Abstract
This pictorial review illustrates CT and MR imaging appearance of gastrointestinal stromal tumor (GIST) of the stomach and other lesions with similar imaging appearance. GIST of the stomach appears as well-defined enhanced masses with characteristics of subeppthial neoplasms. Majority are exophytic growth, but can also be of intra-luminal growth. GIST can growth into a large mass without gastrointestinal tract obstruction. Necrosis is often seen in GIST and results in heterogeneous enhancement and communication with gastrointestinal tract. CT and MRI features of several other neoplasms mimicking GISTs in the stomach are also described in this review.
Key Words: CT, MR, gastrointestinal stromal tumor (GIST)
Gastrointestinal stromal tumor (GIST) is the most common subepithelial neoplasm that can be found throughout the gastrointestinal tract, but most of them occur in the stomach. GIST consists of spinde cells or epithelioid cells, therefore they were misdiagnose as leiomyoma or leiomyosarcoma before their immunohistochemical properties were discovered (1). The uniqueness of CD117 positive can distinguish GIST from other neoplasm with similar optical microscopical appearance (2). There are several subepithelial neoplasms of the stomach which can exhibit similar CT and MRI features as GIST. This review seeks to illustrate CT and MR manifestations of GIST of the stomach.
GIST of stomach
60-70% of GIST occurs in the stomach. They arise from interstitial cells of Cajal, which are pacemaker cells for gut movement (3). Therefore, GIST usually arises from the muscularis propria and exhibit characteristics of subepithelial neoplasms (Figure 1). The tumors can be extraluminal, intraluminal or mixed (dumbbell-shaped) pattern, while 79% of them are exophytic growth (4). GIST typically grows into a well-defined exophytic mass (Figures 1,2), but intraluminal masses can also be seen (Figure 3). Small tumors are often of homogeneous density or signal and large tumors tend to show irregular lobulated margins, mucosal ulceration, central necrosis, hemorrhage, cavitation, and heterogeneous enhancement (3) (Figures 4,5). Extensive necrosis can result in fistula formation with air-fluid level or oral contrast materials in the cavity (Figure 6). Mucosal ulceration can lead to gastrointestinal bleeding. Gastric GIST often has a better survival than small intestinal GIST (5). Large size, hepatic metastasis and presence of wall invasion often suggest a high-grade GIST and predict poor outcome (6) (Figure 7). Malignant GIST commonly metastasizes to the liver or peritoneum, whereas metastases to the lymph nodes and extra-abdominal metastases are rare.
Figure 1.
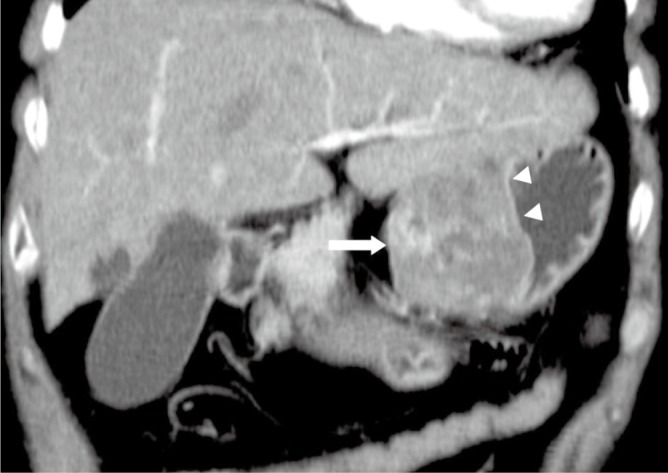
A 64-year-old male with a benign gastric GIST. Coronal multiple planar reformation of contrast enhanced CT shows a well-defined exophtic-growth mass (arrow) with heterogeneous enhancement arising from the small curve of the stomach. Mucosa (arrowheads) covering the tumor remains intact, which implies the mass is submucosa origination
Figure 2.
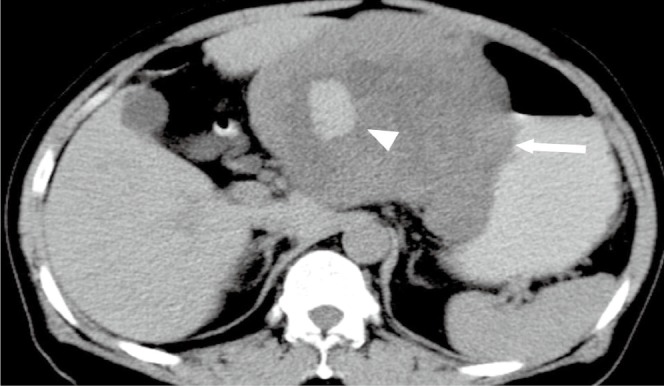
A 57-year-old male with malignant gastric GIST. Axial unenhanced CT image shows a well-defined exophtic-growth homogeneous mass (arrow) with hemorrhage (arrowhead) of the stomach
Figure 3.
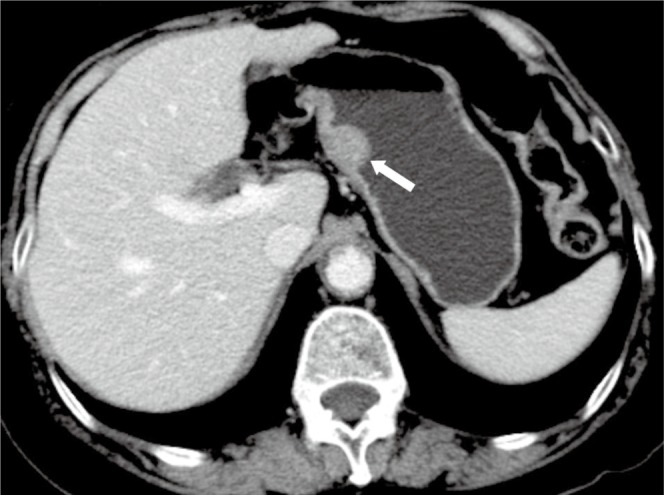
A 72-year-old male with benign gastric GIST. Axial enhanced CT image shows an introphytic homogeneously enhancing nodule (arrow) of the stomach
Figure 4.
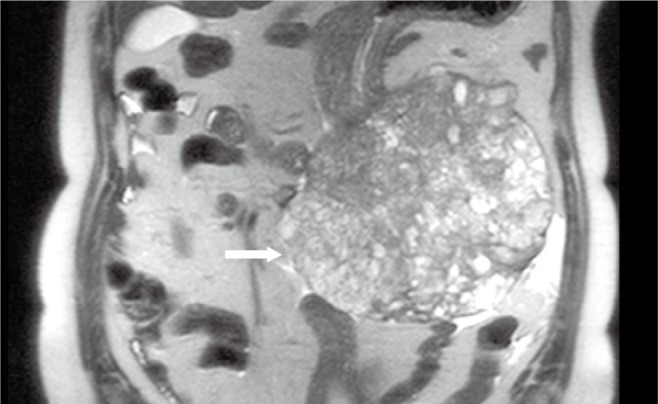
A 68-year-old male with malignant gastric GIST. Coronal true FISP image shows a large extraluminal heterogeneous signal mass from the large curve of stomach (arrow)
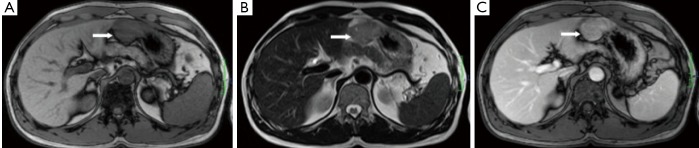
Figure 5.
A 43-year-old female with benign gastric GIST. A. Axial T1-weighted image shows a homogeneous iso-intensity mass from the fundus of stomach; B. Axial T2-weighted image shows the mass is of homogeneous medium signal intensity; C. Axial enhanced T1-weighted image shows homogeneous moderate enhancement
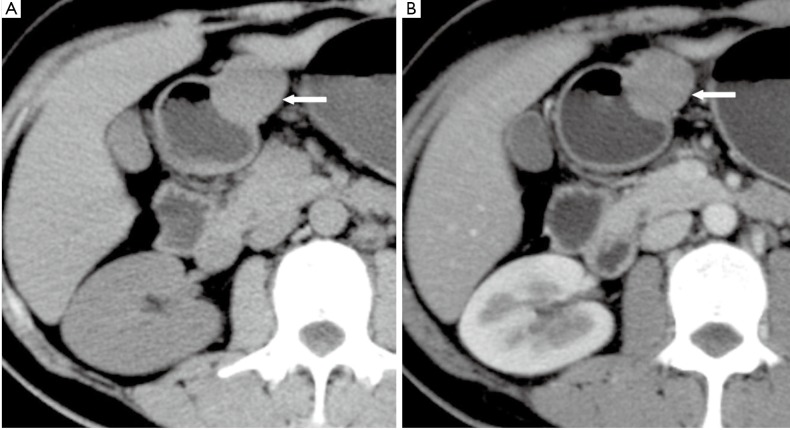
Figure 6.
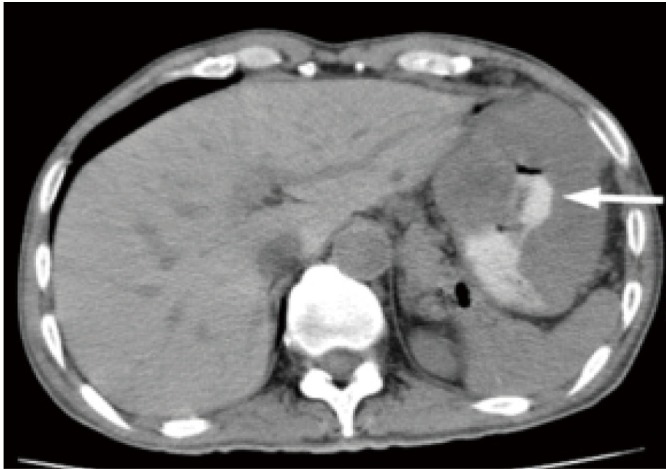
A 52-year-old female with malignant gastric GIST. Axial unenhanced CT image shows an extraluminal mass with necrosis cavity and communication with gastric luminal. Oral contrast materials is seen in the necrosis cavity (arrow)
Figure 7.
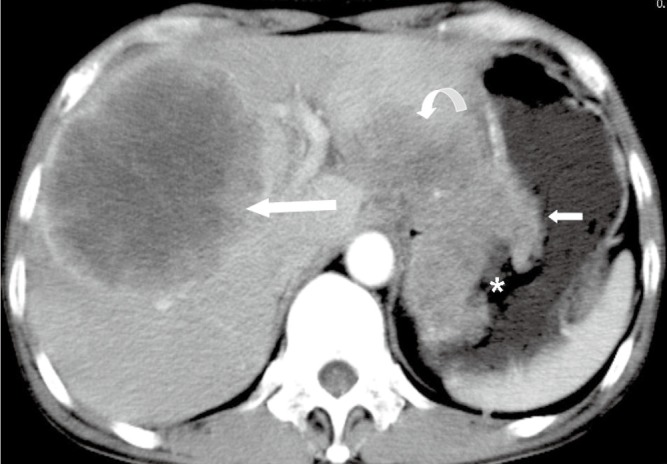
A 50-year-old male with malignant gastric GIST. Axial enhanced CT image shows an extraluminal mass with heterogeneous enhancement (short arrow), mural ulcer (star) and directly invading the left lobe of liver (curve arrow). A heterogeneous enhanced metastasis (long arrow) is demonstrated in the right lobe of liver
Leiomyoma
Leiomyoma originates from either the muscularis mucosae or muscularis propria. They are composed of well-differentiated smooth muscle cells and are rare in the stomach. Although they show similar optical microscopy appearances with GIST, positive staining for a-smooth muscle actin and desmin and negative staining for CD117, CD34, and s100 proteins can distinguish them from GIST at immunohistochemical examination. Leiomyoma appears as subepithelial masses with enhancement at CT and MRI (Figure 8). Because their imaging findings overlap with GIST, it is impossible to distinguish the two entities. The final diagnosis is established by immunohistochemical examination.
Figure 8.
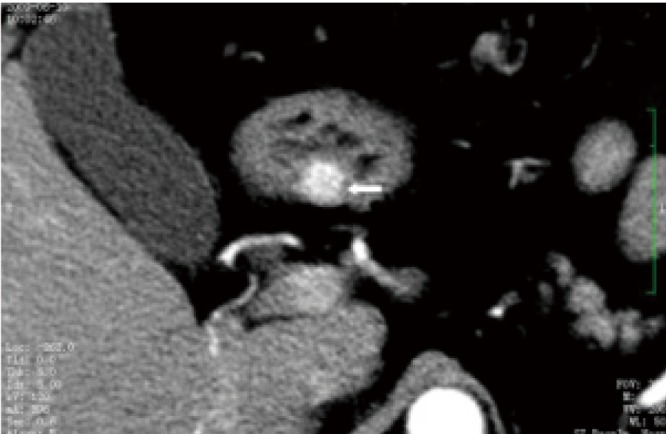
A 42-year-old male with gastric leiomyomas. Enhanced CT shows a subepithelial node in the gastric antrum with avid enhancement
Nerve sheath tumors
Gastric schwannoma arises from the Schwann cells of the neural plexus within the stomach wall. They manifest as subepithelial masses with minimal enhancement during the arterial phase and delayed enhancement during the equilibrium phase (Figure 9). Malignant nerve sheath tumors may show necrosis and heterogeneous enhancement (Figure 10).
Figure 9.
A 41-year-old female with a Schwannoma of the stomach. A. Unenhanced CT shows a well-defined extraluminal soft tissue mass with homogeneous density; B. Enhanced CT shows the neoplasm is of homogeneous enhancement
Figure 10.
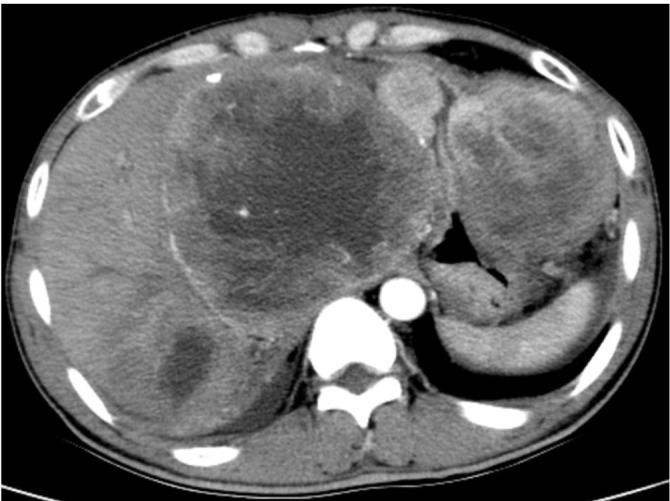
A 63-year-old male with malignant nerve sheath tumor. Enhance CT shows an extrophy growth mass in the large curve of the stomach with heterogeneous enhancement. Multiple metastases are noted in the liver
Neuroendocrine tumors
Most neuroendocrine tumors occur in the appendix, followed by small bowel, but more gastric neuroendocrine tumors are founded recently due to endoscopic examinations. These entities manifest as subepithelial masses with avid enhancement after iv contrast materials (Figure 11).
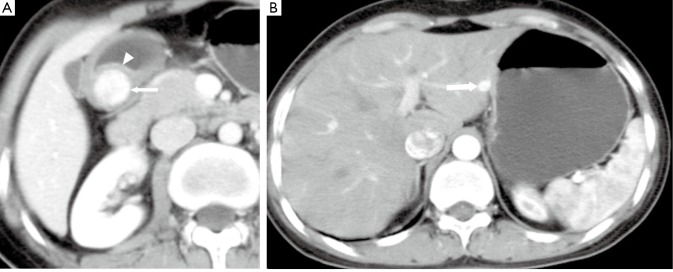
Figure 11.
A 36-year-old female with malignant gastrinoma. A. Enhanced CT shows a well-defined subepithelial node with avid enhancement (arrow). The enhanced mucosa (arrow head) is intact, which is the typical characteristics of subepithelial neoplasms; B. A marked enhanced metastasis (arrow) in the liver is revealed
Lymphoma
Gastric lymphomas account for 1-5% malignant tumors involving the stomach (7). Due to the characteristic of submucosal spread, gastric lymphomas often appears as abnormally thickened gastric walls with perigastric lymph adenopathy (8). Sometimes, gastric lymphoma can form a focal mass which mimics a subepithelial tumor (Figure 12).
Figure 12.
A 62-year-old female with primary gastric lymphoma. CT images show an irregular subepithelial mass in the gastric antrum (arrow in A) and lymph adenopathy (arrow in B)
Miscellaneous masses
Castleman disease, solitary fibrous tumor (SFT), inflammatory myofibroblastic tumor, and schwannomas from peritoneal or retroperitoneal, as well as GIST arising from the mesentery and omentum can be adjacent to the stomach and can mimic extraluminal gastric GIST. Tumors from liver or spleen can invade or compress the stomach, which can also mimic gastric GIST (Figures 13,14)
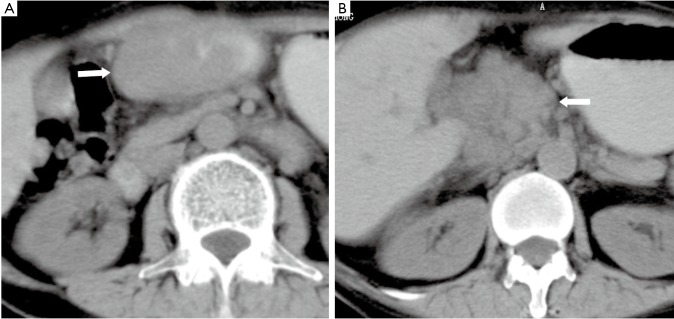
Figure 13.
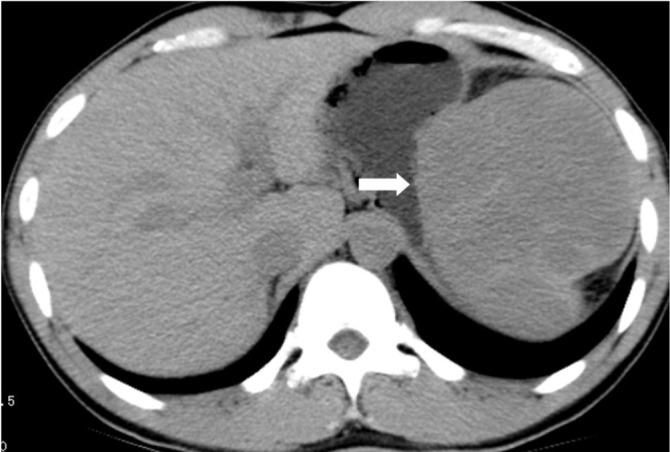
A 28-year-old male with spleen NHL. Unenhanced CT shows a mass compressing the greater curvature of the gastric body, which mimics a subepithelial tumor of the stomach
Figure 14.
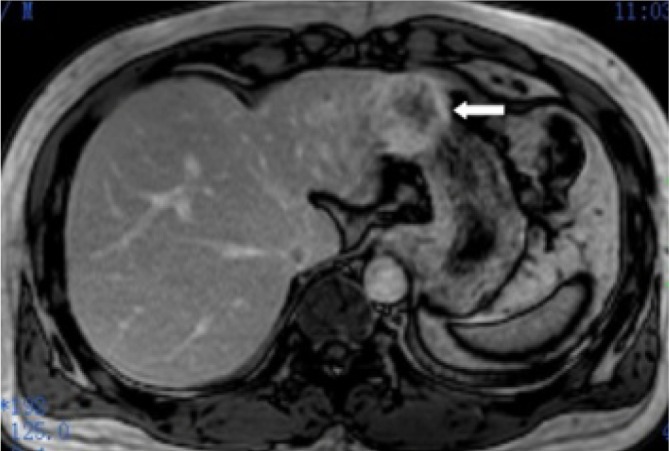
A 46-year-old male with hepatocellular carcinoma of the left lobe of liver. Contrast enhanced MR image shows a periphery enhanced mass invading the stomach and mimicking a gastric subepithelial mass (arrow)
Conclusions
When a mass of the stomach without characteristics of epithial tumors is encountered at CT or MRI, GIST should be considered first in the differential diagnosis. Majority of gastric GIST manifests as an extramural growth soft tissue mass. Moderate to intense enhancement can be found after iv contrast materials. Small masses often appear as homogeneous textures or enhancement, while large masses tend to show heterogeneous texture or enhancement. Extensive necrosis, even fistula between gastric lumen and necrosis cavity, can be seen. Unlike the epithial tumors, GIST seldom causes GI tract obstruction, ascites and metastasis to lymph nodes. Malignant GIST often metastasizes to the liver and mesentery. Several gastric subepithelial tumors may mimic GIST and their imaging manifestations overlap. Due to their benign biological characteristics, GIST tends to grow into a large mass when the mass products symptom and the patient seeks medical services. Therefore, when a large subepithal mass of the stomach is found at CT or MRI, it more like to be a GIST, especially when it poses extensive necrosis, fistula formation and hepatic or/and mesentery metastasis without ascites, lymph node enlargement and GI tract obstruct sign.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7:507-19 [DOI] [PubMed] [Google Scholar]
- 2.Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, et al. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol 1998;11:728-34 [PubMed] [Google Scholar]
- 3.Lee NK, Kim S, Kim GH, et al. Hypervascular subepithelial gastrointestinal masses: CT-pathologic correlation. Radiographics 2010;30:1915-34 [DOI] [PubMed] [Google Scholar]
- 4.Sandrasegaran K, Rajesh A, Rushing DA, et al. Gastrointestinal stromal tumors: CT and MRI findings. Eur Radiol 2005;15:1407-14 [DOI] [PubMed] [Google Scholar]
- 5.Humphris JL, Jones DB. Subepithelial mass lesions in the upper gastrointestinal tract. J Gastroenterol Hepatol 2008;23:556-66 [DOI] [PubMed] [Google Scholar]
- 6.Tateishi U, Hasegawa T, Satake M, et al. Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr 2003;27:792-8 [DOI] [PubMed] [Google Scholar]
- 7.Brady LW, Asbell SO. Malignant lymphoma of the gastrointestinal tract. Erskine Memorial Lecture, 1979. Radiology 1980;137:291-8 [DOI] [PubMed] [Google Scholar]
- 8.Buy JN, Moss AA. Computed tomography of gastric lymphoma. AJR Am J Roentgenol 1982;138:859-65 [DOI] [PubMed] [Google Scholar]