Abstract
Paecilomyces lilacinus (Thom) Samson LPS 876, a locally isolated fungal strain, was grown on minimal mineral medium containing “hair waste,” a residue from the hair-saving unhairing process, and produced a protease with keratinolytic activity. This enzyme was biochemically characterized. The optimum reaction conditions, determined with a response surface methodology, were 60°C and pH 6.0. It was remarkably stable in a wide range of pHs and temperatures. Addition of Ca2+, Mg2+, or sorbitol was found to be effective in increasing thermal stability of the protease. PMSF and Hg2+ inhibited the proteolytic activity indicating the presence of a thiol-dependent serine protease. It showed high stability toward surfactants, bleaching agents, and solvents. It was also compatible with commercial detergents (7 mg/mL) such as Ariel, Skip, Drive, and Ace, retaining more than 70% of its proteolytic activity in all detergents after 1 h of incubation at 40°C. Wash performance analysis revealed that this protease could effectively remove blood stains. From these properties, this enzyme may be considered as a potential candidate for future use in biotechnological processes, as well as in the formulation of laundry detergents.
1. Introduction
Microbial proteases are the most widely exploited industrial enzymes with major application in detergent formulations [1, 2]. These enzymes are being widely used in detergent industry since their introduction in 1914 as detergent additive. Over the past 30 years, the importance of proteases in detergents has changed from being the minor additives to being the key ingredients. The main areas where use of proteases has expanded are household laundry, automatic dishwashers, and industrial and institutional cleaning. In laundry detergents, protein stains such as grass, blood, food, and human swear, are removed through proteolysis. The performance of proteases is influenced by several factors such as pH of detergent, ionic strength, wash temperature, detergent composition, bleach systems, and mechanical handling. Thus, the key challenge for the use of enzymes in detergents is their stability. Various attempts have been made to enhance stability of alkaline proteases by site-directed mutagenesis [3] and protein engineering. “Subtilisin Carlsberg” has been protein engineered to obtain a bleach-stable, alkaline protease by molecular modification [4], but still, there is always a need for newer thermostable alkaline proteases which can withstand bleaching agents present in detergent. Among these different proteases, keratinases constitute a group of enzymes capable of disrupting the highly stable keratin structure consisting of disulfide, hydrogen, and hydrophobic bonds in the form of α-helices and β-sheets [5].
Argentine's economy has traditionally been based on agriculture and related industries. Livestock (cattle, sheep, and poultry) and grains have long been the bulwark of its wealth; its cattle herds are among the world's finest. There are more than 50 million of livestock which generate large amounts of waste including insoluble keratin-containing animal material such as feather, hair, wool, nails claws, hooves, horns, and beaks.
Although hair-saving unhairing processes reduce the organic load from beamhouse liquid effluent, a new solid residue called “hair waste” is generated, its appropriate disposal being then necessary. In a hair saving unhairing process almost 10% (wet basis) in weight of each salted bovine hide become hair waste. Because of tanning industry in Argentina processes, on average, more than 100 ton of salted hide per day, more than 10 ton of solid waste are generated per day, generating an environmental problem of considerable magnitud [6]. Since it is a protein waste, it deserves special attention in order to be utilized for practical purposes.
The fungal biotransformation of the “hair waste” implies considering it as a raw material instead of the present idea of disposability. Thus, hair waste would be the substrate, on which the fungus would act, giving rise to a (partially) hydrolyzed protein with different potential uses (i.e., as animal feed, fertilizer, etc.). In addition, the fungus would produce a proteolytic (keratinolytic) extract of biotechnological interest with a variety of potential applications (cosmetics, textiles, detergent industries, etc.). This paper deals with a particularly case of the second aspect above mentioned.
A series of studies dealing with the bioconversion of keratin waste resulted in the discovery of a novel keratinase activity in a culture supernatant of a fungal strain (Paecilomyces lilacinus (Thom) Samson LPS 876) grown on chicken feather as a sole of carbon, nitrogen, and energy source [7]. In this paper, we report the biochemical characterization, including the effect of some surfactants and bleaching agents on enzyme stability, its compatibility with various commercial liquid and solid detergents and a study of an efficient stabilization method toward heat inactivation, of the keratinase produced by Paecilomyces lilacinus growing on hair waste substrate.
A wash performance was also done with particular emphasis on its potential application as an enzyme ingredient for the formulation of laundry detergents.
2. Material and Methods
2.1. Microorganism and Identification as a Keratinolytic Fungus
Paecilomyces lilacinus (Thom) Samson LPS 876, a nonpathogenic fungal strain locally isolated from alkaline forest soils, was used. It was selected from Spegazzini Institute Fungal Culture Collection (La Plata National University, Argentina) after a preliminary screening for keratinolytic fungal strains on feather meal agar containing (per liter) the following: defatted chicken feather meal, 15 g; NaCl, 0.5 g; K2HPO4, 0.3 g; KH2PO4, 0.4 g; agar, 15 g, pH 7.2. The strain selected was punctual streaked and incubated at 28°C for 15 days. The growth of the colony and the clear zone formation around it were daily studied. The ability to degrade keratin was determined according to the presence or absence of hydrolysis halo [8].
2.2. Culture Conditions and Enzyme Production
Production of protease by P. lilacinus was carried out in a minimal mineral medium containing (per liter) the following: 10 g hair waste, 0.496 g NaH2PO4, 2.486 g K2HPO4, 0.016 g FeCl3·6 H2O, 0.013 g ZnCl2, 0.010 g MgCl2, and 0.11 mg CaCl2. Hair waste, obtained from a local tannery, was washed extensively with tap water, dried at 60°C for 2 days, and then kept at room temperature until used. In all cultures, it was a sole carbon, nitrogen, and energy source. The pH was adjusted to 7.0 previous to sterilization [9]. Cultures were performed at 28°C and 200 rpm for 10 days in an orbital shaker, in 500 mL Erlenmeyer flasks containing 200 mL of medium, inoculated with 2 × 106 conidia per mL. Samples of 5 mL were withdrawn at regular intervals, centrifuged (3,000 ×g, 10 min, 4°C), and the supernatant was used for pH, protein content, and enzyme activities determinations.
2.3. Protein Determination
The protein content was determined by Bradford's method using bovine albumin fraction V (SIGMA) as a standard [10].
2.4. Protease Activity
Proteolytic activity was measured as described by Liggieri et al. [11] with some modifications. Reaction mixture containing 100 μL of appropriately diluted enzyme preparation and 250 μL of 1% (w/v) azocasein solution in 0.1 M Tris-HCl buffer (pH: 9) was incubated for 30 min at 37°C. Reaction was stopped by precipitation of the residual substrate with 1 mL of trichloroacetic acid (TCA, 10%). The mixture was kept at room temperature for 15 min and then centrifuged at 3,000 ×g (10 min, 20°C). One mL of 1 M NaOH was added to 1 mL of the supernatant, and absorbance was measured at 440 nm. Measurement was made in triplicate using a blank with a heat inactivated enzyme solution. One unit of proteolytic activity (UC) was defined as the amount of enzyme that, under test conditions, causes an increase of 0.1 units in the absorbance at 440 nm per minute. Azocasein was synthesized as described by Riffel et al. [12].
2.5. Keratinase Activity
Keratinolytic activity was assayed as described by Joshi et al. [13] with some modifications: 800 μL 0.1 M Tris-HCl buffer (pH: 9) was added to 30 mg of azokeratin, and mixture was stirred for 15 min at room temperature until the azokeratin was completely suspended. Appropriately diluted enzyme preparation (100 μL) was added and incubated for 25 min at 37°C with individual magnetic stirring. Reaction was then stopped by the addition of 200 μL of TCA (10%) and centrifuged at 3,000 ×g (10 min, 20°C). The absorbance of the supernatant was measured at 440 nm. A blank was prepared using heat-inactivated enzyme preparation. One unit of keratinase activity (UK) was defined as the amount of enzyme that, under test conditions, causes an increase of 0.01 units in the absorbance at 440 nm per minute. Azokeratin was synthesized as described by Joshi et al. [13] using defatted feather meal as keratin source.
The relationship between keratinolytic and proteinolytic activity (called K : C ratio) is widely used as a parameter for evaluation of the keratinolytic potential of proteases [14, 15]. K : C ratio of P. lilacinus' keratinase was compared with those of several commercial proteases such as proteinase-K (Promega), Alcamax (Cergen), and papain (FLUKA). Stock solutions of these commercial enzymes, in concentration of 1 mg/mL, were prepared in distilled water. They were diluted adequately, and both enzyme activities (keratinase and protease) were determined as described above.
2.6. Biochemical Characterization
A supernatant of a 5-day-old culture was used as crude enzyme preparation for the biochemical characterization of the keratinases produced by P. lilacinus (2.5 UC/mL). It is worth to mention that, for practical reasons, protease activity was used in our research to represent keratinase activity, since keratinolytic (azokeratin) and proteolytic (azocasein) activities are directly related [16], a fact that was also later confirmed for P. lilacinus keratinase (see below).
2.7. Effect of pH and Temperature on Enzyme Stability and Activity
The effect of pH and temperature on enzyme activity and stability was studied using a Response Surface Methodology (RSM) based on the use of a matrix of experiments by which the simultaneous variations of the factors can be studied. Uniform shell design proposed by Doehlert was selected for design the response surface [17]. The main advantage of this procedure lies in the possibility of extending this uniform net in any direction and increasing the number of factors in the study. The real values of the independent variables were coded based on a linear functionality between codified (Z) and actual values (X) according to:
| (1) |
where X 0 is the real value of the central point and ΔX and ΔZ are the difference between the highest and lowest values of real and coded numbers, respectively.
Multiple regression analysis based on the least square method was performed using Mathcad 2001 software [17]. For both determinations, the central values (zero level) for the experimental designs were pH 7.5 and temperature 40°C.
The pH and thermal stability were evaluated incubating the enzyme preparation for 2 h at each chosen experimental condition. The residual protease activity was determined under standard conditions and expressed as percentage of residual activity relative to a control (measured at 0 h of incubation). Temperature varied between 20 and 60°C and pH between 3.0 and 12.0, using a mixture of buffers (Glicine, MES and Tris, 20 mM each).
The protective effect of CaCl2 and MgCl2 (5 mM, each) and sorbitol (10% w/v) on heat inactivation was also studied. The crude enzyme was incubated at 50–60°C with and without the chemicals mentioned above, and residual enzyme activity was measured at regular intervals under standard assay conditions.
The effect of pH and temperature on enzyme activity was determined at each condition set by the Doehlert's design. In this case, temperature varied between 20 and 60°C and pH between 6.0 and 12.0 using the same mixture of buffers described above.
2.8. Effect of Inhibitors, Metal Ions, and Organic Solvents on Enzyme Activity
In order to investigate the effect of different inhibitors of proteases, metal ions, and organic solvents on enzyme activity, the crude enzyme was preincubated for 1 h at room temperature with different reagents. Residual enzyme activity was determined and expressed as percentage relative to a reaction control (no addition). The different reagents tested were phenylmethanesulphonyl fluoride (PMSF, 2 mM), iodoacetate (10 mM), ethylenediaminetetraacetate (EDTA, 5 mM), 1,10-Phenantroline (1 mM), and Pepstatin A (100 μg/mL), Ca2+, Mg2+, Zn2+, and Hg2+ (1 mM each). The solvents tested were DMSO, ethanol, methanol, and isopropanol (1% v/v each).
2.9. Effect of Surfactants and Bleaching Agents on Enzyme Stability
The suitability of the crude protease of P. lilacinus as a detergent additive was determined by testing its stability in presence of some surfactants such as SDS (sodium dodecyl sulphate), Triton X-100, and Tween 20, and bleaching agents such as hydrogen peroxide (H2O2) and sodium perborate. The crude protease was incubated with different concentrations of these additives for 1 h at room temperature (22°C), and then the residual proteolytic activity was measured under standard conditions against a control without any additives, which was taken as 100%.
2.10. Detergent Compatibility
The compatibility of the protease activity in crude extract with commercial solid and liquid laundry detergents (locally available) was also studied. The solid detergents tested were Ariel (Procter & Gamble), Drive, Skip, and Ala matic (Unilever), and the liquid ones were Ace and Ariel (Procter & Gamble); also a prewashed liquid named Mr Musculo (SC Johnson & son) was tested.
Solid detergents were diluted in tap water to give a final concentration of 7 mg/mL, and liquid detergents and prewashed were diluted 100-fold to simulate washing conditions [18]. The endogenous enzymes contained in laundry detergents were inactivated by heating the diluted detergents for 1 h at 65°C prior the addition of an aliquot of crude protease. The corresponding reactions mixture were incubated in each detergents mentioned above for 1 h at different temperatures (30–50°C), and the remaining activities were determined under standard conditions. The enzyme activity of a control, incubated under the similar conditions without detergent, was taken as 100%.
2.11. Evaluation of Washing Performance
Clean cotton cloth pieces (2.5 cm × 2.5 cm) were soiled with blood: 100 μL of blood without pretreatment was applied to cloth piece and then dried. The stained cloth pieces were subjected to wash treatments with commercial solid detergent (Skip, a solid detergent available in Argentineans' market) diluted in tap water at 7 mg/mL, supplemented with and without crude enzyme. When the wash treatment was with the supplementation of the crude enzyme, endogenous enzymes contained in laundry detergents were inactivated by heating the diluted detergents for 1 h at 65°C prior to the addition of an aliquot of crude protease.
Two stained cloth pieces were taken in separate flasks, with 50 mL as final volume, as indicated above: flask with tape water, only, flask with tap water and commercial detergent at final concentration of 7 mg/mL, and flask with tap water, commercial detergent, and crude enzyme of P. lilacinus (62.5 UC/50 mL).
Each flask was incubated at two temperatures: 30 and 40°C for 30 and 60 min under agitation (200 rpm). After incubation, cloth pieces were taken out, rinsed with water, and dried. Visual examination of various pieces showed the effect of the crude enzyme in the removal of stains [19].
2.12. Statistical Analysis
All analyses were performed at least in triplicate, and data were expressed as means ± standard deviations.
3. Results and Discussion
3.1. Identification of P. lilacinus as Keratinolytic Fungus
A series of fungal strains of the Spegazzini Institute Fungal Culture Collection (La Plata National University, Argentina) were preliminary screened for their keratinolytic potential using feather meal agar. In our case, P. lilacinus (Thom) Samson LPS 876 was selected because, after 15 days of incubation at 28°C, a hydrolysis halo was observed indicating the keratinolytic capability of this strain (Figure 1). The use of this technique in a preliminary screening with a similar purpose was reported by Wawrzkiewicz et al. [20]. Where, among the 16 strains of dermatophytes tested, only Trichophyton verrucosum showed tiny fungal colonies surrounded by a wide clear zone of solubilized keratin.
Figure 1.
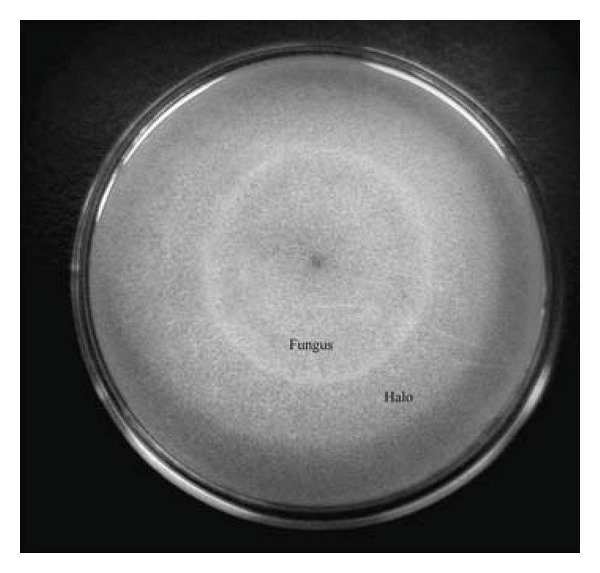
Qualitative test in feather meal agar plates for keratin degrading enzyme activity. A degradation halo surrounding the colony of P. lilacinus is observed.
3.2. Grow Profile of P. lilacinus on Hair Waste Medium
P. lilacinus LPS 876 grew well in a minimal mineral medium containing salts and hair waste as sole carbon, nitrogen, and energy source. As can be seen in Figure 2, extracellular enzyme activities (protease and keratinase) were associated with both an increment in soluble protein concentration as well as with a continuous increase in pH values in culture broth. These facts were reported by several authors for other microorganisms with high keratinolytic activity growing on this kind of substrates. The increment in pH value has been pointed out as an important indicator of the keratinolytic potential in microorganisms because of high level of deamination, with the concomitant ammonium accumulation in culture medium [21]. Moreover, Korniłłowicz-Kowlaska and Bohacz [22] concluded that substrate mass loss, a release of peptides and ammonia, sulfate excretion, and substrate alkalinization should be recognized as homogenized (due to mutual correlation) assessment parameters of the keratinolytic activity of fungi.
Figure 2.
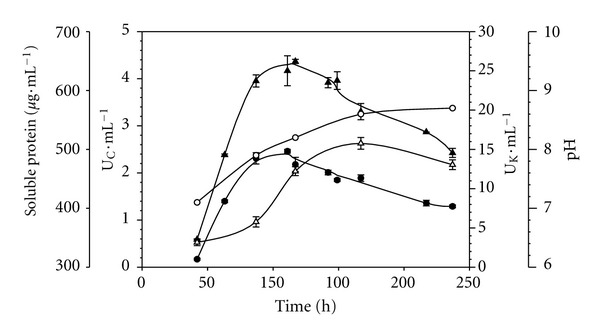
Time course of P. lilacinus culture using a minimal mineral medium containing 10 g L−1 of hair waste (pH: 7). (●) proteolytic activity; (▲) keratinolytic activity; (▵) soluble protein; (◯) pH.
Enzyme activities were evaluated during the culture time course. Maximum protease and keratinase activities concurred at around 111–117 h of cultivation, (Figure 2). The ratio observed between both enzyme activities (K : C ratio) at different culture times was quite constant (11.28 ± 1.06). Therefore, the presence of a single enzyme responsible of both activities was tentatively postulated. Using the same enzyme assay conditions, it is assumed that a protease with K : C ratio higher than 5 is a true keratinase [23]. Generally, reported keratinases have a K : C ratio ranged from 5 to 20 [14, 15, 24].
The hydrolysis of azokeratin and azocasein by the proteases produced by P. lilacinus growing on hair waste was compared with commercial enzymes. The K : C was chosen as criterion for enzyme specificity for keratinous substrates (Table 1). As can be seen, our crude enzyme preparation was superior for hydrolysis of keratin substrate compared with other commercially available proteases. Similar results were reported by Cheng et al. [25] for the keratinase of Bacillus licheniformis and by Gradišar et al. [14] for the keratinases of P. marquandii and Doratomyces microsporus.
Table 1.
Specific proteolytic activity (UC/mg), keratinolytic activity (UK/mg), and K : C ratio of crude extract and commercial proteases.
| Proteolytic activity (UC/mg) |
Keratinolytic activity (UK/mg) |
K : C ratio | |
|---|---|---|---|
| Enzyme preparation | 15.67 | 159.3 | 10.17 |
| Proteinas K | 33.9 | 218.7 | 6.45 |
| Alcamax | 4.6 | 27.4 | 5.96 |
| Papain | 7.6 | 3.9 | 0.51 |
3.3. Effect of pH and Temperature on Enzyme Stability
In general, all detergent compatible enzymes are alkaline thermostable in nature with a high pH optima because the pH of laundry detergents is generally in the range of 9–12 and varying thermostability at laundry temperatures, (50−60°C) [26–28]. For the study of the effect of pH and temperature a RSM was used. The pH and temperature values used in Doehlert's design for enzyme stability determination are shown in Table 2. The central point was replicated three times in order to determine the experimental error. Data presented in Table 2 were converted into second-order polynomial equation.
Table 2.
Actual values (experimental data) and residual activity attained in Doehlert's design for pH and temperature stability.
| pH | Temperature (°C) | Residual activity (%) | |
|---|---|---|---|
| Experimental data | |||
| A | 7.5 | 40 | 93.4 |
| A | 7.5 | 40 | 89.1 |
| A | 7.5 | 40 | 92.2 |
| B | 12 | 40 | 45.1 |
| C | 9.75 | 60 | 3.25 |
| D | 5.25 | 60 | 4.5 |
| E | 3 | 40 | 104.4 |
| F | 5.25 | 20 | 106.4 |
| G | 9.75 | 20 | 104.4 |
Statistical analysis of the results revealed that, in the range studied, the two variables, as well as their interactions, have a significant effect on protease (keratinase) stability.
The following regression equation was obtained to calculate the percentage of residual enzyme activity (% R.A.) after 2 h of incubation:
| (2) |
where pH and T are given as codified data. The r 2 value was equal to 0.91, indicating that only 9% of the total variation was not explained by the model. The contour graph of the proteolytic activity observed as a response to the interaction of pH versus temperature is shown in Figure 3. These results indicate that the enzyme is stable in a wide range of pH and temperatures, preserving more than 60% of its activity after 2 h of incubation at pH 11 and 45°C. A serine proteinase purified from P. lilacinus (Thom) Samson VKM F-3891 displayed 40% of residual activity after 3 h incubation at 60°C [29]. However, in other cases, such as the serine proteinase from Aspergillus chrysogenum, enzyme stability in the alkaline range is substantially reduced.
Figure 3.
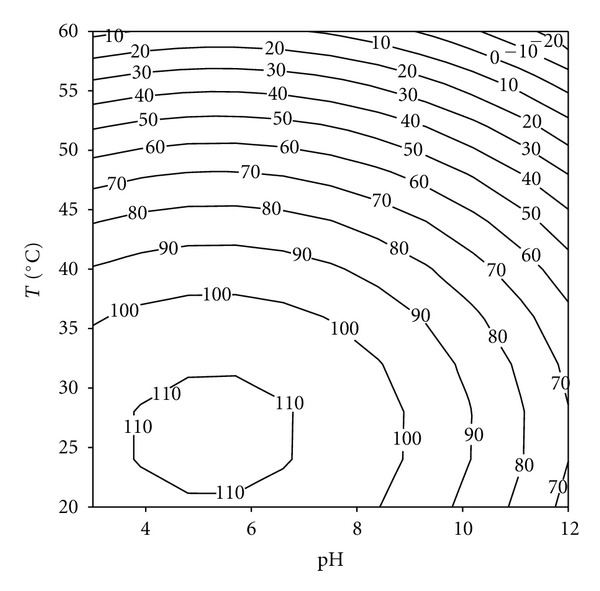
Response surface plot for the effect of pH and temperature on enzyme stability.
Studies on thermostability of the enzyme at 50, 55, and 60°C revealed that heat inactivation displays a typical first order kinetic (Figure 4(a)). The enzyme exhibited half lives of 62, 29, and 10 min at 50, 55, and 60°C, respectively. Addition of metal ions such as CaCl2 and MgCl2 (5 mM) individually, improved the thermostability of the enzyme (Figures 4(b) to 4(d)). It can be seen in Figure 4(b), the apparent half-life of the enzyme increased by 1.3-fold, 1.2-fold, and 1.1-fold by the addition of Mg2+, Ca2+, and sorbitol, respectively. Similar results were observed at 55 and 60°C where sorbitol increased half-life by 1.2-fold. In the case of Mg2+, this metal increased the apparent half-life by 1.1-fold and 1.3 fold at 55 and 60°C, respectively. In general, all chemicals tested here produced a slight increase in the thermal stability of the enzyme.
Figure 4.
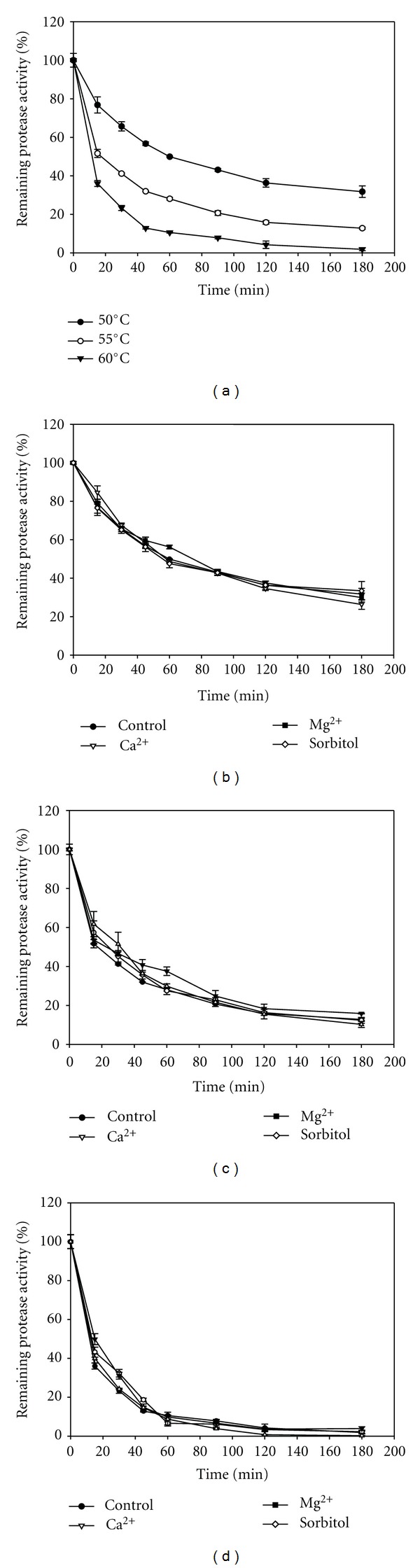
(a) Effect of temperature on protease stability (●) 50°C; (◯) 55°C; (▾) 60°C. (b) Effect of stabilizers on heat inactivation at 50°C. (c) Effect of stabilizers on heat inactivation at 55°C. (d) Effect of stabilizers on heat inactivation at 60°C. For (b), (c), and (d) the original activity before preincubation was taken as 100%. Values are means of three independent determinations.
It had been reported that the addition of Ca2+ or polyhydric alcohols, such as glycerol and polyethylene glycol caused an increase in thermal stability of alkaline proteases. The addition of sorbitol improved the thermal stability for an alkaline protease from B. cereus BG1, which increased its thermal stability by approximately 2-fold at 60°C [30]. Kelkar and Deshpande [31] studied the influence of various polyols on the thermostability of pullulan-hydrolysing activity from Sclerotium rolfsii. The half-life of the enzyme activity at 60°C was about 30 min, and in presence of xylitol and sorbitol (3 M) they reported a significant enhancement in the thermostability of the enzyme with retention of 100% activity after incubation for 7 h at 60°C. Ghorbel et al. [30] reported an alkaline protease that, in the presence of 10mM Ca2+, retained 100, 93, and 26% of its initial activity after heating for 15min at 55, 60, and 70°C, respectively. However, the enzyme was completely inactivated when incubated at 55°C for 15 min in the absence of calcium. On the contrary, the enzyme reported here in presence of 5 mM Ca2+ retained about 57 and 43% of its initial activity after heating 15 min at 55 and 60°C, respectively, but the enzyme in absence of calcium retained more than 25% of its initial activity after 60 min of incubation at 55°C.
Several reports showed that the addition of various additives such as polyols and PEG could enhance enzymes' thermal stability [2, 18]. The increase in the thermal stability by adding these such as additives was probably due to the reinforcement of the hydrophobic interactions among nonpolar amino acids inside the enzyme molecules and thus increased their resistance to inactivation, since it had been reported that polyols modify the structure of water and/or strengthen hydrophobic interactions among nonpolar amino acids inside the protein molecules [32].
The effect of Ca2+ on the improvement of thermal stability against heat inactivation may be explained by the strengthening of interactions inside proteases molecules and by binding of this metal to the autolysis site. The activity of B. mojavensis protease was enhanced not only by Ca2+ but by Fe2+ and Mn2+. It is believed that metal ions protect the enzyme against thermal denaturation and play a vital role in maintaining the active conformation of the enzyme at higher temperatures [33].
3.4. Effect of pH and Temperature on Enzyme Activity
The pH and temperature values used in Doehlert's design for determination of the effect of pH and temperature on enzyme activity are given in Table 3.
Table 3.
Actual values (experimental data) and enzyme activity (UC·mL−1) attained in Doehlert's design for the study of the effect of pH and Temperature on enzyme activity.
| pH | Temperature (°C) | UC·mL−1 | |
|---|---|---|---|
| Experimental data | |||
| A | 9 | 40 | 6.5 |
| A | 9 | 40 | 6.4 |
| A | 9 | 40 | 6.5 |
| B | 12 | 40 | 6.6 |
| C | 10.5 | 60 | 5 |
| D | 7.5 | 60 | 11.6 |
| E | 6 | 40 | 6 |
| F | 7.5 | 20 | 1.2 |
| G | 10.5 | 20 | 1.2 |
The following regression equation was obtained to calculate the enzyme activity (E.A):
| (3) |
where pH and T are given as codified data. The r 2 value was equal to 0.89, indicating that only 11% of the total variation was not explained by the model. The contour graph of the keratinolytic activity observed as a response to the interaction of pH versus temperature is shown in Figure 5. These results indicate an optimal pH and temperature of 6.0 and 60°C, respectively. Kotlova et al. [29] reported a thiol-dependent serine proteinase from P. lilacinus (Thom) Samson VKM F-3891 with an optimal pH into the alkaline range. Such pH dependence is also reported by Bonants et al. [34] for another proteinase isolated from a different strain of the fungus, P. lilacinus (Thom) Samson (CBS 143.75). These proteinases seem to belong to the subgroup of subtilisin-like enzymes with an optimum pH in the alkaline range (pH 10–12).
Figure 5.
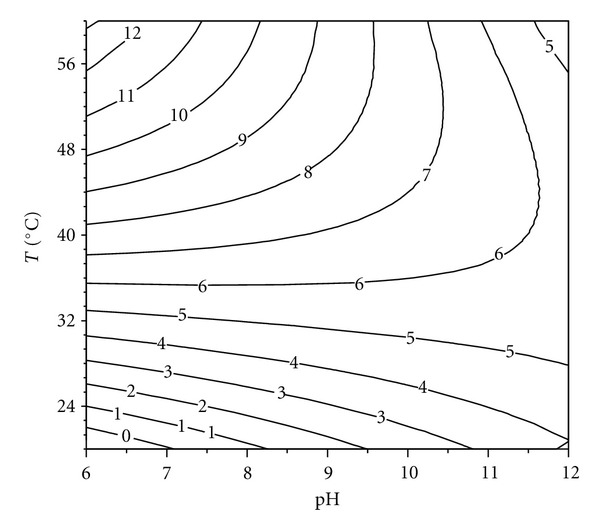
Response surface plot for the effect of pH and temperature on enzyme activity.
In our case, the protease present in the enzyme extract belongs to the group of enzymes, with an optimum pH in a neutral range (pH 6–8) at an assay temperature of 60°C. Although optimal temperature for keratin degradation is 60°C, the stability of the enzyme under this condition is not so high. Because of that, a temperature of 55°C, where the enzyme retains more than 90% of the optimal activity, is proposed for practical applications.
3.5. Effect of Protease Inhibitors, Metal Ions, and Organic Solvents on Enzyme Activity
The effect of various chemical reagents and metal ions on enzyme activity with azocasein as substrate is shown in Table 4. The enzyme activity was strongly inhibited by PMSF (93% of inhibition), a well-known inhibitor of serine proteinases, in particular subtilisins serine proteinases [35]. It was slightly inhibited by Pepstatin A but neither by iodoacetate, EDTA, nor by 1,10-Phenantroline. These facts suggest that in our case, the enzyme produced by P. lilacinus corresponds to a serine protease. Actually, most keratinases described until now are classified into this category [36]. The stability of the enzyme in presence of EDTA is advantageous for its use as a detergent additive. An enzyme, which is to be used as a detergent additive, should not have requirement for a metal cofactor. This is because detergent formulations contain high amounts of chelating agents, which specifically bind to and chelate metal ions making them unavailable in the detergent solution. The chelating agents remove the divalent cations responsible for water hardness and also assist in stain removal.
Table 4.
Effect of protease inhibitors, metal ions, detergents, and solvents on protease activity (data are given as Residual activity (%) ± SD).
| Chemical none | Concentration | Residual activity (%) 100 |
|---|---|---|
| Inhibitor | ||
| PMSF | 2 mM | 7.0 ± 0.0 |
| Iodoacetate | 10 mM | 95.1 ± 4.7 |
| EDTA | 5 mM | 99.6 ± 6.3 |
| 1,10-Phenantroline | 1 mM | 100 ± 0.2 |
| Pepstatin A | 100 μg/mL | 87.5 ± 5.5 |
| Metal ion | ||
| Mg2+ | 1 mM | 105.0 ± 1.2 |
| Zn2+ | 1 mM | 92.8 ± 1.4 |
| Ca2+ | 1 mM | 102.9 ± 0.5 |
| Hg2+ | 1 mM | 6.0 ± 0.6 |
| Detergents | ||
| Triton X-100 | 0.5% (v/v) | 97.7 ± 2.7 |
| Tween 20 | 0.5% (v/v) | 98.5 ± 2.0 |
| SDS | 0.5% (v/v) | 75.9 ± 3.4 |
| Bleaching agent | ||
| 1% (w/v) | 140 ± 2.3 | |
| H2O2 | 2% | 137 ± 0.3 |
| 3% | 122.7 ± 4.0 | |
| 0.2% (w/v) | 99.7 ± 2.4 | |
| Sodium perborate | 0.5% | 97.6 ± 0.9 |
| 1.0% | 90.8 ± 2.0 | |
| Solvent | ||
| DMSO | 1% (v/v) | 106.0 ± 1.6 |
| Ethanol | 1% (v/v) | 105.4 ± 4.0 |
| Methanol | 1% (v/v) | 117.0 ± 2.9 |
| Isopropanol | 1% (v/v) | 98.9 ± 1.7 |
Among the metal ions tested, Hg2+ strongly inhibited proteolytic activity (94% of inhibition), whereas Ca2+ and Mg2+ caused slight activation. Hg2+ is recognized as an oxidant agent of thiol-groups, and the enzyme inhibition by this ion suggests the presence of an important-SH group (such as free cysteine) at/or near the active site [37]. In addition, the strong inactivation by Hg2+ is typical for proteinases belonging to the thermitase and proteinase K subgroups [38]. This feature makes our P. lilacinus keratinase substantially different to the true bacillary subtilisins, as well as to the serine proteinase from P. lilacinus isolated by Bonants et al. [34], which is not inactivated by Hg2+. Based on the presence of functionally important sulfhydryl groups, our keratinase resembles proteinase K and bacillary thiol-dependent subtilisins much more than other fungal serine proteases.
Divalent metal ions such as Ca2+ and Mg2+ slightly activated the enzyme. This fact could be explained because of that they could act as salt or ions bridges stabilizing the enzyme under its active conformation, and thus they might protect the enzyme against thermal denaturation [39, 40].
Crude enzyme preparation showed to be highly stable in presence of different organic solvents such as DMSO, ethanol, methanol, and isopropanol (Table 4), a positive fact considering the potential practical application.
3.6. Effect of Surfactants and Bleaching Agents on Enzyme Stability
The above-mentioned characteristics of our P. lilacinus protease suggested its potential use in different applications like laundry detergent formulation. In order to be effective during washing, a good detergent protease must be compatible and stable with all commonly used detergent compounds such as surfactants, bleaching agents, and other additives, which might be present in detergent formulation [1]. In our case, a crude protease extract was incubated 60 min at room temperature in presence of several additives, and then the residual protease activity was assayed under standard conditions.
As can be seen in Table 4, crude protease was highly stable in presence of nonionic surfactants. It retained near 100% of its initial activity in presence of 0.5% Triton X-100 and 0.5% Tween 20. In presence of 0.5% of SDS, a strong anionic surfactant, it exhibited moderated stability (75%) after 1 h of incubation. SDS is known to be a strong denaturant of proteins including alkaline proteases. It could unfold most proteins through the interaction between the charged head group of SDS and the positively charged amino acid side chains of proteins and between the alkyl chain of SDS and the nonpolar parts on the surface as well as in the interior of proteins [41]. The retention of protease activity by our enzyme preparation in presence of SDS was higher than that of a protease from Aspergillus clavatus ES1, which retained only 33% of its activity under the same stability assay conditions [42].
On the other hand, our enzyme preparation showed excellent stability toward bleaching agents such as H2O2 and sodium perborate (Table 4). It showed an stability similar to proteases from B. licheniformis NH1, which retained 85% and 80% of its activity after incubation with 0.5% H2O2 [19] and resulted in being more stable than an alkaline protease from B. licheniformis RP1 [43] which is less stable against bleaching agents; it's just retained 68% and 48% of its activity after 1 h incubation at 40°C in presence of 2% H2O2 and 0.2% sodium perborate, respectively. Bleaching agents inactivate proteins oxidatively, being Met the primary site for oxidative inactivation. All subtilisins contain a Met residue next to the catalytic Ser residue, so that many of them tend to undergo oxidative inactivation in presence of a bleaching agent such as hydrogen peroxide. Thus, many of available alkaline proteases exhibited low activity and stability toward oxidants, which are common ingredients in modern bleach-based detergents. To overcome these shortcomings, several attempts have been made to enhance enzyme stability by protein engineering [44]. That is why it is important to obtain enzymes with high stability against surfactants and oxidants for practical applications. Detergent applications for keratinases have been also suggested [36]. These include removal of keratinous dirt that are often encountered in the laundry, such as collar of shirts and used as additives for cleaning up drains clogged with keratinous waste.
3.7. Detergent Compatibility
All the commercial detergents contain hydrolytic enzymes, and these enzymes-based detergents known as “green chemicals” find a wide range of applications in laundry, dishwashing, textile, and other related industries [45].
In order to check the compatibility with liquid and solid detergents, the crude enzyme was preincubated in presence of various commercial laundry detergents for 1 h at 30, 40, and 50°C. Solid detergents were diluted in tap water to a final concentration of 7 mg/mL, and the liquid ones were diluted 100-fold to simulate washing conditions. As can be seen in Figure 6(a) the crude enzyme was very stable towards all solid detergents tested, even at 50°C after 1 h of incubation, it retained more than 60% of its activity in presence of Ariel, Ace, and Skip. In presence of Drive, it retained about 57% of its activity being more stable than an alkaline protease from Vibrio fluvialis strain VM10 reported by Venugopal and Saramma [46], which retained just 42% of its activity in presence of Ariel as well as an alkaline serine protease from Bacillus sp. SSR1 reported by Singh et al. [47] which retain lees than 40% of its activity after 1 h of incubation in presence of Ariel at 40°C. Interestingly, it was more stable than the commercial protease named Maxacal, which retained less than 60% after 1 h of incubation in presence of Ariel at 40°C [47]. Similarly, proteases from B. mojavensis A21 [48] and from B. licheniformis RP1 [43] are shown to retain more than 40 and 80% of their activity in presence of Dixan after 1 h of incubation at 50°C, respectively.
Figure 6.
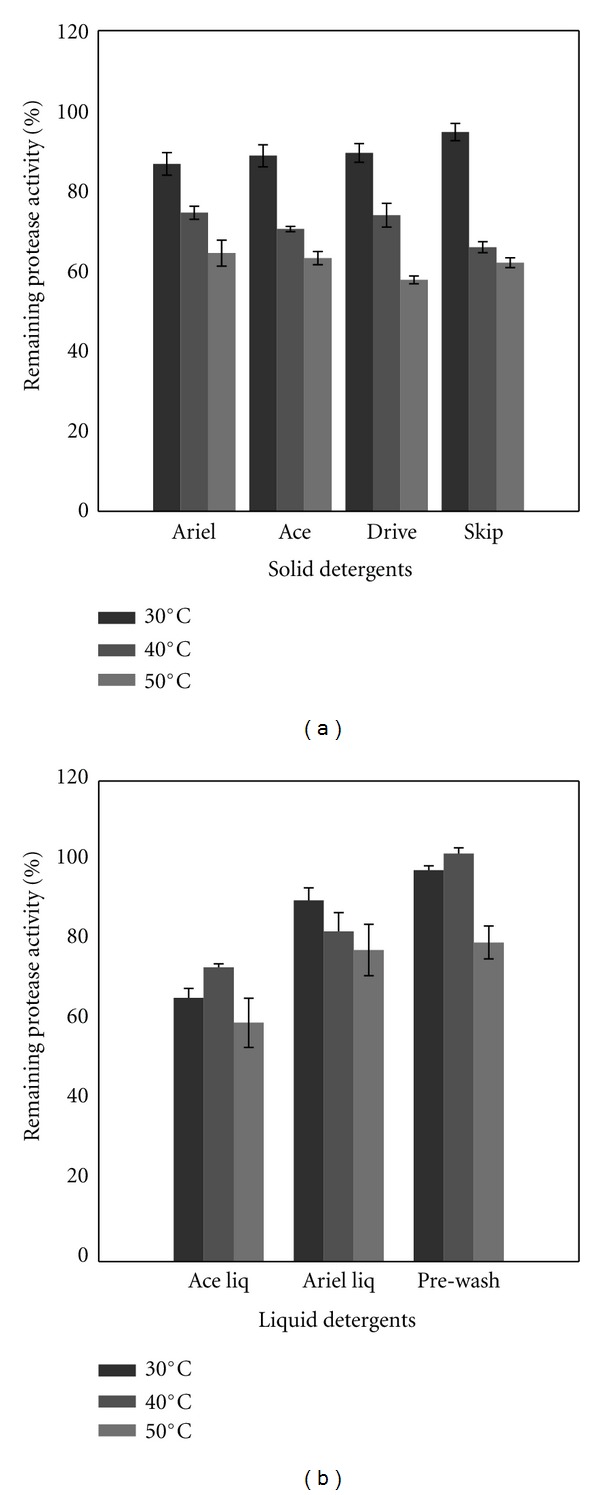
Stability of the crude enzyme in the presence of various commercial solid (a) and liquid detergents (b). CE was incubated in each detergent mentioned for 1 h at different temperatures (30–50°C), and the remaining activities were determined under standard conditions. The enzyme activity of a control, incubated under similar conditions without detergent, was taken as 100%.
In presence of liquid detergents, the crude enzyme retained more than 75% and 50% of its initial activity in presence of Ariel and Ace, respectively, after 1 h of incubation at 50°C (Figure 6(b)).
From the results presented here about the compatibility and stability whit commercial detergents at different temperatures, it can be concluded that our protease will be more effective at temperature from 30 to 40°C for long washing cycles (60 min) and at 50°C for short washing cycles (10–30 min). But with Ariel liq. long washing cycles could be done at 55°C too, because it retained about 78% of its original stability.
3.8. Wash Performance Analysis
The wash performances of the protease present in the crude extract were assessed by its ability to remove blood stain from white cotton cloth (Figure 7). Enzyme in combination of the commercial detergent Skip was tried. The visual comparison of the washed cloth revealed that washing with distilled water at temperatures of 30 and 40°C removed some amount of blood stain from the cotton cloth (Figures 7(a), 7(d), 7(g), and 7(j)). As can be seen, the replacement of the enzymes present in the commercial detergent by the crude enzyme gave a complete blood stain elimination at both temperatures and times, such as the endogenous enzymes from the commercial detergent has done (Figures 7(c), 7(f), 7(i), 7(l)). These results show the efficiency of P. lilacinus protease in proteinaceous stain removal efficiency.
Figure 7.
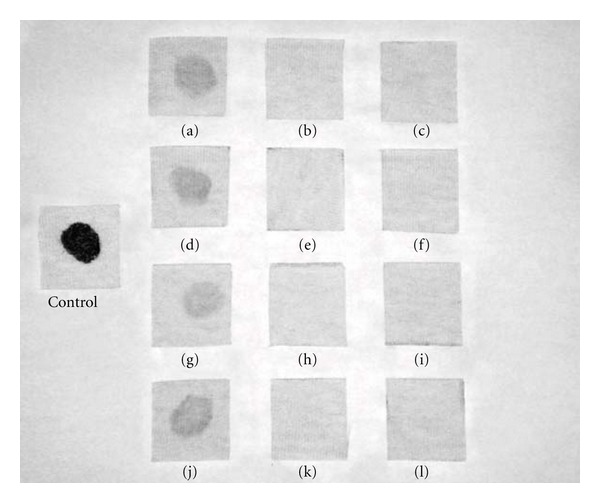
Washing performance analysis of the P. lilacinus enzyme preparation in the presence of the commercial detergent Skip. Analysis were done at 30°C ((a)–(f)) and 40°C ((g)–(l)), for 30 m ((a)–(c) and (g)–(i)) and 60 m ((d)–(f) and (j)–(l)). Cloth washed with tap water ((a), (d), (g), and (j)); ((b), (e), (h), and (k)) cloth washed with Skip; ((c), (f), (i), and (l)) cloth washed with Skip added with crude enzyme of the P. lilacinus protease.
Abidi et al. [49] showed also the significant improvement of the supplementation of proteolytic preparation of Botrytis cinerea, in a laundry detergent (Henkel-Alki), in the elimination of blood, egg yolk, and chocolate stains on fabric. Jellouli et al. [50] and Savitha et al. [51] reported similar results in their wash performance tests. Therefore, P. lilacinus crude extracts containing protease activity could be considered as a potential candidate for use as cleaning additive in detergents to facilitate the release of proteinaceous stains.
4. Conclusions
A locally isolated P. lilacinus strain produces an extracellular protease with keratinase activity when grown on hair waste, the main solid-wastes produced in tanneries, as substrate in submerged cultures.
The protease was characterized, and it exhibited remarkable stability toward surfactants, bleaching agents, and detergent additives like EDTA and sodium perborate. This property of the enzyme is very essential for its application as detergent additive. Our study shows that the extracellular proteolytic enzyme produced by this strain could have an industrial application in detergent industries. Moreover, the enzyme was compatible with most of the laundry detergents tested and showed a good washing performance.
Acknowledgment
This research work was supported by CONICET (Argentina National Council of Scientific and Technical Research).
References
- 1.Gupta R, Beg Q, Lorenz P. Bacterial alkaline proteases: molecular approaches and industrial applications. Applied Microbiology and Biotechnology. 2002;59(1):15–32. doi: 10.1007/s00253-002-0975-y. [DOI] [PubMed] [Google Scholar]
- 2.Rao MB, Tanksale AM, Ghatge MS, Deshpande VV. Molecular and biotechnological aspects of microbial proteases. Microbiology and Molecular Biology Reviews. 1998;62(3):597–635. doi: 10.1128/mmbr.62.3.597-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuchiya K, Nakamura Y, Sakashita H, Kimura T. Purification and characterization of a thermostable alkaline protease from alkalophilic Thermoactinomyces sp. HS 682. Bioscience, Biotechnology, and Biochemistry. 1992;56(2):246–250. doi: 10.1271/bbb.56.246. [DOI] [PubMed] [Google Scholar]
- 4.Wolff AM, Showell MS, Venegas MG, et al. Laundry performance of subtilisin proteases. In: Bott R, Betzel C, editors. Subtilisin Enzymes: Practical Protein Engineering. New York, NY, USA: Springer; 1996. pp. 113–120. [DOI] [PubMed] [Google Scholar]
- 5.Parry DAD, North ACT. Hard α-keratin intermediate filament chains: substructure of the N-and C-terminal domains and the predicted structure and function of the C-terminal domains of type I and type II chains. Journal of Structural Biology. 1998;122(1-2):67–75. doi: 10.1006/jsbi.1998.3967. [DOI] [PubMed] [Google Scholar]
- 6.Galarza BC, Cavello I, Greco CA, Hours R, Schuldt MM, Cantera CS. Alternative technologies for adding value to bovine hair waste. Journal of the Society of Leather Technologies and Chemists. 2010;94(1):26–32. [Google Scholar]
- 7.Cavello I, Cavalitto S, Hours R. Biodegradation of a keratin waste and the concomitant production of detergent stable serine proteases from Paecilomyces lilacinus . Applied Biochemistry and Biotechnology. 2012;167(5):945–958. doi: 10.1007/s12010-012-9650-7. [DOI] [PubMed] [Google Scholar]
- 8.Sangali S, Brandelli A. Isolation and characterization of a novel feather-degrading bacterial strain. Applied Biochemistry and Biotechnology A. 2000;87(1):17–24. doi: 10.1385/abab:87:1:17. [DOI] [PubMed] [Google Scholar]
- 9.Galarza BC, Goya LM, Cantera CS, Garro ML, Reinos HE, López LMI. Fungal biotransformation of bovine hair part 1: isolation of fungus with keratinolytic activity. Journal of the Society of Leather Technologies and Chemists. 2004;88(3):93–98. [Google Scholar]
- 10.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 11.Liggieri C, Arribére MC, Trejo SA, Canals F, Avilés FX, Priolo NS. Purification and biochemical characterization of asclepain c I from the latex of Asclepias curassavica L. Protein Journal. 2004;23(6):403–411. doi: 10.1023/b:jopc.0000039554.18157.69. [DOI] [PubMed] [Google Scholar]
- 12.Riffel A, Lucas F, Heeb P, Brandelli A. Characterization of a new keratinolytic bacterium that completely degrades native feather keratin. Archives of Microbiology. 2003;179(4):258–265. doi: 10.1007/s00203-003-0525-8. [DOI] [PubMed] [Google Scholar]
- 13.Joshi SG, Tejashwini MM, Revati N, et al. Isolation, identification and characterization of a feather degrading bacterium. International Journal of Poultry Science. 2007;6(9):689–693. [Google Scholar]
- 14.Gradišar H, Friedrich J, Krizaj I, et al. Similarities and specificities of fungal keratinolytic proteases: comparison of keratinases of paecilomyces marquandii and doratomyces microsporus to some known proteases. Applied and Environmental Microbiology. 2005;71(7):3420–3426. doi: 10.1128/AEM.71.7.3420-3426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangali S, Brandelli A. Feather keratin hydrolysis by a Vibrio sp. strain kr2. Journal of Applied Microbiology. 2000;89(5):735–743. doi: 10.1046/j.1365-2672.2000.01173.x. [DOI] [PubMed] [Google Scholar]
- 16.Corrêa APF, Daroit DJ, Brandelli A. Characterization of a keratinase produced by Bacillus sp. P7 isolated from an Amazonian environment. International Biodeterioration and Biodegradation. 2010;64(1):1–6. [Google Scholar]
- 17.Cavalitto SF, Mignone CF. Application of factorial and Doehlert designs for optimization of protopectinase production by a Geotrichum klebahnii strain. Process Biochemistry. 2007;42(2):175–179. [Google Scholar]
- 18.Haddar A, Sellami-Kamoun A, Fakhfakh-Zouari N, Hmidet N, Nasri M. Characterization of detergent stable and feather degrading serine proteases from Bacillus mojavensis A21. Biochemical Engineering Journal. 2010;51(1-2):53–63. [Google Scholar]
- 19.Hmidet N, El-Hadj Ali N, Haddar A, Kanoun S, Alya SK, Nasri M. Alkaline proteases and thermostable α-amylase co-produced by Bacillus licheniformis NH1: characterization and potential application as detergent additive. Biochemical Engineering Journal. 2009;47(1–3):71–79. [Google Scholar]
- 20.Wawrzkiewicz K, Wolski T, Łobarzewski J. Screening the keratinolytic activity of dermatophytes in vitro. Mycopathologia. 1991;114(1):1–8. doi: 10.1007/BF00436684. [DOI] [PubMed] [Google Scholar]
- 21.Riffel A, Brandelli A. Keratinolytic bacteria isolated from feather waste. Brazilian Journal of Microbiology. 2006;37(3):395–399. [Google Scholar]
- 22.Korniłłowicz-Kowalska T, Bohacz J. Biodegradation of keratin waste: theory and practical aspects. Waste Management. 2011;31(8):1689–1701. doi: 10.1016/j.wasman.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Sharma R, Gupta R. Thermostable, thiol activated keratinases from Pseudomonas aeruginosa KS-1 for prospective application in prion decontamination. Research Journal of Microbiology. 2010;5(10):954–965. [Google Scholar]
- 24.Yamamura S, Morita Y, Hasan Q, Yokoyama K, Tamiya E. Keratin degradation: a cooperative action of two enzymes from Stenotrophomonas sp. Biochemical and Biophysical Research Communications. 2002;294(5):1138–1143. doi: 10.1016/S0006-291X(02)00580-6. [DOI] [PubMed] [Google Scholar]
- 25.Cheng SW, Hu HM, Shen SW, Takagi H, Asano M, Tsai YC. Production and characterization of keratinase of a feather-degrading Bacillus licheniformis PWD-1. Bioscience, Biotechnology, and Biochemistry. 1995;59(12):2239–2243. doi: 10.1271/bbb.59.2239. [DOI] [PubMed] [Google Scholar]
- 26.Takami H, Akiba T, Horikoshi K. Production of extremely thermostable alkaline protease from Bacillus sp. no. AH-101. Applied Microbiology and Biotechnology. 1989;30(2):120–124. [Google Scholar]
- 27.Bhosale SH, Rao MB, Deshpande VV, Srinivasan MC. Thermostability of high-activity alkaline protease from Conidiobolus coronatus (NCL 86.8.20) Enzyme and Microbial Technology. 1995;17(2):136–139. [Google Scholar]
- 28.Banerjee UC, Sani RK, Azmi W, Soni R. Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochemistry. 1999;35(1-2):213–219. [Google Scholar]
- 29.Kotlova EK, Ivanova NM, Yusupova MP, Voyushina TL, Ivanushkina NE, Chestukhina GG. Thiol-dependent serine proteinase from Paecilomyces lilacinus: purification and catalytic properties. Biochemistry. 2007;72(1):117–123. doi: 10.1134/s0006297907010142. [DOI] [PubMed] [Google Scholar]
- 30.Ghorbel B, Sellami-Kamoun A, Nasri M. Stability studies of protease from Bacillus cereus BG1. Enzyme and Microbial Technology. 2003;32(5):513–518. [Google Scholar]
- 31.Kelkar HS, Deshpande MV. Effect of polyols on the thermostability of pullulan-hydrolysing activity from Sclerotium rolfsii . Biotechnology Letters. 1991;13(12):901–906. [Google Scholar]
- 32.Back JF, Oakenfull D, Smith MB. Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry. 1979;18(23):5191–5196. doi: 10.1021/bi00590a025. [DOI] [PubMed] [Google Scholar]
- 33.Durham DR, Stewart DB, Stellwag EJ. Novel alkaline- and heat-stable serine proteases from alkalophilic Bacillus sp. strain GX6638. Journal of Bacteriology. 1987;169(6):2762–2768. doi: 10.1128/jb.169.6.2762-2768.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonants PJM, Fitters PFL, Thijs H, den Belder E, Waalwijk C, Henfling JWDM. A basic serine protease from Paecilomyces lilacinus with biological activity against Meloidogyne hapla eggs. Microbiology. 1995;141(4):775–784. doi: 10.1099/13500872-141-4-775. [DOI] [PubMed] [Google Scholar]
- 35.Siezen RJ, Leunissen JAM. Subtilases: the superfamily of subtilisin-like serine proteases. Protein Science. 1997;6(3):501–523. doi: 10.1002/pro.5560060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta R, Ramnani P. Microbial keratinases and their prospective applications: an overview. Applied Microbiology and Biotechnology. 2006;70(1):21–33. doi: 10.1007/s00253-005-0239-8. [DOI] [PubMed] [Google Scholar]
- 37.Daroit DJ, Simonetti A, Hertz PF, Brandelli A. Purification and characterization of an extracellular β-glucosidase from Monascus purpureus . Journal of Microbiology and Biotechnology. 2008;18(5):933–941. [PubMed] [Google Scholar]
- 38.Ballinger M, Wells JA. Subtilisin. In: Barrett A, Rawlings ND, Woessner JF, editors. Handbook of Proteases. 1998. pp. 289–294. [Google Scholar]
- 39.Balaji S, Kumar MS, Karthikeyan R, et al. Purification and characterization of an extracellular keratinase from a hornmeal-degrading Bacillus subtilis MTCC (9102) World Journal of Microbiology and Biotechnology. 2008;24(11):2741–2745. [Google Scholar]
- 40.Sareen R, Mishra P. Purification and characterization of organic solvent stable protease from Bacillus licheniformis RSP-09-37. Applied Microbiology and Biotechnology. 2008;79(3):399–405. doi: 10.1007/s00253-008-1429-y. [DOI] [PubMed] [Google Scholar]
- 41.Otzen DE. Protein unfolding in detergents: effect of micelle structure, ionic strength, pH, and temperature. Biophysical Journal. 2002;83(4):2219–2230. doi: 10.1016/S0006-3495(02)73982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajji M, Kanoun S, Nasri M, Gharsallah N. Purification and characterization of an alkaline serine-protease produced by a new isolated Aspergillus clavatus ES1. Process Biochemistry. 2007;42(5):791–797. [Google Scholar]
- 43.Sellami-Kamoun A, Haddar A, Ali NEH, Ghorbel-Frikha B, Kanoun S, Nasri M. Stability of thermostable alkaline protease from Bacillus licheniformis RP1 in commercial solid laundry detergent formulations. Microbiological Research. 2008;163(3):299–306. doi: 10.1016/j.micres.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Estell DA, Graycar TP, Wells JA. Engineering an enzyme by site-directed mutagenesis to be resistant to chemical oxidation. The Journal of Biological Chemistry. 1985;260(11):6518–6521. [PubMed] [Google Scholar]
- 45.Mukherjee AK, Borah M, Rai SK. To study the influence of different components of fermentable substrates on induction of extracellular α-amylase synthesis by Bacillus subtilis DM-03 in solid-state fermentation and exploration of feasibility for inclusion of α-amylase in laundry detergent formulations. Biochemical Engineering Journal. 2009;43(2):149–156. [Google Scholar]
- 46.Venugopal M, Saramma AV. Characterization of alkaline protease from Vibrio fluvialis strain VM10 isolated from a mangrove sediment sample and its application as a laundry detergent additive. Process Biochemistry. 2006;41(6):1239–1243. [Google Scholar]
- 47.Singh J, Batra N, Sobti RC. Serine alkaline protease from a newly isolated Bacillus sp. SSR1. Process Biochemistry. 2001;36(8-9):781–785. [Google Scholar]
- 48.Fakhfakh-Zouari N, Haddar A, Hmidet N, Frikha F, Nasri M. Application of statistical experimental design for optimization of keratinases production by Bacillus pumilus A1 grown on chicken feather and some biochemical properties. Process Biochemistry. 2010;45(5):617–626. [Google Scholar]
- 49.Abidi F, Limam F, Nejib MM. Production of alkaline proteases by Botrytis cinerea using economic raw materials: assay as biodetergent. Process Biochemistry. 2008;43(11):1202–1208. [Google Scholar]
- 50.Jellouli K, Ghorbel-Bellaaj O, Ayed HB, Manni L, Agrebi R, Nasri M. Alkaline-protease from Bacillus licheniformis MP1: purification, characterization and potential application as a detergent additive and for shrimp waste deproteinization. Process Biochemistry. 2011;46(6):1248–1256. [Google Scholar]
- 51.Savitha S, Sadhasivam S, Swaminathan K, Lin FH. Fungal protease: production, purification and compatibility with laundry detergents and their wash performance. Journal of the Taiwan Institute of Chemical Engineers. 2011;42(2):298–304. [Google Scholar]


