used two different strains from both N. oneida and N. giraulti for the behavioral analysis to rule out the possibility that features unique to these two taxa are not strain specific but are characteristic of the species. These two N. oneida strains were used against two different N. giraulti strains, NGRV2X (G1) which is the standard strain for the species and a recently collected strain called NGNY6A5, which was obtained in 2005 from New York (G2). For N. vitripennis and N. longicornis, strains AsymCX and NLCA9304 were used. For phylogenetic analysis of the nuclear data we used multiple strains from all the four species (summarized in Supplementary table 1). O1 was cured of its Wolbachia infection to produce the strain O1U and was used for hybridization studies with strains from the other three species.
Evolutionary and Phylogenetic Analysis of DNA Sequences
DNA was extracted from a single female insect per strain using the DNAeasy kit (Qiagen, Valencia, CA). Nine nuclear genes were sequenced for this study: casein kinase, lipase, arp 2/3 complex, opsin I, phosphoglucose isomerase, cAMP-dependant protein kinase, ATP synthase coupling factor F, fumarylacetoacetate and a sugar transporter. The primers sequences and conditions are described in Raychoudhury et al. (2009). To test for divergence in mitochondria, the cox1 region was used with primers and conditions described in Oliveira et al. (2008). NCBI accession numbers for each fragment from all the strains are summarized in supplementary table 1. To clean the reactions before sequencing, amplified reactions (8uL) were incubated with 0.5 U shrimp alkaline phosphatase and 1.0 U of exonuclease I (Amersham, Piscataway, NJ) along with the supplied buffer. Sequencing was performed directly from the amplified products using a BigDye v3.0 terminator sequencing kit and an ABI 3700 or 3730xl (Applied Biosystems, Foster City, CA) automated sequencer. The chromatograms generated were manually inspected and cleaned with Sequencher (Gene Code Corporation, Ann Arbor, MI) and the sequences were aligned with Bioedit vs 7.0.1 (Hall, 1999). The entire sets of nuclear sequences were concatenated and indels removed. Bayesian maximum likelihood trees were constructed using this concatenated data for the nuclear genes with Mr. Bayes v 3.1.2 (Ronquist and Huelsenbeck, 2003). We used the web based application Find Model (http://hcv.lanl.gov/content/hcvdb/findmodel/findmodel.html) to find the best fitting model for sequence evolution, which was the general time reversible model with gamma distributed rate variation (GTR+Γ). For phylogenetic analysis with Mr. Bayes, the Markov chains were run for at least 1,000,000 generations, sampling every 10 generations until the standard deviation of the split frequencies were below 0.01. The first 25% of the generations were discarded as burn-in and the resultant 50% majority-rule trees were visualized in Treeview v1.6.6 (Page, 1996). Analyses of the different molecular genetic parameters were performed using DNAsp vs 4.10.2 (Rozas et al., 2003). A median-joining network of sequences was constructed with Network (http://www.fluxus-engineering.com/sharenet.htm) for the cox1 sequences as well as the concatenated data set for the nuclear markers.
Mate Discrimination
A series of no-choice assays with N. oneida females were performed to establish the levels of pre-mating isolation with the other three species. Single males and females of fixed age (1-2 days post-emergence) were put together in 12mm glass culture vials for ten minutes in a no-choice trial and mating were observed directly. Female rejection was scored when the male initiated courtship display but the female did not become receptive (Velthuis et al., 2004). If, during the ten minutes of observation, no courtship display was performed by the males, then that data was removed from the final analysis. We also tested the mate preference of interspecies hybrid females. F1 hybrid females were produced by interspecies crosses between males from O1U and females from the other three species and vice versa. These reciprocal crosses resulted in F1 hybrid females with the cytoplasm of both N. oneida and each of the other three species. Since the acceptance of N. oneida females for heterospecific males was very low, we used Fisher’s Exact Test, in preference to Chi Square, for statistical analysis of these data.
F2 hybrid male breakdown and Wolbachia induced post-zygotic incompatibility
In Nasonia there have been previous reports of F2 hybrid male mortality due to interspecific genetic incompatibility (Breeuwer & Werren, 1995). To test whether such incompatibility exists with N. oneida and the other species, we conducted reciprocal hybrid crosses between N. oneida and the other three species and quantified the number of F2 males. Because of its haplo-diploid sex determination, Nasonia females produce only haploid sons as virgins. Since these males are haploid, any recessive hybrid incompatibility loci causing inviability will result in a reduction in the number of F2 male offspring. Thus, the mean number of adult male offspring was used to determine whether N. oneida show any F2 hybrid male breakdown with the other species. Females of O1U who successfully mated with the other three species in the mate discrimination assay were hosted individually. F1 hybrid virgin females from these crosses were each hosted singly with two Sarcophaga bullata pupae for three days and the F2 males were allowed to emerge and were then counted.
We tested whether Wolbachia induces any post-zygotic incompatibility between N. oneida and N. giraulti, as it does with the other species of Nasonia (Breeuwer & Werren, 1990; Bordenstein et al., 2001). Infected males and females from both the species were crossed and the females which successfully mated were hosted individually with two Sarcophaga bullata pupae for two days. The females were then removed and the progeny allowed to emerge and were then counted. All the Nasonia strains were maintained in standard lab conditions of 25o C, twenty-four hours light for two weeks.
Morphological measurements
N. oneida males and females were examined for species specific morphological features. Females of all Nasonia species are difficult to distinguish, whereas males are morphologically distinct (Darling & Werren, 1990). This is also true for N. oneida because male antennae and wings are relatively easy to distinguish. Wing measurements were done with a protocol refined from Weston et al. (1999). Individuals were reared in uncrowded conditions and single females were provided with two Sarcophaga bullata hosts for 48 hours. Forewings were dissected at the hinge and mounted dry on glass slides under cover slips. Heads were mounted on double-sided cellophane tape on the mandible. Wings were digitized using a Zeiss AxioImager Z1 and Zeiss AxioVision 4.6 software (Carl Zeiss) at 10x as mosaic images. Heads were digitized at 4x taking advantage of green autofluorescence of the ocelli (Loehlin et al., in review).
Forewing length was measured from digital images as the distance between the notch that forms at the proximal edge of the hinge and the distal tip of the wing. Width was measured as the width of a box drawn perpendicular to the length axis that contained the most anterior and posterior points of the forewing. Head width, an approximation of body size (Weston et al., 1999), was measured as the distance between the inner edges of the compound eyes at the posterior edge of the paired ocelli. A composite measurement, the normalized wing multiple, avoids the observed correlation between wing size and body size (Weston et al., 1999; Gadau et al., 2002). Normalized wing multiple is calculated as (wing length * wing width / head width). ). Length to width ratios of male antennal flagella were also measured.
Cuticular hydrocarbon profiling with gas chromatography/mass spectrometry (GC-MS)
Cuticular hydrocarbons (CHC), secreted on the insect cuticle primarily for desiccation prevention (Gibbs 1998), have been shown to play major roles in inter- and intraspecific chemical communication in various insect species (Ayasse et al., 2001; Howard & Blomquist, 2005). In many cases, CHC profiles clearly show sex- and species-specific differences, and distinct CHC components serve as signals for species recognition (Singer, 1998; Thomas & Simmons, 2008) and sexual communication (Ferveur, 2005). We tested the CHC profiles in both sexes of N. oneida and N. giraulti in an effort to determine whether the profiles support separation of these two as distinct species. Males and females from 3 strains of each species (N. oneida strains: NONYBR6A, NONYBR6B, NONY11/32; N. giraulti strains: NGNY6X, NGNY7B, NGVA7B) were analyzed amounting to 119 individuals in total. The wasps were freeze-killed and stored individually in glass vials at −20°C after emergence from their Sarcophaga bullata hosts. For cuticular hydrocarbon extraction, each wasp was placed for 10 minutes in 10μl hexane. Obtained extracts were then transferred into a fresh vial and reduced by evaporating the solvent with gaseous nitrogen to ~ 1μl. The concentrated extracts were subsequently injected into a gas chromatograph coupled with a mass spectrometer (GC: 7890A; MS: 5975C; Agilent Technologies, Waldbronn, Germany) operating in electron impact ionisation mode. The entire sample was injected in a split less injector in the split less mode for 60 seconds with an injector temperature of 250°C. Separation of compounds was performed on a fused silica capillary column (HP-5ms; Agilent Technologies, Waldbronn, Germany) coated with a 0.25μm (5%-phenyl)-methylpolysiloxane stationary phase with temperature program starting from 60°C and increasing by 40°C per min to 200°C, followed by an increase of 5°C per min to 320°C. Peak area integration and calculation was performed using the data analysis software from Enhanced Chemstation G1701AA, Version A.03.00 (Hewlett-Packard Company, Palo Alto, CA).
For data analysis the absolute peak area values were divided by the total sum of all peaks (44 CHC peaks detected in total) to normalize the data and eliminate the effects of the fluctuations in the extracted quantities. Thus, peak ratios relative to the total CHC peak area sum were obtained for each peak in each individual, which were used for all subsequent analysis. A discriminant analysis (DA) was performed using SPSS 11.01 for windows (SPSS, Chicago, IL) to access the potential of the CHC profile differences to discriminate strains, species and sexes, amounting to 12 predefined groups. Wilks’ lambda and the percentage of correct assignments of individuals to the respective groups as well as the percentage of correct classification in leave-one-out cross-validation were used as measurement of the quality of the DA.
Results
Population genetics and divergence
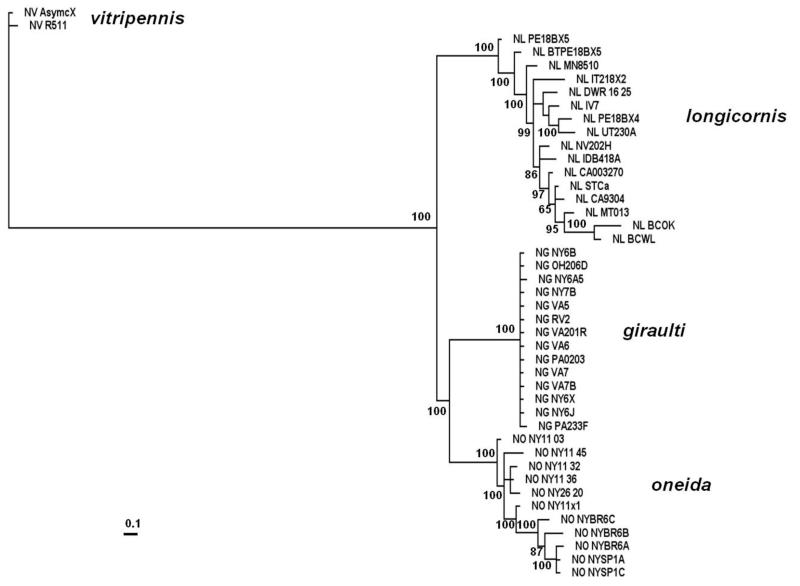
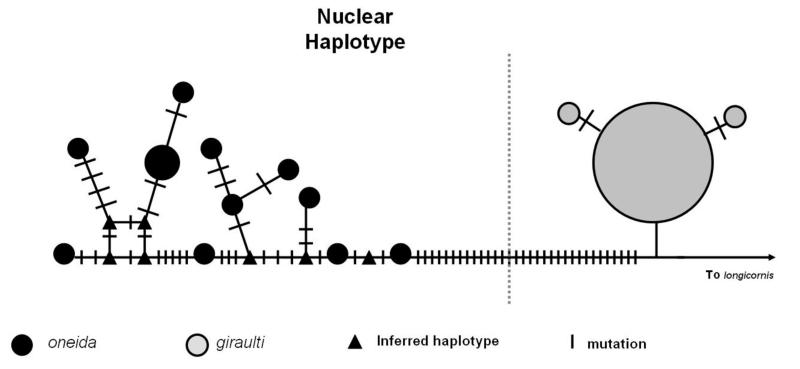
Nuclear sequences were obtained from eleven different N. oneida strains, thirteen different N. giraulti strains from four different states in the US along with sixteen different N. longicornis strains and two N. vitrpennis strains (Supplementary table 1). Sequences from nine nuclear markers were concatenated to give a dataset of 5408 base pairs. The tree of these concatenated sequences (figure 1) clearly shows N. oneida to be a distinct, reciprocally monophyletic lineage of Nasonia, most closely related to N. giraulti (posterior probability 100). Moreover, the nuclear haplotype network also clearly delineates N. oneida from its closest relative N. giraulti (figure 2A). We estimated the total pairwise divergence between N. oneida and N. giraulti to be 0.80%, between N. oneida and N. longicornis to be 0.88% and between N. vitripennis and N. oneida to be 2.65% (summarized in table 1).
Figure 1.
The MrBayes tree of the concatenated nuclear data set comprising of nine genes (5408 base pairs), showing N. oneida to be an independent but closely related lineage of Nasonia.
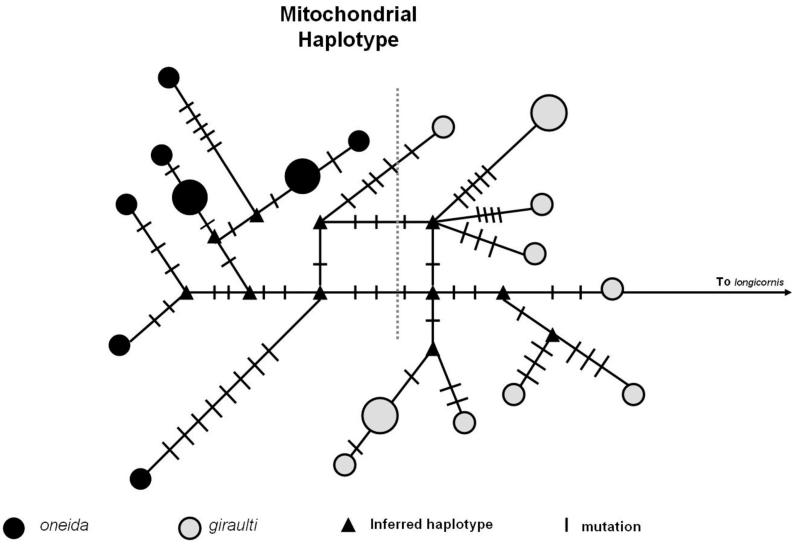
Figure 2.
Haplotype networks of concatenated sequences for the nuclear genes (2A) and the mitochondrial cox1 gene (2B). The size of each node represents its frequency (not to scale). The haplotypes representing the two species are separated by a dashed line.
Table 1.
Measure of the divergence of N. oneida with the other three species in synonymous, non-synonymous and intronic nucleotides for both nuclear and mitochondrial DNA. Results are based on 2652 base pairs of coding sequences and 2424 base pairs of intronic sequences obtained from nine nuclear markers and 1000 base pairs of the mitochondrial cox1 gene. All values are corrected with Jukes and Cantor method (Jukes & Cantor 1969). [O= N. oneida, G= N. giraulti, L= N. Longicornis, V= N. Vitripennis]
| O/V | O/L | O/G | ||
|---|---|---|---|---|
| Synonymous sites | 0.0470 | 0.0101 | 0.0091 | |
| Nuclear genes |
Non-synonymous
sites |
0.0053 | 0.0088 | 0.0036 |
| Intronic sites | 0.0474 | 0.0125 | 0.0126 | |
| All sites | 0.0265 | 0.0088 | 0.0080 | |
|
Mitochondrial
COI |
Synonymous sites | 0.6963 | 0.4660 | 0.0570 |
|
Non-synonymous
sites |
0.0148 | 0.0054 | 0.0001 | |
| All sites | 0.1231 | 0.0869 | 0.0124 |
The Nasonia lineage is presumed to have split relatively recently. Campbell et al. (1993) used the differences in the ITS2 region of the 28S rDNA locus and estimated the divergence between N. longicornis and N. giraulti to be 0.4 million years ago (MYA). Raychoudhury et al. (2009) used a rate of 2.2% synonymous divergence per million years (from Tamura et al., 2004) and estimated the same split to be 0.51 MYA. Using the same rate of synonymous divergence, the N. oneida and N. giraulti split is estimated to be around 0.41 MYA and the N. oneida and N. longicornis split is estimated to be 0.46 MYA. Clearly, these three are closely related species with relatively recent divergences.
The total pair wise divergence in the mitochondria between N. oneida and N. longicornis is 8.69% whereas the difference between N. oneida and N. giraulti is low, 1.24% (table 1). There is evidence of a prior interspecies mitochondrial introgression between N. oneida and N. giraulti (Raychoudhury et al., 2009). Nevertheless, mitochondrial haplotypes can be sorted by species (figure 2B) and there are no shared mitochondrial haplotypes between the two species. Therefore, there has been sufficient time since the mitochondrial introgression for unique changes to accumulate in the N. oneida mitochondria including six different synonymous changes that are fixed between the species.
Differences in wing size and male antennae
N. oneida forewings are slightly different in size from N. giraulti, in a pattern of elevated sexual dimorphism. Using the composite measurement of normalized wing multiple, which corrects wing area for head size, N. oneida male forewings are significantly smaller than N. giraulti males (n=20, Student’s t, p < 0.01), with a mean difference of 5%. In contrast, N. oneida female forewings are 3% larger than N. giraulti (n=20, Student’s t, p< 0.01). This result is consistent with the pattern of differential sexual dimorphism for forewing size observed between the other Nasonia species (Darling & Werren 1990). N. oneida male antennal flagella also differed significantly in their length to width ratios from those of N. giraulti (n=12, Student’s t, p < 0.0001). While the mean ± 1 standard deviation for N. oneida was 10.43±0.52, while for N. giraulti it was 8.30±0.27.
Cuticular Hydrocarbon (CHC) Profiles
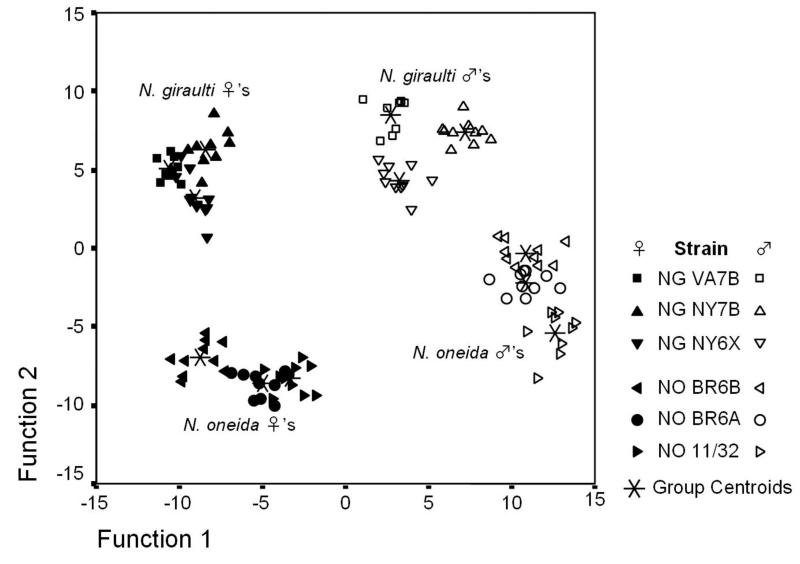
We conducted a CHC profile comparison of males and females from N. giraulti and N. oneida using three different strains from each species. The CHCs of N. oneida and N. giraulti show a strong separation in both sexes (summarized in figure 3).
Figure 3.
Discriminant analysis of cuticular hydrocarbon profiling of N. oneida and N. giraulti, showing clear separation by sex and species.
The discriminant analysis performed on the relative abundances of all identified 44 CHC compounds significantly differentiated the 12 pre-defined groups according to sex and species (Wilk’s λ < 0.0001, χ2 = 1671.79, p < 0.0001). Function 1 accounted for 54 % of the total variance, clearly discriminating the sexes of each species, while function 2, accounting for 29.1 % of the total variance, separated the six different strains into sharply distinguishable species clusters (figure 3).
Mate Discrimination
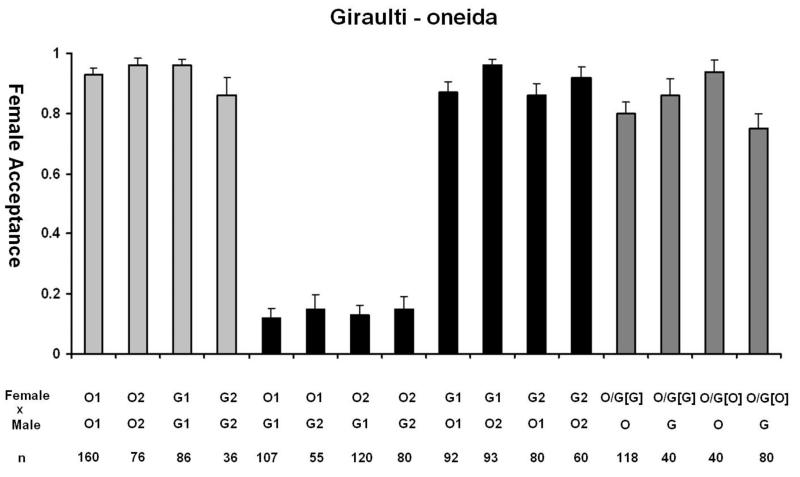
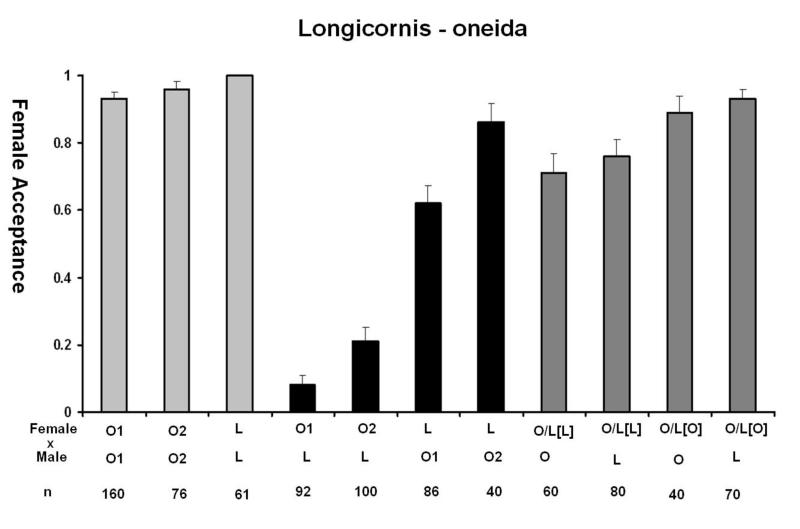
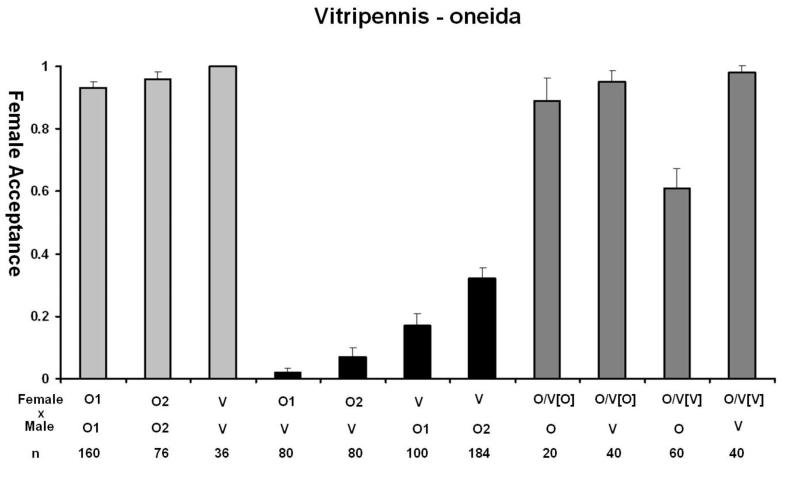
One of the primary evidences that speciation has occurred is the presence of pre-mating isolation, which in most cases, is the first barrier to evolve (Coyne and Orr, 2004). N. oneida shows a distinct pattern of female mate discrimination against the males of the other species. This was the first character that alerted us to its difference with N. giraulti. In figure 4A we summarize the data for the behavioural isolation seen with N. giraulti. Females show a strong sexual isolation against heterospecific males. O1 and O2 females accept only 12% and 13% of G1 males, significantly less than in the reciprocal cross, where G1 females accept 87% and 96% of O1 and O2 males (Fisher’s Exact test, p<10−6). Acceptance of G2 males by the two N. oneida strains was also low, 12% and 15%, while G2 female accepted 86% and 92% males from O1 and O2, respectively (compared to within-species controls, FET in both p< 10−6). Thus, N. oneida females show strong sexual isolation against N. giraulti males but N. giraulti females do not. Similarly, with males of N. longicornis and N. vitripennis, O1 and O2 also show strong discrimination and only accept 8% and 21% of N. longicornis males and 2% and 7% of N. vitripennis males, respectively (Figure 4B and 4C). Therefore, N. oneida females show strong sexual isolation against the males of the other three species. In its native range in New York State, N. oneida is sympatric with N. vitripennis and N. giraulti, and this high level of mate discrimination probably plays a role in maintaining genetic integrity of the species.
Figure 4.
Behavioral observations of N. oneida (O) with A) N. giraulti (G), B) N. longicornis (L) and C) N. vitripennis (V), showing N. oneida females to be strongly isolated from the other three species. F1 hybrids between species have significantly reduced levels of mate discrimination. Hybrid crosses were done in both directions and cytoplasm of F1 hybrids are indicated by []. Two different N. oneida and N. giraulti strains were used for these observations (O1, O2 and G1, G2, respectively) while hybrid females were established only with the Wolbachia-free strain O1U. (Light grey bars: acceptance of conspecific males. Black bars: acceptance of heterospecific males. Dark grey bars: hybrid female acceptance.) See text for details.
The genetic nature of the mate discrimination phenotype of N. oneida females was further investigated using F1 females from successful inter-species crosses. Results indicate that F1 hybrid females lose their discriminating phenotype. F1 hybrid females (with N. oneida cytoplasm) accept N. giraulti males 75% of the time, significantly greater than the 12% acceptance seen in N. oneida females (compared to the acceptance of N. oneida, FET, p< 10−6). Hybrid females with N. giraulti cytoplasm also do not discriminate against N. giraulti males. Similarly, F1 females with both types of cytoplasm did not discriminate significantly against N. oneida males. This indicates that the mate discrimination phenotype is recessive because female discrimination against heterospecific males significantly decreases in heterozygotes. A similar pattern is also seen with N. longicornis and N. vitripennis (summarized in figure 4B and 4C, respectively).
Post-zygotic F2 male hybrid breakdown
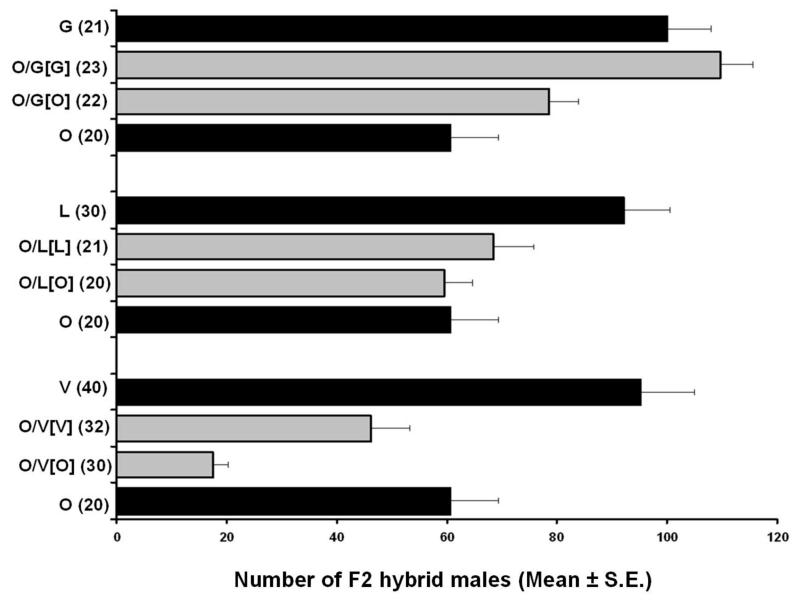
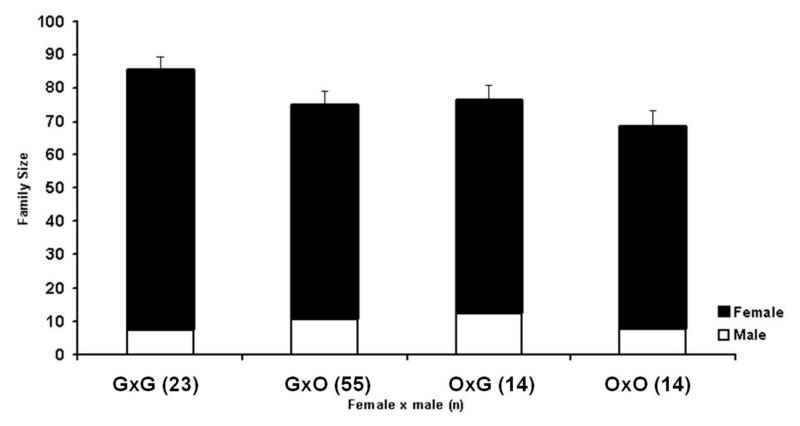
Nasonia species, although mostly inter-fertile after treatment with antibiotics to cure the resident Wolbachia infections, do show partial F2 hybrid male breakdown (Breeuwer & Werren, 1995) and thus some degree of intrinsic post-zygotic isolation. Post-zygotic isolation is most pronounced between N. vitripennis and N. giraulti (Breeuwer & Werren, 1995) and is visible as a reduction in the number of F2 male progeny of the hybrid females. Post-zygotic isolation of N. oneida with all the other three species was investigated by crossing Wolbachia-free O1U females with Wolbachia-free males of the other three species and vice-versa, and then checking for reduction in the mean number of F2 hybrid sons of the hybrid virgin females (figure 5). There is significant hybrid breakdown between N. vitripennis and N. oneida irrespective of the cytoplasm of the F1 females (Mann Whitney U-test, for N. oneida cytoplasm U=599, p<0.001, and in N. vitripennis cytoplasm, U=1225.5, p<0.001).With N. giraulti, there was significant difference in mean number of adult hybrid males relative to the control (MWU, for N. oneida cytoplasm U=351.5, p<0.001, and for N. giraulti cytoplasm, U=478, p<0.001). However, the effect was in the opposite direction from what was expected, as there was a significant increase in the number of hybrid males relative to the within species controls. Crosses between N. longicornis and N. oneida show an asymmetric effect on the F2 offspring number. No reduction in hybrid F2 male number is seen in the N. oneida cytoplasm relative to pure N. oneida virgins (MWU, U=210, p=0.59), but in the N. longicornis cytoplasm there is a significant reduction in F2 hybrid males relative to pure N. longicornis (MWU, U=531, p<0.001). Thus, we can conclude that N. oneida has significant levels of intrinsic post-zygotic isolation with N. vitripennis, asymmetric and unidirectional isolation with N. longicornis and little or none with N. giraulti.
Figure 5.
Mean numbers of male offspring obtained from virgins of the four species and their hybrids. Family sizes of hybrid females are indicated with grey bars. (V, G, L, and O are N. vitripennis, N. giraulti, N. longicornis and N. oneida respectively).
Hybridizations were done in both directions and cytoplasm is indicated by []. See text for details.
Wolbachia induced post-zygotic isolation
One of the main isolating barriers among Nasonia species is the endosymbiont Wolbachia which causes post-mating isolation amongst the species (Breeuwer et al., 1992; Bordenstein et al., 2001). Raychoudhury et al. (2009) previously showed that the three strains of Wolbachia in N. oneida are identical in sequence to N. giraulti, and probably were introduced by hybrid introgression between the species. We tested for Wolbachia-induced cytoplasmic incompatibility by crossing infected N. oneida and N. giraulti. As expected there is no detectable level of incompatibility (figure 6) due to the presence of Wolbachia (MWU, U=606, p=0.07). This indicates that Wolbachia induced cytoplasmic incompatibility does not play a major role in post-zygotic isolation between N. oneida and N. giraulti, at least under laboratory conditions.
Figure 6.
Crosses between Wolbachia infected N. oneida (O) and N. giraulti (G) showing no effects of cytoplasmic incompatibility between the species.
Nasonia oneida (Desjardins and Raychoudhury): Species Diagnosis
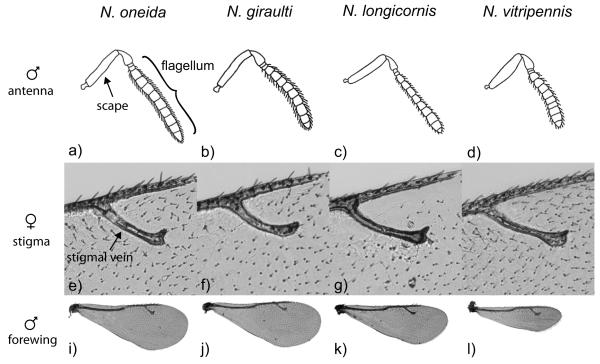
Both sexes of N. oneida differ from N. vitripennis and N. longicornis by antennal structure; N. oneida has an angulate antennal scape (as seen in figure 7 of male antennae) similar to N. giraulti, while N. vitripennis has a spindle-shaped scape and N. longicornis has a cylindrical scape. Females of N. oneida can also be distinguished from N. vitripennis by a lack of setae on the stigma and distal margin of the forewing, although N. giraulti and N. longicornis also lack these setae to varying extents. Females of N. oneida are difficult to distinguish from its closest sister species N. giraulti by morphology. N. oneida females tend to have a slightly curved stigmal vein, which is more similar to the straight stigmal vein of N. vitripennis than to the strongly arched stigmal vein of N. giraulti or the elbowed stigmal vein of N. longicornis (see figure 7), although limited overlap does occur between all the three species. Males of N. oneida are easily distinguished from N. vitripennis by their broad and rounded forewings, whereas N. vitripennis has small forewings and N. longicornis has triangular forewings (see figure 7). N. oneida males can be additionally distinguished from N. longicornis by their long funicular setae (see figure 7). N. oneida males can also be distinguished by their long and slender antennal flagellum (>9.5X as long as wide), whereas N. giraulti and N. vitripennis male flagellum are shorter and wider (<8.8X and <8.5X as long as wide, respectively; see figure 7). N. longicornis males have an intermediate flagellum which is difficult to distinguish from any of the other species. N. oneida males can also be distinguished from N. giraulti by the coloration of the dorsal surface of the mesosoma, which is green in N. oneida and reddish in N. giraulti, although the colors sometimes fade after death.
Figure 7.
Morphological differences between N. oneida and the other Nasonia species. N. oneida has an angulate scape (a) as does N. giraulti (b), while N. vitripennis has a spindle-shaped scape and N. longicornis has a cylindrical scape. N. oneida has the narrowest male antennal flagellum (a), with a length to width ratio of ~10.4:1 (a). N. longicornis, N. giraulti and N. vitripennis have progressively wider antennal flagella, with length to width ratios of ~9.5:1, ~8.3:1, and ~8.0:1, respectively (b, c, and d). N. oneida females tend to have a slighty curved stigmal vein on the forewing (e), which is more similar to the straight stigmal vein of N. vitripennis (h) than to the stongly arched stigmal vein of N. giraulti (f) or the elbowed stigmal vein of N. longicornis (g). The forewings of N. oneida males are broad and rounded (i), similar to those of N. giraulti (j), while the forewings of N. longicornis are triangular (k) and those of N. vitripennis are short and narrow.
Type Material
All type material comes from strain NONYSP1C, which was derived from female wasps which emerged from a single Protocalliphora pupa collected from a tree swallow nest in Springville, New York, on 08/01/08 by Richard Wells of the New York State Bluebird Society. One holotype female and seven paratype females and four paratype males have been deposited in the United States National Museum in Washington, DC. Two paratype females and one paratype male are deposited at each of the British Museum of Natural History, London, UK and the Royal Ontario Museum, Toronto, Canada.
Etymology
Named for Lake Oneida, on the shores of which the first specimens of the species were collected.
N. oneida type description
A detailed description of genus-level characters is given in Darling and Werren (1990). Female: Length: 2.3 mm. Head: Antenna: scape cylindrical in lateral view; scape: pedicel: F1:F2: club: flagellum 5.9:2.1:1.2:1:2.7:9.7, F1 and F2 0.7x as long as wide. Mesosoma: dorsellum with upper margin not scalloped, sinuately emarginated along midline. Forewing : 2.2x as long as wide, with marginal setae only along posterior wing margin, marginal vein: postmarginal vein: stigmal vein 1.8:1.2:1, stigmal vein relatively straight, only slightly arched toward postmarginal vein, distance from stigma to postmarginal vein 0.6x length of stigmal vein. Male: as female except: Length: 2.2 mm. Head: Antenna: scape:pedicel:F1:F2:club:flagellum 4.6:1.5:1:1:2.8:9.4, flagellum 10.5x as long as wide, club 3x as long as wide, antennal setae 0.7x as long as F2. Malar space expanded lateroventrally, cheek-like. Mesosoma: dorsally with greenish coloration. Forewing: 2.4x as long as wide, marginal vein: postmarginal vein: stigmal vein 2:1.7:1.
Discussion
In this study we establish that N. oneida is a distinct species in the genus Nasonia, and is a sister species to N. giraulti. N. oneida can be distinguished by genetic divergence from the other three species (figure 1), distinct cuticular hydrocarbon profiles in both sexes (figure 3) separating it from N. giraulti, subtle morphological differences (figure 7) and asymmetric mate discrimination against the other species (figure 4).
A distinctive feature of N. oneida is strong, but mostly, asymmetric pre-mating isolation. Even in no-choice situations N. oneida females strongly discriminate against N. giraulti males, while N. giraulti females readily mate with N. oneida males. One of the ways such isolation could have evolved is selection against interspecific hybrids, also known as reinforcement (Butlin, 1989). Evidence that hybridization between these two species has occurred in the past comes from the fact that they share similar mitochondria which could have been a result of Wolbachia-mediated introgression (Raychoudhury et al., 2009). Moreover, N. oneida is sympatric with N. vitripennis and N. giraulti in its native range and now also with N. longicornis (Raychoudhury & Werren, unpublished). Thus, it seems plausible that such a sympatric distribution increased the probability of hybridization, setting the stage for reinforcement to occur. However, we could not detect any intrinsic post-zygotic isolation (hybrid breakdown) with N. giraulti, at least under laboratory conditions. Moreover, Wolbachia does not seem to be a cause of post-mating isolation between N. oneida and N. giraulti and can be ruled out as a causal feature currently selecting for reinforcement, in contrast to the role of Wolbachia demonstrated in Drosophila recens and D. subquinaria (Jaenike et al., 2006). Therefore, at least in laboratory conditions, we could not detect a significant cost to hybridization with N. giraulti. This does not rule out significant levels of reduced hybrid fitness in the ecological context, which would not be detected under laboratory conditions. Several studies indicate that ecological isolation can precede intrinsic isolating effects such as hybrid sterility and inviability (Schluter, 2001).
Unlike N. oneida females, N. giraulti females do not show a pronounced mate discrimination against N. oneida males. G1 females accept O1 and O2 males 87% and 96% of the time, while G2 females accept them 86% and 92% of the time (FET, p=0.48). This lack of mate discrimination is consistent with earlier studies between N. giraulti and N. longicornis (Bordenstein et al., 2001). This perhaps, can be explained by a unique biological feature of N. giraulti. Mating typically takes place inside the host (Drapaeu & Werren, 1999), and therefore there may not be strong selection for female mate discrimination to evolve because opportunities for heterospecific mating are low. More information regarding the biology of N. oneida needs to be established before a comprehensive hypothesis is put forward to explain the evolution of this strong mate discrimination.
Despite its recent discovery and collection from only two sites in New York, there is considerably more genetic variation in N. oneida than in all N. giraulti collected from throughout the northeastern United States. As figure 2A indicates there is a very low level of variation in N. giraulti compared to N. oneida. This perhaps is an indication of N. giraulti going through a severe bottleneck sometime in its recent past. In contrast, there is some variation in N. giraulti mitochondria as indicated by figure 2B. This can be explained by the rapid mutation rates in Nasonia mitochondria relative to the nucleus. Oliveira et al. (2008) found that Nasonia mitochondrial genes evolve at a rate that is 35 times greater than the nuclear genes. Therefore, looking at the pattern of nuclear and mitochondrial haplotype networks, what we can surmise is that N. giraulti experienced a bottleneck sufficiently long ago to allow the accumulation of some level of variation in its mitochondria but not in its nuclear genes. Severe inbreeding within the host may have maintained an effectively clonal species of N. giraulti subsequent to the bottleneck. The data also indicate that N. oneida is not an artificial (anthropogenic) introduction in New York, as such an event would produce a genetic haplotype structure with significantly less variation than observed. Levels of nuclear variation in N. oneida (figures 1 and 2A) in just two locations in New York are similar to that found in N. vitripennis and N. longicornis (Werren et al., 2009) indicating comparable effective population sizes. In contrast N. giraulti, despite its widespread geographic distribution in eastern North America, appears to be effectively a clonal population.
Raychoudhury et al. (2009) estimated the synonymous divergence between N. longicornis and N. giraulti to be 1.12% and in this study we estimate the synonymous divergence between N. longicornis and N. oneida to be 1.01% and that between N. giraulti and N. oneida to be 0.91%. Thus, the levels of synonymous divergence between these three species are very similar. This indicates that these species diverged at approximately the same time. Nevertheless, phylogenetic analysis indicates that N. oneida and N. giraulti are sister species with strong bootstrap support (figure 1), due to a number of shared fixed mutations present in N. oneida and N. giraulti but absent in N. longicornis. A slight asymmetric F2 hybrid breakdown also occurs between N. oneida and N. longicornis but not between N. oneida and N. giraulti (figure 5), which is further evidence that N. oneida is biologically closer to N. giraulti than it is to N. longicornis. It is possible that the similarity between N. oneida and N. giraulti is due to an ancient hybridization event between these species. A recent hybridization event between N. longicornis and either N. oneida or N. giraulti to create a the third “hybrid species” is unlikely because 7 of nine genes show higher similarity between N. oneida and N. giraulti, one is ambiguous (due to low level of polymorphism) and only one joins N. oneida and N. longicornis. In no cases do individual gene sequences from N. oneida and N. giraulti fall within the N. longicornis clade, which would be expected if hybridization occurred recently. We conclude that the nuclear sequence information indicates that these three species diverged approximately at the same time (0.4 – 0.5 MYA), but that N. giraulti and N. oneida are sister species.
In contrast to the nuclear data the mitochondrial data suggest a more recent mitochondrial sweep between N. oneida and N. giraulti, probably due to Wolbachia induced cytoplasmic incompatibility. There are no divergence among 6 Wolbachia genes of these three species and the mitochondrial sequences are similar (Raychoudhury et al., 2009). This Wolbachia-mitochondrial hybridization event is estimated to have occurred approximately 0.06 – 0.07 MYA, based on rates of synonymous mitochondrial divergence for Nasonia (76.3 – 94.9 % per MY, Raychoudhury et al., 2009) and level of synonymous mitochondrial divergence between the species (5.7%, table 1). We conclude that the hybridization leading to the mitochondrial-Wolbachia sweep did not lead to a general nuclear genetic admixture of the species, as also argued in Raychoudhury et al. (2009). Given the strong cytoplasmic drive caused by Wolbachia, the sweep could readily have occurred from a single or few hybridization events, which would result in little genetic admixture. However, complete genome sequencing of N. oneida (underway) will reveal whether there are signatures of low levels of hybridization within the genome.
A key question about the biology of N. oneida is ‘where did it come from?’ The first specimens were found in Brewerton, New York, in 2005, a site that has been relatively well investigated by field collections for Nasonia since 1987. Therefore, the sudden appearance of N. oneida in the Brewerton collection site is surprising. This suggests that this species is a recent migrant to the area. An alternate explanation is that we had previously incorrectly identified N. oneida collected from Brewerton as N. giraulti because, as mentioned above, these two species have similar morphological features. However, no existing N. giraulti field strains in the laboratory, collected prior to 2005, are N. oneida, indicating that N. oneida was not collected before 2005. Field collections done in New York in 2005 yielded both species, but since then we have failed to obtain N. giraulti from New York. This also coincides with the discovery of N. longicornis as an introduced population in New York (Raychoudhury & Werren, unpublished). Thus, there is a complex scenario of Nasonia distribution in New York. All the four species now seem to be sympatric here, contrary to the earlier estimated presence of just two, N. vitripennis and N. giraulti (Darling & Werren, 1990). Whether the introduction of N. longicornis had any role to play in our inability to obtain N. giraulti and the discovery of N. oneida is difficult to say but these two phenomena may not be coincidental. A thorough field study in upstate New York extending to Canada and other areas is necessary to determine the geographic range and history of N. oneida.
Nasonia belongs in the family Pteromalidae which has more than 3,500 described species (Noyes, 2003) and is a member of the super family Chalcidoidea, a group containing over 22,000 described species (Noyes, 2003). So, it is not surprising that new species are being found. But what is counter-intuitive is that studies of Nasonia in such well investigated sites, such as those in New York State, can still yield such novelties. Our understanding of the distribution and diversity of Nasonia is clearly incomplete and is in flux. New species are being found in previously well investigated sites and past distributions are being challenged. Upstate New York remains a well characterized area for Nasonia field studies but future work must investigate areas that have not been as well investigated. These sites are Mid-Western USA extending to Canada, South and South-eastern USA and Eurasia.
Nasonia is fast emerging as a model for the evolutionary genetics of speciation (Breeuwer & Werren, 1995; Werren & Loehlin, in press), and has been further facilitated by the recent sequencing of genomes of three species, N. giraulti, N. longicornis and N. vitripennis (Werren et al.2009); N. oneida sequencing is now underway. The ability to cross species boundaries by genetic crosses is a particularly useful tool for evolutionary genetic investigations, allowing the introgression, fine scale mapping and cloning of genes involved in phenotypic differences between the species (Loehlin et al., in review; Werren et al., 2009). The discovery of N. oneida opens up further opportunities for these microevolutionary genetic investigations. For example, N. oneida differs from the other Nasonia in cuticular hydrocarbons, male mating behavior, antennal and wing morphology, diapause tendency, and female mate preference. N. oneida is characterized by strong female mate discrimination that acts as a recessive character to acceptance of heterospecific males. Introgression of a N. oneida male wing-size gene into an N. vitripennis background has already been done successfully (Loehlin et al., in review). The search for genes for mate preference has had a long and tortuous history (Ritchie 1992). Besides Drosophila, where some answers to the genetic basis of mate preference are forthcoming (Doi et al., 2001), very little is known in other species. N. oneida, with its haplodiploidy, interfertility and a strong mate preference phenotype represents a strong new candidate to investigate the genetic basis of female mate discrimination.
Supplementary Material
Supplementary table 1: List of all the strains used from the four Nasonia species with their localities. The NCBI accession numbers for each genetic fragment for each strain is also given.
Acknowledgments
We thank M. E. Clark and R. Sen for helpful comments on the manuscript. We also thank members of the New York Blue Bird Society for help in field collection of bird nest boxes, in particular J. Rogers and R. Wells. This project was funded by the U. S. National Institutes of Health grant R24 GM084917 to JHW. Jan Buellesbach was funded by the Excellence Initiative of the German Research Foundation (GSC-4, Spemann Graduate School).
Reference
- Ayasse M, Paxton RJ, Tengo J. Mating behavior and chemical communication in the order hymenoptera. Annual Review of Entomology. 2001;46:31–78. doi: 10.1146/annurev.ento.46.1.31. [DOI] [PubMed] [Google Scholar]
- Beukeboom L, van den Assem J. Courtship and mating behaviour of interspecific Nasonia hybrids (Hymenoptera: Pteromalidae): a grandfather effect. Behav Genet. 2002;31:167–177. doi: 10.1023/a:1010201427204. [DOI] [PubMed] [Google Scholar]
- Bordenstein SR, Marshall ML, Fry AJ, Kim U, Wernegreen JJ. The tripartite associations between bacteriophage, Wolbachia, and arthropods. Plos Pathogens. 2006;2:384–393. doi: 10.1371/journal.ppat.0020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein SR, O’Hara FP, Werren JH. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature. 2001;409(6821):707–710. doi: 10.1038/35055543. [DOI] [PubMed] [Google Scholar]
- Breeuwer JA, Stouthamer R, Barns SM, Pelletier DA, Weisburg WG, Werren JH. Phylogeny of cytoplasmic incompatibility micro-organisms in the parasitoid wasp genus Nasonia (Hymenoptera: Pteromalidae) based on 16S ribosomal DNA sequences. Insect Mol Biol. 1992;1(1):25–36. doi: 10.1111/j.1365-2583.1993.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Breeuwer JA, Werren JH. Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature. 1990;346(6284):558–560. doi: 10.1038/346558a0. [DOI] [PubMed] [Google Scholar]
- Breeuwer JAJ, Werren JH. Hybrid breakdown between two haplodiploid species-the role of nuclear and cytoplasmic genes. Evolution. 1995;49(4):705–717. doi: 10.1111/j.1558-5646.1995.tb02307.x. [DOI] [PubMed] [Google Scholar]
- Burton-Chellew MN, Beukeboom LW, West SA, Shuker DM. Laboratory evolution of polyandry in the parasitoid wasp Nasonia vitripennis. Animal Behaviour. 2007;74:1147–1154. [Google Scholar]
- Butlin RK. Reinforcement of premating isolation. In: Otte D, Endler JA, editors. Speciation and its Consequences. Sinauer Associates; Sunderland, MA: 1989. pp. 158–179. [Google Scholar]
- Campbell BC, Steffen-Campbell JD, Werren JH. Phylogeny of the Nasonia species complex (Hymenoptera: Pteromalidae) inferred from an internal transcribed spacer (ITS2) and 28S rDNA sequences. Insect Mol Biol. 1993;2(4):225–237. doi: 10.1111/j.1365-2583.1994.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Clark ME, O’Hara FP, Chawla A, Werren JH. Behavioural and spermatogenic hybrid male breakdown in Nasonia. Heredity. doi: 10.1038/hdy.2009.152. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sinaur Associates; Sunderland, MA: 2004. pp. 276–281. [Google Scholar]
- Darling DC, Werren JH. Biosystematics of Nasonia (Hymenoptera, Pteromalidae) - 2 New species reared from birds nests in North-America. Ann Entom Soc America. 1990;83(3):352–370. [Google Scholar]
- Doi M, Matsuda M, Tomaru M, Matsubayashi H, Oguma Y. A locus for female discrimination behavior causing sexual isolation in Drosophila. Proc Natl Acad Sci USA. 2001;98(12):6714–6719. doi: 10.1073/pnas.091421598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferveur JF. Cuticular hydrocarbons: Their evolution and roles in Drosophila pheromonal communication. Behavior Genetics. 2005;35(3):279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- Gadau J, Page RE, Jr., Werren JH. Mapping of hybrid incompatibility loci in Nasonia. Genetics. 1999;153(4):1731–1741. doi: 10.1093/genetics/153.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadau J, Page RE, Werren JH. The genetic basis of the interspecific differences in wing size in Nasonia (Hymenoptera; Pteromalidae): major quantitative trait loci and epistasis. Genetics. 2002;161(2):673–684. doi: 10.1093/genetics/161.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs AG. Water-proofing properties of cuticular lipids. American Zoologist. 1998;38:471–482. [Google Scholar]
- Hall TA. Bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Howard RW, Blomquist GJ. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annual Review of Entomology. 2005;50:371–393. doi: 10.1146/annurev.ento.50.071803.130359. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Dyer KA, Cornish C, Minhas MS. Asymmetrical reinforcement and Wolbachia infection in Drosophila. PLoS Biol. 2006;4:1852–1862. doi: 10.1371/journal.pbio.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes TH, Cantor CR. Evolution of protein molecules. In: Munro HN, editor. Mammalian Protein Metabolism. Academic Press; New York: 1969. pp. 21–123. [Google Scholar]
- Loehlin DW, Enders LS, Werren JH. Evolution of sex-specific wing shape at the widerwing locus in four species of Nasonia. Heredity. doi: 10.1038/hdy.2009.146. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JA, Brent AE, Leaf DS, Pultz MA, Desplan C. Localized maternal orthodenticle patterns anterior and posterior in the long germ wasp Nasonia. Nature. 2006;439(7077):728–732. doi: 10.1038/nature04445. [DOI] [PubMed] [Google Scholar]
- Lynch JA, Desplan C. A method for parental RNA interference in the wasp Nasonia vitripennis. Nat Protoc. 2006;1(1):486–494. doi: 10.1038/nprot.2006.70. [DOI] [PubMed] [Google Scholar]
- Niehuis O, Judson AK, Gadau J. Cytonuclear genic incompatibilities cause increased mortality in male F-2 hybrids of Nasonia giraulti and N. vitripennis. Genetics. 2008;178(1):413–426. doi: 10.1534/genetics.107.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes JS. Universal Chalcidoidea database. 2003 wwwnhmacuk/entomology/chalcidoids/indexhtml.
- Oliveira D, Raychoudhury R, Lavrov DV, Werren JH. Rapidly evolving mitochondrial genome and directional selection in mitochondrial genes in the parasitic wasp Nasonia (Hymenoptera : Pteromalidae) Mol Biol Evol. 2008;25(10):2167–2180. doi: 10.1093/molbev/msn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RDM. TreeView: An application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences. 1996;12(4):357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Raychoudhury R, Baldo L, Oliveira DCSG, Werren JH. Modes of acquisition of Wolbachia : Horizontal transfer, hybrid introgression and co-divergence in the Nasonia species complex. Evolution. 2009;63(1):165–183. doi: 10.1111/j.1558-5646.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- Ritchie MG. Setbacks in the search for mate-preference genes. Trends in Ecol Evol. 1992;7(10):328–329. doi: 10.1016/0169-5347(92)90123-S. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rosenberg MI, Lynch JA, Desplan C. Heads and tails: Evolution of antero-posterior patterning in insects. Biochimica Et Biophysica Acta-Gene Regulatory Mechanisms. 2009;1789(4):333–342. doi: 10.1016/j.bbagrm.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19(18):2496–2497. doi: 10.1093/bioinformatics/btg359. [DOI] [PubMed] [Google Scholar]
- Schluter D. Ecology and the origin of species. Trends in Ecology & Evolution. 2001;16(7):372–380. doi: 10.1016/s0169-5347(01)02198-x. [DOI] [PubMed] [Google Scholar]
- Singer TL. Roles of hydrocarbons in the recognition systems of insects. American Zoologist. 1998;38:394–405. [Google Scholar]
- Skinner SW. Maternally Inherited Sex Ratio in the Parasitoid Wasp Nasonia vitripennis. Science. 1982;215(4536):1133–1134. doi: 10.1126/science.215.4536.1133. [DOI] [PubMed] [Google Scholar]
- Tamura K, Subramanian S, Kumar S. Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol Biol Evol. 2004;21(1):36–44. doi: 10.1093/molbev/msg236. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Simmons LW. Sexual dimorphism in cuticular hydrocarbons of the Australian field cricket Teleogryllus oceanicus (Orthoptera : Gryllidae) Journal of Insect Physiology. 2008;54:1081–1089. doi: 10.1016/j.jinsphys.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Velthuis BJ, Yang W, van Opijnen T, Werren JH. Intra-specific variation in sexual isolation: Genetics of female mate discrimination in Nasonia longicornis (Darling) Animal Behavior. 2004;69(5):1107–1120. [Google Scholar]
- Werren JH. Sex ratio adaptations to local mate competition in a parasitic wasp. Science. 1980;208(4448):1157–1159. doi: 10.1126/science.208.4448.1157. [DOI] [PubMed] [Google Scholar]
- Werren JH, Loehlin DW. Nasonia: An emerging model system with haplodiploid genetics. Emerging Model Organisms 2. Cold Spring Harbor laboratory Press; Cold Spring Harbor, New York: (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Richards S, Desjardins CA, Niehuis O, Gadau J, Colbourne JK, et al. Functional and evolutionary insights from the genomes of three parasitoid Nasonia species. Science. 2009 doi: 10.1126/science.1178028. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston RF, Qureshi I, Werren JH. Genetics of a wing size difference between two Nasonia species. J Evol Biol. 1999;12:586–595. [Google Scholar]
- Whiting AR. The biology of the parasitic wasp Mormoniella vitripennis. [=Nasonia brevicornis] (Walker) Quarterly Review of Biology. 1967;42:333–406. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1: List of all the strains used from the four Nasonia species with their localities. The NCBI accession numbers for each genetic fragment for each strain is also given.