Abstract
Alteration of the ERα/ERβ balance is a critical step in breast cancer development and progression, and selective restoration of the activity of estrogen receptors has been proposed as one of the major therapeutic approaches for breast cancer. In this issue of JCI, Cheng et al. show that, by differentially modulating the stability of ERα and ERβ, PES1 increases the ERα/ERβ ratio and triggers breast tumor growth. These findings highlight PES1 as a potential target for the treatment of breast cancer.
Alteration in the expression of estrogen receptors (ERs) has been detected in breast cancer tissues, and differences in the levels of ERs are correlated with the clinical outcome (1, 2). Insights into the mechanisms that regulate expression and activity of ERs have suggested novel approaches for the prognosis and treatment of breast cancer. Apart from the development of selective ERα and ERβ agonists and antagonists that stimulate or inhibit the activity of the receptors, current research is focusing on alternative strategies that target the signaling of ERs beyond the ligand-ER interaction. Several factors that control the expression and turnover of ERs, including methylases, microRNAs, kinases, and ubiquitin ligases, are under investigation (3). These studies aim to discover novel biomarkers that can complement ER status in the prognosis and prediction of therapeutic response as well as identify new drug targets for restoring the levels of ERs in cancer tissues.
Estrogens mediate their effects on cell growth and differentiation within the mammary gland by signaling through ERα and ERβ (4, 5). After activation in response to estrogen binding, ERs can elicit different transcriptional responses by acting as transcription factors themselves and/or interacting with and regulating the activity of other transcription factors. They can also interact with and alter the activity of one another. Their ligand-binding domains and activating function 1 domains (AF1 domains), which interact with coactivators, share a medium and low degree of homology, respectively. This explains their different affinity to ligands and coregulatory proteins and, at least partly, their distinct biological actions (6). It is well known that ERα expression is associated with aberrant proliferation and the development of malignancy, and ERα level is the principal predictor for the response of breast cancers to endocrine therapy. In contrast, ERβ has been shown to inhibit breast cancer cell proliferation, migration, and invasion (3, 7). Although there is still a controversy regarding the prognostic and predictive role of ERβ expression in breast cancer, most of the studies that have analyzed a large number of samples with well-validated antibodies have shown correlations of wild-type receptor (ERβ1) with better clinical outcome (8, 9).
PES1 governs the ER subtypes
In this issue of JCI, Cheng et al. show that Pescadillo ribosomal biogenesis factor (PES1), a breast cancer–associated gene 1 (BRCA1) C-terminal (BRCT) domain-containing protein, may play a crucial role in the development and the response of breast cancer to systemic therapy (10). It has previously been reported that PES1 is expressed at higher levels in primary breast cancers compared with PES1 expression in normal mammary tissues (11). Furthermore, knockdown of PES1 slows down the proliferation of breast cancer cells. PES1 stimulates cell proliferation by promoting both ribosome biogenesis and cell cycle progression (11–13). Now Cheng et al. hypothesize that PES1 induces breast tumor growth by regulating steroid hormone signaling through control of the turnover of both ERα and ERβ. They found that PES1 enhances the stability of ERα while simultaneously targeting ERβ for degradation, thereby increasing the levels of ERα and decreasing those of ERβ. The authors believe that this alteration in the ERα/ERβ expression ratio explains the correlation between increased PES1 levels and increased breast tumor growth and the better response to tamoxifen treatment.
Cheng et al. manipulated PES1 expression in a series of breast cancer cell lines that were positive for both ERα and ERβ, positive only for ERα, or negative for both receptors. Treatment of these cells with selective ERα or ERβ agonists revealed that PES1 exerted differential actions on the transcriptional responses of the ER subtypes; it increased the transcriptional activity of ERα and decreased that of ERβ, resulting in increased expression of estrogen-responsive genes that are known to promote cell proliferation and survival. The authors found that PES1 altered the transcriptional activity of the ERs by regulating their protein stability. Ubiquitylation assays and treatment of the cells with proteasome inhibitors showed that increased levels of PES1 protected ERα from proteasome-mediated breakdown while targeting ERβ for degradation through the same pathway. Furthermore, the authors found that the E3 ubiquitin ligase carboxyl terminus of Hsc70-interacting protein (CHIP) is responsible for the PES1-mediated alteration in the ubiquitylation and degradation of the ERs (Figure 1 and ref. 10).
Figure 1. A model of PES1 activity in breast cancer.
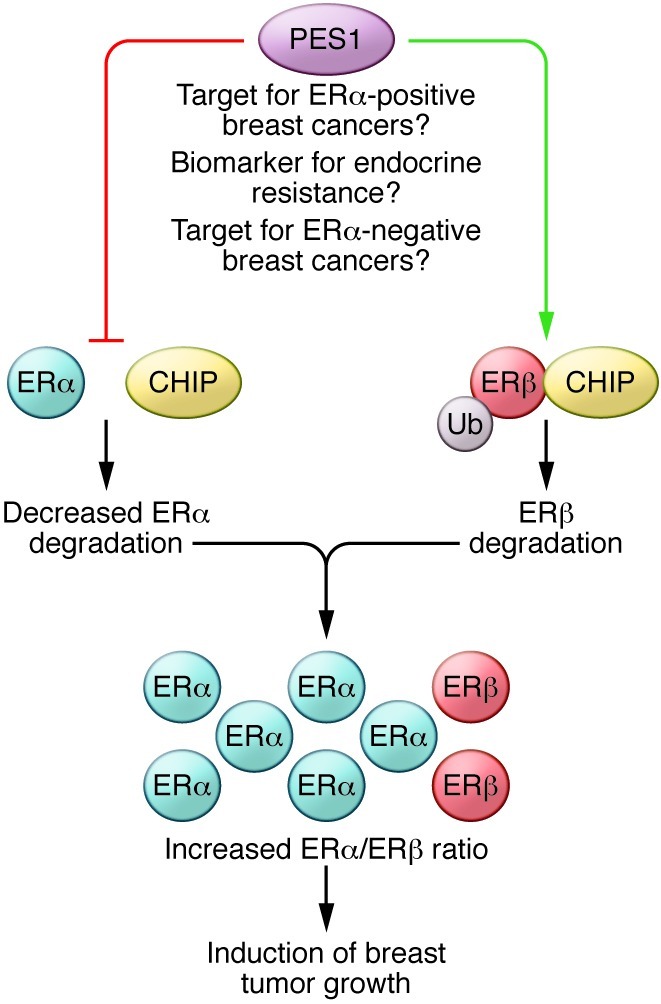
PES1 promotes mammary tumor growth and plays a role in sensitivity to endocrine therapy by regulating the stability of ERs. PES1 reduces the interaction of ERα with the E3 ubiquitin ligase CHIP, thereby blocking ubiquitylation and degradation of ERα. In contrast, by inducing the interaction of CHIP with ERβ, PES1 targets ERβ for degradation. This leads to an increase in the ERα/ERβ protein ratio that has been shown to influence breast tumor growth. Since ERα levels determine tamoxifen sensitivity in breast cancer, PES1 may have a role in predicting response to endocrine therapy. These findings also suggest that PES1 may represent a novel therapeutic target in breast cancer and may serve as a useful biomarker for endocrine therapy resistance. Ub, ubiquitin.
To further understand the function of PES1, the authors carried out pull-down and coimmunoprecipitation experiments with mutants with different domain deletions of PES1, ERα, and ERβ and identified the ER AF1 and AF2 domains as those responsible for the PES1 interaction. Using the same mutants, they identified the regions of PES1 responsible for the CHIP-dependent alteration of ERα and ERβ degradation. The authors also showed that the PES1-mediated increase in the ERα/ERβ expression ratio is associated with enhanced proliferation and tamoxifen sensitivity of breast cancer cells when they grow either in vitro or in vivo in mice (10).
PES1 as biomarker for endocrine resistance
Cheng et al. investigated the clinical significance of PES1 by analyzing protein levels of PES1 and other biomarkers in normal and breast cancer tissues. They found that PES1 levels correlate positively with ERα and negatively with ERβ. Increased expression of PES1 was also associated with better survival in patients with breast cancer who received tamoxifen treatment. Although a role for PES1 in predicting endocrine resistance emerges from the analysis of the breast cancer samples of this study, further investigation of the patterns of expression of PES1 and correlation with ERα expression and clinical outcome in the ERα-positive tamoxifen-treated patients as well as multivariate analysis would be required to indicate the utility of PES1 as a biomarker. In addition, a comparison of PES1 expression between ERα-positive and ERα-negative breast cancers would help to clarify whether lack of PES1 is associated with the loss of ERα in a significant proportion of breast cancers.
Multiple actions of PES1 in breast cancer
The authors provide substantial evidence that strengthens their major conclusion that PES1 promotes mammary cell growth by regulating the stability of ERs and increasing the ERα/ERβ ratio in the tissue. Given that increased proliferation of the mammary cells is implicated in breast cancer initiation, tumor growth, and response to systemic therapy, the study by Cheng et al. proposes a new target for the development of drugs, which through a selective restoration of ERα and ERβ level and activity, can prevent cancer development and progression and improve existing endocrine therapy.
In contrast to other factors that have previously been shown to influence breast cancer development and response to therapy by regulating only one of the two ER subtypes, PES1 is unique in regulating the expression and activity of both receptors. At first glance, this implies that PES1 influences specific biological responses in which both ER subtypes are involved. However, the distinct biological functions elicited by ERα and ERβ and the ER subtype–specific expression patterns detected in breast cancer imply multiple roles for PES1 in breast cancer biology and therapy, depending on the breast cancer subtype and the disease stage. ERβ has been shown to regulate migration and invasion and is expressed in ERα-negative breast cancers (9, 14). Therefore, it is of clinical interest to clarify whether PES1 can impact metastasis and survival in triple-negative cancers by downregulating ERβ. In addition, since both ERs have been reported to regulate the expression of members of the ERBB family of receptor tyrosine kinases, including human epidermal growth factor receptor-2 (ERBB2, also known as HER2) (9, 15, 16), it is important to elucidate whether PES1 might affect the levels of HER2 and thus have a clinical role as biomarker in predicting the response of HER2-positive breast cancers to HER2-specific antibodies and inhibitors.
The study by Cheng et al. also shows an inverse correlation between PES1 and ERβ in breast cancers, in which PES1 is correlated with the response to tamoxifen treatment. Honma et al. have associated ERβ with survival in patients who received tamoxifen (8). However, the role of ERβ in endocrine therapy in breast cancer still remains unclear. Further studies will clarify whether ERβ is involved in PES1-dependent alteration of the tamoxifen response in breast cancers.
Finally, it remains to be elucidated whether the ability of PES1 to affect tumor growth by regulating the ERα/ERβ expression ratio influences other tissues, such as prostate and colon, in which perturbation of the balance between ERα and ERβ has been associated with increased incidence of cancer (14, 17).
Acknowledgments
Jan-Åke Gustafsson is indebted to the Robert A. Welch Foundation for an endowment.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2012;122(8):2771–2773. doi:10.1172/JCI65133.
See the related article beginning on page 2857.
References
- 1.Roger P, et al. Dissociated overexpression of cathepsin D and estrogen receptor alpha in preinvasive mammary tumors. Hum Pathol. 2000;31(5):593–600. doi: 10.1053/hp.2000.6687. [DOI] [PubMed] [Google Scholar]
- 2.Shaaban AM, et al. Declining estrogen receptor-beta expression defines malignant progression of human breast neoplasia. Am J Surg Pathol. 2003;27(12):1502–1512. doi: 10.1097/00000478-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11(8):597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 4.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walter P, et al. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82(23):7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heldring N, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 7.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9(9):631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 8.Honma N, et al. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J Clin Oncol. 2008;26(22):3727–3734. doi: 10.1200/JCO.2007.14.2968. [DOI] [PubMed] [Google Scholar]
- 9.Marotti JD, Collins LC, Hu R, Tamimi RM. Estrogen receptor-beta expression in invasive breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2010;23(2):197–204. doi: 10.1038/modpathol.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng L, et al. PES1 promotes breast cancer by differentially regulating ERα and ERβ. J Clin Invest. 2012;122(8):2857–2870. doi: 10.1172/JCI62676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, et al. Down-regulation of pescadillo inhibits proliferation and tumorigenicity of breast cancer cells. Cancer Sci. 2009;100(12):2255–2260. doi: 10.1111/j.1349-7006.2009.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maiorana A, Tu X, Cheng G, Baserga R. Role of pescadillo in the transformation and immortalization of mammalian cells. Oncogene. 2004;23(42):7116–7124. doi: 10.1038/sj.onc.1207916. [DOI] [PubMed] [Google Scholar]
- 13.Prisco M, et al. Role of pescadillo and upstream binding factor in the proliferation and differentiation of murine myeloid cells. Mol Cell Biol. 2004;24(12):5421–5433. doi: 10.1128/MCB.24.12.5421-5433.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mak P, et al. ERbeta impedes prostate cancer EMT by destabilizing HIF-1alpha and inhibiting VEGF-mediated snail nuclear localization: implications for Gleason grading. Cancer Cell. 2010;17(4):319–332. doi: 10.1016/j.ccr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurtado A, et al. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008;456(7222):663–693. doi: 10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindberg K, Helguero LA, Omoto Y, Gustafsson JA, Haldosen LA. Estrogen receptor beta represses Akt signaling in breast cancer cells via downregulation of HER2/HER3 and upregulation of PTEN: implications for tamoxifen sensitivity. Breast Cancer Res. 2011;13(2):R43. doi: 10.1186/bcr2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong NA, Malcomson RD, Jodrell DI, Groome NP, Harrison DJ, Saunders PT. ERbeta isoform expression in colorectal carcinoma: an in vivo and in vitro study of clinicopathological and molecular correlates. J Pathol. 2005;207(1):53–60. doi: 10.1002/path.1807. [DOI] [PubMed] [Google Scholar]


