Abstract
Background
The evolutionary history and relationships of the mud shrimps (Crustacea: Decapoda: Gebiidea and Axiidea) are contentious, with previous attempts revealing mixed results. The mud shrimps were once classified in the infraorder Thalassinidea. Recent molecular phylogenetic analyses, however, suggest separation of the group into two individual infraorders, Gebiidea and Axiidea. Mitochondrial (mt) genome sequence and structure can be especially powerful in resolving higher systematic relationships that may offer new insights into the phylogeny of the mud shrimps and the other decapod infraorders, and test the hypothesis of dividing the mud shrimps into two infraorders.
Results
We present the complete mitochondrial genome sequences of five mud shrimps, Austinogebia edulis, Upogebia major, Thalassina kelanang (Gebiidea), Nihonotrypaea thermophilus and Neaxius glyptocercus (Axiidea). All five genomes encode a standard set of 13 protein-coding genes, two ribosomal RNA genes, 22 transfer RNA genes and a putative control region. Except for T. kelanang, mud shrimp mitochondrial genomes exhibited rearrangements and novel patterns compared to the pancrustacean ground pattern. Each of the two Gebiidea species (A. edulis and U. major) and two Axiidea species (N. glyptocercus and N. thermophiles) share unique gene order specific to their infraorders and analyses further suggest these two derived gene orders have evolved independently. Phylogenetic analyses based on the concatenated nucleotide and amino acid sequences of 13 protein-coding genes indicate the possible polyphyly of mud shrimps, supporting the division of the group into two infraorders. However, the infraordinal relationships among the Gebiidea and Axiidea, and other reptants are poorly resolved. The inclusion of mt genome from more taxa, in particular the reptant infraorders Polychelida and Glypheidea is required in further analysis.
Conclusions
Phylogenetic analyses on the mt genome sequences and the distinct gene orders provide further evidences for the divergence between the two mud shrimp infraorders, Gebiidea and Axiidea, corroborating previous molecular phylogeny and justifying their infraordinal status. Mitochondrial genome sequences appear to be promising markers for resolving phylogenetic issues concerning decapod crustaceans that warrant further investigations and our present study has also provided further information concerning the mt genome evolution of the Decapoda.
Keywords: Mud shrimps, Mitochondrial genome, Gene order, Evolution, Phylogenetics
Background
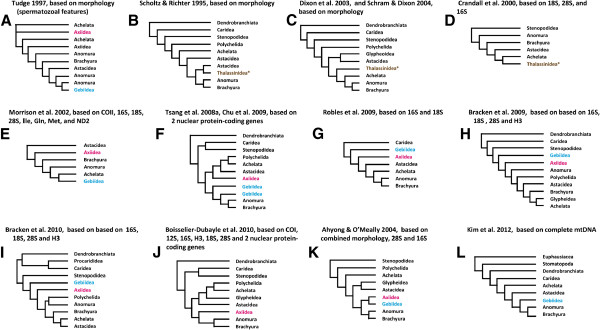
Decapoda is one of the most diverse groups of crustaceans, with over 15,000 extant species in 180 families [1]. Seven main groups (with the ranks of sub- or infraorder) are generally recognized in Decapoda. They are Dendrobranchiata (e.g. penaeoid shrimps and allies), Caridea (caridean shrimps), Stenopodidea (stenopodid shrimps), lobsters (Macrura Reptantia), mud shrimps or ghost shrimps (Thalassinidea or Gebiidea + Axiidea), Anomura (hermit crabs and allies) and Brachyura (true crabs). The phylogenetic relationships amongst these groups within the Decapoda and even the monophyletic status of these groups have long been debated amongst carcinologists and general consensus has yet to be reached, with recent morphological cladistic and molecular analyses still showing contrasting results (Figure 1). One the most controversial recent findings is that the mud shrimps are not monophyletic [1-8], with some of them representing the sister taxon of the other Reptantia (= non-shrimp like decapod crustaceans).
Figure 1.
Hypotheses of higher-level decapod relationships based on recent morphology analyses (A-C), molecular data (D-J), combined morphology and molecular data (K) and latest complete mtDNA sequence (L). Taxa name following De Grave et al. [1] instead of original usages. *Thalassinidea refers to treat members of Gebiidea and Axiidea as forming a monophyletic group. Traditionally, lobsters (Macrura Reptantia) refer to members of Astacidea, Achelata, Glypheidea and Polychelida, while Procarididea is included in Caridea.
Mud shrimps have a worldwide distribution from shallow to deep waters, and more than 600 extant species have been described to date [1]. The classification scheme of mud shrimps have been in flux at all levels. They are often considered to be a monophyletic group up to the rank of infraorder, i.e., Thalassinidea [9-16]. According to different authors, these animals have been treated under Anomura [17-21], as an independent group within the Reptantia [10,11,14,22,23], or aligned with the lobsters [24]. While some authors [25-29] had long questioned the monophyly of Thalassinidea and divided it into two groups (namely Gebiidea and Axiidea), the monophyly of Thalassinidea has been supported by some morphological cladistic analyses [9,10,12,14,16], molecular data [11,30] or combined morphological and molecular analysis [15]. Nevertheless, the latest molecular analyses [3-8] mostly concur in the separation of Thalassinidea.
In most molecular analyses, partial DNA sequences are used to resolve the phylogenetic relationships of decapod crustaceans [3,11,15,22,31-34], but they are often too short to contain a sufficient amount of genetic variation for resolving higher systematics [5,35]. In the previous studies involving mud shrimps [3,4,6,7,11,15,30], the total length of partial sequences used is less than 5300 bp. The animal mitochondrial (mt) DNA is a small, extrachromosomal, and circular double-stranded DNA molecule of 12–20 kb in size, and usually contains the same set of 37 genes, including 13 protein-coding genes, two ribosomal RNA genes and 22 transfer RNA genes [36-38]. Recent advances in DNA sequencing technology have allowed rapid, cost-effective sequencing of the complete mtDNA genome. And it has become increasingly popular in studies of molecular evolution, phylogeography, and phylogenetic relationships at various taxonomic levels [38-43], mainly because of its maternal inheritance, the presence of strictly orthologous genes evolving at different rates, and lack of genetic recombination [38,44-46]. Complete mtDNA sequences provide sets of genome-level characteristics, such as gene rearrangement, which is rather conserved within some major metazoan lineages, and therefore can be especially powerful in resolving systematic relationships among higher taxa [3,40,42,47-49].
Complete mitochondrial genome sequences are now available for 37 decapod crustaceans (April, 2012; http://www.ncbi.nlm.nih.gov, with a sergesteid species Actetes chinensis with mitogenome reported [50] but not yet available from GenBank) that represent all the main groups. However, the latest phylogenetic reconstruction of decapod crustaceans using complete mitochondrial genome sequences [51,52] still has low resolution in most of the deep branches, notably with the status of Stenopodidea, lobsters and mud shrimps unresolved. Moreover, only a single species of mud shrimp collected from Korea, namely Upogebia major (De Haan, 1841) belonged to Gebiidea, has been sequenced for mitochondrial genome [53].
In this paper, we report the complete mitochondrial genomes of five thalassinidean species with representatives from both Gebiidea and Axiidea. They are Austinogebia edulis (Ngoc-Ho and Chan 1992), Upogebia major (from China) and Thalassina kelanang Moh and Chong, 2009 of Gebiidea, and Nihonotrypaea thermophilus Lin, Komai and Chan, 2007 and Neaxius glyptocercus (Von Martens, 1868) of Axiidea. Considering the difference in sampling location and sequence variation, we only included the mitochondrial genome of Upogebia major we sequenced in the analysis. The mitochondrial genome structure of these five mud shrimps were compared with those of other decapods. The gene rearrangement occurred in mud shrimps were identified. Moreover, the infraorder status of Axiidea and Gebiidea was analyzed based on all 50 malacostracan mitochondrial genomes currently available.
Results
Genome composition
The complete mtDNA sequences of A. edulis, U. major, T. kelanang, N. glyptocercus and N. thermophilus were determined to be 15,761, 16,143, 15,528, 14,909 and 15,240 bp long, respectively (Additional file 1). They all contained 13 protein-coding genes (PCGs), two ribosomal RNA genes (rRNA), 22 transfer RNA genes (tRNA) and a putative control region as in other metazoans (Figure 2; Additional files 2, 3, 4, 5, 6). The structural organizations of the five mitochondrial genomes are shown in Figure 2.
Figure 2.
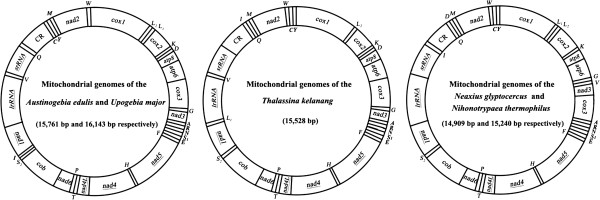
Gene maps of the mitochondrial genomes of Austinogebia edulis,Upogebia major,Thalassina kelanang,Neaxius glyptocercus and Nihonotrypaea thermophilus. Genes encoded on the heavy or light strands are shown outside or inside the circular gene map, respectively. The putative control region is denoted by “CR”. The tRNA genes are designated by single-letter amino acid codes except those encoding leucine and serine, L1, L2, S1 and S2 denote tRNALeu(CUN), tRNALeu(UUR), tRNASer(AGN), and tRNASer(UCN) genes, respectively.
The overall A + T content of A. edulis mtDNA is 73.6%, higher than that of other decapod species except Scylla tranquebarica (73.8%) (see Additional file 1). The overall A + T content of U. major, T. kelanang, N. glyptocercus and N. thermophilus ranged from 66.3-70.7%, similar to other decapods (see Additional file 1). This pattern of base composition in five mud shrimps held for the protein-coding, rRNA, tRNA genes, and the control region when considered separately.
For the 13 PCGs of five mitochondrial genomes, nine protein-coding genes (atp6, atp8, cox1, cox2, cox3, cob, nad2, nad3, and nad6) were encoded on the H-strand, while the remaining four (nad1, nad4, nad4L, and nad5) were encoded on the L-strand (Additional files 2, 34, 5, 6). This transcriptional polarity is identical in all reported decapod mitochondrial genomes. Moreover, they all contained two reading frames overlapped on the same strand: atp6 and atp8, nad4 and nad4L each shared seven nucleotides. No notable reduction or extension of gene length as compared to other decapods was observed.
In A. edulis, U. major and T. kelanang mitochondrial genomes, lrRNA and srRNA were separated by tRNAVal, while the two rRNA genes in N. glyptocercus and N. thermophilus mtDNA were adjacent to each other (Figure 2). The rRNAs were both coded on L-strand. All five mitochondrial genomes had typical 22 tRNA genes, which ranged from 61 to 73 bp in length (Additional files 2, 34, 5, 6), and all of them (except tRNASer(AGN)) formed a typical cloverleaf secondary structure. The tRNASer(AGN) lacked DHC arm, a feature commonly observed in metazoan mtDNAs [54].
The non-coding regions of A. edulis, U. major, T. kelanang, N. glyptocercus and N. thermophilus mtDNAs were 845, 1,188, 784, 162, 581 bp, respectively (see Additional files 2, 34, 5, 6). Of these regions, the largest non-coding region in each genome was assumed to be the control region (CR) with high A + T composition (Additional file 1). The mtDNA of N. glyptocercus had the shortest CR (91 bp) among decapods, and its A + T content was the lowest (59.3%) (Additional file 1). The remaining non-coding regions of the five mitochondrial genomes were considered to be intergenic spacers. Most intergenic spacers contained a few nucleotides (1–56 bp) (Additional file 2, 34, 5, 6). However, a relatively large spacer, 177 bp in length, was found between srRNA and tRNAGl in the U. major mtDNA (Figure 2 and Additional file 3). Further analyses showed that this large region had an A + T content of 89.8%, higher than that in control region (85.2%).
Gene order
The complete genome arrangements of five mud shrimps were depicted in Figures 3 and 4. The gene order of T. kelanang mtDNA was identical to that of the pancrustacean (Crustacea + Hexapoda) ground pattern [55], while the genomic organization of four other mud shrimps showed two novel gene orders compared to other mt genomes in the MitoZoa database. Specifically, the mitochondrial genomes of A. edulis and U. major, and N. glyptocercus and N. thermophilus, shared the same gene order, respectively.
Figure 3.
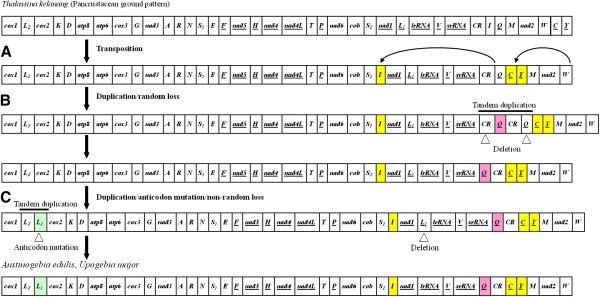
Proposed mechanism for the mitochondrial gene arrangement of Austinogebia edulis, Upogebia major (Decapoda: Gebiidea). Gene order of Thalassina kelanang is identical to that of pancrustaceans ground pattern. Arrows and shaded boxes indicate rearranged genes. Gene segments are not drawn to scale. All genes are transcribed from left to right except for those underlined that exhibit opposite transcriptional orientation.
Figure 4.
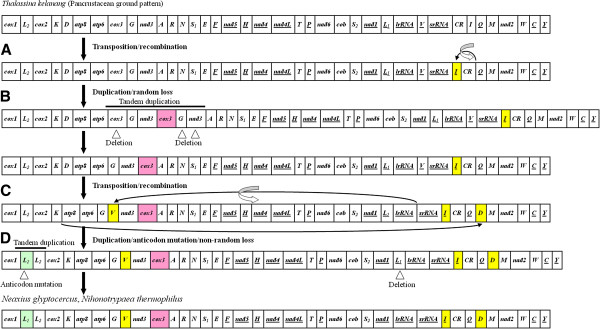
Proposed mechanism for the mitochondrial gene arrangement of Neaxius glyptocercus and Nihonotrypaea thermophilus (Decapoda: Axiidea). Arrows and shaded boxes indicate rearranged genes. The circling arrow indicates inversion. Gene segments are not drawn to scale. All genes are transcribed from left to right except for those underlined that exhibit opposite transcriptional orientation.
Compared with the pancrustacean ground pattern, at least five genes were rearranged in each of the mt genome of A. edulis, U. major, N. glyptocercus and N. thermophilus (Figures 3 and 4). The tRNALeu(CUN) (L1), located between nad1 and lrRNA in other arthropod mtDNAs, was found between tRNALeu(UUR) (L2) and cox2 in A. edulis and U. major, and between cox1 and tRNALeu(UUR) (L1) in N. glyptocercus and N. thermophilus. The tRNAIle (I) was located between tRNASer(UCN) (S2) and nad1 in A. edulis and U. major, and between srRNA and CR in N. glyptocercus and N. thermophilus. In A. edulis and U. major, tRNAGln (Q) moved upstream to the putative control region, and tRNACys (C) and tRNATyr (Y) moved to the location between CR and tRNAMet (M). Additionally, in N. glyptocercus and N. thermophilus mtDNAs, the tRNA gene tRNAVal (V) changed to downstream of tRNAGly (G), tRNAAsp (D) moved to upstream of tRNAMet (M), and only one protein-coding gene cox3 was involved in the rearrangement. The cox3 located between atp6 and tRNAGly (G) in other crustaceans moved upstream to tRNAAla (A) in N. glyptocercus and N. thermophilus. All these genes rearranged in the same orientation as the mitochondrial gene arrangement of pancrustacean ground pattern with the exception of tRNALeu(CUN) (L1) in the four mud shrimps, and tRNAIle (I) and tRNAVal (V) in N. glyptocercus and N. thermophilus. Noticeably, the two tRNALeu sequences in each mt genome of the four mud shrimps shared significant identity with each other, and the similarity was 86% in A. edulis, 80% in U. major, 77% in N. glyptocercus and 93% in N. thermophilus.
Phylogenetic analysis
The concatenated alignments of nucleotide and amino acid data from all 13 protein-coding genes were used to investigate the phylogenetic relationships among major lineages of Decapoda. For each dataset, the BI and ML analyses generated nearly identical tree topology except for two branches denoted by open arrowheads, which strongly supported the monophyly of Decapoda (Figure 5A and B). Values of nodal support were typically congruent between the two trees. Both the nucleotide and amino acid phylogenies indicated strong support (BPP/ML bootstrap value in nucleotide phylogeny = 0.99/93, BPP/ML bootstrap value in amino acid phylogeny =0.99/74) for the separation of two suborders Dendrobranchiata and Pleocyemata in Decapoda. The placing of Caridea at the base of Pleocyemata was well supported (0.80/93, 0.99/69). The remaining natant decapod Stenopodidea was sister with Reptantia with a strong support in amino acid phylogeny (BPP/ML bootstrap value =0.98/79) but only moderate support in nucleotide phylogeny (BPP =0.70). Reptantia was strongly supported to be a monophyletic group (0.99/100, 1.00/100).
Figure 5.
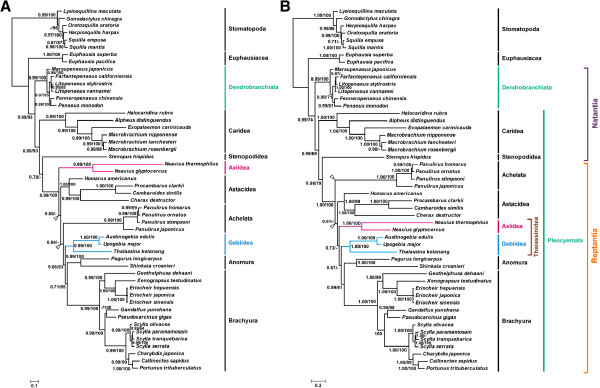
Phylogenetic trees of the nucleotide (A) and amino acid sequence datasets (B) derived from Decapoda using Bayesian inference and maximum likelihood analysis, respectively. Branch lengths and topologies came from Bayesian analyses. Numbers in each branch indicated Bayesian posterior probabilities (BPP)/maximum likelihood (ML) bootstrap values. The minus signs represent the bootstrap values of below 60 %. The topological incongruity between Bayesian and ML analyses is denoted by open arrowheads.
Within Repantia, the Brachyura and Anomura (i.e. Mieura) were reciprocally monophyletic (0.99/100, 1.00/100), and their sister relationship also received supported (0.71/85, 99/61). The monophyly of Thalassinidea (Gebiidea + Axiidea) was not supported in the nucleotide phylogeny or the amino acid phylogeny. Yet AU test could not reject monophyly of Thalassinidea. Gebiidea and Axiidea were both shown to be well supported clades (0.99/100, 1.00/100), with moderate support (0.66/63, 0.87/-) for the sister relationship between Gebiidea and Mieura. However, the position of Axiidea in Reptantia was incongruent between the nucleotide and amino acid trees. Similarly, there was no support for the monophyly of the lobsters (Achelata + Astacidea).
Discussion
Molecular features of mitochondrial genomes in mud shrimps
Features of decapod mitochondrial genomes include a high A + T content and rearranged gene structure [56-58]. These features are also apparent in the complete mtDNA sequences of four of the mud shrimps studied, i.e., A. edulis, U. major, N. glyptocercus and N. thermophilus. Together with T. kelanang mtDNA, all five mitochondrial genomes have the same gene number as other pancrustaceans (13 PCGs, 2 rRNAs, 22 tRNAs). However, the U. major mtDNA annotated by Kim et al. [53] has an extra tRNALeu(CUN) between nad1 and lrRNA. The different annotation is due to amphibolous beginning of lrRNA and identification of tRNALeu(CUN). No anticodon and tRNA-like secondary structure of tRNALeu(CUN) is identified between nad1 and lrRNA in the U. major mtDNA we sequenced, as in the one sequenced by Kim et al. [53].
Most variations in size in mitochondrial genomes are caused by sequences in non-coding regions [53,58]. N. glyptocercus mtDNA has the shortest control region among the decapod mtDNA published, while in U. major mtDNA, a relative large intergetic spacer (177 bp) with high A + T content is discovered. Such a large AT-rich region other than CR rarely occurs in malacostracan species and has only been reported in the stomatopods Oratosquilla oratoria[59] and Squilla mantis[60]. But the length and position of the second AT-rich region are different among the above three species. Moreover, such an AT-rich region is notably absent in the other four mud shrimps, indicating that it is not a conserved feature of thalassinidean mtDNA.
The pancrustacean ground pattern is well retained in T. kelanang mitogenome, suggesting that the ancestor of mud shrimps (or at least the Gebiidea) had a typical pancrustacean mtDNA gene order. However, the other four mud shrimps, A. edulis, U. major, N. glyptocercus and N. thermophilus have rearranged mitochondrial genomes compared to the pancrustacean ground pattern (Figure 3) [56,57,61,62]. Further searches in the MitoZoa database show that mitochondrial sequences of A. edulis and U. major, N. glyptocercus and N. thermophilus exhibit two novel genome structures, respectively. Except for tRNALeu(CUN), the rearranged genes of A. edulis and U. major belonged to Gebiidea occur at tRNAIle—tRNATyr junction. However, the rearranged genes of the two axiids N. glyptocercus and N. thermophilus occur more dispersedly.
Possible mechanisms for gene rearrangement
Two major categories of mechanisms have been suggested to explain mitochondrial gene rearrangement: (1) tandem duplication followed by random or non-random deletion [63,64], and (2) non-homologous recombination [65,66]. The first mechanism may explain many or most of the observed rearrangements, while the second one has been proposed to explain gene translocation and inversion [56,67]. Combined the above mechanisms and the results from CREx (Additional files 7 and 8), the rearrangement of four mud shrimps mtDNAs can be depicted as three or four steps (Figures 3 and 4).
For A. edulis and U. major mtDNA, firstly, transposition of tRNAIle (I), tRNACys (C) and tRNATyr (Y) occurred before duplication. If this event occurred after duplication, more genes were duplicated and longer distance translocation were required (Figure 3A). Secondly, an independent duplication/random loss events occurred to account for the translocation of tRNAGln (Q) (Figure 3B). Thirdly, a duplication/anticodon mutation/non-random loss event [52,68] is expected to account for translocation of tRNALeu(CUN) (L1) (Figure 3C). A duplication of tRNALeu(UUR) (L2) on H-strand might have happened. One of the duplicated tRNALeu(UUR) (L2) changed into tRNALeu(CUN) (L1) by anticodon mutation. Subsequently the ancestral tRNALeu(CUN) (L1) lost the function and eventually is deleted from L-strand.
For N. glyptocercus and N. thermophilus mtDNA, transposition of tRNAIle (I) occurred first followed by one recombination event (Figure 4A). Then an independent duplication/random loss event occurred to account for the translocation of cox3 (Figure 4B). This was followed by transposition of tRNAAsp (D) and tRNAVal (V) and a recombination event (Figure 4C). Finally, a duplication/anticodon mutation/non-random loss event [52,68] occurred to account for the translocation of tRNALeu(CUN) (L1) (Figure 4D).
Another possibility for the translocation of tRNALeu(CUN) (L1) predicted by CREx is based on “duplication/random loss model” [63]. This interpretation seems less likely given that there is a long distance between tRNALeu(UUR) (L2) and tRNALeu(CUN) (L1) in the ancient arrangement, as well as the presence of inversion in tRNALeu(CUN) (L1) [52]. Moreover, the sequence homology between tRNALeu(UUR) (L2) and tRNALeu(CUN) (L1) in A. edulis, U. major, N. glyptocercus and N. thermophilus is higher than any two other randomly chosen tRNAs. It seems to be more possible that two tRNALeu arose from duplication followed by anticodon mutation. The similar duplication/anticodon mutation events have also been reported in other crustaceans, for example in amphipods Caprella scaura[69] and Gammarus duebeni[70], and decapods Geothelphusa dehaani[71] and Stenopus hispidus[52].
Under the above models, including the random and nonrandom loss, incomplete deletion or partial retention of duplication resulted in the formation of the multiple intergenic spacers (Additional files 2, 34, 5, 6). These results indicate that intergenic spacers might serve as a guide in deducing the generation of gene rearrangement [56]. Moreover, the distinct rearrangement processes suggest that Gebiidea and Axiidea evolved independently from the pancrustacean ground pattern.
Phylogenetic relationships of the major clades in Decapoda
With higher taxon samplings and the inclusion of all the major groups of decapod crustaceans, the present complete mitochondrial genomic analysis strongly supports that the Caridea is sister to the other Pleocyemata. Similar to the results of Shi et al. [52], Stenopodidae is revealed to be a sister clade of Reptantia. While this relationship is only weakly supported in Shi et al. [52], this grouping is strongly supported in our tree based on amino acid sequences.
Within the Reptantia, the sister relationship between Brachyura and Anomura (i.e. the Meiura) has always received very high support in complete mitochondrial genomic analyses [51,52,62]. The present result suggests that Gebiidea is the sister group of Meiura though only with moderate support. In general, the topology of the currently most extensive complete mitochondrial genomic tree of decapod crustaceans (particularly the one based on amino acid sequences) is most similar to the most recent mt genome analyses by Kim, Park, et al. [51], and those of Scholtz and Richter [10] and Ahyong and O’Meally [15] deduced from morphology and combined morphology and molecular (16S and 28S) data, respectively. The trees of Scholtz and Richter [10] and Ahyong and O’Meally [15] are essentially the same except for the identity of the sister clade of Thalassinidea (i.e. Gebiidea + Axiidea), which is considered to be monophyletic. Thalassinidea is sister to Mieura in Ahyong and O’Meally [15] but in Scholtz and Richter [10] it is sister to the clawed lobster Astacidea which is shown to be polyphyletic. The main difference between the present result with these two analyses is that Gebiidea and Axiidea do not make up a monophyletic group while the position of Astacidea is unresolved. The mitogenome tree of Kim et al. [50] includes only one mud shrimp species belonged to Gebiidea. Adding two more species and genera of Gebiidea in the present work reveals a similar topology of Gebiidea being sister to Mieura. However, the other mud shrimp group Axiidea does not cluster with Gebiidea.
As the results of complete mitochondrial genome sequence analysis are now rather consistent with the conclusions deduced from some morphology and partial gene sequence data, it seems to be promising in using complete mtDNA sequence to reconstruct the evolutionary history of decapod crustaceans. Nevertheless, more taxon sampling, particularly the inclusion of certain key groups such as the sergesteid shrimp (supposed to be sister to Penaeoidea in Dendrobranchiata), blind lobsters Polychelida [4], primitive cave shrimp Procarididea [7], the living fossil lobster Glypheidea [23], the enigmatic shrimp Luciferidae [72] and the various bizarre anomuran groups [73], will be necessary to achieve this goal.
Conclusions
This study presents five complete mitochondrial genomes of mud shrimps, Austinogebia edulis, Upogebia major and Thalassina kelanang of Gebiidea, and Nihonotrypaea thermophilus and Neaxius glyptocercus of Axiidea. The contents of individual mt genes in these five mud shrimps are similar to that in other decapods. The U. major mt genome contain a relative large intergenic spacer with higher A + T content than that in control region. The N. glyptocercus mt genome, the shortest decapod mtDNA known, has the shortest control region. Except for T. kelanang, the other four mud shrimps have rearranged mt genomes compared to pancrustacean ground pattern. A duplication/loss (random and nonrandom) and recombination model may result in their mt gene order. The different gene arrangement process suggests the derived gene orders of Gebiidea and Axiidea might have evolved independently. Phylogenetic analyses do not support the monophyly of mud shrimps, while the positions of Gebiidea and Axiidea in Reptantia are poorly resolved.
Methods
Sample collection and DNA extraction
The collecting sites of the mud shrimp specimens used in the present study are A. edulis from Starfish Bay, Hong Kong, U. major from Qingdao No.1 Bathing Beach, China, T. kelanang from Kelanang Beach, Selangor, Malaysia, N. glyptocercus from Kenting, Taiwan and N. thermophilus from Kueishan Island, Taiwan. The specimens obtained were stored in 75-95% ethanol. Total genomic DNA for all species was extracted from tissues by using a DNeasy tissue kit (Qiagen) following the manufacturer’s protocol.
PCR and sequence determination of A. edulis and U. major mitochondrial genomes
Four short fragments of the genes cox1, nad5, lrRNA and cob were first determined by PCR with the universal primer sets of LCO1490/HCO2198 [74], nad5F/nad5R [75], 16S1471/16S1472 [76] and cobF424/ cobR876 [77]. PCR products were purified using the Qiaquick Gel extraction Kit (Qiagen) and directly sequenced with ABI 3730xl DNA Analyzer.
Based on the sequences obtained above, long PCR primers were designed to amplify the entire A. edulis (AE) and U. major (UM) mitochondrial genomes in four fragments: AE/UMcox1F-AE/UMnad5R, AE/UMnad5F-AE/UMcobR, and AE/UMcobF-AE/UM16SR, AE/UM16SR-AE/UMcox1R, with the PCR products of approximate 5.5 kb, 3.7 kb, 1.9 and 4.5 kb in length, respectively (Additional file 9). PCR reactions were carried out in 25 μl reaction mixtures containing 18.8 μl of sterile distilled H2O, 2.5 μl of 10× LA PCR buffer II (Mg2+ plus, Takara), 0.5 μl of dNTP (10 mM each), 1 μl of each primer (5 μM), 0.2 μl of LA Taq polymerase (5 unit/μl, Takara), and 1 μl of DNA template (approximate 30 ng). The amplifications were performed on TaKaRa PCR Thermal Cycler Dice Model TP600 (Takara Bio Inc.) with an initial denaturation at 94° for 3 min, followed by 34 cycles of denaturation at 94° for 20 s, annealing at 50-52° for 50 s, extension at 68° for for 1 min/kb, and a final extension at 68° for 10 min. Long PCR products were purified using the Qiaquick Gel extraction Kit (Qiagen) and bidirectionally sequenced using a primer-walking strategy on ABI 3730xl DNA Analyzer.
PCR and sequence determination of T. kelanang, N. glyptocercus and N. thermophilus mitochondrial genomes
Four partial fragments of the genes cob, cox1cox2 (2 kb), cox3, srRNA were first determined by PCR with the primer sets of Cyb1/Cyb2 [78] crust-cox1f [79]/CCO2Rv1 [80], Scox3-F(GCCCCTTCAGTNGAAATTGG)/Scox3-R (ACTACATCDACRAAATGTCAATATCA), and srRNA-F (AAATTTAATTCAACATCGAGGTCGCAAACT)/srRNA-R (TTGACYGTGCRAAGGTAGCATAATAATTAG). Additional three partial mitochondrial sequences (nad4, nad5 and 12S) of T. kelanang were also determined with PCR primer sets of L11424-ND4/H11534-ND4M [81], crust-nd5f/crust-nd5r and crust-12Sf/crust-12Sr [79].
Based on the sequences obtained above, long PCR primers were designed to amplify the entire T. kelanang (TK), N. glyptocercus (NG) and N. thermophilus (NT) mitochondrial genomes in several fragments: TKbs-R/H11534-ND4M [81], TKc1s-R/TK12s-R, TKc2s-F/TKd5s-R, NGbs-F/NGrRs-R, NGc3s-F/NGbs-R, NGc1s-R/NGrRs-F, NGc2s-F/NGc2s-R, NTbs-R/NTc2s-F, and NTc1s-R/NTrRs-F, with the PCR products of approximate 2 kb, 3.7 kb, 3.7 kb, 2.3 kb, 5.6 kb, 3.6 kb, 2.5 kb, 7.5 kb and 4.2 kb in length, respectively (Additional file 10). PCR reaction and sequencing were generally the same as described in A. edulis and U. major mitochondrial genomes.
Sequence analysis
Base calling was processed using Phred [82,83] and sequence reads were assembled using Phrap with default parameters. All assembled sequences were manually checked using CONSED to remove misassembles [84].
The locations of 13 protein-coding genes and two rRNAs were initially identified by DOGMA [85] with default settings, and refined by alignment with mitochondrial genomes of Panulirus japonicus (NC_004251) and Squilla mantis (NC_006081). A majority of tRNA genes was identified by the tRNAscan-SE 1.21 [86] in default search mode using mitochondrial/chloroplast DNA as the source and invertebrate mitochondrial genetic code for tRNA structure prediction. The remaining tRNA genes were identified by inspecting sequences for tRNA-like secondary structures and anticodons. The complete mtDNA sequences of A. edulis, U. major, T. kelanang, N. glyptocercus and N. thermophilus were deposited with GenBank under accession numbers JN897376-JN897380, respectively.
The inferred mitochondrial gene orders of the above five mud shrimps were compared with that of the 527 other arthropod species included in the MitoZoa database [87] (http://mi.caspur.it/mitozoa/index.php, Release 10, 14-Dec-2011). The genome rearrangement steps were predicted by algorithms implanted in CREx server [88] together with gene rearrangement models reported in previous arthropod mitochondrial genomes [56].
Phylogenetic analysis
Along with the complete mtDNA sequences from A. edulis, U. major, T. kelanang, N. glyptocercus and N. thermophilus, all currently available 37 decapod complete mitochondrial sequences (see Additional file 1) were used in phylogenetic analysis. The six stomatopods Gonodactylus chiragra (GenBank accession number: NC_007442), Harpiosquilla harpax (NC_006916), Lysiosquilla harpax (NC_007443), Oratosquilla oratoria (NC_014342), Squilla empusa (NC_007444) and Squilla mantis (NC_006081), and two euphausians Euphausia pacifica (NC_016184) and Euphausia superba (EU583500) served as outgroups. Both nucleotides and amino acids of 13 protein-coding genes were subjected to concatenated alignments using ClustalX 1.83 with the default settings [89]. For the nucleotides, we omitted the third codon position before alignment, according to the result of a saturation analysis [90] by DAMBE version 5.0.32 [91]. The final nucleotide and amino acid datasets consisted of 7,577 and 3,781 sites, respectively. Phylogenetic trees were built by two approaches including Bayesian inference (BI) analysis using Phylobayes 3.3b [92] and maximum-likelihood (ML) analysis using RaxML 7.0.4 [93].
For the nucleotide dataset, the model GTR + I + G was selected by JMODELTEST 0.1.1 [94]. The model MtRev + I + G + F was chosen as the best-fit model for the amino acid dataset by ProtTest version 2.4 [95]. According to preliminary analysis, the categories model with GTR (CAT-GTR) and CAT-Poisson models [96,97] fit the data best and were used for BI and ML analysis of the nucleotide and amino acid data, respectively. For BI analysis, two independent MCMC chains were run simultaneously to determine whether the searching reached stabilization, and were stopped when all chains converged (maxdiff less than 0.1). For ML analysis, 1000 bootstraps were used to estimate the node reliability. Topology testing was performed using Consel [98] for the approximately unbiased (AU) test [99].
Abbreviations
atp6 and 8: ATPase subunit 6 and 8; bp: Base pair (s); BI: Bayesian inference; BP: Bootstrap; cox1-3: Cytochrome c oxidase subunits I-III; cob: Cytochrome b; lrRNA: 16S ribosomal RNA; ML: Maximum likelihood; Mt: Mitochondrial; mtDNA: Mitochondrial DNA; nt: Nucleotide (s); nad1-6 and 4 L: NADH dehydrogenase subunits 1–6 and 4 L; ORF: Open reading frame; PCG: Protein coding gene; PCR: Polymerase chain reaction; BPP: Bayesian posterior probabilities; rRNA: Ribosomal RNA; srRNA: 12S ribosomal RNA; tRNA: Transfer RNA; tRNA gene: trnX (where X is replaced by single letter amino acid code of the corresponding amino acid).
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TYC, KHC and ZC contributed to the conception and design of the study. FJL and YL performed the laboratory works of A. edulis, T. kelanang, N. glyptocercus and N. thermophilus. ZS and RL conducted the work on U. major. YL and LMT performed bioinformatics analyses of nucleotide and protein sequences. FJL and YL cooperated with the writing of the manuscript. ZC supervised the study and wrote the final draft of the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Genomic characteristics of decapod mitochondrial genomes.
Location of genes in the mitochondrial genome of Austinogebia edulis.
Location of genes in the mitochondrial genome of Upogebia major.
Location of genes in the mitochondrial genome of Thalassina kelanang.
Location of genes in the mitochondrial genome of Neaxius glyptocercus.
Location of genes in the mitochondrial genome of Nihonotrypaea thermophilus.
Mitochondrial gene order rearrangement scenario of Austinogebia edulis, Upogebia major (Decapoda: Gebiidea) inferred by CREx. The elements in the blue shaded boxes are lost in the second copy, therefore the remaining copies are moved to the front. The elements in the red shaded boxes are lost in the first copy, therefore the remaining copies are moved to the back.
Mitochondrial gene order rearrangement scenario of Neaxius glyptocercus and Nihonotrypaea thermophilus (Decapoda: Axiidea) inferred by CREx. The elements in the blue shaded boxes are lost in the second copy, therefore the remaining copies are moved to the front. The elements in the red shaded boxes are lost in the first copy, therefore the remaining copies are moved to the back.
Specific primers used in amplification of Austinogebia edulis and Upogebia major mitochondrial genomes.
Specific primers used to amplification of the fragments which covered the gaps after sequencingThalassina kelanang, Neaxius glyptocercus and Nihonotrypaea thermophilus mitochondrial genomes.
Contributor Information
Feng-Jiau Lin, Email: fjlin@mail.ncku.edu.tw.
Yuan Liu, Email: liuyuan@qdio.ac.cn.
Zhongli Sha, Email: shazl@qdio.ac.cn.
Ling Ming Tsang, Email: kiryusky@gmail.com.
Ka Hou Chu, Email: kahouchu@cuhk.edu.hk.
Tin-Yam Chan, Email: tychan@mail.ntou.edu.tw.
Ruiyu Liu, Email: jyliu@qdio.ac.cn.
Zhaoxia Cui, Email: zhxcui@qdio.ac.cn.
Acknowledgements
Sincerely thanks are extended to H.H. Moh and V.C. Chong of the University of Malaya for kindly providing us some specimens of Thalassinia kelanang for the present study. We also thank Dr. MinXiao Wang of Institute of Oceanology, Chinese Academy of Sciences for his assistance in bioinformatics analyses. This work was supported by grants from the National Science Council, Taiwan to Tin-Yam Chan, National Natural Science Foundation of China 31172054 to Dr. Zhongli Sha, and Chinese National ‘863’ Project (No. 2012AA10A409) and National Natural Science Foundation of China (41276165) to Dr. Zhaoxia Cui.
References
- De Grave S, Deam Pentcheff N, Ahyong ST, Chan TY, Crandall KA, Dworschak PC, Felder DL, Feldmann RM, Fransen CHJM, Goulding LYD. et al. A classification of living and fossil genera of decapod crustaceans. The Raffles Bulletin of Zoology. 2009;21:1–109. [Google Scholar]
- Tudge CC. Phylogeny of the Anomura (Decapoda, Crustacea): Spermatozoa and spermatophore morphological evidence. Contrib Zool. 1997;67(2):125–141. [Google Scholar]
- Morrison CL, Harvey AW, Lavery S, Tieu K, Huang Y, Cunningham CW. Mitochondrial gene rearrangements confirm the parallel evolution of the crab-like form. P Roy Soc Lond B Bio. 2002;269(1489):345–350. doi: 10.1098/rspb.2001.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang LM, Ma KY, Ahyong ST, Chan TY, Chu KH. Phylogeny of Decapoda using two nuclear protein-coding genes: origin and evolution of the Reptantia. Mol Phylogenet Evol. 2008;48(1):359–368. doi: 10.1016/j.ympev.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Chu KH, Tsang LM, Ma KY, Chan TY, Ng PKL. In: Decapod Crustacean Phylogenetics. Martin JW, Crandall KA, Felder DL, editor. New York: CRC Press; 2009. Decapod phylogeny: what can protein-coding genes tell us; pp. 89–99. [Google Scholar]
- Bracken HD, Toon A, Felder DL, Martin JW, Finley M, Rasmussen J, Palero F, Crandall KA. The decapod tree of life: compiling the data and moving toward a consensus of decapod evolution. Arthropod Systematics & Phylogeny. 2009;67(1):99–116. [Google Scholar]
- Bracken HD, De Grave S, Toon A, Felder DL, Crandall KA. Phylogenetic position, systematic status, and divergence time of the Procarididea (Crustacea: Decapoda) Zoologica Scripta. 2010;39(2):198–212. doi: 10.1111/j.1463-6409.2009.00410.x. [DOI] [Google Scholar]
- Robles R, Tudge CC, Dworschak PC, Poore GCB, Felder DL. In: Decapod Crustacean Phylogenetics. Martin JW, Crandall KA, Felder DL, editor. New York: CRC Press; 2009. Molecular phylogeny of the Thalassinidea based on nuclear and mitochondrial genes; pp. 309–326. [Google Scholar]
- Poore GCB. A phylogeny of the families of Thalassinidea (Crustacea: Decapoda) with keys to families and genera. Memoirs of the Museum of Victoria. 1994;54:79–120. [Google Scholar]
- Scholtz G, Richter S. Phylogenetic systematics of the reptantian Decapoda (Crustacea, Malacostraca) Zool J Linn Soc-Lond. 1995;113(3):289–328. [Google Scholar]
- Crandall KA, Harris DJ, Fetzner JW. The monophyletic origin of freshwater crayfish estimated from nuclear and mitochondrial DNA sequences. P Roy Soc Lond B Bio. 2000;267(1453):1679–1686. doi: 10.1098/rspb.2000.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schram FR. Phylogeny of decapods: moving towards a consensus. Hydrobiologia. 2001;449(1–3):1–20. [Google Scholar]
- Martin JW, Davis GE. An updated classification of the recent crustacea. Natural History Museum of Los Angeles County: Science Series. 2001;39:1–124. [Google Scholar]
- Dixon CJ, Ahyong ST, Schram FR. A new hypothesis of decapod phylogeny. Crustaceana. 2003;76:935–975. doi: 10.1163/156854003771997846. [DOI] [Google Scholar]
- Ahyong ST, O'Meally D. Phylogeny of the Decapoda reptantia: Resolution using three molecular loci and morphology. Raffles B Zool. 2004;52(2):673–693. [Google Scholar]
- Schram FR, Dixon CJ. Decapod phylogeny: addition of fossil evidence to a robust morphological cladistic data set. Bulletin of the Mizunami Fossil Museum. 2004;31:1–19. [Google Scholar]
- Borradaile LA. On the classification of the Thalassinidea. The Annals and Magazine of Natural History, series 7. 1903;12:534–551. doi: 10.1080/00222930308678891. [DOI] [Google Scholar]
- Borradaile LA. On the classification of the decapod crustaceans. The Annals and Magazine of Natural History, series 7. 1907;19:457–486. doi: 10.1080/00222930709487277. [DOI] [Google Scholar]
- De Man JG. The Decapoda of the Siboga-expedition. Part 7. The Thalassinidae and Callianassidae collected by the Siboga-expedition with some remarks on the Laomediidae. Siboga Expéditie. 1928;39(A6):1–187. [Google Scholar]
- Bouvier E-L. Décapodes marcheurs. Faune de France. 1940;37:1–407. [Google Scholar]
- Zariquiey-Álvarez R. Crustáceos decápodos ibéricos. Investig Pesq. 1968;32:1–510. [Google Scholar]
- Porter ML, Perez-Losada M, Crandall KA. Model-based multi-locus estimation of decapods phylogeny and divergence times. Mol Phylogenet Evol. 2005;37:355–369. doi: 10.1016/j.ympev.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Boisselier-Dubayle MC, Bonillo C, Cruaud C, Couloux A, de Forges BR, Vidal N. The phylogenetic position of the 'living fossils' Neoglyphea and Laurentaeglyphea (Decapoda: Glypheidea) Comptes Rendus Biologies. 2010;333(10):755–759. doi: 10.1016/j.crvi.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Holthuis LB. FAO Fisheries Synopsis. vol. 125. Rome: Food and Agriculture Organization of the United Nations; 1991. FAO species catalog. Vol. 13. Marine lobsters of the world. An annotated and illustrated catalogue of species of interest to fisheries known to date; p. 292. [Google Scholar]
- Gurney R. Larvae of decapod crustacea. Part 5. Nephropsidea and thalassinidea. Discovery Reports. 1938;17:291–344. [Google Scholar]
- Gurney R. Larvae of decapod Crustacea. London: Ray Society; 1942. [Google Scholar]
- De Saint Laurent M. Versune nouvelle classification des Crustaces Decapodes Reptantia. Bulletin de I'Office National des Peches Republique Tunisienne, Ministere de UAgriculture. 1979;3:15–31. [Google Scholar]
- De Saint Laurent M. Sur la classification et la phylogenie des Thalassinides: definitions de la superfamille des Axioidea, de la sous-famille des Thomassiniinae et de deux genres nouveaux (Crustacea Decapoda) C R Hebd Acad Sci. 1979;288:1395–1397. [Google Scholar]
- Tudge C. In: Advances in Spermatozoal Phylogeny and Taxonomy. Jamieson BGM, Ausio J, Justin JL, editor. Paris: Mémoires du Muséum National d’Histoire Naturelle; 1995. Ultrastructure and phylogeny of the spermatozoa of the infraorders Thalassinidea and Anomura (Decapoda, Crustacea) pp. 251–263. vol. 166. [Google Scholar]
- Tsang LM, Lin FJ, Chu KH, Chan TY. Phylogeny of Thalassinidea (Crustacea, Decapoda) inferred from three rDNA sequences: implications for morphological evolution and superfamily classification. J Zool Syst Evol Res. 2008;46(3):216–223. doi: 10.1111/j.1439-0469.2008.00459.x. [DOI] [Google Scholar]
- Harrison MK, Crespi BJ. Phylogenetics of Cancer crabs (Crustacea: Decapoda: Brachyura) Mol Phylogenet Evol. 1999;12(2):186–199. doi: 10.1006/mpev.1998.0608. [DOI] [PubMed] [Google Scholar]
- Ptacek MB, Sarver SK, Childress MJ, Herrnkind WF. Molecular phylogeny of the spiny lobster genusPanulirus(Decapoda: Palinuridae). Mar Freshwater Res. 2001;52(8):1037–1047. doi: 10.1071/MF01070. [DOI] [Google Scholar]
- Munasinghe DHN, Burridge CP, Austin CM. Molecular phylogeny of the spiny lobster genusPanulirus(Decapoda: Palinuridae). Biol J Linn Soc. 2004;81(4):553–563. doi: 10.1111/j.1095-8312.2003.00299.x. [DOI] [Google Scholar]
- Shih HT, Ng PKL, Schubart CD, Chang HW. Phylogeny and phylogeography of the genus Geothelphusa (Crustacea: Decapoda, Brachyura, Potamidae) in southwestern Taiwan based on two mitochondrial genes. Zool Sci. 2007;24(1):57–66. doi: 10.2108/zsj.24.57. [DOI] [PubMed] [Google Scholar]
- Roe AD, Sperling FAH. Patterns of evolution of mitochondrial cytochrome c oxidase I and II DNA and implications for DNA barcoding. Mol Phylogenet Evol. 2007;44(1):325–345. doi: 10.1016/j.ympev.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Boore JL. Animal mitochondrial genomes. Nucleic Acids Res. 1999;27(8):1767–1780. doi: 10.1093/nar/27.8.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpert F, Podsiadlowski L. The complete mitochondrial genome of the common sea slater, Ligia oceanica (Crustacea, Isopoda) bears a novel gene order and unusual control region features. BMC Genomics. 2006;7:241–258. doi: 10.1186/1471-2164-7-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissi C, Iannelli F, Pesole G. Evolution of the mitochondrial genome of Metazoa as exemplified by comparison of congeneric species. Heredity. 2008;101(4):301–320. doi: 10.1038/hdy.2008.62. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Cann RL, Carr SM, George M, Gyllensten UB, Helmbychowski KM, Higuchi RG, Palumbi SR, Prager EM, Sage RD. et al. Mitochondrial DNA and two perspectives on evolutionary genetics. Biol J Linn Soc. 1985;26:375–400. doi: 10.1111/j.1095-8312.1985.tb02048.x. [DOI] [Google Scholar]
- Boore JL, Collins TM, Stanton D, Daehler LL, Brown WM. Deducing the pattern of arthropod phylogeny from mitochondrial DNA rearrangements. Nature. 1995;376(6536):163–165. doi: 10.1038/376163a0. [DOI] [PubMed] [Google Scholar]
- Avise JC. Phylogeography: The history and formation of species. Cambridge, MA: Harvard Univ Press; 2000. [Google Scholar]
- Boore JL, Macey JR, Medina M. Sequencing and comparing whole mitochondrial genomes of animals. Molecular Evolution: Producing the Biochemical Data, Part B. 2005;395:311–348. doi: 10.1016/S0076-6879(05)95019-2. [DOI] [PubMed] [Google Scholar]
- Cui ZX, Liu YA, Li CP, Chu KH. Species delineation in Pampus (Perciformes) and the phylogenetic status of the Stromateoidei based on mitogenomics. Mol Biol Rep. 2011;38(2):1103–1114. doi: 10.1007/s11033-010-0207-y. [DOI] [PubMed] [Google Scholar]
- Yamauchi MM, Miya MU, Machida RJ, Nishida M. PCR-based approach for sequencing mitochondrial genomes of decapod crustaceans, with a practical example from kuruma prawn (Marsupenaeus japonicus) Mar Biotechnol. 2004;6(5):419–429. doi: 10.1007/s10126-003-0036-2. [DOI] [PubMed] [Google Scholar]
- Elson JL, Lightowlers RN. Mitochondrial DNA clonality in the dock: can surveillance swing the case? Trends Genet. 2006;22(11):603–607. doi: 10.1016/j.tig.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Qian G, Zhao Q, Wang AN, Zhu LIN, Zhou K, Sun H. Two new decapod (Crustacea, Malacostraca) complete mitochondrial genomes: bearings on the phylogenetic relationships within the Decapoda. Zool J Linn Soc-Lond. 2011;162(3):471–481. doi: 10.1111/j.1096-3642.2010.00686.x. [DOI] [Google Scholar]
- Boore JL, Brown WM. Big trees from little genomes: mitochondrial gene order as a phylogenetic tool. Curr Opin Genet Dev. 1998;8(6):668–674. doi: 10.1016/S0959-437X(98)80035-X. [DOI] [PubMed] [Google Scholar]
- Dowton M. Relationships among the cyclostome braconid (Hymenoptera: Braconidae) subfamilies inferred from a mitochondrial tRNA gene rearrangement. Mol Phylogenet Evol. 1999;11(2):283–287. doi: 10.1006/mpev.1998.0580. [DOI] [PubMed] [Google Scholar]
- Simon C, Buckley TR, Frati F, Stewart JB, Beckenbach AT. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu Rev Ecol Evol S. 2006;37:545–579. doi: 10.1146/annurev.ecolsys.37.091305.110018. [DOI] [Google Scholar]
- Kim S, Kim J, Choi HG, Park JK, Min GS. Complete mitochondrial genome of the northern mauxia shrimp Acetes chinensis (Decapoda, Dendrobranchiata, Sergestoidae) Mitochondrial DNA. 2012;23(1):28–30. doi: 10.3109/19401736.2011.643878. [DOI] [PubMed] [Google Scholar]
- Kim S, Park MH, Jung JH, Ahn DH, Sultana T, Park JK, Choi HG, Min GS. The mitochondrial genomes of Cambaroides similis and Procambarus clarkii (Decapoda: Astacidea: Cambaridae): the phylogenetic implications for Reptantia. Zoologica Scripta. 2012;41(3):281–292. doi: 10.1111/j.1463-6409.2012.00534.x. [DOI] [Google Scholar]
- Shi H, Liu R, Sha Z, Ma J. Complete mitochondrial DNA sequence of Stenopus hispidus (Crustacea: Decapoda: Stenopodidea) and a novel tRNA gene cluster. Marine Genomics. 2012;6:7–15. doi: 10.1016/j.margen.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim T, Choi HG, Park JK, Ahn DH, Min GS. The complete mitochondrial genome of the Japanese mud shrimp Upogebia major (Crustacea, Decapoda) Mitochondrial DNA. 2011;22(4):94–96. doi: 10.3109/19401736.2011.624609. [DOI] [PubMed] [Google Scholar]
- Wolstenholme DR. Animal mitochondrial DNA: Structure and Evolution. Int Rev Cytol. 1992;141:173–216. doi: 10.1016/s0074-7696(08)62066-5. [DOI] [PubMed] [Google Scholar]
- Boore JL, Lavrov DV, Brown WM. Gene translocation links insects and crustaceans. Nature. 1998;392(6677):667–668. doi: 10.1038/33577. [DOI] [PubMed] [Google Scholar]
- Sun H, Zhou K, Song D. Mitochondrial genome of the Chinese mitten crab Eriocheir japonica sinenesis (Brachyura: Thoracotremata: Grapsoidea) reveals a novel gene order and two target regions of gene rearrangements. Gene. 2005;349:207–217. doi: 10.1016/j.gene.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Yang JS, Nagasawa H, Fujiwara Y, Tsuchida S, Yang WJ. The complete mitochondrial genome sequence of the hydrothermal vent galatheid crab Shinkaia crosnieri (Crustacea: Decapoda: Anomura): a novel arrangement and incomplete tRNA suite. BMC Genomics. 2008;9:257. doi: 10.1186/1471-2164-9-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki JS, Dahms HU, Hwang JS, Lee JS. The complete mitogenome of the hydrothermal vent crab Xenograpsus testudinatus (Decapoda, Brachyura) and comparison with brachyuran crabs. Comp Biochem Physiol Part D Genomics Proteomics. 2009;4(4):290–299. doi: 10.1016/j.cbd.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cui Z. The complete mitochondrial genome of the mantid shrimp Oratosquilla oratoria (Crustacea: Malacostraca: Stomatopoda): Novel non-coding regions features and phylogenetic implications of the Stomatopoda. Comp Biochem Physiol Part D Genomics Proteomics. 2010;5(3):190–198. doi: 10.1016/j.cbd.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Cook CE, Yue QY, Akam M. Mitochondrial genomes suggest that hexapods and crustaceans are mutually paraphyletic. Proceedings of the Royal Society B-Biological Sciences. 2005;272(1569):1295–1304. doi: 10.1098/rspb.2004.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi MM, Miya MU, Nishida M. Complete mitochondrial DNA sequence of the Japanese spiny lobster,Panulirus japonicus(Crustacea: Decapoda). Gene. 2002;295(1):89–96. doi: 10.1016/S0378-1119(02)00824-7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cui Z. Complete mitochondrial genome of the Chinese spiny lobster Panulirus stimpsoni (Crustacea: Decapoda): genome characterization and phylogenetic considerations. Mol Biol Rep. 2011;38(1):403–410. doi: 10.1007/s11033-010-0122-2. [DOI] [PubMed] [Google Scholar]
- Boore JL. In: Comparative genomics, computational biology series Volume 1. Sankoff D, Nadeau J, editor. Kluwer, Dordrecht: Academic Publishers; 2000. The duplication/random loss model for gene rearrangement exemplified by mitochondrial genomes of deuterostome animals; pp. 133–147. [Google Scholar]
- Lavrov DV, Boore JL, Brown WM. Complete mtDNA sequences of two millipedes suggest a new model for mitochondrial gene rearrangements: Duplication and nonrandom loss. Mol Biol Evol. 2002;19(2):163–169. doi: 10.1093/oxfordjournals.molbev.a004068. [DOI] [PubMed] [Google Scholar]
- Lunt DH, Hyman BC. Animal mitochondrial DNA recombination. Nature. 1997;387(6630):247. doi: 10.1038/387247a0. [DOI] [PubMed] [Google Scholar]
- Shao RF, Barker SC. The highly rearranged mitochondrial genome of the plague thrips,Thrips imaginis(Insecta: thysanoptera): Convergence of two novel gene boundaries and an extraordinary arrangement of rRNA genes. Mol Biol Evol. 2003;20(3):362–370. doi: 10.1093/molbev/msg045. [DOI] [PubMed] [Google Scholar]
- Wang MX, Sun S, Li CL, Shen X. Distinctive mitochondrial genome of Calanoid copepodCalanus sinicuswith multiple large non-coding regions and reshuffled gene order: Useful molecular markers for phylogenetic and population studies. BMC Genomics. 2011;12:73. doi: 10.1186/1471-2164-12-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs PG, Jameson D, Jow H, Rattray M. The evolution of tRNA-Leu genes in animal mitochondrial genomes. J Mol Evol. 2003;57(4):435–445. doi: 10.1007/s00239-003-2494-6. [DOI] [PubMed] [Google Scholar]
- Krebes L, Bastrop R. The mitogenome of Gammarus duebeni (Crustacea Amphipoda): A new gene order and non-neutral sequence evolution of tandem repeats in the control region. Comp Biochem Phys D. 2012;7(2):201–211. doi: 10.1016/j.cbd.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Ito A, Aoki MN, Yokobori S, Wada H. The complete mitochondrial genome of Caprella scaura (Crustacea, Amphipoda, Caprellidea), with emphasis on the unique gene order pattern and duplicated control region. Mitochondrial DNA. 2010;21(5):183–190. doi: 10.3109/19401736.2010.517834. [DOI] [PubMed] [Google Scholar]
- Segawa RD, Aotsuka T. The mitochondrial genome of the Japanese freshwater crab, Geothelphusa dehaani (Crustacea: Brachyura): Evidence for its evolution via gene duplication. Gene. 2005;355:28–39. doi: 10.1016/j.gene.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Tavares C, Serejo C, Martin JW. In: Decapod Crustacean Phylogenetics. Martin JW, Crandall KA, Felder DL, Boca R, editor. London, New York: CRC Press, Taylor & Francis Group; 2009. A preliminary phylogenetic anaylsis of the Dendrobranchiata based on morphological characters; pp. 261–279. [Google Scholar]
- Tsang LM, Chan TY, Ahyong ST, Chu KH. Hermit to king, or hermit to all: multiple transitions to crab-like forms from hermit crab ancestors. Syst Biol. 2011;60(5):616–629. doi: 10.1093/sysbio/syr063. [DOI] [PubMed] [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3(5):294–299. [PubMed] [Google Scholar]
- Lavrov DV, Brown WM, Boore JL. Phylogenetic position of the Pentastomida and (pan)crustacean relationships. P Roy Soc Lond B Bio. 2004;271(1538):537–544. doi: 10.1098/rspb.2003.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubart CD, Neigel JE, Felder DL. Use of the mitochondrial 16S rRNA gene for phylogenetic and population studies of Crustacea. Crustacean Iss. 2000;12:817–830. [Google Scholar]
- Boore JL, Brown WM. Mitochondrial genomes of Galathealinum, Helobdella, and Platynereis: Sequence and gene arrangement comparisons indicate that Pogonophora is not a phylum and Annelida and Arthropoda are not sister taxa. Mol Biol Evol. 2000;17(1):87–106. doi: 10.1093/oxfordjournals.molbev.a026241. [DOI] [PubMed] [Google Scholar]
- Hunter RL, Webb MS, Iliffe TM, Bremer JRA. Phylogeny and historical biogeography of the cave-adapted shrimp genus Typhlatya (Atyidae) in the Caribbean Sea and western Atlantic. J Biogeogr. 2008;35(1):65–75. [Google Scholar]
- Podsiadlowski L, Bartolomaeus T. Organization of the mitochondrial genome of mantis shrimp Pseudosquilla ciliata (Crustacea: Stomatopoda) Mar Biotechnol. 2005;7(6):618–624. doi: 10.1007/s10126-005-0017-8. [DOI] [PubMed] [Google Scholar]
- Peregrino-Uriarte AB, Varela-Romero A, Muhlia-Almazan A, Anduro-Corona I, Vega-Heredia S, Gutierrez-Millan LE, De la Rosa-Velez J, Yepiz-Plascencia G. The complete mitochondrial genomes of the yellowleg shrimp Farfantepenaeus californiensis and the blue shrimp Litopenaeus stylirostris (Crustacea: Decapoda) Comp Biochem Physiol Part D Genomics Proteomics. 2009;4(1):45–53. doi: 10.1016/j.cbd.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Miya M, Pietsch TW, Orr JW, Arnold RJ, Satoh TP, Shedlock AM, Ho HC, Shimazaki M, Yabe M, Nishida M. Evolutionary history of anglerfishes (Teleostei: Lophiiformes): a mitogenomic perspective. BMC Evol Biol. 2010;10:58. doi: 10.1186/1471-2148-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8(3):186–194. [PubMed] [Google Scholar]
- Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998;8(3):175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- Gordon D, Abajian C, Green P. Consed: a graphical tool for sequence finishing. Genome Res. 1998;8:195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- Wyman SK, Jansen RK, Boore JL. Automatic annotation of organellar genomes with DOGMA. Bioinformatics. 2004;20(17):3252–3255. doi: 10.1093/bioinformatics/bth352. [DOI] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25(5):955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi R, de Meo PD, Picardi E, D'Antonio M, Paoletti D, Castrignano T, Pesole G, Gissi C. MitoZoa: A curated mitochondrial genome database of metazoans for comparative genomics studies. Mitochondrion. 2010;10(2):192–199. doi: 10.1016/j.mito.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Bernt M, Merkle D, Ramsch K, Fritzsch G, Perseke M, Bernhard D, Schlegel M, Stadler PF, Middendorf M. CREx: inferring genomic rearrangements based on common intervals. Bioinformatics. 2007;23(21):2957–2958. doi: 10.1093/bioinformatics/btm468. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XH, Xie Z, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Mol Phylogenet Evol. 2003;26(1):1–7. doi: 10.1016/S1055-7903(02)00326-3. [DOI] [PubMed] [Google Scholar]
- Xia X, Xie Z. DAMBE software package for data analysis in molecular biology and evolution. J Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]
- Lartillot N, Lepage T, Blanquart S. PhyloBayes 3: a Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics. 2009;25(17):2286–2288. doi: 10.1093/bioinformatics/btp368. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008;25(7):1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Abascal F, Zardoya R, Posada D. ProtTest: selection of best-fit models of protein evolution. Bioinformatics. 2005;21(9):2104–2105. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
- Lartillot N, Philippe H. A bayesian mixture model for across-site heterogeneities in the amino-acid replacement process. Mol Biol Evol. 2004;21(6):1095–1109. doi: 10.1093/molbev/msh112. [DOI] [PubMed] [Google Scholar]
- Lartillot N, Brinkmann H, Philippe H. Suppression of long-branch attraction artefacts in the animal phylogeny using a site-heterogeneous model. BMC Evol Biol. 2007;7(1):S4. doi: 10.1186/1471-2148-7-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira H, Hasegawa M. CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics. 2001;17(12):1246–1247. doi: 10.1093/bioinformatics/17.12.1246. [DOI] [PubMed] [Google Scholar]
- Shimodaira H. An approximately unbiased test of phylogenetic tree selection. Syst Biol. 2002;51(3):492–508. doi: 10.1080/10635150290069913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genomic characteristics of decapod mitochondrial genomes.
Location of genes in the mitochondrial genome of Austinogebia edulis.
Location of genes in the mitochondrial genome of Upogebia major.
Location of genes in the mitochondrial genome of Thalassina kelanang.
Location of genes in the mitochondrial genome of Neaxius glyptocercus.
Location of genes in the mitochondrial genome of Nihonotrypaea thermophilus.
Mitochondrial gene order rearrangement scenario of Austinogebia edulis, Upogebia major (Decapoda: Gebiidea) inferred by CREx. The elements in the blue shaded boxes are lost in the second copy, therefore the remaining copies are moved to the front. The elements in the red shaded boxes are lost in the first copy, therefore the remaining copies are moved to the back.
Mitochondrial gene order rearrangement scenario of Neaxius glyptocercus and Nihonotrypaea thermophilus (Decapoda: Axiidea) inferred by CREx. The elements in the blue shaded boxes are lost in the second copy, therefore the remaining copies are moved to the front. The elements in the red shaded boxes are lost in the first copy, therefore the remaining copies are moved to the back.
Specific primers used in amplification of Austinogebia edulis and Upogebia major mitochondrial genomes.
Specific primers used to amplification of the fragments which covered the gaps after sequencingThalassina kelanang, Neaxius glyptocercus and Nihonotrypaea thermophilus mitochondrial genomes.