Abstract
Optical fluorescence imaging with the right combination of imaging modality and targeted contrast agents offers tremendous improvement in intraoperative imaging and clinical output (i.e., image-guided cancer surgery). Therefore, it is of paramount importance to gain an in-depth knowledge in the design of targeted contrast agents to meet clinical requirements. Currently, there are several clinically approved contrast agents available; however, none perform optimally in vivo by providing optimum sensitivity, stability, specificity, and safety for target imaging, diagnosis, and therapy. In this review, we discuss basic design considerations for targeted contrast agents in terms of optical and physicochemical properties, biological and physiological interactions, and biodistribution and targeting.
Key Words: Near-infrared imaging, optical imaging, targeted contrast agent, biodistribution
Introduction
Surgery is a complicated procedure, and is often performed without any image guidance, relying only on a surgeon’s palpation and direct visualization of surface features. Recently, many biomedical imaging modalities have been developed to image tissues and detect architectural abnormalities prior to surgical procedures [reviewed in (1-3)]. However, most of these imaging modalities cannot provide tissue- and disease-specific contrast in real time (3-9). The development of real-time image guidance, where it provides a surgeon direct image guidance to the target of the interest, is needed to avoid many surgical problems such as incomplete resection of cancerous tissues or damage to nerves or blood vessels. Optical imaging provides real-time visualization of the surgical field, allowing intraoperative image-guided surgery (10-18). The implications of image-guided surgery are significant in offering the opportunity for greatly improved surgical outcomes and benefits to the large population of patients undergoing surgical procedures. Imaging in the near-infrared (NIR) window (700-900 nm), also known as the “therapeutic window” has tremendous potential by offering low absorbance and scattering in tissues while providing maximum depth of light penetration (19). As a result, with the right combination of an NIR fluorescence imaging system and a targeted contrast agent, a high-quality target image can be obtained to assist real-time intraoperative surgery without changing the surgical field-of-view (20,21). In addition, it is cost-efficient and allows for simple detection with high spatial resolution and sensitivity, and low risk to the living subject because of the use of nonionizing radiation (22).
It is important to note that the ability to visualize a target tissue mainly depends on the optical contrast, resulted from the difference in the amount of molecular uptake into the target and normal tissues, i.e., target-to-background ratio (TBR) (2,23,24). However, achieving a high TBR with high sensitivity, specificity, and selectivity is difficult due to the limited number of biomarkers and targeting moieties. Indeed, only two NIR probes, methylene blue (MB) and indocyanine green (ICG), have been approved by the FDA for clinical use. Therefore, in a clinical discipline, an NIR contrast agent of well-defined physicochemical and in vivo properties is an unmet need for early phase diagnosis with accurate targeting (25-29).
In this review, we discuss the basic criteria required for the development of clinically applicable contrast agents in perspective of biodistribution and targeting. We also propose “4S design considerations: stability, sensitivity, specificity, and safety” to optimize physicochemical and optical properties of contrast agents in addition to their biological, physiological, and targeting properties. The criteria discussed in this review can serve as general design considerations that can be applied to develop almost any type of targeted contrast agents.
Stability: nature of optical contrast agents
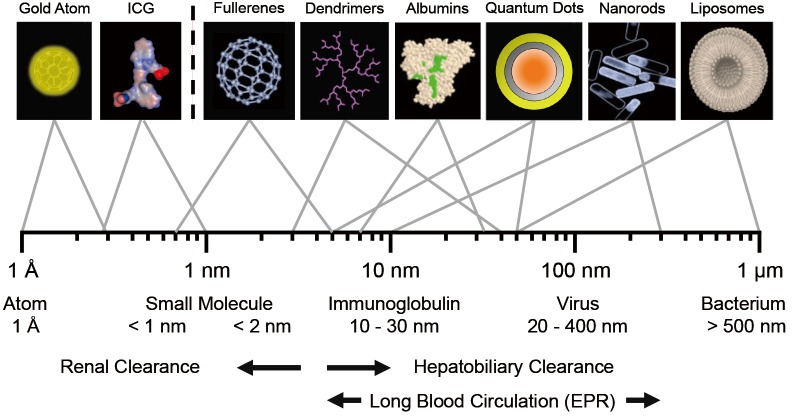
Over the past 50 years, various contrast agents from small molecules to nanoparticles have been developed for molecular imaging, diagnosis, and therapeutic purposes (Figure 1). To design contrast agents, the first consideration should be “stability” of a molecule in vitro and in vivo. Physicochemical properties such as hydrodynamic diameter (HD), chemical composition, shape, hydrophilicity/lipophilicity, and surface charge distribution are the key factors to determine intra- and intermolecular interactions such as solubility, degradation, and aggregation in vitro [reviewed in (9,12)]. These properties are also important to determine the fate of injected contrast agents in vivo by associating with serum proteins and nonspecific uptake in normal tissues and organs (12,22). As shown in Figure 1, the threshold for rapid renal clearance is 5.5 nm in HD, and a contrast agent larger than 8 nm is excreted via hepatobiliary clearance route. In order to get the enhanced permeation and retention (EPR) effect, the HD of a contrast agent needs to be in the range of 10 and 200 nm with a hydrophilic (polar or zwitterionic) surface coating to have a long blood circulation while avoiding immune responses.
Figure 1.
Relative sizes of contrast agents: HD ranges for contrast agents useful for biomedical imaging (top row) and naturally occurring materials (bottom row). ICG = indocyanine green. The threshold between rapid renal clearance and hepatobiliary clearance is about 5.5 nm in HD. EPR (enhanced permeation and retention) is efficient when the size of an injected molecule is between 10 nm and 200 nm in HD. [Modified from Ref (12); Copyright permission from Decker Publishing]
Sensitivity: detectability and optical properties
“Sensitivity” is the ability to detect either the probe signal at the target (direct response) or change in a signal, depending on the quantity of probes at the target (indirect response) (30). Optical fluorescence imaging provides a high signal-to-background ratio (SBR) only when the physicochemical and optical properties of a contrast agent are optimized to have high signal at the target and ultralow background in the NIR window (1,3). Thus, the optical contrast agents for in vivo imaging should be designed to have excitation and emission wavelengths in the NIR window so that the maximum number of photons travels deep into the target tissue and return to the detector of choice. Imaging in the NIR window also reduces a significant amount of autofluorescence (1,3). To ensure high signal in the target, the contrast agent should have efficient optical properties including high extinction coefficient and quantum yield without having undesired properties such as photobleaching, photodegradation, and quenching (9).
Specificity: biodistribution and targeting (organ- vs. cellular-specificity)
“Specificity” is the ability to distinguish the target from non-target processes or tissues (30). Specific targeting improves image resolution by enhancing the target signal and reducing background signals (concentration of contrast agents per unit volume of target tissue determines the signal strength). Several important physiological properties required for targeted contrast agents include reasonable blood half-life, selective binding to the desired epitope, low nonspecific uptake into normal tissues, and efficient elimination from the body (18,31). The injected contrast agents need rapid and complete biodistribution in order to achieve site-specific targeting and complete elimination of unbound molecules from the target tissue as well as from the body in a reasonable period time.
As shown in Figure 2, the biological half-life can be derived from the curve-fitting model. Plasma half-life (t1/2) is an important pharmacokinetic parameter and defined as the time it takes for the concentration of the injected molecule in the plasma to decrease by 50%. The alpha and beta half-lives are predominately associated with distribution phase (purple) and elimination phase (blue), respectively: the initial rapid decline after the maximal concentration mainly attributes to the distribution of contrast agent from intravascular space to other compartments such as extravascular fluid and tissues (distribution phase; t1/2,α) and the slower decline is mainly resulted from the elimination phase (t1/2,β), where the concentration in plasma gradually decreases due to cellular metabolism and excretion (32-34). The beta half-life mainly reflects the terminal half-life. The degree and rate of the distribution are influenced by various factors including HD, formulations, surface charge distribution, administration route, injection dose, protein binding, blood flow, and blood pH (Figure 2).
Figure 2.
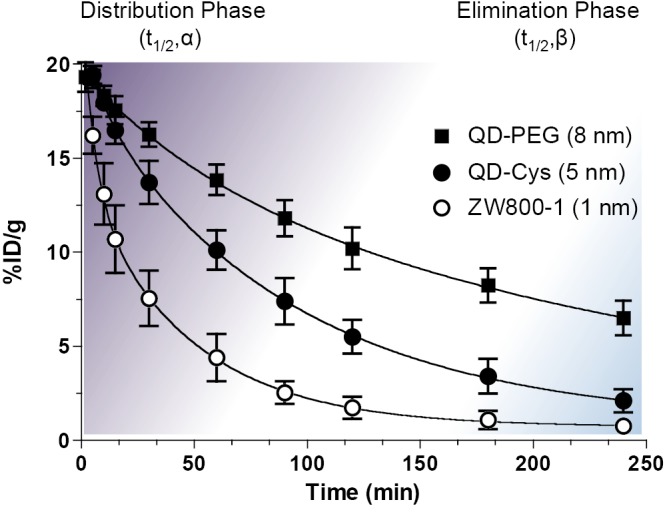
Pharmacokinetics: the plasma half-life is composed of distribution phase (t1/2,α) and elimination phase (t1/2,β) as indicated in purple and blue colors, respectively. Shown are blood half-life examples obtained from ZW800-1 (28) (a small molecule, 1 nm in HD), QD-Cys (25) (a small nanoparticle, 5 nm in HD), and QD-PEG (26) (a typical-sized nanoparticle, 8 nm in HD)
Contrast agents accumulate in the specific tissues during the distribution-equilibrium phase (biodistribution), and reach particular subcellular compartments or targeting cells (targeting). The transition between distribution and elimination phases plays a critical role in the accumulation of contrast agents in the specific tissue, which can be divided into two distinct classes: organ-specific targeting and subcellular-specific targeting.
Organ-specific targeting (mode of action)
A major challenge in molecular targeting is that, regardless of molecular level affinity, many probes cannot reach the site of interest when they are administrated intravenously. Therefore, it is important to understand the in vivo fate of contrast agents (organ-specific targeting) to achieve subcellular-specific targeting (4,19,23,35). Organ-specific targeting is defined as the degree of partitioning to tissue when the living organism is exposed to molecular probes during or after systemic circulation. This is firmly associated with (I) macroscopic tissue properties, (II) tissue compartment concentration, (III) intrinsic physicochemical properties, and (IV) physiological dynamic behavior of the contrast agent. Macroscopic tissue properties, such as tissue cortical tension and adhesion, viscosity, and infiltration tendency, ensure that a significant fraction of injection dose is scattered towards response of intrinsic physicochemical properties. For example, when delivered into lungs, a small cationic charged nanoparticle quickly associates with endogenous proteins and causes inflammation (27). Therefore, as physiological dynamic behavior is annotated to tissue properties, each contrast agent can be cited to desired biological locations on the target, where biochemical interactions or systemic accumulations occur.
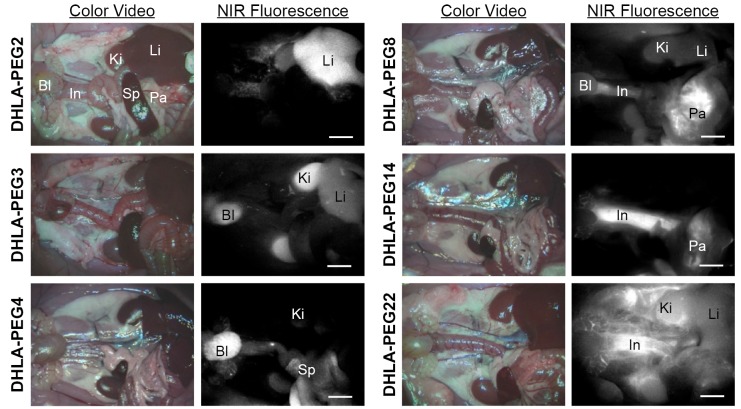
Figure 3 shows organ- and tissue-selective biodistribution and elimination of PEGylated nanoparticles (26). By introducing various lengths of neutral and hydrophilic polymer chains (DHLA-PEG), nanoparticles could avoid serum protein binding and achieve enhanced contrast at the specific location of the body such as liver, kidneys, bladder, pancreas, and long blood circulation. Among the key determinants (i.e., chemical composition, HD, shape, and surface charge), the HD after being stabilized in serum showed a profound effect on the biodistribution and clearance of nanoparticles (Figure 3).
Figure 3.
In vivo NIR fluorescence imaging of nanoparticles in Sprague-Dawley rats. 20 pmol/g of PEGylated InAs(ZnS) quantum dots were injected intravenously 4 h prior to imaging. Abbreviations used are: DHLA, dihydrolipoic acid; PEG, polyethylene glycol; Ki, kidneys; Bl, bladder; Li, liver; Pa, pancreas; Sp, spleen; and In, intestine. λExc=667±11 nm; λEm=720 nm long pass. NIR fluorescence images have identical exposure times and normalizations. Scale bars =500 µm. [Adapted from Ref (26); Copyright permission from ACS Publications]
Naked contrast agents without employing a targeting ligand could serve to differentiate disease sites compared to surrounding background tissues. By capitalizing on the extravasation of ICG through the leaky tumor vasculature, they could enhance contrast to distinguish malignant from benign breast lesions (16). However, building a targeted contrast agent by rendering bifunctional ligands on a fluorophore to light up the target cell is a more general approach for achieving high TBR with specificity and selectivity. As an example, we applied this basic principle for tumor-specific targeting using a non-sticky zwitterionic NIR fluorophore ZW800-1 (28,29) exhibiting no serum protein binding and ultralow background tissue uptake by conjugating with an integrin-specific cRGD ligand (36). The cRGD-ZW800-1 conjugate successfully reached the target cancer site (integrin αvβ3-positive cancer) through efficient biodistribution (organ-specific targeting), and the unbound fluorophores excreted to urine in 4 h, which increased the TBR significantly (36).
Cellular-specific targeting (mechanism of action)
The contrast agents, then, take a large step toward intracellular compartments, in which molecular mechanism is mainly operating for affinity interactions (37-39). The cellular-specificity allows the targeted region to be detected, even at the microscopic level. However, the intracellular interaction is a complex process including cellular uptake, retention and distribution in the cell, and efflux from the cell. This primarily requires attacking tissue permeability with avoidance of serum protein binding. Capillary pore in normal tissue is about 5 nm; therefore, a molecule sized greater than 5 nm cannot rapidly equilibrate between intravascular and extravascular spaces, affecting their blood half-life (12). In addition, highly charged molecules nonspecifically adsorb serum proteins, which increases the overall size significantly and changes the surface functionality.
The cellular-specificity often refers to a specific biochemical ligand that interacts with single or multiple molecular targets including enzymes or receptors. Therefore the cellular-specificity is rather related to active (31,36) or activatable targeting (40-42), while organ-specificity is more likely resulted from physicochemical properties of contrast agents and thus applicable to passive targeting (11,43-48). In general, active targeting could be achieved by conjugating a probe with targeting ligands, such as small molecules, peptides, antibodies, and aptamers that are specific to cell surface markers present or overexpressed on cancer cells (12). To promote desired biodistribution and clearance, the modular chemistry needs to be thoroughly designed by balancing between the targeting moiety and effector domain with consideration of organ-specificity (2).
Safety: potential toxicity
Potential toxicity is a major hurdle for the development of many contrast agents in the consideration of clinical translation [reviewed in (12)]. The proposed toxicity evaluations by the FDA include developmental and reproductive toxicity, immunotoxicity, neurotoxicity, respiratory toxicity, carcinogenicity, acute/chronic toxicology, and genotoxicity. Such toxicities may be resulted from the intact molecular properties or the decomposed components released during degradation in vivo. The major determining factors are physicochemical properties of the injected molecule including size, surface chemistry and physics, and formulation. The dosage and route of administration, and absorption, distribution, metabolism, and elimination (ADME) are also important considerations. Most inorganic nanoparticles are limited for clinical use because of nonbiocompatible elements, which are known to have acute or chronic toxicity in vertebrates (49). For example, contrast agents with lipophilic and unbalanced surface charges are likely to associate with serum proteins, increasing retention time in the body and risk of the reticuloendothelial system (RES) uptake into the liver, spleen, and bone marrow (12,25). Health risks of inorganic agents also include cytotoxicity to organelles and membranes, induced apoptosis, and peroxidative stress. Therefore, the design of biocompatible and biodegradable contrast agents that have little or non-toxicity is highly recommended (25). However, even organic nanoparticles or small molecules, if they result in improper biodistribution and incomplete clearance, can cause potential toxicity such as reproductive risks, immunotoxicity, and carcinogenicity through biological interactions in the body (50). Rapid biodistribution and complete clearance are therefore required for the targeted contrast agent to be successful.
4S design considerations for optical contrast agents
For the clinical use, a contrast agent needs to be designed by considering physicochemical and optical stability, reasonable blood half-life, selective binding to desired epitopes, low nonspecific uptake into normal tissues, efficient elimination from the body, and non-toxicity (18,31). Here we summarize the basic design considerations for optical contrast agents in terms of “4S criteria - stability, sensitivity, specificity, and safety” (Table 1).
Table 1. 4S design considerations for targeted contrast agents.
| 4S criteria determining properties | Definition, considerations, and requirements |
|---|---|
| Stability: physicochemical, optical, and biological properties | Ability to resist changes in chemical, physical, and optical properties in biological and physiological conditions over time |
| Determinable factor for solubility, aggregation, degradation, serum protein binding, and nonspecific uptake in vivo | |
| Physicochemical stability (solubility) is governed by chemical composition, HD, hydrophilicity/lipophilicity, and surface charge | |
| Optical stability: photobleaching, photodegradation, and quenching | |
| Sensitivity: detectability and optical properties | Ability to detect either the probe signal at the target (direct response) or change in a signal, depending on the quantity of probes at the target (indirect response) |
| Maximum excitation and emission wavelength in the NIR range for deep tissue imaging | |
| Require high extinction coefficient and high quantum yield | |
| Specificity: targeting properties (Organ vs. Cellular) | Ability to distinguish the target from non-target processes or tissues (enhanced contrast) |
| Require high concentration of contrast agents per unit volume of target tissue: targeting improves image resolution by enhancing the target signal and reducing background signals. | |
| Organ-specific targeting (mode of action): effective delivery to the target tissue (i.e., biodistribution, pharmacokinetics, and clearance) | |
| Cellular-specific targeting (mechanism of action): active and activatable targeting | |
| Safety: physicochemical, biological, and physiological properties | Ability to resist innate immune defenses or undesirable consequences related to toxicity |
| Affecting factors are molecule’s size, surface chemistry, formulation, surface physics, dosage and route of administration, and ADME (absorption, distribution, metabolism, elimination). | |
| Require nontoxic, biocompatible, or biodegradable properties |
Conclusions
Optical imaging holds great potential, and clinical translation of optical contrast agents has a significant impact on surgery outcomes and patient care. Therefore, design of an optimum contrast agent targeted to specific cancers is desperately needed for efficient imaging, diagnosis, and therapy. This review offers a guideline for designing tissue-specific contrast agents to maximize targetability and functionality in in vivo imaging and image-guided surgery. We believe that comprehensive understanding of molecular properties, tissue properties, biodistribution, and targeting mechanisms would help the widespread translation of currently developing numerous contrast agents into the clinic.
Acknowledgements
We thank Drs. John V. Frangioni (BIDMC) and Summer L. Gibbs (OHSU) for many helpful discussions. We also thank David Burrington, Jr. for editing and Eugenia Trabucchi for administrative assistance. This work was supported by the National Institutes of Health (NIBIB) grants #R01-EB-010022 and #R01-EB-011523, and the Dana Foundation Program in Brain and Immuno-Imaging.
Disclosure: The authors declare no conflict of interest.
References
- 1.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 2003;7:626-34 [DOI] [PubMed] [Google Scholar]
- 2.Frangioni JV. New technologies for human cancer imaging. J Clin Oncol 2008;26:4012-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging 2010;9:237-55 [PMC free article] [PubMed] [Google Scholar]
- 4.Jang B, Park S, Kang SH, et al. Gold nanorods for target selective SPECT/CT imaging and photothermal therapy in vivo. Quant Imaging Med Surg 2012;2:1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneko OF, Willmann JK. Ultrasound for molecular imaging and therapy in cancer. Quant Imaging Med Surg 2012;2:87-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Pang Y, Vigneron D, et al. Investigation of multichannel phased array performance for fetal MR imaging on 1.5T clinical MR system. Quant Imaging Med Surg 2011;1:24-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pysz MA, Guracar I, Tian L, et al. Fast microbubble dwell-time based ultrasonic molecular imaging approach for quantification and monitoring of angiogenesis in cancer. Quant imaging Med Surg 2012;2:68-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan J, Mei CS, Panych LP, et al. Towards fast and accurate temperature mapping with proton resonance frequency-based MR thermometry. Quant Imaging Med Surg 2012;2:21-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbs SL. Near infrared fluorescence for image-guided surgery. Quant Imaging Med Surg 2012;2:177-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissleder R, Tung CH, Mahmood U, et al. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol 1999;17:375-8 [DOI] [PubMed] [Google Scholar]
- 11.Weissleder R.A clearer vision for in vivo imaging. Nat Biotechnol 2001;19:316-7 [DOI] [PubMed] [Google Scholar]
- 12.Choi HS, Frangioni JV. Nanoparticles for biomedical imaging: fundamentals of clinical translation. Mol Imaging 2010;9:291-310 [PMC free article] [PubMed] [Google Scholar]
- 13.Ashitate Y, Tanaka E, Stockdale A, et al. Near-infrared fluorescence imaging of thoracic duct anatomy and function in open surgery and video-assisted thoracic surgery. J Thorac Cardiovasc Surg 2011;142:31-8.e1-2. [DOI] [PMC free article] [PubMed]
- 14.Hutteman M, Choi HS, Mieog JS, et al. Clinical translation of ex vivo sentinel lymph node mapping for colorectal cancer using invisible near-infrared fluorescence light. Ann Surg Oncol 2011;18:1006-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashitate Y, Stockdale A, Choi HS, et al. Real-time simultaneous near-infrared fluorescence imaging of bile duct and arterial anatomy. J Surg Res 2012;176:7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poellinger A.Near-infrared imaging of breast cancer using optical contrast agents. J Biophotonics 2012;5:815-26 [DOI] [PubMed] [Google Scholar]
- 17.Hadjipanayis CG, Jiang H, Roberts DW, et al. Current and future clinical applications for optical imaging of cancer: from intraoperative surgical guidance to cancer screening. Semin Oncol 2011;38:109-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovar JL, Simpson MA, Schutz-Geschwender A, et al. A systematic approach to the development of fluorescent contrast agents for optical imaging of mouse cancer models. Anal Biochem 2007;367:1-12 [DOI] [PubMed] [Google Scholar]
- 19.Frangioni JV. The problem is background, not signal. Mol Imaging 2009;8:303-4 [PubMed] [Google Scholar]
- 20.Hawrysz DJ, Sevick-Muraca EM. Developments toward diagnostic breast cancer imaging using near-infrared optical measurements and fluorescent contrast agents. Neoplasia 2000;2:388-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Licha K, Riefke B, Ebert B, et al. Cyanine dyes as contrast agents in biomedical optical imaging. Acad Radiol 2002;9:S320-2 [DOI] [PubMed] [Google Scholar]
- 22.Cassidy PJ, Radda GK. Molecular imaging perspectives. J R Soc Interface 2005;2:133-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbs-Strauss SL, Nasr KA, Fish KM, et al. Nerve-highlighting fluorescent contrast agents for image-guided surgery. Mol Imaging 2011;10:91-101 [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbs-Strauss SL, Vooght C, Fish KM, et al. Molecular imaging agents specific for the annulus fibrosus of the intervertebral disk. Mol Imaging 2010;9:128-40 [PubMed] [Google Scholar]
- 25.Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol 2007;25:1165-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi HS, Ipe BI, Misra P, et al. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett 2009;9:2354-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi HS, Ashitate Y, Lee JH, et al. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol 2010;28:1300-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi HS, Nasr K, Alyabyev S, et al. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew Chem Int Ed Engl 2011;50:6258-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyun H, Bordo MW, Nasr K, et al. cGMP-Compatible preparative scale synthesis of near-infrared fluorophores. Contrast Media Mol Imaging 2012;7:516-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeGrado TR, Turkington TG, Williams JJ, et al. Performance characteristics of a whole-body PET scanner. J Nucl Med 1994;35:1398-406 [PubMed] [Google Scholar]
- 31.Choi HS, Liu W, Liu F, et al. Design considerations for tumour-targeted nanoparticles. Nat Nanotechnol 2010;5:42-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ariëns EJ. Drug levels in the target tissue and effect. Clin Pharmacol Ther 1974;16:155-75 [DOI] [PubMed] [Google Scholar]
- 33.Nightingale CH, Carver P. Basic principles of pharmacokinetics. Clin Lab Med 1987;7:267-78 [PubMed] [Google Scholar]
- 34.Marko-Varga G, Végvári A, Welinder C, et al. Standardization and utilization of biobank resources in clinical protein science with examples of emerging applications. J Proteome Res 2012;11:5124-34 [DOI] [PubMed] [Google Scholar]
- 35.Schroeder A, Heller DA, Winslow MM, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer 2011;12:39-50 [DOI] [PubMed] [Google Scholar]
- 36.Choi HS, Gibbs SL, Lee JH, et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol. 2013 doi: 10.1038/nbt.2468. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: first in-human results. Nat Med 2011;17:1315-9 [DOI] [PubMed] [Google Scholar]
- 38.Reticker-Flynn NE, Malta DF, Winslow MM, et al. A combinatorial extracellular matrix platform identifies cell-extracellular matrix interactions that correlate with metastasis. Nat Commun 2012;3:1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandersius SA, Weijer CJ, Newman TJ. Emergent cell and tissue dynamics from subcellular modeling of active biomechanical processes. Phys Biol 2011;8:045007. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi H, Ogawa M, Alford R, et al. New strategies for fluorescent probe design in medical diagnostic imaging. Chem Rev 2010;110:2620-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi T, Urano Y, Kamiya M, et al. Highly activatable and rapidly releasable caged fluorescein derivatives. J Am Chem Soc 2007;129:6696-7 [DOI] [PubMed] [Google Scholar]
- 42.Urano Y, Asanuma D, Hama Y, et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med 2009;15:104-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura S, Kobayashi T, Nakamura M, et al. Enhanced in vivo responses of osteoblasts in electrostatically activated zones by hydroxyapatite electrets. J Mater Sci Mater Med 2009;20:99-103 [DOI] [PubMed] [Google Scholar]
- 44.Hellebust A, Richards-Kortum R.Advances in molecular imaging: targeted optical contrast agents for cancer diagnostics. Nanomedicine (Lond) 2012;7:429-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pierce MC, Javier DJ, Richards-Kortum R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int J Cancer 2008;123:1979-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiyose K, Hanaoka K, Oushiki D, et al. Hypoxia-sensitive fluorescent probes for in vivo real-time fluorescence imaging of acute ischemia. J Am Chem Soc 2010;132:15846-8 [DOI] [PubMed] [Google Scholar]
- 47.Ogawa M, Kosaka N, Choyke PL, et al. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res 2009;69:1268-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa M, Kosaka N, Longmire MR, et al. Fluorophore-quencher based activatable targeted optical probes for detecting in vivo cancer metastases. Mol Pharm 2009;6:386-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klaassen CD. eds. Casarett & Doull's Toxicology: The Basic Science of Poisons. New York: McGraw-Hill, 2001. [Google Scholar]
- 50.Oberdörster G.Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health 2001;74:1-8 [DOI] [PubMed] [Google Scholar]