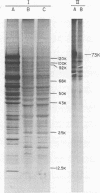
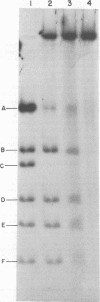
Abstract
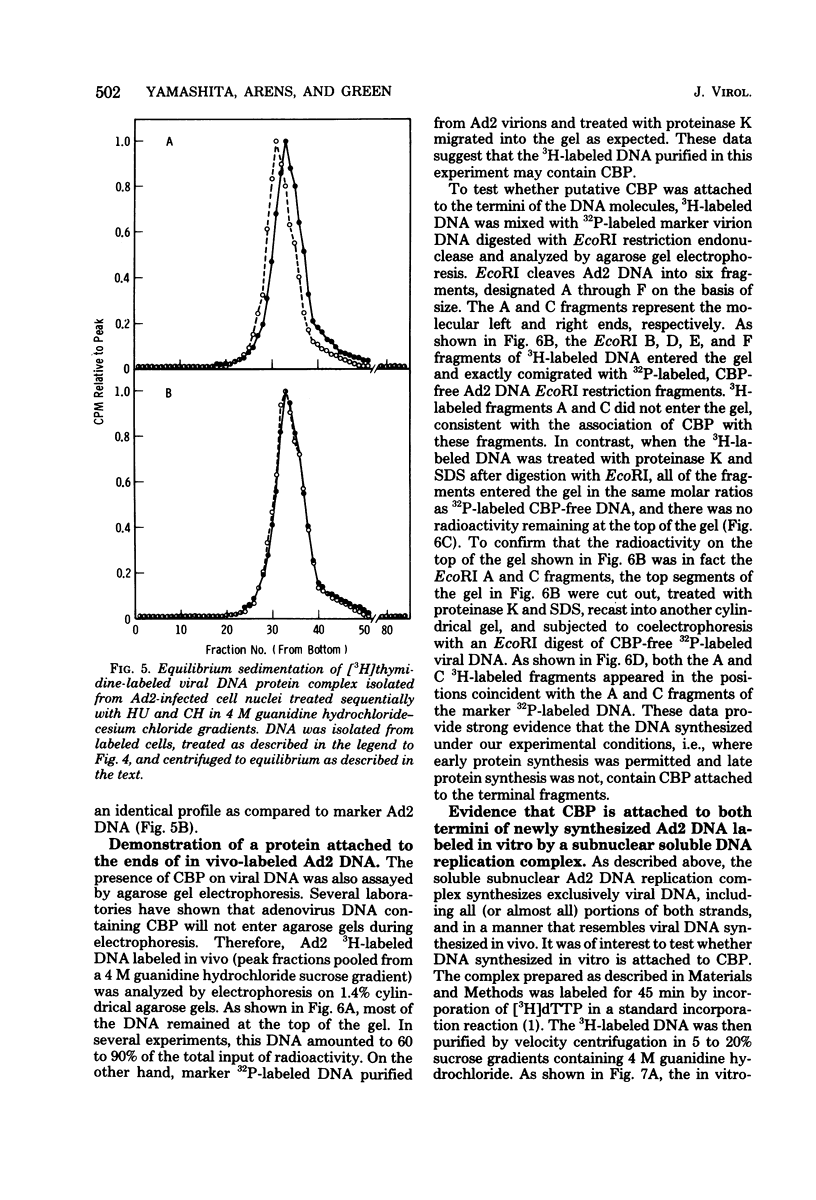
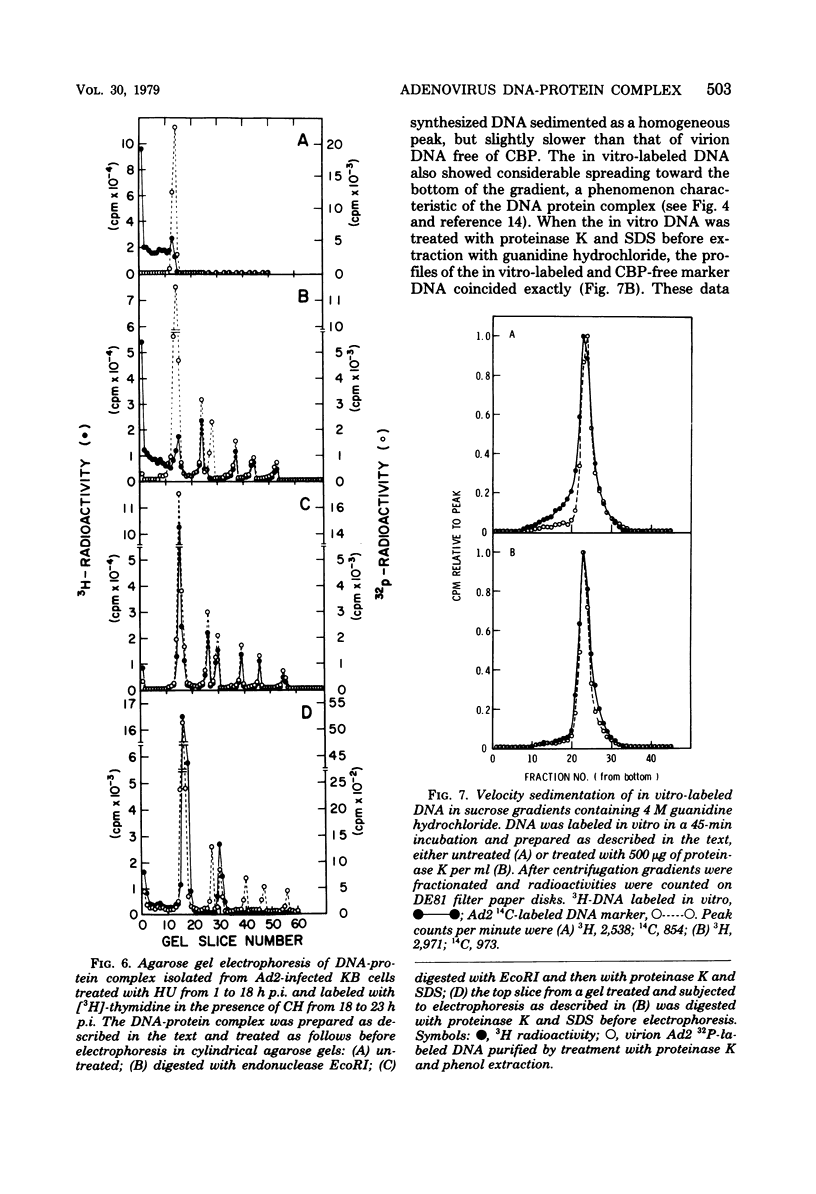
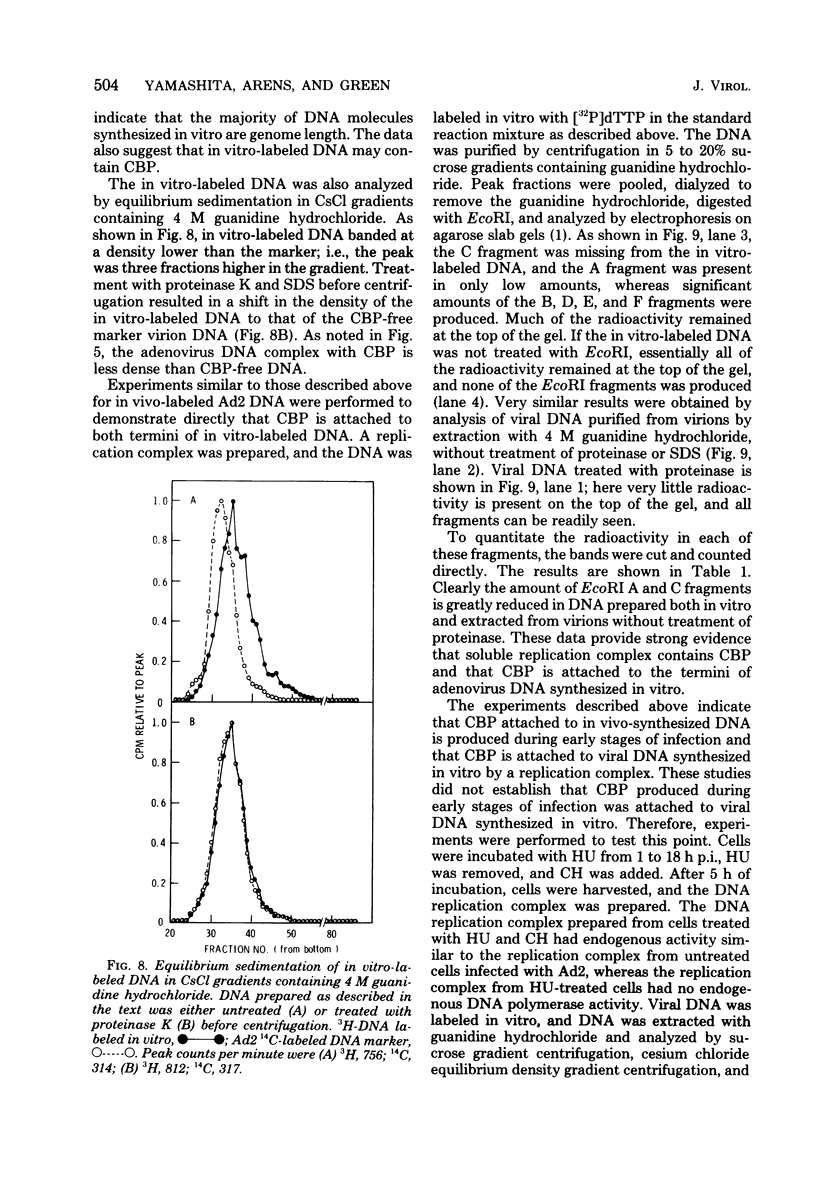
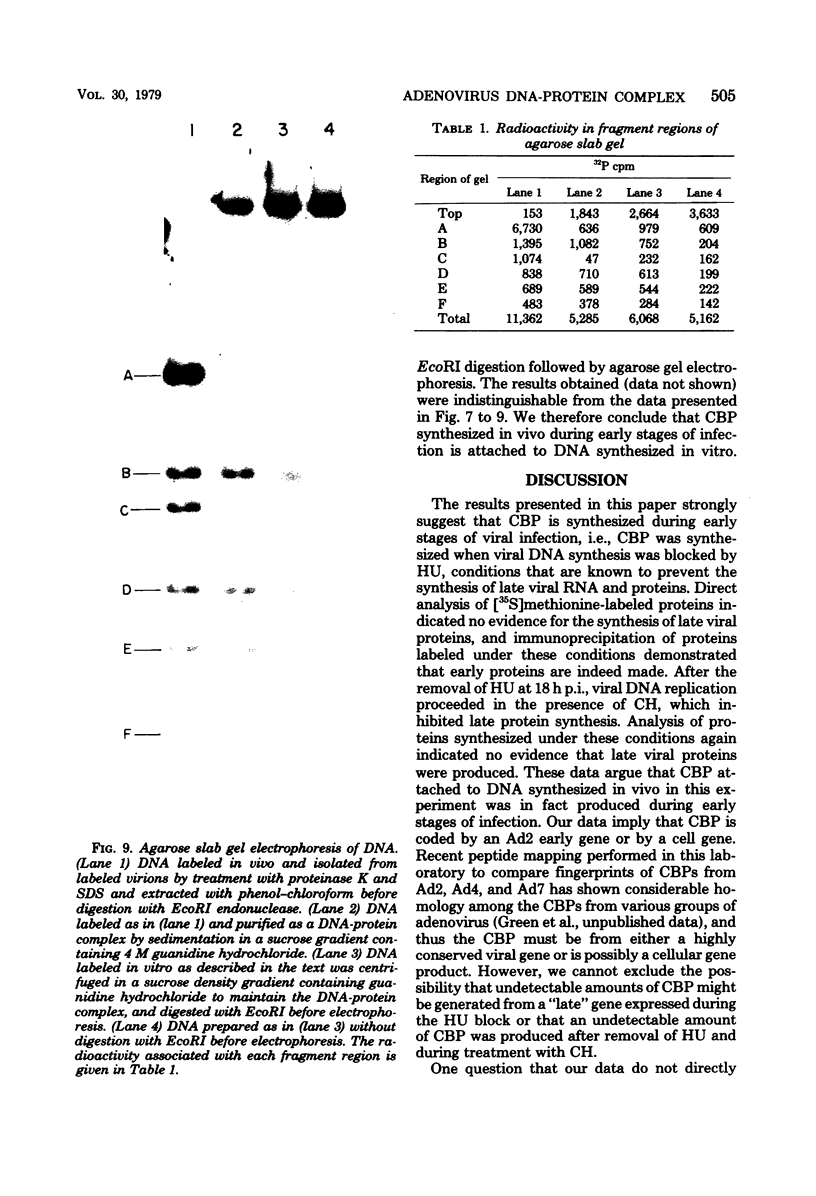
The human adenovirus DNA genome contains a protein (CBP, or covalently bound protein) linked to each 5' terminus. To assess whether CBP is synthesized early, infected cells were incubated with hydroxyurea from 1 to 18 h postinfection, the hydroxyurea was removed, cycloheximide was added, and viral DNA was labeled with [3H]thymidine from 18 to 23 h postinfection. Removal of hydroxyurea at 18 h postinfection permits the synthesis of viral DNA, whereas cycloheximide maintains the block in late viral protein synthesis. Three lines of evidence are presented to show that viral 3H-labeled DNA prepared by this procedure was linked to CBP: (I) the DNA sedimented more rapidly than protein-free DNA (i.e., protinase treated) in neutral sucrose gradients containing guanidine hydrochloride; (ii) the DNA banded at a lower density than protein-free DNA in CsCl gradients containing guanidine hydrochloride; and (iii) neither the 3H-labeled DNA nor the end fragments produced by EcoRI digestion entered a 1.4% agarose gel during electrophoresis. These experiments are strong evidence that CBP is not a product of a late viral gene and is therefore the product of either an early viral gene or a cell gene. Experiments were performed to test whether CBP is attached to viral DNA synthesized in vitro by a soluble complex that synthesizes exclusively viral DNA as completed viral genomes in vitro. In vitro-labeled DNA was analyzed by velocity sedimentation, equilibrium sedimentation, and agarose gel electrophoresis as described above. Our results indicate that the majority of in vitro-synthesized DNA molecules were attached to CBP. These results, which indicate that CBP is synthesized early after infection and is attached to viral DNA labeled in vitro by a soluble replication complex, are consistent with the idea that CBP may play a role in viral DNA replication.
Full text
PDF


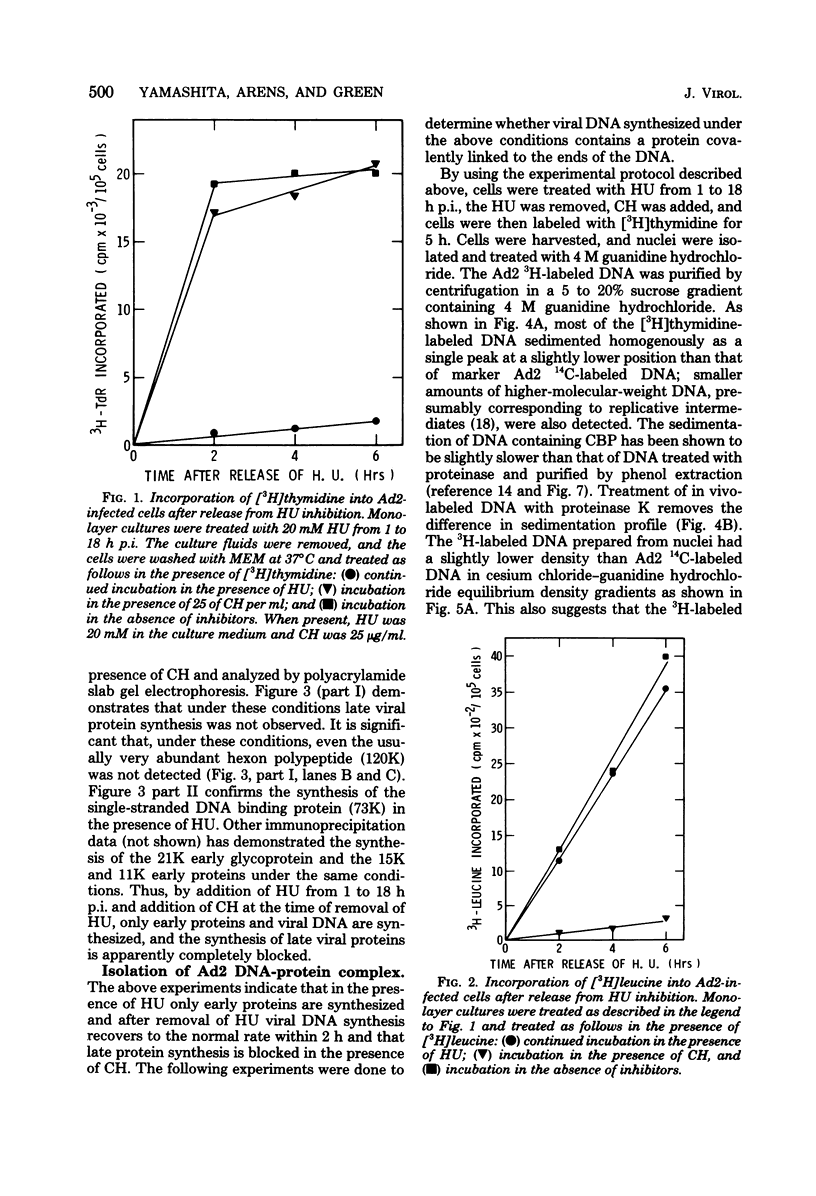
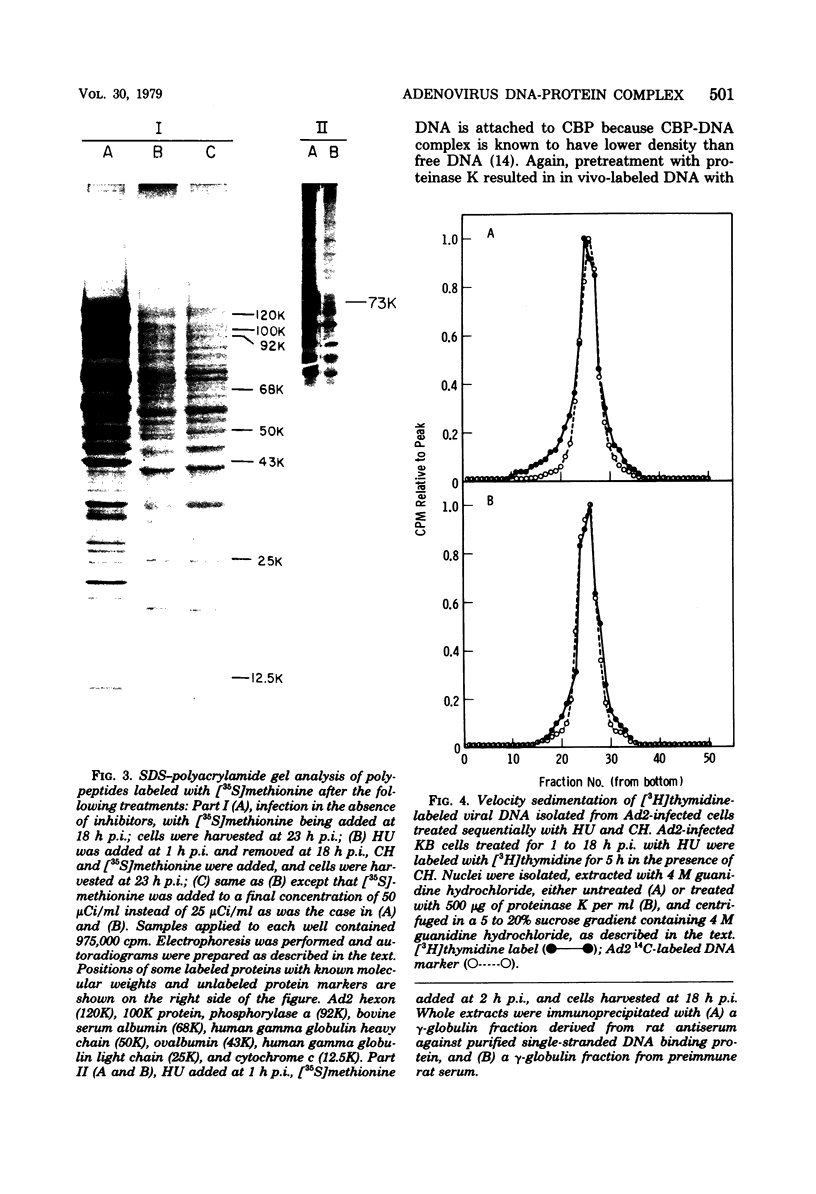


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arens M., Yamashita T. In vitro termination of adenovirus DNA synthesis by a soluble replication complex. J Virol. 1978 Feb;25(2):698–702. doi: 10.1128/jvi.25.2.698-702.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arens M., Yamashita T., Padmanabhan R., Tsuruo T., Green M. Adenovirus deoxyribonucleic acid replication. Characterization of the enzyme activities of a soluble replication system. J Biol Chem. 1977 Nov 25;252(22):7947–7954. [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Brayton C., Baum S. G. Synthesis of type 2 adenovirus DNA in the presence of cycloheximide. J Virol. 1973 Apr;11(4):544–551. doi: 10.1128/jvi.11.4.544-551.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra W., Van Wielink P. S., Sussenbach J. S. The visualization of a circular DNA-protein complex from adenovirions. Virology. 1977 Jan;76(1):444–447. doi: 10.1016/0042-6822(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R., Padmanabhan R. V. Specific interaction of a protein(s) at or near the termini of adenovirus 2 DNA. Biochem Biophys Res Commun. 1977 Apr 25;75(4):955–964. doi: 10.1016/0006-291x(77)91475-9. [DOI] [PubMed] [Google Scholar]
- Pearson G. D. Intermediate in adenovirus type 2 replication. J Virol. 1975 Jul;16(1):17–26. doi: 10.1128/jvi.16.1.17-26.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Rho H. M., Jeng Y. H., Wold W. S., Green M. Association of adenovirus type 2 early proteins with a soluble complex that synthesizes adenovirus DNA in vitro. Biochem Biophys Res Commun. 1977 Nov 21;79(2):422–428. doi: 10.1016/0006-291x(77)90175-9. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Bellett J. D. A circular DNA-protein complex adenoviruses and its possible role in DNA replication. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):523–531. doi: 10.1101/sqb.1974.039.01.064. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Blair G. E. Polypeptide phosphorylation in adenovirus-infected cells. J Gen Virol. 1977 Jan;34(1):19–35. doi: 10.1099/0022-1317-34-1-19. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Sugawara K., Gilead Z., Wold W. S., Green M. Immunofluorescence study of the adenovirus type 2 single-stranded DNA binding protein in infected and transformed cells. J Virol. 1977 May;22(2):527–539. doi: 10.1128/jvi.22.2.527-539.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., Ellens D. J., Jansz H. S. Studies on the mechanism of replication of adenovirus DNA. II. The nature of single-stranded DNA in replicative intermediates. J Virol. 1973 Nov;12(5):1131–1138. doi: 10.1128/jvi.12.5.1131-1138.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C. Studies on the mechanism of replication of adenovirus DNA. I. The effect of hydroxyurea. Virology. 1973 Jul;54(1):299–303. doi: 10.1016/0042-6822(73)90142-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Shiroki K., Shimojo H. The relationship between the formation of inclusions and viral DNA synthesis in adenovirus 12-infected cells. Virology. 1977 Jul 1;80(1):136–148. doi: 10.1016/0042-6822(77)90386-5. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Arens M., Green M. Adenovirus deoxyribonucleic acid replication. II. Synthesis of viral deoxyribonucleic acid in vitro by a nuclear membrane fraction from infected KB cells. J Biol Chem. 1975 May 10;250(9):3273–3279. [PubMed] [Google Scholar]
- Yamashita T., Arens M., Green M. Adenovirus deoxyribonucleic acid replication. Isolation of a soluble replication system and analysis of the in vitro DNA product. J Biol Chem. 1977 Nov 25;252(22):7940–7946. [PubMed] [Google Scholar]
- Yamashita T., Green M. Adenovirus DNA replication. I. Requirement for protein synthesis and isolation of nuclear membrane fractions containing newly synthesized viral DNA and proteins. J Virol. 1974 Sep;14(3):412–420. doi: 10.1128/jvi.14.3.412-420.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]